Translate this page into:
Utilizing photothermally induced oscillation damping parameters for the determination of bacterial load suspended in microfluidic resonators
⁎Corresponding author at: Department of Physics and Astronomy, College of Science, King Saud University, Riyadh 11451, Saudi Arabia. aalodhayb@ksu.edu.sa (Abdullah Alodhayb)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Microchannel resonators containing a miniaturized volume of a solution can have various applications in different fields. In this study, a microchannel cantilever was loaded with a solution containing a very small number of Pseudomonas fluorescens bacteria suspended in M9 growth medium. The liquid-filled microchannel cantilever was irradiated with a 532-nm laser. The shift in the frequency of the cantilever due to varying bacterial loads is less reliable; therefore, it could not be used for monitoring the bacterial concentration. The energy loss of the cantilever extracted from the quality factor exhibited reliable results and a very strong correlation with the bacterial concentration. The results showed a linear relation between the damping factor of the cantilever and the bacterial concentration. Accordingly, these findings were expected because the bacteria inside the solution can be considered as particles acting against the cantilever motion due to the solution’s viscosity. Thus, more bacteria caused more damping, in agreement with the experimental observations. A semiquantitative experiment was conducted with a heat source (i.e., laser beam) that focused at the cantilever tip to demonstrate the redistribution of the bacterial load within the solution due to the thermal gradient.
Keywords
Microfluidic resonators
Oscillation
Damping parameters
Bacteria
Thermal gradient
1 Introduction
Temperature can influence cell morphology, metabolism, and death of living organisms. At a molecular level, temperature can be manifested through changes in the protein structure and DNA/RNA functions. Recently, the thermal activity of biological cells has attracted increasing interest and huge efforts have been devoted for developing simple and low-cost thermal sensors to monitor it (Raiteri et al., 2001, Lee et al., 2009, Chen and Chatterjee 2013, Lee et al., 2014, Koren and Kühl 2015, Ricciardi et al., 2015, Lee et al., 2016, Maduraiveeran et al., 2018, Guo et al., 2019, Materón et al., 2019). It is possible to determine the working of cells by measuring their energy consumption. The average readout signal of measurements performed on a large number of the same cells are frequently employed to represent the thermal activity of a single cell through bulk measurements (Nedergaard et al., 1977, Clark et al., 1986). Thus, the caloric production of individual cells is inaccurately measured due to conflicting factors from surrounding cells (Inomata et al., 2016). However, by detecting metabolic release within single cells, researchers can significantly reduce the research cost, time, and resulting waste. Therefore, it is important to monitor the thermal behavior of single cells in laboratories in the fields of medicine and biotechnology (Kucsko et al., 2013, Park et al., 2013).
Various components and nanomaterials have been used to measure the heat produced by individual cells, such as chemical sensors, fluorescent nanogels (Gota et al., 2009), thermocouples, quantum dots (Yang et al., 2011), multiwalled carbon nanotubes (Vyalikh et al., 2008), thermosensitive dyes (Zohar et al., 1998), and green fluorescent proteins (Verhaegen et al., 2000). These components are extremely sensitive (Kucsko et al., 2013); however, they require the insertion of a thermometer into the cell. Researchers have used microfabricated thermal nanocalorimeters for measuring the heat produced by individual cells (Verhaegen et al., 2000, Johannessen et al., 2002). Such devices involve the immersion of cells in water, which reduces the sensitivity of the devices due to liquid damping. Microfluidic techniques, particularly droplet-configuration microfluidic techniques, have been used to overcome the limitations associated with the immersion of cells in water (Yeo et al., 2011, Inomata et al., 2012, Inomata et al., 2016). These techniques have attracted increasing attention owing to their extensive applicability in DNA amplification, protein analysis, single-cell assays, and chemical synthesis (Yeo et al., 2011). In principle, the microfluidic calorimeters measure the frequency of the resonator to determine the amount of heat generated by the attached cells. Additionally, experiments are usually performed in vacuum, resulting in minimal heat loss and damping. Meanwhile, microcantilever calorimeters are appealing owing to their compactness and high sensitivity (Barnes et al., 1994, Toda et al., 2010). In particular, metal–dielectric cantilevers are preferred, where the metal layer reflects the incident light and the dielectric layer absorbs it. Silicon nitride is commonly used as the absorbing dielectric layer owing to its high absorption across the associated infrared (IR) spectrum, whereas aluminum is commonly used as the metallic layer owing to its high thermal expansion coefficient. As a result, silicon nitride–aluminum is the most desirable material for microcantilever calorimeters. Due to the different thermal expansion coefficients of the component layers, minor temperature variations cause a bimaterial microcantilever (BMC) to suffer from mechanical deflection (Miriyala et al., 2016). Nevertheless, owing to the ultrasensitivity of the BMC, it is used in various applications as sensing platforms (Singamaneni et al., 2008, Canetta and Narayanaswamy 2013). Toda et al. developed a temperature-sensing BMC and were able to measure the local heat produced by a single mammalian cell in situ (Toda et al., 2017). Their technique promoted the lab-on-a-chip technology; thus, the combination of optics and microfluidics has recently attracted considerable attention. Nanomechanical IR spectroscopy has been used in several sensing applications (Rahimi et al., 2015, Etayash et al., 2016). In this technique, a BMC undergoes mechanical deflection in response to the heat produced by IR radiation. To successfully measure the heat capacities of five volatile organic compounds at varying temperatures, Khan et al. demonstrated the use of BMCs as optically heated closed-chamber calorimeters (Khan et al., 2016). Online measurement of the thermal properties of a small amount of liquid at quite a reasonable level of precision (23 mj/gK) is obtained using the reported device. This system combines the high thermal sensitivity of a BMC and optical heating via infrared radiation to overcome the limitations of resistive heating while showing high application potential for performing thermal measurements. BMCs with a temperature resolution of 10−5 K and a power of 40 pW are renowned for their high thermal sensitivity (Varesi et al., 1997). Alodhayb used COMSOL Multiphysics to develop a heat transfer model for calculating the heat capacity of a biological cell resting on a microcantilever surface by calculating the thermal time constant from the BMC deflection curve (Alodhayb 2020).
In this study, bacterial cells were placed in a microcantilever channel. Subsequently, typical changes in the cantilever dynamic parameters were identified and correlated with the bacterial load concentration in the microcantilever. We validated the conclusions by performing a semiquantitative assessment of bacterial diffusion in a liquid by measuring the thermal gradient in the liquid inside the channel created through the precise application of a laser to the tip of the cantilever. The results were compared with those having no laser shone on them.
2 Experimental setup
Experiments were conducted using a U-shaped microchannel fabricated on the top of a 500-µm microfluidic cantilever (MC 516, Fourien Inc. Edmonton Canada). The U-shaped channel was 16-µm wide and 3-µm high. Fig. 1(a) shows a top view of the cantilever. The dashed lines represent fluidic ports (500 µm × 500 µm) at the microchip’s bottom side (5 mm × 5 mm). Fig. 1(b) shows an angled view of the silicon microchip. The silicon nitride cantilever is present on the top side of the microchip, whereas the fluidic ports are present on the bottom, allowing for leak-free solution loading into the microchannel.The measurements were performed using an analytical instrument (Picomeasure, Fourien Inc.). The instrument comprised a microfluidic flow cell, a vacuum chamber, optical components, and an integrated spectrum analyzer. Detailed discussion about the Picomeasure system can be found in Alzahrani et al. (2021).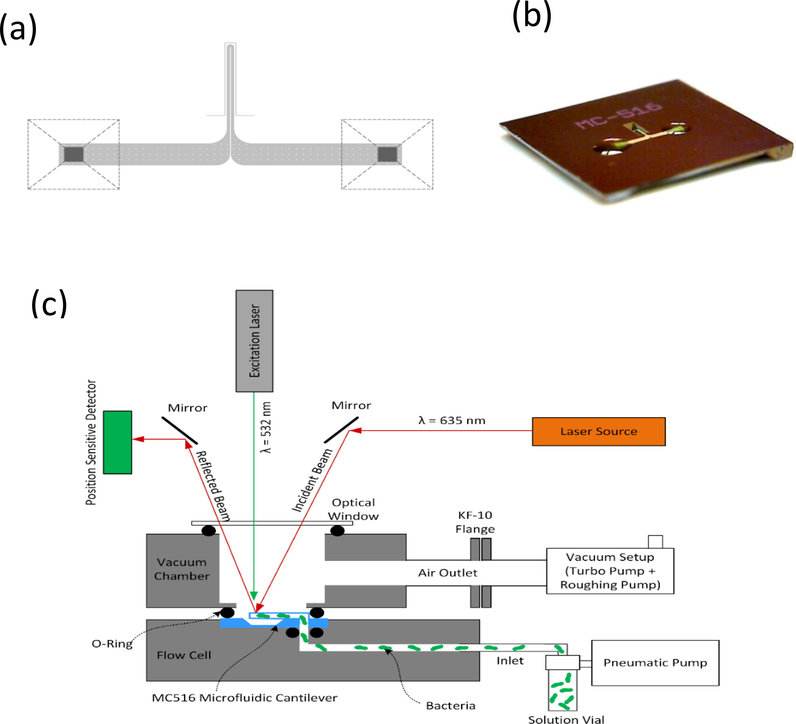
(a) Top-view schematic of the microfluidic cantilever. (b) Angled view of the silicon microchip hosting a microfluidic cantilever. (c) Schematic of the Picomeasure instrument showing the placement of the optics, vacuum chamber, and flow cell for loading the bacterial solution into the microfluidic cantilever MC516.
In this study, we used Pseudomonas fluorescens as the bacterial model. The measurements were performed using an empty microchannel and a microchannel loaded with M9 (minimal—microbial-growth medium) (control sample). A bacteria-containing M9 solution was injected into the microchannel using a pneumatic microfluidic pump. A polytetrafluoroethylene tube was used to connect the pump to the flow cell. Because the flow cell was made of polyetheretherketone (PEEK), it was resistant to solvents and acids, making it suitable for sterilization. The microchip was placed inside the flow cell using micro o-rings, allowing the loading of the dense bacterial solution into the microchannel under high pressure.
During the measurements, the excitation laser (wavelength = 532 nm) was turned ON and OFF at a specific interval to heat and cool the contents inside the microchannel. To reduce convective heat loss and vibration damping, the experiments were conducted in vacuum at a pressure of 4.5 × 10−5 mbar, which was achieved using a combination of turbo and roughing pumps. The operation of the excitation laser was controlled using the software of the Picomeasure instrument.
3 Results and discussion
Fig. 2 presents the changes in the resonance frequency of the microchannel cantilever as a function of the bacterial concentration in the M9 solution, when the laser is OFF. There is no clear correlation between the microchannel cantilever’s frequency and the bacterial concentration.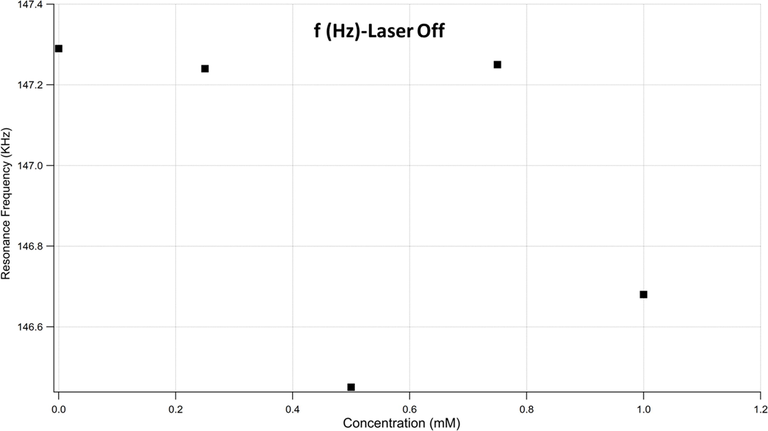
Microchannel cantilever’s frequency as a function of the bacterial concentration.
This finding was expected because the bacterial load is suspended within the M9 solution, the effect on cantilever’s stiffness and the change in mass would be minimal. Thus, searching for a parameter that can make a significant contribution to a measurable physical quantity is of interest. Therefore, the quality factor (Q-factor) has been extracted from the data to explore how the bacterial load alters the cantilever’s mechanical response.
Fig. 3 shows the effect of the bacterial load within the solution on the Q-factor. Interestingly, the Q-factor decreases with increasing bacterial concentration, indicating increasing energy loss due to increasing bacterial load. This, in turn, prompts a reexamination of the effect of increased bacterial density on the energy loss when the damping force is considered. The effect of the coexistence of semisolid microparticles (bacteria) within an M9 solution is observed when viscosity is considered.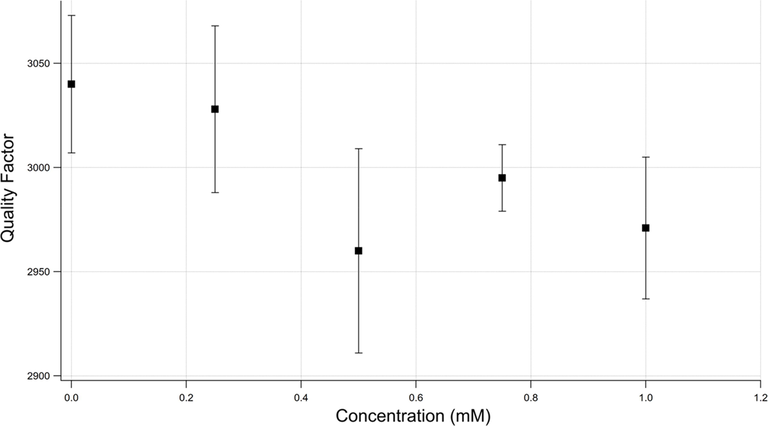
Quality factor as a function of the bacterial concentration.
In vibrational systems, the Q-factor depends on the variable viscosity-damping parameter, as indicated in Eq. (1):
Fig. 4 shows the relationship between
and the bacterial concentration. Under the assumption of negligible mass change, it is expected that c is proportional to
.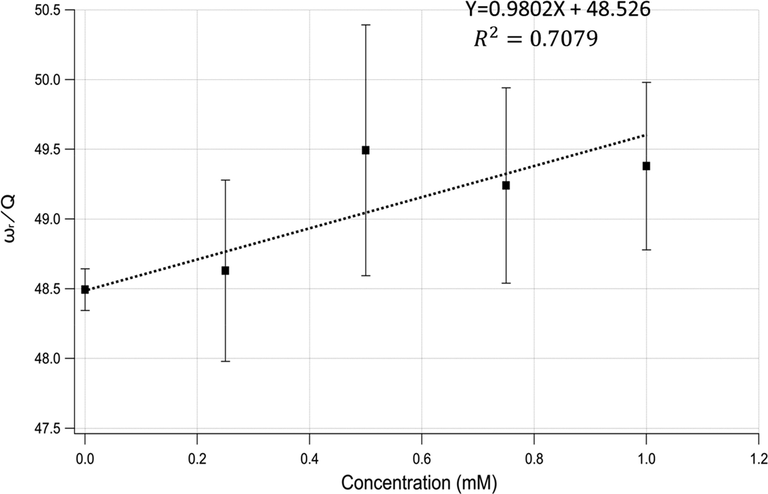
ωr/Q vs. bacterial concentration, showing an increasing damping force with increasing bacterial concentration.
Fig. 4 shows the practical linear relationship between c and the bacterial concentration. The greater the bacterial concentration in the solution is, the greater is the damping force acting against the cantilever oscillation. The damping is inevitable because the semisolid bacteria will be out of phase with the solution as the cantilever oscillates. Explicitly, increasing the bacterial load increases the surface area, causing the damping force to become strong; it is expected to behave linearly, as shown in the Fig. 4.
To verify the previous finding, the tip of the cantilever was irradiated with a laser beam to investigate the thermal effect. In principle, the heat created by the laser beam should affect the stiffness of the oscillator, changing its resonance frequency. However, this may cause some reorganization of the bacterial distribution along the microchannel. Due to the thermodynamic equilibrium that maintains a balance between the thermal and concentration distributions, the hot tip of the cantilever is expected to have less bacterial concentration, whereas the cold side is expected to have more. Thus, there will be a shift in mass uniformity as in this case, the most sensitive part of the cantilever will be lighter than that at equilibrium, causing the resonance frequency to increase.
Fig. 5 shows the resonance frequency shifts at each concentration and for the empty microchannel when the laser beam is ON. As can be seen from the figure, the resonance frequency increases in all the cases, in agreement with the conclusion from Fig. 4. Indeed, the thermal effect of laser-induced heating on the empty cell and the one with zero bacterial concentration leads to frequency shifts; this is due to the thermal effect of the laser-induced heating on the cantilever itself and the liquid inside the channel. Because the solutions with bacterial loads exhibit larger frequency shifts, it can be inferred that the bacterial rearrangement is also one of the main parameters affecting the cantilever dynamic properties.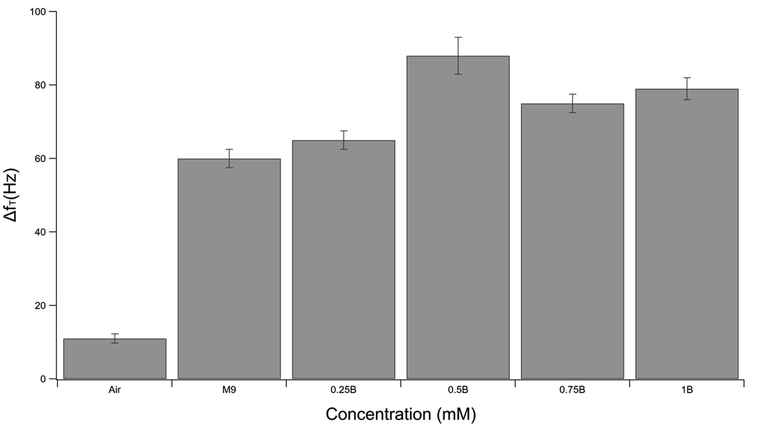
Frequency shifts due to the laser beam heating of the free tip of the cantilever in the empty microchannel and microchannel filled with different bacterial concentrations.
4 Conclusion
In this study, microchannel cantilevers were used to detect the bacterial concentrations. The measurements showed a strong correlation between the cantilever’s c and the bacterial concentrations. This can be explained by considering the inertia of the bacteria acting against the cantilever motion. The conclusion was further verified through gradient heating induced via laser irradiation. Hence, the damping parameter can be used as one of the sensing means for microchannel sensors. The results reported here can be potentially used for industrial, environmental, and medical applications to minimize the cost and time involved in using conventional techniques.
Acknowledgement
The authors are grateful to the Deanship of Scientific Research, King Saud University, for funding through the Vice Deanship of Scientific Research Chairs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Modeling of an optically heated MEMS-based micromechanical bimaterial sensor for heat capacitance measurements of single biological cells. Sensors. 2020;20(1):215.
- [Google Scholar]
- Nanomechanical detection of bacteria-bacteriophage interactions using microchannel microcantilevers. J. Electrochem. Soc.. 2021;168(8):087509
- [CrossRef] [Google Scholar]
- A femtojoule calorimeter using micromechanical sensors. Rev. Sci. Instrum.. 1994;65(12):3793-3798.
- [Google Scholar]
- Sub-picowatt resolution calorimetry with a bi-material microcantilever sensor. Appl. Phys. Lett.. 2013;102(10):103112
- [CrossRef] [Google Scholar]
- Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev.. 2013;42(12):5425-5438.
- [CrossRef] [Google Scholar]
- Microcalorimetric measurements of heat production in brown adipocytes from control and cafeteria-fed rats. Biochem. J.. 1986;235(2):337-342.
- [CrossRef] [Google Scholar]
- Microfluidic cantilever detects bacteria and measures their susceptibility to antibiotics in small confined volumes. Nat. Commun.. 2016;7(1):12947.
- [CrossRef] [Google Scholar]
- Hydrophilic fluorescent nanogel thermometer for intracellular thermometry. J. Am. Chem. Soc.. 2009;131(8):2766-2767.
- [Google Scholar]
- A highly selective and sensitive dual-mode sensor for colorimetric and turn-on fluorescent detection of cyanide in water, agro-products and living cells. Anal. Chim. Acta. 2019;1065:113-123.
- [Google Scholar]
- Highly sensitive thermometer using a vacuum-packed Si resonator in a microfluidic chip for the thermal measurement of single cells. Lab Chip. 2016;16(18):3597-3603.
- [CrossRef] [Google Scholar]
- Pico calorimeter for detection of heat produced in an individual brown fat cell. Appl. Phys. Lett.. 2012;100(15) 154104
- [CrossRef] [Google Scholar]
- Heat conduction nanocalorimeter for pl-scale single cell measurements. Appl. Phys. Lett.. 2002;80(11):2029-2031.
- [Google Scholar]
- Heat capacity measurements of sub-nanoliter volumes of liquids using bimaterial microchannel cantilevers. Appl. Phys. Lett.. 2016;108(21) 211906
- [CrossRef] [Google Scholar]
- A simple laminated paper-based sensor for temperature sensing and imaging. Sens. Actuators, B. 2015;210:124-128.
- [CrossRef] [Google Scholar]
- Ion-sensitive field-effect transistor for biological sensing. Sensors. 2009;9(9):7111-7131.
- [Google Scholar]
- Various on-chip sensors with microfluidics for biological applications. Sensors. 2014;14(9):17008-17036.
- [Google Scholar]
- Biosensors based on graphene oxide and its biomedical application. Adv. Drug Deliv. Rev.. 2016;105:275-287.
- [CrossRef] [Google Scholar]
- Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron.. 2018;103:113-129.
- [CrossRef] [Google Scholar]
- Chapter 13 - Graphene-containing microfluidic and chip-based sensor devices for biomolecules. In: Pandikumar A., Rameshkumar P., eds. graphene-based electrochemical sensors for biomolecules. Elsevier; 2019. p. :321-336.
- [Google Scholar]
- Thermomechanical behavior of a bimaterial microchannel cantilever subjected to periodic IR radiation. Sens. Actuators, B. 2016;235:273-279.
- [CrossRef] [Google Scholar]
- Microcalorimetry of isolated mammalian cells. Nature. 1977;267(5611):518-520.
- [CrossRef] [Google Scholar]
- Thermal conductivity of single biological cells and relation with cell viability. Appl. Phys. Lett.. 2013;102(20) 203702
- [CrossRef] [Google Scholar]
- Methane sensing at room temperature using photothermal cantilever deflection spectroscopy. Sens. Actuators, B. 2015;221:564-569.
- [Google Scholar]
- Micromechanical cantilever-based biosensors. Sens. Actuators, B. 2001;79(2):115-126.
- [CrossRef] [Google Scholar]
- Lab-on-fiber technology: a new vision for chemical and biological sensing. Analyst. 2015;140(24):8068-8079.
- [Google Scholar]
- Bimaterial microcantilevers as a hybrid sensing platform. Adv. Mater.. 2008;20(4):653-680.
- [Google Scholar]
- Cantilever beam temperature sensors for biological applications. IEEJ Trans. Electr. Electron. Eng.. 2017;12(2):153-160.
- [CrossRef] [Google Scholar]
- Evaluation of bimaterial cantilever beam for heat sensing at atmospheric pressure. Rev Sci Instrum.. 2010;81(5) 055104
- [CrossRef] [Google Scholar]
- Photothermal measurements at picowatt resolution using uncooled micro-optomechanical sensors. Appl. Phys. Lett.. 1997;71(3):306-308.
- [Google Scholar]
- A high-throughput silicon microphysiometer. Sens. Actuators, A. 2000;82(1):186-190.
- [CrossRef] [Google Scholar]
- A carbon-wrapped nanoscaled thermometer for temperature control in biological environments. Nanomedicine (Lond). 2008;3(3):321-327.
- [Google Scholar]
- Quantum dot nano thermometers reveal heterogeneous local thermogenesis in living cells. ACS Nano. 2011;5(6):5067-5071.
- [CrossRef] [Google Scholar]
- Thermal imaging of receptor-activated heat production in single cells. Biophys. J.. 1998;74(1):82-89.
- [Google Scholar]







