Translate this page into:
Ultramorphological study of immature stages and male genitalia of forensically significant flesh fly Sarcophaga dux thomson, 1868 (Diptera: Sarchophagidae)
⁎Corresponding author. madhubaladhakane@gmail.com (Madhu Bala)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Sarcophaga dux Thomson is a well-known flesh fly species of medicinal and forensic importance and remain active throughout the year. Female of this fly lays first instars directly onto the carcass or on wounds of living host as it causes myiasis. Use of S. dux in forensic investigations is limited because identification of immature stages as well as adults by conventional means is very challenging.
Methods
To overcome this limitation, the adults of S. dux were collected and reared under laboratory conditions. Immature stages were analyzed by using scanning electron microscopy and male genitalic characters were analyzed by light microscopy. Adults were identified on the basis of male genitalic characters whereas, immature stages like larva and pupa were identified on the basis of various taxonomic characters i.e. cephaloskeleton, anterior and posterior spiracles, appearance of spiracular cavity, papillae on the caudal segments and shape/arrangements of spines.
Results and Conclusion
Through present study an effort was made for accurate identification of immatures and adults of this flesh fly species of medical and forensic importance. Illustrations presented here will help in forensic investigations in cases involving this flesh fly species.
Keywords
Chrysomya megacephala
Forensic Entomology
Sarcophaga dux
Larvae
1 Introduction
Flies belonging to the Family Sarcophagidae are important forensic indicators in evaluating human decomposition as they can detect a corpse within few minutes after death at several kilometers (Goff, 2000). Sarcophagidae comprises of 3 subfamilies, Miltogrammatinae, Paramacronychiinae and Sarcophaginae (Nandi, 2002) and represents over 3100 described species belonging to 400 genera and representatives of this family have world wide geographic distribution with most species occurring either in tropical or warm temperate regions (Vairo et al., 2015). In India, Nandi (2002) recorded 163 species of Sarcophagidae under 50 genera. The genus Sarcophaga Meigen of family Sarcophagidae is exceedingly diverse group and comprises about 30% of the total species (Buenaventura and Pape, 2017). It includes about 890 species belonging to 169 sub genera (Buenaventura et al., 2017).
Sarcophaga dux Thomson is a flesh fly species of medical importance in many parts of the world (Cherix et al., 2012). Geographically, it is abundant and found in many part of the world including, India, Nepal, Thailand, Malaysia, Indonesia, Korea, Japan, Saudi Arabia, Egypt, Myanmar, Philippines, Sri Lanka, Taiwan, China; Southern Europe (France), Australia; and Hawaii, USA (Sukontason et al., 2014). Adults of S. dux remain active throughout the year including summer, rainy and winter season (Sukjit, 2011). Generally, it is larviparous insect as adult female lay their 1st instar (larviparity) directly onto the suitable breeding medium but under laboratory conditions they were also found to oviposit their eggs directly on the substrate (Sukontason et al., 2007). S. dux is light grayish in appearance with three longitudinal black strips on the mesonotum and the abdominal segments are covered by checkered pattern (Sukontason et al., 2014). Sinha and Mahato (2016) studied intra-puparial development of Sarcophaga dux under laboratory condition, in which the total development time taken by pupa was about 252 hrs. Siddiki and Zambare (2017) also studied the duration of different life cycle stages from larvae to adults of P. dux for rainy, winter and summer seasons under room temperature. Abd Al Galil et al. (2021) investigated the life cycle stages of the firstly colonizing Sarchophagidae flies on cadavers including Sarcophaga dux Thomson under the effect of chemical dimethoate (organophosphate), which can alter their development rate. S. dux is having both medicinal and forensic importance as it is known to parasitize arthropods [M.martensii (Chinese scorpion)] (Shi et al., 2015) and were also found associated with human corpse (Cherix et al., 2012). Myiasis is the infestation of living tissue of vertebrate animals including human being with larvae of various dipterous flies (Das et al., 2010). It can be differentiated as cutaneous, atrial, wound, intestinal or urinary depending on the location of fly larvae (Aguilera et al., 1999).
This species has both medicinal as well as forensic importance. Larvae of this species are known to cause myiasis in humans and animals (Kaufmann, 1996), and associated with crime investigations (Cherix et al., 2012; Sukontason et al., 2014). The modified mouthhooks of this fly can easily penetrate the skin which supports it to being necrophagous or capable of causing myiasis (Sukontason et al., 2003). As compare to Calliphoridae, Sarcophagidae are not frequently used as evidence in forensic investigations. Sarcophagidae has great potential in forensic investigations but their uses are limited because identification of immature stages as well as adults by conventional methods is very challenging because of lots of similarity in morphology.
Present study was conducted to dispense identification characters of both adults and immature stages of Sarcophaga dux Thomson by using light and scanning electron microscopy (SEM). An effort has been made for accurate identification of this medically and forensically relevant flesh fly species for future forensic investigations. Correct species identification of forensically important insect is a first prerequisite toward Postmortem Interval estimation. Moreover, this species could also be of medical concern as its blade like cutting margin can penetrate easily into the wounds (Sukontason et al., 2003). Therefore, it can cause subcutaneous myiasis in human.
2 Material and methods
Gravid female of Sarcophaga dux was collected by sweeping hand net over 2 kg chicken meats as bait placed in the campus of Punjabi University, Patiala (India). Collected flies were brought to the rearing laboratory in the department of Zoology and Environmental sciences, Punjabi University, Patiala (India) and kept in rearing cage. The females were nourished with powdered sugar and water in a conical flask with wick of blotting sheet and cotton swab on top. Chicken liver (10 g) was placed on wet blotting sheet in petri dish as a larviposition medium. To avoid larviposition at night liver piece was removed from rearing cage at night and placed again next morning. After larviposition, the first instars larvae were transferred to 1L glass rearing jars having moist husk as pupation medium and provided with 15 g of chicken meat as a food source. The rearing jars were sealed with a piece of muslin cloth by using a rubber band to prevent escape of larvae. At regular interval, immature stages (5–6) were obtained from rearing jars and killed by hot water boiling (95 °C) to avoid deformation.
Larvae and pupae were preserved in Kahle’s solution (30 ml of 95% ethanol + 12 ml of formaldehyde + 4 ml of glacial acetic acid + 60 ml of water) for further study (Singh et al., 2012). Rests of the immatures were continued to rear to get adults for further identification. After emergence the male adults were collected and killed by placing in killing jars having ethyl acetate. To identify species, the abdomen of male was detached and soaked in a 10% KOH for 24 hrs. The internal genitalia were dissected from the abdomen and preserved in the clove oil for clearance purpose and later used for identification and photography. Specimen was identified using the keys given by Nandi (2002). Larval instars were dissected to study their cephaloskeleton as well. Photography of cephaloskeleton, anterior and posterior spiracles were done with the help of image processing unit (Leica DM 2000). For studying the detailed external morphology of the immature stages, third instar larvae and pupae were prepared for SEM studies by placing in 2.5% glutaraldehyde for fixation at 4 °C for 24 hrs (Singh et al., 2012). It was then be subjected to post fixation in 1% osmium tetroxide and dehydration in graded alcohol series (Singh et al., 2012). This was followed by immersion in acetone before applying a critical point drying procedure. The larvae were mounted on stubs, coated with gold; ultra-micro graphs were obtained using SEM (JOEL JSM-6510LV). Terminology given by Singh et al. (2012) and Szpila et al. (2015) is followed for larval and pupal characters.
3 Results
Identification of adult fly based on genitalic characters was done with the help of light microscopy. Whereas, the identification of immature stages i.e. larvae and pupae of Sarcophaga dux were analyzed by using both Light and Electron microscopy.
Adult identification: The adults of Sarcophaga dux Thomson are light grayish in color with three black stripes arranged longitudinally on the mesonotum. Adults also have a distinctive black strip with golden or yellowish margins between their eyes. Male are larger than the female. The male is about 10 to 12 mm in size. Morphological characters of adult male, abdomen possess black and silvery checkered pattern (Fig. 1A). Median marginal bristles on tergite 1 + 2 and tergite third of abdomen are absent. But 2 pair of thick bristles are present on lateral margin along with some thin bristles. Third tergite consists of only one pair bristle at lateral margin. Fourth tergite bears 4 pairs of bristles with 1 median pair and 3 at the lateral margin (Fig. 1A, D).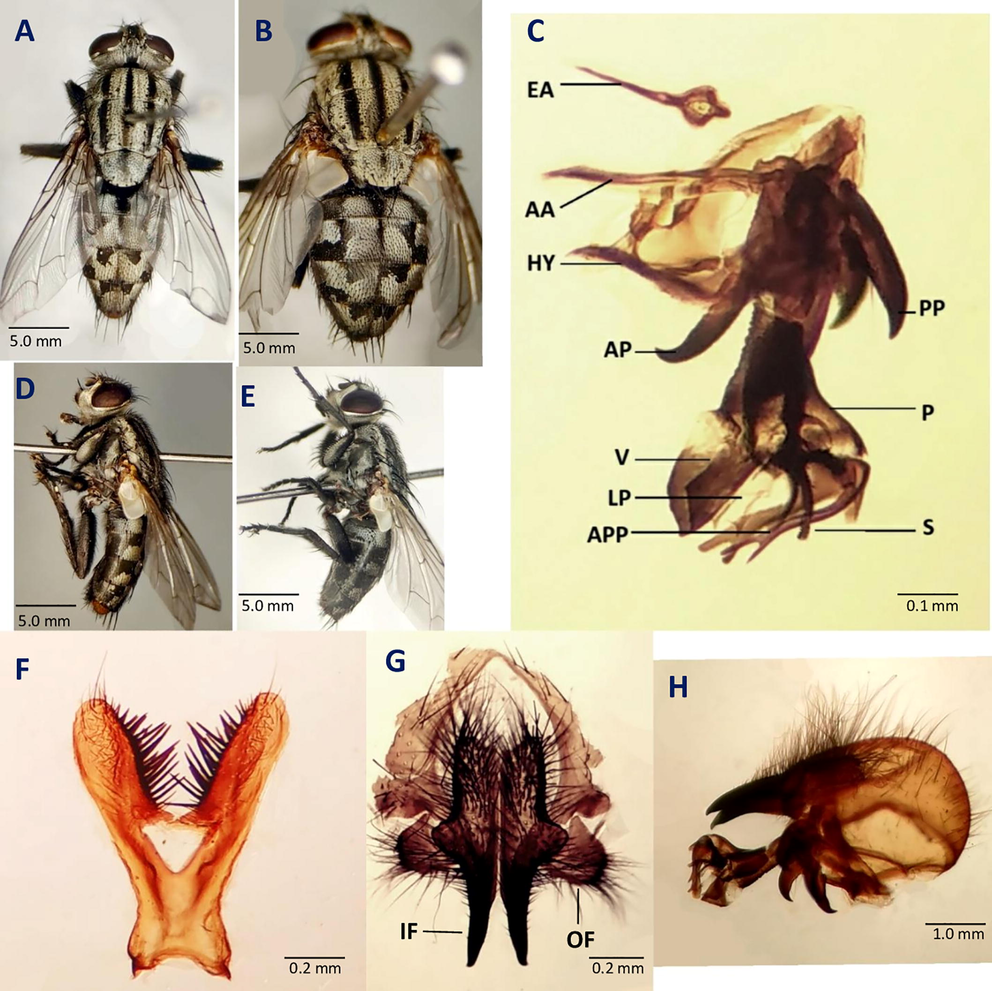
Adults morphology of S. dux. A male dorsal view, B female dorsal view, C penis lateral view, D male lateral view E female lateral view F fifth sternite G outer and inner forceps H last genital segment, epandrium Abbreviations: EA- ejaculatory apodeme, AA- aedeagal apodeme, HY- hypandrium, AP- Anterior paramere, PP- Posterior paramere, P- Paraphallus, V- Ventralia, LP- Lateral plate of paraphallus APP- Apical Plate of paraphallus S- Styli of glans, IF- Inner forceps, OF- Outer forceps.
A row of 14 strong marginal bristles followed by thin bristles are present on 5th tergite (Fig. 1A, D). In male, genital segment (7 + 8) forms syntergosternite a cup shaped structure covered by short and moderate dense hairs. Marginal bristles are absent on Syntergosternite (7 + 8). Epandriun is yellowish-brown in color cover with short hairs (Fig. 1H). Long hairs are present on first and second sternite. Thin bristles and short hairs are present on the fourth and fifth sternite. The male terminalia is the most diagnostic characters used for the species identifications of sarcophagidae which includes various structures.
Fifth sternum is Y shape with open medial window (Fig. 1F). Posterior arms of fifth sternite are short and slender with a long terminal hair (Fig. 1F). Thin hairs are scattered on posterior arms to medial window area. The stout bristles are present on inner surface of arms. Size of bristles reduces laterally from the medial window area toward the terminal. Anterior end is curved with two small horns distinctly projects anterio-laterally from the body (Fig. 1F). Inner forceps are long, almost straight, thin, pointed with slightly curved tips (Fig. 1G). Hairs are present on its whole surface except the basal half without hair. Size of hairs gradually decreases along the length of inner forceps (Fig. 1H). Outer forceps is almost covered by hairs and lobed structure with long hairs on the distal end (Fig. 1G).
Ejaculatory apodeme is pendulum shape and suspends above the Aedeagal apodeme (Fig. 1C). Anterior paramere are straight and slightly curved upward at the end (Fig. 1C). Posterior paramere carries 2–3 hairs, medially broad and apically curved in a sickle shaped structure (Fig. 1C). Paraphallus is wide, tubiform, and larger than theca (Fig. 1C). Paraphallus is connected to the various abruptly arranged projections at base (Fig. 1C). Apical plate of paraphallus originates from base of paraphallus, projects anteriorly bifid structure and parallelly extends with ventralia (Fig. 1C). Ventralia is plate like extensive structure, and anteroventrally stretched (Fig. 1C). Lateral plate of paraphallus is wide and uprightly elongated structure lies below the ventralia (Fig. 1C). The Styli of glan is short and extends below the lateral plate of paraphallus (Fig. 1C). It is a comb like structure, which bears three inwardly curved spines (Fig. 1C).
The female is about 7 to 9 mm long. The abdomen of female of Sarcophaga dux is modified both for oviposition and larviposition (Fig. 1B, E). The abdomen bears 4 visible segments (T1 + 2 to T5) (Fig. 1B). Tergite 6 is slightly sclerotized and appears to have row of 11 pair of bristles (Fig. 1B, E). The last tergite is known as supranal plate that is composed of tergite 9 + 10 (Kurahashi and Samerjai, 2018). The anus lies between the cerci and supra anal plate. Cerci are present above the supra anal plate. The genital pore present between the sternite 8 and subanal plate (Kurahashi and Samerjai, 2018).
Description of Immature Stages: Larvae of Sarcophaga dux Thomson are typical vermiform, elongated, almost cylindrical, which is tapering at anterior end and bulgy at posterior end. Some external organs such as the mouth hooks, the posterior pair of spiracles, the spine bands and the papillae of the perianal cavity are easily visible under microscope. But some organ i.e. the cephaloskelton, anterior spiracles, internal parts of posterior spiracle etc. were removed and slides were prepared for further analysis. All the 12 segments of larvae were easily visible including 1 cephalic, 3 thoracic and 8 abdominal segments.
First instar: The first instars are about 4 mm in size. Mouth hook of first instars is apically sharp and its distal half is slightly curved. Anterior spiracles are not present on thoracic segment. The posterior spiracles are present with undeveloped spiracular slits.
Second instar: The second instars are about 1 cm long. Mouth hook is pointed, and its distal half is curved. Outgrowths of anterior spiracles are visible in second instars. It represents a sclerotized, fan shaped structure at the anterior end. The posterior pair of spiracles is present with two visible oval spiracular slits. Spiracular cavity is undeveloped.
Third instar: The third instar is about 1.4 cm in size. The Cephaloskeleton grows larger in size lies near the anterior end. Mouth hook is gradually curved, distal half bend in a sickle shaped arch, pointing toward ventro-anteriorly. A fully developed anterior pair of spiracles are present with well-arranged lobes. The posterior pair of spiracles are present with three fully developed spiracular slits (Fig. 2E, F). Three slits are located on spiracular plates set inside a deep cavity on the posterior end (Fig. 2D).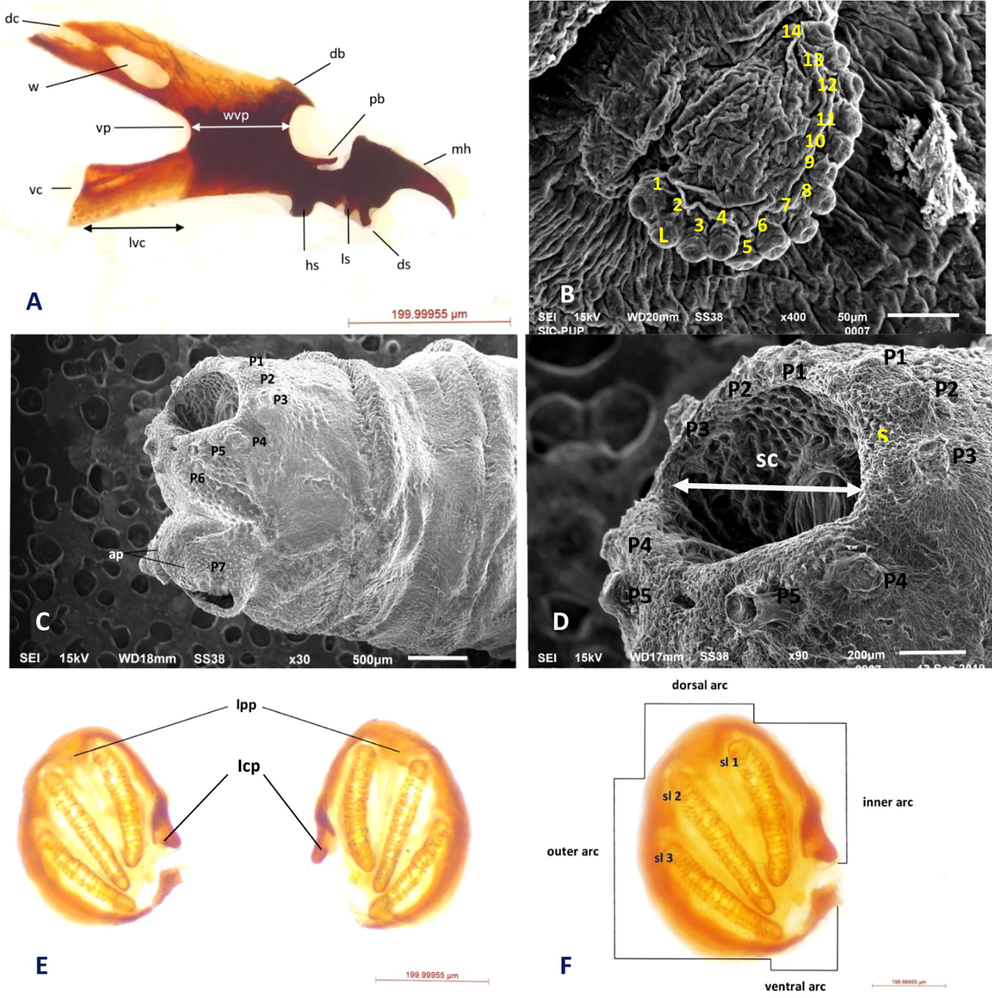
Third instar morphology of S. dux. A Cephaloskeleton, B Anterior spiracle, C Posterior view (3rd instar), D Spiracular cavity E Arrangements of posterior spiracles F Shape of posterior spiracle Abbreviations: db- dorsal bridge, dc- dorsal cornu, lvc- length of ventral cornu, mh- mouthhooks, pb- parastomal bar, sp- posterior spiracles, vc- ventral cornu, vp- vertical plate, w- window, wvp- width of vertical plate, hs- hypostomal sclerite, ds- dental sclerite, ls- labial sclerite, l- spiracular lobes, Icp- incomplete peritreme, Ipp- internal peritremal projections, sc- spiracular cavity, s- spines.
Cephaloskeleton: Mouth hook of third instar is pointed and downwardly curved (Fig. 2D). The dental sclerite (ds) is projected outwards the anterior side (Fig. 2D). The parastomal bars (pb) are well differentiated with the entire length of hypostomal sclerites (hs) slightly curved apically (Fig. 2D). The dorsal bridge (db) is slightly curved and pointed reaches half the length of the parastomal bars (pb) (Fig. 2D). Hypostomal sclerite (hs) is thickened structure joining mouth hooks with the ventral plate (Fig. 2D). Length of ventral cornu is almost equals to the width of
vertical plate (vp) (Fig. 2D). The length of dorsal cornu (ds) is longer than the ventral cornu and width is almost similar (vc) (Fig. 2D). Dorsal cornu (dc) of cephaloskeleton has long elongated window (w) apically, which is easily visible (Fig. 2D). Dorsal cornu (ds) is erect throughout its length and slightly curved at apex (Fig. 2D). Window (w) on ventral cornu (vc) is indistinct (Fig. 2D).
Anterior spiracles: A pair of anterior spiracles is present on both the lateral sides of first thoracic segments (Fig. 3A). It is about 10 ums in size. Anterior spiracles are oval and circular in shape with straight anterior edges. It consists of about 14 spiracular lobes (Fig. 2B). All lobes arranged in form of an arc (Fig. 2B). Lobes of anterior spiracle arranged in single slightly irregular row (Fig. 2B).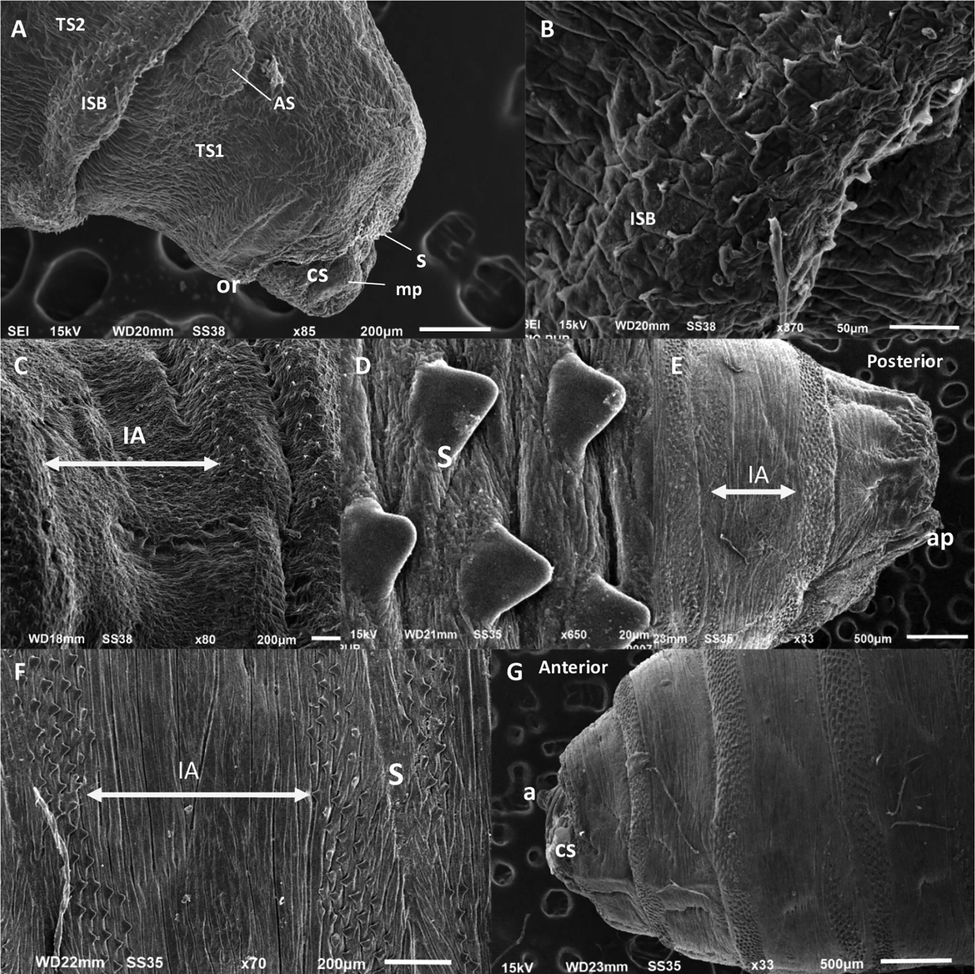
Third instar and puparium morphology of S. dux A. Anterior view (3rd instar) B. Spinose band (3rd instar) C. Inter-band area (3rd instar) D. Spines on spinose band (pupa) E. Posterior view (pupa) F. Inter-band area (pupa) G. Anterior view (pupa) Abbreviations: CS- Cephalic Segment, TS - thoracic segments, AS- anterior spiracle, ISB-intersegmental spine band, IA- Inter-band area, S- spines, CS- cephalic segment, mp- maxillary palps, or- oral ridge, ap- anal pad, s- spines, a- antennae.
Spiracular cavity and accompanying structures: Posterior spiracles are present over the concave spiracular field (Fig. 2D). Spiracular field get fixed in a well-developed spiracular cavity with deep depression (Fig. 2C, D). Entrance of spiracular cavity is wide and slightly semicircular (Fig. 2D). Over the boundary of perianal cavity seven pair of fleshy circumspiracular papilla (P1-P7) is present (Fig. 2C). First and second pair of papillae is smaller in size as compare to other papilla (p3-p5) (Fig. 2D). The papilla (p3-p5) is moderate and similar in size (Fig. 2D). The p6 is smaller in size to all and poorly visible under light microscope. The first three pairs of papillae (p1-p3) lies above the posterior spiracles and arranged dorsally to dorso-lateral direction (Fig. 2D). The other two papillae (p4-p5) lie at ventro-lateral position (Fig. 2D). The 6th pair arranges mid ventrally and seventh pair (p7) lies at ventral position and more robust than any other pair (Fig. 2C). The anal opening is determined by two fleshy lips (Fig. 2C).
Posterior spiracles: Posterior spiracles are D shape, arranged in a pair of large discs, with three distinct, long, narrow, golden-brown, and oval slits (Fig. 2E, F). Two slits (sl2 and sl3) are relatively straight in structure while one (sl1) is slightly curved and coalesce with others near the ventral arc (Fig. 2F). Spiracular slits inside peritreme are not pointing toward the opening (Fig. 2F). The peritreme is heavily sclerotized, incomplete and without button (Fig. 2E, F). The peritreme is slightly projected inwards at two places to form asymmetrical internal peritremal projections (Fig. 2E). A well-defined inner arc with distinctly located ventral arc is present 2F).
Shape and arrangement of spines: Spines are heavly sclerotised and present only over the spinose band. Spines are thick and have a single tip (Fig. 3B, D). Bands of Spine are not present on the inter-band area of thoracic segments (Fig. 3C, F). Spines on the anterior spinose band directed perpendicular related to the surrounding integument (Fig. 3B). Sizes of spines gradually decrease towards the posterior end of the body. The anterior rows of spine within the same spinose band project forwards while the posterior row of spines projects backward (Fig. 3F). A ring of hair like spines is present on the anal division surrounding spiracular cavity (Fig. 2D). Spines are also present on the anal pads (Fig. 2C). Spinose bands consist of 7 to 10 irregular rows of spines (Fig. 3F). Spines are triangular and tapering at the end (Fig. 3B). Spine are not densely packed and arranged in asymmetrical condition without overlapping each other (Fig. 3D). Spines are absent on inter-band area of lateral surface of first thoracic segment but present in between first thoracic and cephalic segment (Fig. 3A).
Pupa: The pupa of S. dux has retracted pseudocephalon alike the other flesh fly species. The 3rd instars larva develops in a hard and compact structure enclosing inside the last larval skin known as pupa. During pupal stage, the cephalic segment and 3 thoracic segments of the larvae constricts in the form of hard and rounded structure anteriorly (Fig. 3G). Pupal size ranges about 9 mm long, barrel shaped and reddish-brown in color. Color tends to be change with the age of the pupa. SEM study revealed that both anterior and posterior parts get heavily scleriotized during pupal stage with small projection appear outwards (Cephalic segments, antennas, papillae, anal pad etc.) (Fig. 3E, G). Also, the arrangements of spines on spinose band are clearly visible on pupa (Fig. 3F). Shape of the spine’s changes from tapering to blunt end (Fig. 3D). The arrangement of spines holds same as they were present in the 3rd instars larvae because the last cuticle of 3rd instars form pupal shell.
4 Discussion
Family Sarcophagidae is an important forensic indicator in evaluating human decomposition as its representatives can detect a dead body within few minutes after death at several kilometers (Goff, 2000). The sarcosaprophagous species larviposit on decaying bodies and within a few hours the larvae begin to develop by feeding off the cadaver while a few species feed on open wounds of vertebrates causing myiasis (Singh and Garg, 2009). Thus, a sound knowledge about the durations of various developmental stages of the flesh fly species found on a corpse has practical application in estimating the time and place of death (Sukontason et al., 2003). The biological data from these species are essential to estimate the minimum Postmortem interval (PMImin), which corresponds to the period of insect activity on corpse (Tomberlin et al., 2011). Therefore, study of larval morphology and adult genitalia will help in the accurate identification of different species of Sarcophagidae. Sarcophagids have medical and forensic importance therefore, their accurate identification is very crucial.
Detailed studies of male genitalia are primarily used for the identifications of sarcophagid species as gentilic characters are species specific. In the subfamily Sarcophaginae the 6th tergite is disappeared, in miltogramminae it is present between 5th tergite and first genital segment, but in paramacronychiinae 6th tergite merged with first genital segment (Kurahashi and Samerjai, 2018). These characters distinguish these subfamilies from each other’s. Moreover, the shape of cercus, serstylus and 5th sternite shows interspecific variations. Structures of Phallus and vesica of penis are also species specific. Chaiwong et al. (2007) studied male genitalia of S. dux with the help of Scanning Electron Microscopy and added various morphological characters for their identification. The shape and arrangement of spines on the fifth Sternite act as an important tool to identify the male of Sarcophagidea (Kurahashi and Samerjai, 2018). Vairo et al. (2011) described identification key for the species of Sarcophagidae based on male terminalia with other morphological characters. Generally, the female genitalia of sarcophagidae are modified to lay larvae (Kurahashi and Samerjai, 2018) and few species also lays eggs (Sukontason et al., 2014). But, in complex species structure the female genitalia also renders good characters for the identification (Kurahashi and Samerjai, 2018). Mostly females appear on the carcasses to lay their larvae, but most of the identification keys are based on the male morphological characters. Therefore, Vairo et al. (2015) provided detailed description of females of Sarcophagidae based on gentilic characters which could be a useful morphological tool to distinguish closely related species or in the case where only the females of each species are available.
Larvae of S. dux are voracious feeder and continuously feed up the carcass until it gets mature. Mostly larvae get collected from the carcass as forensic evidence for death investigations. But, larvae of Sarcophagidae are difficult to identify at the species level due to similarity in their external morphology. A very few sets of characters usually used for the identifications of their larvae. Singh et al. (2012) described various characters commonly used in the larval identification of Sarcophagidae including the shape of cephaloskeleton, organization of anterior spiracles, distribution and arrangements of spines over surface, arrangement and size of papillae around the spiracular cavity, configuration of posterior spiracles. Szpila et al. (2015) also revised the characters of third instars for their identification focusing especially on the configuration of lobes of anterior spiracles, shape, and size of spiracular cavity, arrangement of spines on inter-band area etc. Based on available literature and original material obtained from the rearing of flesh fly S. dux, in present study an effort has been made to describe various identification characters, which could be useful for both the identification of adults and immature stages. Moreover, SEM also revealed many characters which are not visible under standard light microscopy.
The anterior spiracles are external structure and well-known characters for species identification in larvae. Usually, the number, shape, and arrangement of spiracular lobes are used for the larval identification of flesh flies (Ishijima, 1967, Singh et al., 2012). The lobes of anterior spiracles are very minute structure, which is very difficult to extract and visible under light microscope only, therefore for analyzing such minute structure, SEM study plays a significant role in identification. However, the number of lobes on the spiracles in individuals of same species may be different or slight inconsistent (Sukontason et al., 2003; Singh et al., 2012; Szpila et al., 2015). Recently the shape of spiracles (oval or circular) and arrangement of lobes in one or multiple rows are also used as identification characters (Ishijima, 1967; Sukontason et al., 2003, 2010; Velásquez et al., 2010; Szpila et al., 2015). The shape of anterior spiracle of some species (i.e. Ravinia belforti) has a distinct heart-shaped structure (da-Silva-Xavier and de Carvalho Queiroz, 2016). The lobes on the anterior spiracles in S. dux, are remarkably different (i.e. 14–17) from the lobes of other flesh flies within the parasarcophaga genera (Kano et al., 1951; Ishijima, 1967; Tumrasvin and Kano, 1979; Salona Bordas and Gonzalez-Mora, 2005; Singh et al., 2012) (Table. 1).
Species
No. of Spiracular Lobes
Species
No. of Spiracular Lobes
Parasarcophaga ruficornis (Fabricius)
8–12 (Singh et al., 2012)
Parasarcophaga shiritakaensis (Hori)
46–49 (Ishijima,1967)
Parasarcophaga albiceps Meigen
32–38 (Kano et al., 1951)
Parasarcophaga oshimensis (Kano & Field)
38–46 (Ishijima,1967)
Parasarcophaga orchidea (Boettcher)
28–34 (Ishijima, 1967)
Parasarcophaga brevicornis (Ho)
12–15 (Tumrasvin and Kano, 1979)
Parasarcophaga harpax Pandelle
40–44 (Ishijima, 1967)
Parasarcophaga similis (Meade)
24–30 (Ishijima,1967)
Parasarcophaga tsushimae (Senior-White)
33–36 (Ishijima, 1967)
Parasarcophaga aegyptica (Salem)
14–15 (Salona Bordas and Gonzalez-Mora, 2005)
Cephaloskeleton is also used as identification marker for larval identification. Cephaloskeleton is a very minute structure and seems similar to the other sarcophagous species (Rotheray and Gilbert, 1999) but there are some species specific variations in their particular parts i.e. length of ventral cornu, shape and size of the window, shape of the dorsal cornu, shape and size of the dorsal arch and the different width of vertical plate (Singh et al., 2012). Parts of cephaloskelton can be analyzed by using flat and concave slides. But, to make permanent sides of cephaloskelton is very problematic task. As compressing of cephaloskelton on a flat slide by coverslip cause distortion of parts i.e. mouth hooks, dorsal and ventral cornu overlaps (Szpila, 2010; Sukontason et al., 2010; Szpila et al., 2015). Some authors also used concave slides and non-invasive clearing methods, for a better observation of their sclerites. But in these methods, debris between the cornu sometimes makes the dorsal window totally invisible. For proper analysis of cephaloskelton, some authors also used its sagittal section. But the sagittal section causes destruction in its various parts i.e. dorsal bridge, hypostomal sclerite etc. (Richet et al., 2011). To overcome this challenge, we used to cut only dorsal cornu, ventral cornu and mouth hook of one side gently with a sharp needle and then squeezing by cover slip did not dislocate the delicate formations of Cephaloskelton. The dorsal cornu is wider and longer than the ventral cornu in P. ruficornis (Singh et al., 2012). Whereas the length of dorsal cornu (ds) in S. dux is longer than the ventral cornu and width is almost similar. Additionally, some species (i.e. S. (Helicophagella) melanura Meigen) has differential sclerotised structure at the apex of ventral cornu of cephaloskeleton (Szpila et al., 2015) which is absent in S. dux.
Size, shape and arrangements of spines on the surface of immature of necrophagous flies are widely used as identification characters by various authors (Wallman, 2001; Sukontason et al., 2003; Szpila, 2010; Ishijima, 1967; Nandi, 1980; Szpila et al., 2015). Szpila et al. (2015) used the term inter-band area to describe the presence or absence of spines over the central surface of larvae of flesh fly. Presence of spines shows a significant taxonomic character as it shows a little interspecific variation (Wallman, 2001). Moreover, size, shape, distribution, and proportion of spines along the length of larval body also act as peculiar character for its identification (Sukontason et al., 2003; Singh et al., 2012). The distribution of small spines band in the flesh flies may be obstruct by the accessory small spines present over the inter-band area (Szpila et al., 2015). The presence and absence of spine on different integumental sculpture of larvae of flesh fly also shows inter-specific variations. During present study a ring of hair like spines were observed on the anal division around the spiracular cavity which could be a diagnostic character for larval identification in case of S. dux. The shape of thoracic spines is thick, and triple or quadruple tipped in some sarcophagidae species i.e. Ravinia belforti (da-Silva-Xavier and de Carvalho Queiroz, 2016). But in S. dux it is observed thick spines with single tips. Moreover, the Inter-band area of the first thoracic segment of Sarcophaga (Robineauella) caerulescens are covered by spines (Szpila et al., 2015), which is absent in S. dux.
Velásquez et al. (2010) described various characters such as the arrangement of posterior spiracle, shape of spiracular cavity, structures of the anal segment for the larval identification of Sarcophagidae. The shape and arrangements of three spiracular slits on the posterior spiracle are acts as an important character in the identification of sarcophagidae (Cantrell, 1981). Moreover, the degree of sclerotisation and the shape of internal peritremal projection is an intraspecific variation i.e. in P. ruficornis, it is projected inwards in a triangular shape (Singh et al., 2012), But in S. dux it is asymmetrical. In the larvae of Sarcophaga caerulescens ventral arc of posterior spiracle is absent (Ishijima, 1967) which is well defined in S. dux. The peritremes are distantly arranged in S. dux (Fig. 2E), whereas it is absent in some species i.e. Ravinia belforti (da-Silva-Xavier and de Carvalho Queiroz, 2016).
Tubercles of Oxysarcodexia paulistanensis and Ravinia belforti are almost similar and elongated (Lopes and Leite, 1987; da-Silva-Xavier and de Carvalho Queiroz, 2016). The size of the papillae/tubercles at the caudal segment is also a useful identification character (Lopes and Leite, 1987). The size of papillae may be smaller to moderate. The tubercles (p1-p5) of P. ruficornis are moderate and similar in size (Singh et al., 2012), whereas in S. dux (p1- p2) tubercles are smaller in size. Tubercles of Oxysarcodexia paulistanensis and Ravinia belforti is almost similar and elongated (Lopes and Leite, 1987; da-Silva-Xavier and de Carvalho Queiroz, 2016). Lopes and Leite (1987) also observed the outer dorsal papillae of third instar of Oxysarcodexia paulistanensis much more elongated than that of Oxysarcodexia confusa. Therefore, it can act as the useful morphological character to differentiate closely related species of flesh flies (Singh et al., 2012). The pairs of papillae are constant at species level although, its arrangement in regular or irregular rows is useful for species identification (Kano et al., 1951).
The width of entrance and development of spiracular cavity is a useful character for the identification of third instar larvae of flesh fly (Szpila, 2010). In various cases such as the third instar larvae of genus Sarcophaga are simply misidentified with the larvae of genus Wohlfahrtia as both have undeveloped integumental sculpture (Szpila et al., 2015). Therefore, size and development of spiracular cavity could be a useful tool to distinguish such kind of resembling species. Moreover, in third instars larvae of subfamily Paramacronychiinae the entrance of spiracular cavity is precisely smaller than that of sub family Sarcophaginae (Szpila et al., 2015). In flesh fly Phylloteles pictipennis, the Spiracular cavity is not developed, which distinguish it from other species (Szpila et al., 2015). Generally, the key character which is used to identify Sarcophagidae is based mostly on male gentilic characteristics. Lack of identification keys of immature stages make it difficult to identify. Rearing of immature stages up to adults required for correct identification. Still, more work needs to be done for the identification of Sarcophagidae particularly on the immature stages, as they act as important forensic indicators during crime investigation.
Acknowledgements
This study was financed by Taif University Researchers Supporting Project number (TURSP-2020/92), Taif university, Taif, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effects of insecticide dimethoate on the developmental rate of forensic importance sarcophagid flies. Journal of King Saud University –. Science. 2021;33(2):101349.
- [CrossRef] [Google Scholar]
- Multilocus and multiregional phylogeny reconstruction of the genus Sarcophaga (Diptera, Sarcophagidae) Molecular phylogenetic and evolution. 2017;107:619-629.
- [CrossRef] [Google Scholar]
- Molecular phylogeny of the hyperdiverse genus Sarcophaga (Diptera: Sarcophagidae), and comparison between algorithms for identification of rogue taxa. Cladistics. 2017;33:109-133.
- [Google Scholar]
- The immature stages of some Australian Sarcophaginae (Diptera: Sarcophagidae) Journal of Australian Entomological Society. 1981;20(3):237-248.
- [Google Scholar]
- Chaiwong, T., Sukontason, K.L., Chaithong, U., Olson, J.K., Kurahashi, H., Sukontason, K. 2007. Male genitalia of flesh fly ParaSarcophaga dux (Diptera: Sarcophagidae) revealed by scanning electron microscopy. Journal of American Mosquito Control Association, 23:80–83
- Occurrences of flesh flies (Diptera: Sarcophagidae) on human cadavers in Switzerland, and their importance as forensic indicators. Forensic Sci. Int.. 2012;220(1-3):158-163.
- [Google Scholar]
- Accidental intestinal myiasis caused by genus Sarcophaga. Indian Journal of Medical Microbiology.. 2010;28(2):176.
- [CrossRef] [Google Scholar]
- Ultrastructure analysis of the immature stages of Ravinia belforti (Diptera: Sarcophagidae), a species of medical-veterinary and forensic importance, by scanning electron microscopy. Acta Trop.. 2016;159:192-199.
- [Google Scholar]
- A Fly for the Prosecution: Insect Evidence Helps Solving Crimes. London, Cambridge: Harvard University Press; 2000. p. :225.
- Revision of the third stage larvae of synanthropic flies of Japan (Diptera: Anthomyiidae, Muscidae, Calliphoridae and Sarcophagidae) Japanese journal of Sanitization Zoology. 1967;18(2-3):47-100.
- [Google Scholar]
- Notes on the flies of medical importance in Japan (Part II). The larvae of Sarcophaga known in Japan. Japanese Journal of Experimental Medicine. 1951;21:115-131.
- [Google Scholar]
- Parasitic infections of domestic animals: a diagnostic manual. Basel, Switzerland: Birkhauser Verlag; 1996.
- Revised keys to the flesh flies of Thailand, with the establishment of a new genus (Diptera: Sarcophagidae) Medical Entomology and Zoology. 2018;69(2):67-93.
- [CrossRef] [Google Scholar]
- Third contribution to the knowledge of the Raviniini (Diptera, Sarcophagidae), based on observations of the larvae, using scanning electron microscope. Mem Inst Oswaldo Cruz. 1987;82(3):407-413.
- [Google Scholar]
- Studies on the larvae of flesh flies from India (Diptera: Sarcophagidae) Oriental Insects. 1980;14(3):303-323.
- [Google Scholar]
- Fauna of India: Diptera: Sarcophagidae. Published by Zoological Survey of India.. 2002;10:270-274.
- [Google Scholar]
- Sarcophaga of France (Diptera: Sarcophagidae). Sofia: Pensoft Series Faunistica; 2011.
- The phylogeny of palaearctic Syrphidae: evidence from larval stages. Zoological Journal of Linnaeus Society. 1999;127:1-112.
- [Google Scholar]
- Primera cita de Liosarcophaga aegyptica (Salem, 1935) (Diptera, Sarcophagidae) de la Peninsula Iberica, con descripcion de sus fases larvarias II y III, pupario y adultos. Bol Soc Entomology Aragon. 2005;36:251-255.
- [Google Scholar]
- Parasitoidism of the Sarcophaga dux (Diptera: Sarcophagidae) on the Mesobuthus martensii (Scorpiones: Buthidae) and Its Implications. Ann. Entomol. Soc. Am.. 2015;108(6):978-985.
- [Google Scholar]
- Effect of seasonal temperature variation on the duration of life cycle stages of the fly of forensic importance, Parasarcophga dux (Thomson) (Diptera: Sarcophagidae) International journal of advanced research. 2017;5(11):265-269.
- [Google Scholar]
- Studies on the life history of sarcophaga ruificornis (Fab.) at varying temperatures (Diptera: Sarcophagidae) Annu. Rev. Entomol.. 2009;27(1–21):45-48.
- [Google Scholar]
- Ultramorphological characteristics of immature stages of a forensically important fly Parasarcophaga ruficornis (Fabricius) (Diptera: Sarcophagidae) Parasitological Research. 2012;110(2):821-831.
- [Google Scholar]
- Intra-puparial development of flesh fly Sarcophaga dux (Thomson) (Diptera, Sarcophagidae) Current Science Association. 2016;111(6):1063-1107.
- [Google Scholar]
- Sukjit S: Diversity and succession of carrion arthropods on pig Sus scrofa domestica carcasses under different conditions in Nan Province, Thailand. Thailand: Chulalongkorn University; 2011:1–236. Ref Type: Thesis/ Dissertation
- Fine structure of the eggshell of the blow fly, Lucilia cuprina. J. Insect Sci.. 2007;7 Article 9
- [Google Scholar]
- Sarcophaga dux (Diptera: Sarcophagidae): A flesh fly species of medical importance. Biol. Res.. 2014;47(14):1-9.
- [Google Scholar]
- Forensically important flesh fly species in Thailand: Morphology and developmental rate. Parasitol. Res.. 2010;106:1055-1064.
- [Google Scholar]
- Larval ultrastructure of Parasarcophaga dux (Thomson) (Diptera: Sarcophagidae) Micron. 2003;34(8):359-364.
- [Google Scholar]
- Szpila, K. 2010. The first instar of European Miltogramminae (Diptera: Sarcophagidae). Wydwanictwo Naukowe UMK, Toruń, 272 ss.
- Third instar larvae of flesh flies (Diptera: Sarcophagidae) of forensic importance—critical review of characters and key for European species. Parasitol. Res.. 2015;114(6):2279-2289.
- [Google Scholar]
- A roadmap for bridging basic and applied research in forensic entomology. Annual Reviews of Entomology. 2011;56:401-421.
- [Google Scholar]
- Studies on medically important flies in Thailand. VI. Reports on 48 species of sarcophagid flies, including the taxonomic keys (Diptera: Sarcophagidae) Bull. Tokyo Med. Dent. Univ.. 1979;26:149-179.
- [Google Scholar]
- Pictorial identification key for species of Sarcophagidae (Diptera) of potential forensic importance in southern Brazil. Revista Brasileira de Entomologia. 2011;55(3):333-347.
- [Google Scholar]
- Comparative morphology and identification key for females of nine Sarcophagidae species (Diptera) with forensic importance in Southern Brazil. Revista Brasileira de Entomologia. 2015;59(3):177-187.
- [Google Scholar]
- Diptera of forensic importance in the Iberian Peninsula: larval identification key. Med. Vet. Entomol.. 2010;24(3):293-308.
- [CrossRef] [Google Scholar]
- Third instar larvae of common carrion-breeding blowflies of the genus Calliphora (Diptera: Calliphoridae) in South Australia. Invertebrates Taxonomy. 2001;15(1):37.
- [CrossRef] [Google Scholar]







