Use of antioxidants to augment semen efficiency during liquid storage and cryopreservation in livestock animals: A review
⁎Address: Department of Basic Sciences, College of Education, Imam Abdulrahman Bin Faisal University, P.O. Box 2375, Dammam 14513, Saudi Arabia. mgalmutary@iau.edu.sa (Mohsen Ghaleb Al-Mutary)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Despite the widespread use of frozen or refrigerated mammalian spermatozoa, their quality remains low as they are highly sensitivity to injuries during preservation and thawing. Moreover, in vitro conditions during spermatozoa storage may affect sperm quality and thereby oocyte fertilization, cleavage, and blastocyst development. Recently, antioxidants have been employed during semen extenders for livestock in different storage protocols to compensate for the depletion of endogenous antioxidant concentration in seminal plasma due to dilution as well as to counteract in vitro oxidative stress and minimize free radical generation. The present article reviews the most effective enzymatic or non-enzymatic antioxidants used during livestock spermatozoa preservation.
Keywords
Antioxidants
Spermatozoa
Liquid storage
Cryopreservation
Livestock
1 Introduction
Artificial insemination (AI) with preserved semen is the preferred mode of breeding over normal mating for livestock animals. AI has several advantages, such as the prevention of sexually transmitted diseases, establishment of a germplasm bank, and avoidance of male transfer. However, the success of AI largely depends on the efficiency of in vitro semen storage protocols in maintaining spermatozoa quality (Lone et al., 2017; Skidmore et al., 2018). Semen is preserved in an extender containing Tris, glycerol, glucose, citric acid, water, egg yolk, and antibiotics at refrigerated temperatures for several days during liquid storage (Al-Bulushi et al., 2019) and at −198 °C for several months or even years during cryopreservation (Gangwar et al., 2018; Prell et al., 2020). Typically, seminal plasma contains both enzymatic and non-enzymatic antioxidants; however, their protective actions against oxidative stress are significantly weakened following semen dilution in the extender prior to storage (Peris-Frau et al., 2020). Furthermore, high polyunsaturated fatty acid levels render the spermatozoon plasma membrane highly susceptible to oxidative stress and particularly to lipid peroxidation by reactive oxygen species (ROS) (Özer Kaya et al., 2018), due to the imbalance between ROS levels and natural antioxidant activity of sperm (Hamilton et al., 2016). The detrimental effects of ROS on spermatozoa decrease sperm viability and motility, impair fertilization, reduce implantation and pregnancy, minimize the cleavage rate, lower embryo quality, and inhibit blastocyst formation (Selvaraju et al., 2008; Osman et al., 2015; Simon et al., 2017) (Fig. 1). Consequently, the use of exogenous antioxidants to maintain ROS balance and protect spermatozoa from oxidative damage during preservation has recently garnered increased interest (Souza et al., 2019; Al-Mutary et al., 2020). Nonetheless, the use of antioxidants for maintaining sperm quality during semen storage remains debatable. Therefore, the objective of this review is to summarize the most effective and frequently used antioxidants during spermatozoa preservation.
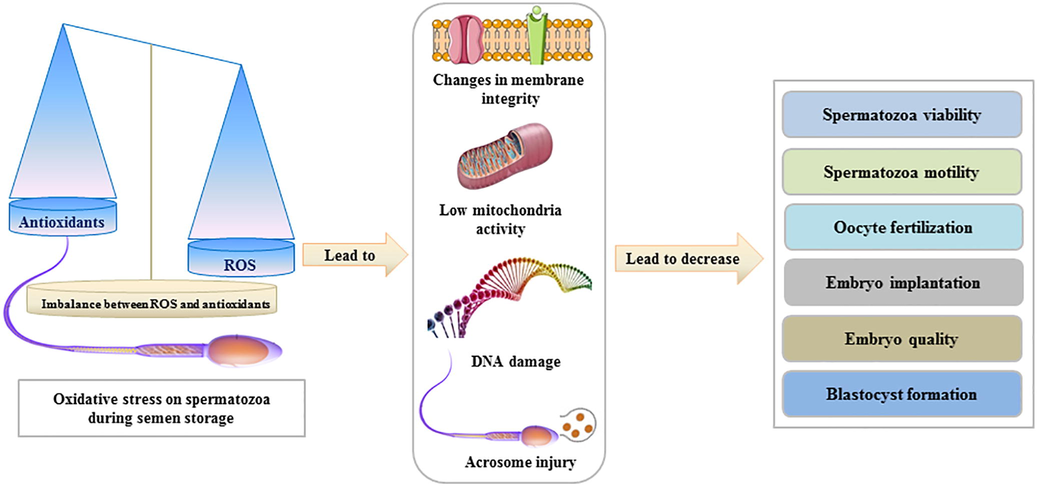
- Effects of oxidative stress during semen storage on spermatozoa quality.
2 Methodology
Experimental studies in the field of semen storage, which reported the use of antioxidants to increase semen efficiency, were searched for in major databases, such as Science Direct, Scopus, PubMed, Google Scholar, and Web of Knowledge. Only publications between January 2000 and June 2020 were included to reflect the latest developments in this field. Combinations of keywords that would represent semen storage and antioxidants, such as “cryopreservation” and “liquid storage with “antioxidants,” were used. The selected studies on livestock animals are summarized in Table 1.
| Antioxidant | Antioxidant Concentration | Storage Type | Species | Primary Effects | References |
|---|---|---|---|---|---|
| Resveratrol (RSV) | 10 and 50 µM RSV | CRY | Goat |
|
Lv et al. (2019) |
| 200 and 400 μM RSV | LIQ | Ram |
|
Al-Mutary (2019), Al-Mutary et al. (2020) | |
| 10, 20, 50, and 100 μM RSV | CRY | Buffalo |
|
Longobardi et al. (2017), Ahmed et al. (2020) | |
| 50 μM RSV | CRY | Boar |
|
Zhu et al. (2019) | |
| 50 μM RSV | LIQ | Boar |
|
Sun et al. (2020) | |
| 150 μM RSV | CRY | Boar | |||
| Iodixanol (Id) | 1.25, 2.5, and 5% Id | CRY | Buffalo |
|
Swami et al. (2017a) |
| 2.5% Id | CRY | Bovine |
|
Saragusty et al. (2009), Chuawongboon et al. (2017) | |
| 10% Id | CRY | Bovine |
|
Marqui et al. (2018) | |
| 5% Id | CRY | Ram |
|
Cirit et al. (2013) | |
| Cysteamine (CYM) | 1 and 2 mM CYM | CRY | Buffalo |
|
Swami et al. (2017b) |
| 5 mM CYM | CRY | Buffalo |
|
||
| 1 and 2 mM CYM | LIQ | Ram |
|
Peker Akalin et al. (2016) | |
| 2.5–7.5 mM CYM | CRY | Bovine |
|
Sarıözkan et al. (2015) | |
| Glutathione (GSH) | 0.5 mM GSH | CRY | Bovine |
|
Gangwar et al. (2018) |
| 10 mM GSH | CRY | Canine |
|
Lucio et al. (2016) | |
| 2 mM GSH | CRY | Boar |
|
Estrada et al. (2017) | |
| 1 and 5 mM GSH | CRY | Deer |
|
Anel-López et al. (2015) | |
| 5 mM GSH | CRY | Boar |
|
Yeste et al. (2014), Giaretta et al. (2015) | |
| 100 and 200 mM GSH | LIQ | Ram |
|
Shi et al. (2020) | |
| Quercetin (QUE) | 200 µM QUE | CRY | Buffalo |
|
Ahmed et al. (2019) |
| 10 µM QUE + Dimethylacetamide | CRY | Goat |
|
Seifi-Jamadi et al. (2017) | |
| 0.1 mM QUE | CRY | Equine |
|
Seifi-Jamadi et al. (2016) | |
| 0.15 mM QUE | CRY | Equine |
|
Gibb et al. (2013) | |
| 25 μg·mL-1 QUE | CRY | Bovine |
|
Avdatek et al. (2018) | |
| 5 and 20 μg·mL-1 QUE | CRY | Ram |
|
Silva et al. (2012) | |
| L-arginine (LA) | 0.5 mM LA | CRY | Ram |
|
Özer Kaya et al. (2018) |
| 1 mM LA | CRY | Buffalo |
|
Siddique and Atreja (2013) | |
| 4 and 6 mM LA | LIQ | Goat |
|
Susilowati et al. (2019) | |
| 1 mM LA | CRY | Bovine |
|
Maciel et al. (2018) | |
| Catalase (CAT) | 500 IU·mL-1 CAT | CRY | Camel |
|
Malo et al. (2019) |
| 15 IU·mL-1 CAT + antioxidants | LIQ | Equine |
|
Del Prete et al. (2019) | |
| 5, 10, 15, and 20 IU·mL-1 CAT | CRY | Bovine |
|
Arslan et al. (2019) | |
| Melatonin (MEL) | 0.05, 0.1, 0.2, and 1 mM | LIQ | Ram |
|
Dai et al. (2019) |
| 1 µM MEL | CRY | Equine |
|
Lançoni et al. (2018) | |
| 1 µM MEL | LIQ | Equine |
|
Affonso et al. (2017) | |
| 1.5 mM MEL | LIQ | Equine |
|
Izadpanah et al. (2015) | |
| 1 mM MEL | LIQ | Ram |
|
Ashrafi et al. (2011) | |
| Cysteine (CYS) | 15 mM CYS | CRY | Ram |
|
Atessahin et al. (2008) |
| 2.0 mM CYS | CRY | Buffalo |
|
Iqbal et al. (2016) | |
| 10 mM CYS | CRY | Boar |
|
Malo et al. (2010) | |
| AI, artificial insemination; CRY, cryopreservation; IVF, in vitro fertilization; LIQ, liquid storage; MDA, malondialdehyde; ROS, reactive oxygen species | |||||
3 The most effective antioxidants used during spermatozoa storage
3.1 Resveratrol (RSV)
RSV is a natural plant polyphenol that is commonly used as an antioxidant and a therapeutic agent (Javkhedkar et al., 2015). Recently, various concentrations of RSV were used during semen storage. Addition of 10 or 50 μM RSV to the semen extender during goat semen cryopreservation improved post-thaw sperm viability, acrosome integrity, mitochondrial activity, membrane integrity, and overall motility by inhibiting ROS generation (Lv et al., 2019). In addition, Al-Mutary (2019) and Al-Mutary et al., (2020) reported that addition of 200 and 400 μM RSV to the Triladyl extender for ram semen during chilled storage improved spermatozoa quality, relieved oxidative stress, enhanced in vitro fertility, and increased longevity. Furthermore, addition of 50 μM RSV to the freezing extender for buffalo semen maintained sperm membrane integrity, decreased oxidative stress, reduced capacitation-like changes, and enhanced in vitro fertility (Longobardi et al., 2017). Furthermore, addition of 50 μM RSV to the boar semen extender protected spermatozoa from post-thaw ROS generation and improved their quality by enhancing AMP-activated protein kinase phosphorylation (Zhu et al., 2019). Addition of 50 and 100 μM RSV to the buffalo semen extender improved post-thaw spermatozoa quality parameters, antioxidant state, and fertilizing potential as well as prevented DNA fragmentation and lipid peroxidation (Ahmed et al., 2020). Recently, Sun et al. (2020) reported the beneficial actions of 50 and 150 µM RSV on boar sperm parameters as well as mitochondrial and oxidative states during liquid storage and cryopreservation. Finally, addition of 50 μM RSV during cryopreservation prevented early apoptosis of buck spermatozoa (Falchi et al., 2020).
3.2 Glutathione (GSH)
GSH minimizes or even prevents ROS-induced intracellular damage (Pompella et al., 2003) and plays pivotal roles in mitochondrial function, cellular membrane stabilization, and oxidative stress alleviation in mammalian spermatozoa (Llavanera et al., 2020). Addition of 0.5 mM GSH to the bull semen extender improved post-thaw spermatozoa motility, live sperm count, and acrosome integrity (Gangwar et al., 2018). Moreover, addition of 10 mM GSH to the canine semen extender protected acrosome integrity following cryopreservation, whereas the addition of 20 mM GSH promoted mitochondrial injury (Lucio et al., 2016). Additionally, supplementation of the freezing medium with 2 mM GSH for boar spermatozoa positively affected sperm motility, membrane integrity, and nuclear stability following freeze–thaw procedures (Estrada et al., 2017). Improved mitochondrial functionality and kinematics of cryopreserved red deer spermatozoa following the addition of 1 and 5 mM GSH to the semen extender were also reported (Anel-López et al., 2015). Similarly, addition of 5 mM GSH improved the cryotolerance of poor freezability ejaculates of boar spermatozoa (Yeste et al., 2014). Moreover, addition of high GSH concentrations (100 and 200 mM) during liquid storage maintained motility, membrane integrity, mitochondrial activity, and antioxidant status of ram spermatozoa (Shi et al., 2020). Giaretta et al., (2015) reported that addition of 5 mM GSH alone or in combination with 100 µM ascorbic acid to the freeze–thaw medium improved the quality of boar spermatozoa.
3.3 Quercetin (QUE)
QUE is a flavonoid that scavenges reactive nitrogen species and ROS (Boots et al., 2008). The addition of QUE at 200 µM level improved post-thaw progressive motility; membrane, acrosome, and DNA integrity; and in vivo fertility of buffalo bull spermatozoa (Ahmed et al., 2019). Addition of QUE (10 µM) in combination with dimethylacetamide during freezing protected goat semen by preventing lipid peroxidation and improving spermatozoa motion kinetics (Seifi-Jamadi et al., 2017). In stallions, addition of 0.1 mM QUE during cryopreservation maintained sperm motility (Seifi-Jamadi et al., 2016). Similarly, Gibb et al. (2013) reported that the use of 0.15 mM QUE during the storage of sex-sorted, frozen stallion spermatozoa enhanced sperm motility and oocyte penetration as well as maintained DNA integrity. However, contrary to the reported benefits, addition of 25 μg·mL−1 QUE to the freezing extender did not improve progressive and overall bull spermatozoa motility, although it positively affected spermatozoa DNA integrity (Avdatek et al., 2018). Conversely, the use of QUE at concentrations of 5–20 µg·mL−1 enhanced the mitochondrial membrane potential of ram spermatozoa (Silva et al., 2012).
3.4 Iodixanol (Id)
Id exhibits antioxidant properties depending on the amount of free radical generation in an extender or a medium (Swami et al., 2017a). Addition of 1.25%, 2.5%, and 5% (v/v) Id during cryopreservation protected buffalo spermatozoa by minimizing antioxidant consumption, which in turn prevented membrane lipid peroxidation (Swami et al., 2017a). Supplementation of the semen extender with 2.5% Id improved progressive motility, viability, and plasma membrane and acrosome integrity of frozen bull spermatozoa, although it did not affect the efficiency of IVF and AI (Chuawongboon et al., 2017). However, addition of Id at a high concentration (10%) to the bull semen freezing medium protected the plasma membrane (Marqui et al., 2018). Moreover, addition of 5% Id improved the post-thaw parameters of ram spermatozoa (Cirit et al., 2013). Id likely altered the ice crystal structure and increased glass transition temperature during cryopreservation of bovine spermatozoa (Saragusty et al., 2009).
3.5 Cysteamine (CYM)
CYM functions by promoting GSH production in cells to protect them from oxidative stress (Maher et al., 2008). Peker Akalin et al. (2016) reported that 1 and 2 mM CYM enhanced ram sperm motility and viability as well as oxidative and mitochondrial states following liquid storage. Moreover, Sarıözkan et al., (2015) demonstrated that addition of 2.5 or 7.5 mM CYM to the freezing and thawing solutions for bull semen decreased DNA damage and malondialdehyde (MDA) content of spermatozoa as well as activated antioxidant enzymes (e.g., superoxide dismutase and glutathione peroxidase). However, Swami et al. (2017b) reported that low CYM concentrations (1 and 2 mM) did not protect buffalo semen from cryopreservation effects, while high CYM concentrations produced detrimental effects (5 mM).
3.6 L-arginine (LA)
The use of LA is based on its potential to regulate superoxide and hydrogen peroxide levels (Scott and Bolton, 2000). In vitro addition of 0.5 mM LA during cryopreservation protected the membrane of ram spermatozoa and increased arginase activity in seminal plasma following freezing (Özer Kaya et al., 2018). Moreover, addition of 1 mM LA protected buffalo spermatozoa from lipid peroxidation as well as enhanced their motility and viability (Siddique and Atreja, 2013). In addition, storage of goat semen in a skim milk extender supplemented with 4 and 6 mM LA for 5 days improved spermatozoa quality by maintaining viability, membrane integrity, and motility; inhibiting MDA production; and decreasing apoptotic rate (Susilowati et al., 2019). Furthermore, supplementation of 1 mM LA to an in vitro capacitation medium for frozen–thawed bovine spermatozoa altered proteome abundance (Maciel et al., 2018).
3.7 Catalase (CAT)
CAT is a sensitive enzyme that can reduce hydrogen peroxide intoxication within cells by splitting it into water and oxygen molecules (Rubio-Riquelme et al., 2020). CAT supplementation (500 IU·mL−1) during thawing of dromedary camel semen extended spermatozoa survival, although it did not affect fertilization rate following AI (Malo et al., 2019). Addition of CAT (15 IU·mL−1) in combination with specific antioxidants to the chilled extender of stallion spermatozoa inactivated caspase-3 and improved motility and viability after 72 h of storage (Del Prete et al., 2019). Similarly, addition of various concentrations of CAT (5, 10, 15, and 20 IU·mL−1) to the semen extender during freezing improved bull spermatozoa quality following cryopreservation, although it did not affect IVF rate and embryo development (Arslan et al., 2019).
3.8 Melatonin (MEL)
MEL protects human spermatozoa by reducing nitric oxide levels (Du Plessis et al., 2010). In equines, addition of 1 µM MEL to the semen extender improved post-thaw mitochondrial functioning of spermatozoa (Lançoni et al., 2018). Moreover, MEL (at 1 µM) protected equine spermatozoa acrosome, mitochondria, and cellular membrane integrity after 8 h of refrigerated storage (Affonso et al., 2017). During refrigerated storage of stallion semen, 1.5 mM MEL improved sperm motility by decreasing lipid peroxidation after 48 h (Izadpanah et al., 2015). Addition of MEL (1 mM) to the ram semen extender improved spermatozoa motility parameters after storage at 5 °C for 48 h (Ashrafi et al., 2011). The best sperm motility, plasma membrane integrity, mitochondrial activity, and total antioxidant capacity and the lowest MDA content were observed following liquid preservation of ram semen in the presence of MEL (0.05, 0.1, and 0.2 mM) (Dai et al., 2019).
3.9 Cysteine (CYS)
CYS is an intracellular GSH precursor and contains a thiol group that can penetrate the plasma membrane of spermatozoa. It also act as an antioxidant (Coyan et al., 2011). The use of CYS during spermatozoa storage is limited compared to that of the other antioxidants. At 5 mM, CYS protected ram spermatozoa and enhanced their motility after thawing (Atessahin et al., 2008). Iqbal et al. (2016) reported that addition of 2.0 mM CYS during cryopreservation of buffalo spermatozoa activated the antioxidant system as well as enhanced sperm motility and in vivo fertility. Furthermore, higher CYS concentrations (10 mM) positively affected the viability and acrosome integrity of boar spermatozoa following cryopreservation (Malo et al., 2010).
4 Conclusion and future perspective
The present review emphasizes the positive effects of using RSV, GSH, QUE, Id, CYM, LA, CAT, MEL, and CYS on sperm quality of livestock animals during liquid storage or cryopreservation. However, the concentration of each antioxidant exclusively depends on species, semen extender or preservation medium composition, storage type, and in vitro stress conditions. Therefore, further studies on optimizing the conditions of in vitro semen storage, composition of the preservation medium, and procedures for maintaining the optimum quality of stored spermatozoa are warranted. Moreover, studies examining the fertility of stored spermatozoa (either in vivo or in vitro) would advance the field of AI. Specific studies investigating the precise mechanisms of action of the abovementioned antioxidants during semen storage are also imperative. Finally, the field of sperm cryobiology would benefit from studies on altered expression patterns of specific genes in persevered spermatozoa following antioxidant supplementation and their potential use as markers for sperm quality.
Acknowledgments
The author would like to thank Dr. Mohammed Alshamlih for reviewing this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Addition of antioxidants myoinositol, ferulic acid, and melatonin and their effects on sperm motility, membrane integrity, and reactive oxygen species production in cooled equine semen. J. Equine Vet. Sci.. 2017;59:57-63.
- [CrossRef] [Google Scholar]
- Stimulating effects of Quercetin (QUE) in tris citric acid extender on post thaw quality and in vivo fertility of buffalo (Bubalus bubalis) bull spermatozoa. Theriogenology. 2019;134:18-23.
- [CrossRef] [Google Scholar]
- The addition of resveratrol in tris citric acid extender ameliorates post-thaw quality parameters, antioxidant enzymes levels, and fertilizing capability of buffalo (Bubalus bubalis) bull spermatozoa. Theriogenology. 2020;152:106-113.
- [CrossRef] [Google Scholar]
- Liquid storage of dromedary camel semen in different extenders. Animal Reprod. Sci.. 2019;207:95-106.
- [CrossRef] [Google Scholar]
- Effect of resveratrol supplementation to Triladyl® on the quality of ram chilled semen. Indian J. Animal Sci.. 2019;89:923-937.
- [Google Scholar]
- Effect of different concentrations of resveratrol on the quality and in vitro fertilizing ability of ram semen stored at 5 °C for up to 168 h. Theriogenology. 2020;152:139-146.
- [CrossRef] [Google Scholar]
- Reduced glutathione addition improves both the kinematics and physiological quality of post-thawed red deer sperm. Animal Reprod. Sci.. 2015;162:73-79.
- [CrossRef] [Google Scholar]
- “Effect of the addition of different catalase concentrations to a TRIS-egg yolk extender on quality and in vitro fertilization rate of frozen-thawed bull sperm’’. Cryobiology. 2019;91:40-52.
- [CrossRef] [Google Scholar]
- Effects of anti-oxidant additives on microscopic and oxidative parameters of Angora goat semen following the freeze–thawing process. Small Ruminant Res.. 2008;77(1):38-44.
- [CrossRef] [Google Scholar]
- Supplementation of quercetin for advanced DNA integrity in bull semen cryopreservation. Andrologia. 2018;50(4):e12975.
- [CrossRef] [Google Scholar]
- Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol.. 2008;585:325-337.
- [Google Scholar]
- Effects of supplementation of iodixanol to semen extender on quality and fertilization ability of frozen–thawed Thai native bull sperm. Animal Sci. J.. 2017;88:1311-1320.
- [Google Scholar]
- Comparison of cryoprotective effects of iodixanol, trehalose and cysteamine on ram semen. Animal Reprod. Sci.. 2013;139:38-44.
- [Google Scholar]
- Effects of cysteine and ergothioneine on post-thawed Merino ram sperm and biochemical parameters. Cryobiology. 2011;63:1-6.
- [Google Scholar]
- Effect of addition of melatonin on liquid storage of ram semen at 4°C. Andrologia. 2019;51:e13236
- [Google Scholar]
- Combined addition of superoxide dismutase, catalase and glutathione peroxidase improves quality of cooled stored stallion semen. Animal Reprod. Sci.. 2019;210:106195
- [Google Scholar]
- The in vitro effects of melatonin on human sperm function and its scavenging activities on NO and ROS. Andrologia. 2010;42:112-116.
- [Google Scholar]
- The addition of reduced glutathione to cryopreservation media induces changes in the structure of motile subpopulations of frozen-thawed boar sperm. Cryobiology. 2017;78:56-64.
- [Google Scholar]
- Resveratrol supplementation and cryopreservation of buck semen. Cryobiology. 2020;95:60-67.
- [Google Scholar]
- Effect of reduced glutathione supplementation on cryopreservation induced sperm cryoinjuries in Murrah bull semen. Animal Reprod. Sci.. 2018;192:171-178.
- [Google Scholar]
- Combining reduced glutathione and ascorbic acid has supplementary beneficial effects on boar sperm cryotolerance. Theriogenology. 2015;83:399-407.
- [Google Scholar]
- Quercetin improves the postthaw characteristics of cryopreserved sex-sorted and nonsorted stallion sperm. Theriogenology. 2013;79:1001-1009.
- [Google Scholar]
- Induced lipid peroxidation in ram sperm: semen profile, DNA fragmentation and antioxidant status. Reproduction. 2016;151:379-390.
- [Google Scholar]
- l-Cysteine improves antioxidant enzyme activity, post-thaw quality and fertility of Nili-Ravi buffalo (Bubalus bubalis) bull spermatozoa. Andrologia. 2016;48:943-949.
- [Google Scholar]
- Iraj Ashrafi, Hamid Kohram, Hamid Naijian, Bahreini, M., Poorhamdollah, M., 2011. Protective effect of melatonin on sperm motility parameters on liquid storage of ram semen at 5°C. Afr. J. Biotechnol. 10, 6670–6674.
- Melatonin has a beneficial effect on stallion sperm quality in cool condition. J. Equine Vet. Sci.. 2015;35:555-559.
- [Google Scholar]
- Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol.. 2015;308:R840-R846.
- [Google Scholar]
- Melatonin added to cryopreservation extenders improves the mitochondrial membrane potential of postthawed equine sperm. J. Equine Vet. Sci.. 2018;69:78-83.
- [Google Scholar]
- Barranco, I., 2020. Glutathione S-transferases play a crucial role in mitochondrial function, plasma membrane stability and oxidative regulation of mammalian sperm. Antioxidants (Basel) 9.
- Effect of dilution on cryosurvival of low sperm doses: a review. Cryo Lett.. 2017;38:471-476.
- [Google Scholar]
- Resveratrol prevents capacitation-like changes and improves in vitro fertilizing capability of buffalo frozen-thawed sperm. Theriogenology. 2017;88:1-8.
- [Google Scholar]
- Effect of reduced glutathione (GSH) in canine sperm cryopreservation: In vitro and in vivo evaluation. Cryobiology. 2016;72:135-140.
- [Google Scholar]
- Improving the quality of cryopreserved goat semen with a commercial bull extender supplemented with resveratrol. Animal Reprod. Sci.. 2019;208:106127
- [Google Scholar]
- l-arginine alters the proteome of frozen-thawed bovine sperm during in vitro capacitation. Theriogenology. 2018;119:1-9.
- [Google Scholar]
- A novel approach to enhancing cellular glutathione levels. J. Neurochem.. 2008;107:690-700.
- [Google Scholar]
- Anti-oxidant supplementation improves boar sperm characteristics and fertility after cryopreservation: Comparison between cysteine and rosemary (Rosmarinus officinalis) Cryobiology. 2010;61:142-147.
- [Google Scholar]
- Individual male dependent improvement in post-thaw dromedary camel sperm quality after addition of catalase. Animal Reprod. Sci.. 2019;209:106168
- [Google Scholar]
- Marqui, F.N., Martins, A., da Cruz, T.E., Berton, T.I.U., de Paula Freitas-Dell’Aqua, C., Júnior, J.A.D.A., Oba, E., 2018. Addition of iodixanol in bull freezing extender improves the sperm membranes integrity. Animal Reprod. Sci. 194, e26–e27.
- The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod. Biomed. Online. 2015;30:120-127.
- [Google Scholar]
- Effect of l-arginine addition on long-term storability of ram semen. Andrologia. 2018;50:e12945
- [Google Scholar]
- Peker Akalin, P., Bucak, M.N., GÜNgÖR, Ş., BaŞPinar, N., ÇOyan, K., Dursun, Ş., İLİ, P., Aksoy, A., KaraŞÖR, Ö.F., BİLgİLİ, A., SariÖZkan, S., Yenİ, D., 2016. Influence of lycopene and cysteamine on sperm and oxidative stress parameters during liquid storage of ram semen at 5°C. Small Ruminant Res. 137, 117–123.
- Sperm cryodamage in ruminants: understanding the molecular changes induced by the cryopreservation process to optimize sperm quality. Int. J. Mol. Sci.. 2020;21:2781.
- [Google Scholar]
- The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol.. 2003;66:1499-1503.
- [Google Scholar]
- Motility and fertility evaluation of thawed frozen stallion semen after 24 hours of cooled storage. J. Equine Vet. Sci.. 2020;102983
- [Google Scholar]
- Catalase as a molecular target for male infertility diagnosis and monitoring: an overview. Antioxidants (Basel, Switzerland). 2020;9:78.
- [Google Scholar]
- Protective effects of iodixanol during bovine sperm cryopreservation. Theriogenology. 2009;71:1425-1432.
- [Google Scholar]
- Antioxidative effects of cysteamine, hyaluronan and fetuin on post-thaw semen quality, DNA integrity and oxidative stress parameters in the Brown Swiss bull. Andrologia. 2015;47:138-147.
- [Google Scholar]
- L-arginine modifies free radical production and the development of experimental allergic encephalomyelitis. Inflamm. Res.. 2000;49:720-726.
- [Google Scholar]
- Antioxidant effect of quercetin in an extender containing DMA or glycerol on freezing capacity of goat semen. Cryobiology. 2017;75:15-20.
- [Google Scholar]
- Quercetin ameliorate motility in frozen-thawed turkmen stallions sperm. J. Equine Vet. Sci.. 2016;45:73-77.
- [Google Scholar]
- Evaluation of sperm functional attributes in relation to in vitro sperm-zona pellucida binding ability and cleavage rate in assessing frozen thawed buffalo (Bubalus bubalis) semen quality. Animal Reprod. Sci.. 2008;106:311-321.
- [Google Scholar]
- Effects of reduced glutathione on ram sperm parameters, antioxidant status, mitochondrial activity and the abundance of hexose transporters during liquid storage at 5 ℃. Small Ruminant Res.. 2020;189:106139
- [Google Scholar]
- Effect of l-Arginine and spermine-NONOate on motility, viability, membrane integrity and lipid peroxidation of Murrah buffalo (Bubalus bubalis) spermatozoa. Livestock Sci.. 2013;153:147-153.
- [Google Scholar]
- Effect of antioxidants resveratrol and quercetin on in vitro evaluation of frozen ram sperm. Theriogenology. 2012;77:1722-1726.
- [Google Scholar]
- A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J. Androl.. 2017;19:80.
- [Google Scholar]
- An update on semen collection, preservation and artificial insemination in the dromedary camel (Camelus dromedarius) Animal Reprod. Sci.. 2018;194:11-18.
- [Google Scholar]
- Effect of different concentrations of l-carnitine in extender for semen cryopreservation in sheep. Cryobiology. 2019;89:104-108.
- [Google Scholar]
- Resveratrol protects boar sperm in vitro via its antioxidant capacity. Zygote 2020:1-8.
- [Google Scholar]
- Addition of L-arginine in skim milk extender maintains goat spermatozoa quality in chilled temperature for five days. Vet. World. 2019;12:1784-1789.
- [Google Scholar]
- The cryoprotective effect of iodixanol in buffalo semen cryopreservation. Animal Reprod. Sci.. 2017;179:20-26.
- [Google Scholar]
- Cysteamine supplementation revealed detrimental effect on cryosurvival of buffalo sperm based on computer-assisted semen analysis and oxidative parameters. Animal Reprod. Sci.. 2017;177:56-64.
- [Google Scholar]
- The improving effect of reduced glutathione on boar sperm cryotolerance is related with the intrinsic ejaculate freezability. Cryobiology. 2014;68:251-261.
- [Google Scholar]
- Resveratrol improves boar sperm quality via 5'AMP-activated protein kinase activation during cryopreservation. Oxid. Med. Cell Longev.. 2019;2019:5921503.
- [Google Scholar]







