Translate this page into:
Two factor at a time approach by response surface methodology to aggrandize the Bacillus subtilis KLP2015 surfactin lipopeptide to use as antifungal agent
⁎Corresponding author. kanwarss2000@yahoo.com (Shamsher S. Kanwar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A Gram’s positive, rod shaped and endospores-forming ‘bacterial isolate KLP15′ was identified as Bacillus subtilis (Accession number KT459335) by the biochemical tests and 16S rRNA based sequencing. Lipopeptide(s) production from B. subtilis KLP2015 increased from 200.0 to 547.0 mg/l after the optimization of physico-chemical parameters like choice of nitrogen source, fermentation time, fermentation temperature and broth pH by ‘One Factor at a Time’ (OFAT) approach. Further, the yield of lipopeptide(s) increased to 985 mg/l from an earlier value of 547 mg/l when a “Two Factors at a Time (TFAT) approach using Response Surface Methodology (RSM) was attempted. The extracted crude lipopeptide fraction from the cell-free fermentation broth of B. subtilis KLP2015 was purified and characterized by High Performance Liquid Chromatography (HPLC), Thin Layer Chromatography (TLC), UV-Visible Spectrophotometry (UV-vis), Fourier Transform Infrared (FTIR) and MALDI-TOF studies. HPLC and TLC results confirmed the purity of the lipopeptide preparation and its Surfactin-type nature. The FTIR and MALDI-TOF spectra of the purified lipopeptide were found to be similar to that of an authentic Surfactin molecule. The purified lipopeptide showed potent 75.1% and 41.9% antifungal activity against Mucor sp. and Aspergillus niger, respectively on the basis of zone of inhibition of growth of these fungal strains. Moreover, the purified lipopeptide preparation of B. subtilis KLP2015 was most effective against the growth of Mucor sp. as reflected by the recorded MIC values of 6.25 µg/ml while MIC of lipopeptide for the A. niger was 12.5 µg/ml.
Keywords
Antifungal activity
Biocontrol
Lipopeptide(s)
Optimization
Surfactin
1 Introduction
The excessive use of chemical pesticides in the agriculture crops has created an adverse ecological balance that affects the whole ecological environment (Meena et al., 2014; Meena and Kanwar, 2015). Chemical surfactant compounds extensively used in petroleum industries are very toxic, often recalcitrant in nature and cause extensive contamination of ground water (Sujata et al., 2016). The increasing defiance of microorganisms to secondary metabolites/antibiotics is a major debatable concern in the world. To avoid the use of toxic and non-ecofriendly surfactants/ detergents, there is an urgent need to develop eco-friendly new lipopeptide-like antibiotics with higher yield to combat the fungal phytopathogens possessing distinct modes of action (Arik et al., 2006). Surfactin lipopeptides (LPs) are low molecular weight biosurfactants that can effectively emulsify and decrease the surface tension of water (Selene and Gustavo, 2016). Keeping these issues in mind, the present study focussed on the use of B. subtilis LP to inhibit the growth of the fungal phytopathogens of crops like Aspergillus niger and Mucor sp. which cause diseases in the plants. Aspergillus niger is a one of the most prominent species of the fungal genus Aspergillus. It causes a disease called ‘black mold’ on certain fruits and vegetables such as grapes, onions and peanuts and is also a common contaminant of stored foods (Sharma, 2012). Capsicum annuum is a plant of genus Capsicum which originated in the American tropics and has been propagated throughout the world (Pickersgill, 1997). The fruit of Capsicum has variety of names, such as ‘chilli’, ‘chilli pepper’ or ‘pepper’ depending on the place and type of fruit. Chilli (Capsicum sp.), an important economic crop in the whole world (Poulos, 1992) is severely infected by anthracnose fungus which may cause crop yield losses of up to 50% (Pakdeevaraporn et al., 2005) and 10–80% in some of the developing countries (Poonpolgul and Kumphai, 2007). In India, Capsicum spp. are commonly used as vegetables or salad-dressing by the people. The Capsicum spp. are also key earning sources of people of Shimla region in North-India. In the present study, a fungal strain isolated from the roots of diseased Capsicum annuum plant was identified as Mucor sp. by 18S rRNA sequencing. In the earlier reports, Colletotrichum species and some other fungal strains have been reported as phytopathogens but none of the Mucor spp. has been reported as disease causing agent of Capsicum plants. Unfortunately, the use of biological control methods for prevention of chilli plant diseases has not received much attention in the past. Thus, in this study, we have used a Mucor sp. as a phytopathogen to challenge the Capsicum annuum plants. This is the first report of use of Surfactin LP of a B. subtilis strain as an antifungal agent to prevent Mucor sp. manifestation and infection of Capsicum annuum plants. B. subtilis produces chiefly three types of lipopeptides with potent antifungal activities for agriculture, biotechnological and biopharmaceutical applications (Hsieh et al., 2008). Lipopeptides are small molecular mass bioactive molecules (including Surfactin, Iturin, Fengycin, Mycosubtilin etc.) which exhibit surfactant and strong antimicrobial activities (Lee et al., 2006; Kim et al., 2010). To enhance the recovery of lipopeptides from the cell-free fermentation broth, an acid precipitation method used in our study was found to be very cost-effective. As biocontrol agent/ antibiotic, the bacterial LPs may be involved in quorum sensing, cell motility and the formation of biofilm on the animate or inanimate surfaces (Priya and Usharani, 2009). Thus the bacterial LPs may be tried and evaluated to resolve a number of global issues in medicine (Banat et al., 2010), industry (Abdel-Mawgoud et al., 2008) and environmental protection (Meena et al., 2018; Mulligan, 2009). Moreover, the bacterial lipopeptides have lower toxicity for plants and animals, high biodegradability, low irritancy and good compatibility with human skin (Cameotra and Makkar, 2004; Meena et al., 2017). The present study primarily focussed to enhance the yield of the LPs produced by B. subtilis in the fermentation broth using a TFAT-RSM approach.
2 Materials and methods
2.1 Identification of bacterial isolate (KLP15)
The LPs producing bacterial strain was isolated from the sample of rhizosphere soil, of Pisum sativum collected from the Solan, Himachal Pradesh, India. A bacterial isolate KLP15 was subjected to both Gram’s and Endospore staining according to previously reported methods (Oktari et al., 2017). Stained glass slides were mounted with the fresh culture of bacterial strain and observed at 1000 X magnification under a compound microscope. DNA was isolated from the bacterial culture followed by PCR amplification of 16S rDNA by using a forward (785F; GGATTAGATACCCTGGTA) and a reverse primer (907R; CCGTCAATTCTTTAGTTT; Sergio and William, 2009). PCR mixture consisted of Taq DNA polymerase (0.5 µl), dNTPs (200 µM; 1 µl), MgCl2 and reaction buffer (1X; 5 µl) for efficient PCR amplification of DNA templates (20 ng/µl; 1 µl). The PCR was conducted under the following conditions: denaturation at 95 °C for 2 min; 30 cycles of denaturation (30 s at 95 °C), annealing (1 min at 55 °C) and extension at 72 °C for 10 min (Hongoh et al., 2003). The forward (800 bp) and reverse (911 bp) sequenced were further merged and a sequence of 1441 bases was obtained to perform BLAST analysis by using CLUSTAL-W program of MEGA software version 6.0 (Tamura et al., 2007). The gene sequences of closely related bacterial strains were retrieved from a server through the BLAST and the evolutionary dendrogram was deduced by Neighbour-Joining method (Saitou and Nei, 1987). A Phylogenetic tree was constructed by using the CLC drug discovery work bench software (Tamura et al., 2007; Sharma et al., 2017).
2.2 Lipopeptide(s) extraction by acid precipitation methanol extraction (APME) method
For production and extraction of lipopeptides, the bacterial culture was grown at 30 ± 1 °C for 72 h in Erlenmeyer flasks (250 ml capacity) containing 100 ml of Luria Bertani (LB) broth pH 7.0 at 200 rpm in a shaking incubator. After incubation, bacterial cells pellet was removed by centrifugation (10,000×g for 10 min) and the LP containing cell-free fermentation broth was collected. The pH of the cell-free fermentation broth was adjusted to 2.0 by adding 6 N HCl (Grover et al., 2010). The acid precipitated LPs were recovered by centrifugation (12,000×g for 15 min at 4 °C) and were extracted with methanol used in a ratio of 1:20 (mg/µl).
2.3 Optimization of nitrogen source, fermentation time, temperature and pH of the LB-fermentation broth for the lipopeptides production by B. subtilis
The effect of the selected nitrogen sources on the production of LPs by B. subtilis were studied by adding 1% (w/v) of chosen (Ammonium sulphate, peptone, urea, sodium nitrate, ammonium nitrate, beef extract, potassium nitrate, ammonium chloride or yeast extract) nitrogen source individually to the production broth i.e. Luria Bertani (LB) broth containing casein enzyme hydrolysate (10 g/l), yeast extract (5 g/l) and NaCl (5 g/l). The inoculated broths (5% v/v of 7 h seed culture) with pH set to 7.0 were incubated for 48 h at 37 °C under shaking at 200 rpm followed by LPs extraction by APME method. The effect of incubation time (24, 48, 72, 96 and 120 h), temperature (25, 30, 35, 37, 40, 45 and 50 °C) and pH (4, 5, 6, 7, 8, 9, 10, 11 and 12) were tested separately for the production of lipopeptide in the fermentation broth by the B. subtilis. The yield of the crude lipopeptide obtained in each case was determined and recorded.
2.4 Validation of response surface methodology (RSM) approach for the statistical optimization of lipopeptide(s) production by B. subtilis
In Plackett-Burman analysis, three optimized variables [beef extract (10 g/l), production time (72 h) and KH2PO4 (10 mM)] were selected for further screening by Central Composite Design (CCD) analysis. In Plackett-Burman analysis, a total 12 combinations of broth ingredients were generated by the Design expert software (version 7.0) and all these experiments/set-ups were performed. The crude lipopeptides yield was recorded in each case. Plackett-Burman design results obtained from different combinations of the above 3 variables were used to build a Pareto chart to find out the significant variables to test them further in the CCD analysis for the LPs production. Design expert software (version 7.0) was used for Plackett-Burman design to regress analysis of the chosen variables. The p values < 0.05 and positive factors [production time and KH2PO4] observed from the Pareto chart were considered for further optimization for LPs production by B. subtilis KLP2015 strain using Central Composite Design (CCD) model. The K+ ions might play an important role in the bacterium to maintain Na+/K+ ion channels accurately. Generally, B. subtilis uses active potassium transport mechanisms to concentrate intracellular potassium. In CCD, production time range (72, 84 and 96 h) and KH2PO4 concentration (10, 15 and 20 mM) were taken in which middle value was optimized from the Plackett-Burman design. In CCD, a total of 13 experiments were performed and result were recorded.
2.5 Purification and characterization of the lipopeptide of B. subtilis KLP2015
2.5.1 HPLC analysis of the lipopeptide
The lyophilized powder of LP was dissolved in methanol (1 mg/ml) for the HPLC analysis. Lipopeptide sample was analyzed by HPLC using 515 HPLC pump (Waters, USA) equipped with a reverse phase Lichrospher ®100 C18 (5µm) (4.6 mm × 250 mm) column (Merck, Germany) and 2998 photodiode array detector. The stationary phase was a C18 bonded phase on silica with 5 µm particles (Kinsella et al., 2009). The test (LP preparation) and reference compounds were analyzed in isocratic elution processes using a mobile phase of 80: 20 (v/v) acetonitrile: water containing 0.1% trifluoroacetic acid. The flow rate of mobile phase was adjusted at 1 ml/min. Lipopeptide sample (10 µl in methanol) was injected and analyzed at 208 nm wavelength. Lipopeptide of B. subtilis KLP2015 was quantified by comparing the area under peak of the lipopeptide sample with that of the reference lipopeptides i.e., Surfactin and Iturin A. The exact amount of LP present in the test sample was calculated using following formula;
2.5.2 Thin layer chromatography for detection of LP
A solvent system comprising chloroform: methanol: water (65:25:4; v/v; Xiao-Hong et al., 2009) was developed as a mobile phase and active lipopeptide fraction (5 µl) was applied at the point of origin towards the bottom of the TLC plate (SDFCL, Silica gel 60/ UV254, 0.5∗20 cm and thickness: 0.2 mm). As the lipopeptide sample moved up to the top of the plate, the developed TLC plate was removed and air dried. To detect the presence of lipid moieties associated with a protein, the TLC plate was sprayed uniformly with distilled water and kept for drying. However, to detect the amino acids/peptide spots, the TLC plate was sprayed with the ninhydrin (0.25% w/v in methanol). The Rf values of test or reference were calculated by using the following formula;
2.5.3 UV-VIS spectra of the lipopeptide
The ultraviolet absorbance spectrum of the lipopeptide of B. subtilis KLP2015 was measured. UV-vis spectrophotometer (CARY VARIAN, USA) was used for the measurement of absorbance values at wavelengths from 190 to 800 nm (Bechard et al., 1998). Spectra of Iturin A and Surfactin were also taken as reference profiles.
2.5.4 FTIR analysis of the purified lipopeptide of B. subtilis KLP2015
The FTIR was used to determine the functional groups and the chemical bonds present in the purified LP of B. subtilis KLP2015 (Das et al., 2008). The infrared spectrum of the authentic LP ‘Surfactin’ (Sigma Aldrich, USA) and ’Iturin A’ (Sigma Aldrich, USA) were also obtained for comparison with spectra of purified LP of B. subtilis KLP2015. The spectrum was generated in the range of 400–4000 cm−1 with a resolution of 4 cm−1 which was further processed with IR analytical software.
2.5.5 MALDI TOF-MS/MS of the purified lipopeptide of B. subtilis KLP2015
The purified LP was mixed with the cyno-4 hydroxycinnamic acid (10 mg/ml) in 50% (v/v) of acetonitrile in 0.1% trifluoroacetic acid followed by spotting onto a target plate for the LP analysis. Mass spectra were recorded on a Bruker Omniflex instrument (USA) in reflectron mode. Ion ‘source 1’ and ‘source 2’ were set to 19.0 and 14.0 kV, respectively (Neil et al., 2007). Samples were dried under a lamp onto a conventional stainless-steel target. Two hundred-nanosecond pulsed ion extraction was used with matrix suppression up to 200 Da. Results of the MALDI were observed and recorded for the analysis of purified LP of B. subtilis KLP2015.
2.6 Application of the purified lipopeptide of B. subtilis KLP2015
2.6.1 Antifungal activity of purified lipopeptide against selected fungal pathogens
The purified Surfactin of B. subtilis KLP2015 dissolved in sodium phosphate buffer (10 mg/ml; 50 µl) was bio-assayed on Potato dextrose agar (PDA) medium taken in a Petri plate against each of the test pathogens that included Mucor sp. and A. niger by Well-diffusion method (Tagg and McGiven, 1971). The percent (%) growth inhibition of inoculated fungal stain was calculated as follows;
Where Dc = Average diameter of mycelial colony control; Dt = Average diameter of treated sets
Purified Surfactin LP preparation [5 ml; 10 mg/ml prepared in 20 mM phosphate buffer (pH 7.5)] of B. subtilis KLP2015 was also checked for its antimucor activity on Capsicum annuum plants experimentally infected with Mucor sp. The fungal (5 ml) mycelial suspension of spores (2.5 × 105 spores/ml) of Mucor sp. inoculated in the Capsicum annuum roots was taken as a control group. The simultaneous fungal and Surfactin LP inoculation in the Capsicum annuum roots was kept as a test for observation on 10, 20 and 30 days post inoculation. After 10, 20 and 30 days, lipopeptide-treated and placebo-treated [20 mM phosphate buffer; pH 7.5] Capsicum annuum plants challenged with selected fungal Mucor sp. were observed and results were recorded.
2.6.2 Minimum inhibitory concentration (MIC) assay for antifungal activity of LP by the broth micro dilution method
The MIC is the lowest concentration of the any antimicrobial agent that completely inhibits growth of the test organism (Meir et al., 2017). Autoclaved Potato dextrose (PD) broth (100 µl) was dispensed into the wells of a 96-wells micro titre plate. The LP (98 µl; 100 µg/ml) of B. subtilis KLP2015 was also added followed by its 2-fold dilution. After adding the LP (98 µl) in the upper most wells, the volume was diluted subsequently by transferring 100 µl in the succeeding wells. Thereafter, 2 µl of the each of the selected fungal spore suspension (Mucor sp, and A. niger) was inoculated in the wells. The fungal inoculated micro titre plates were incubated for the 48 h at 30 °C. Thereafter, the results were observed and recorded.
2.6.3 Lactophenol staining of fungal (Mucor sp.) phytopathogen
Remel lactophenol is a dye used for qualitative procedures to prepare fungal specimens for microscopic examination (Linder, 1929). Fungal cells (10 µl; OD540 ∼ 1.0) of the Mucor sp. were directly incubated with lipopeptide (300 µl; 10 mg/ml) for 24 h. Lactophenol stain was added (5 µl) into the suspension of fungal cells and LP, and this suspension was examined under the microscope (1000× magnification).
3 Results
3.1 Identification of bacterial isolate KLP15
Gram’s staining results showed Gram positive, purple colored, rod shaped bacterial cells in the culture smear after examining under the microscope (Fig. 1A). This bacterial isolate also produced (Malachite stained green colored) endospores (Fig. 1B). The bacterial isolate KLP15 was characterized by 16S rRNA analysis. The evolutionary dendrogram was deduced by using the CLC drug discovery work bench software to analyze its genus and species (Fig. 1D). The bacterial isolate KLP15 was identified as a Bacillus subtilis strain KLP2015 (NCBI Accession number KT459335).![Identification of bacterial isolate. (A) Gram’s staining image; (B) Endospore staining image of the Bacterial isolate KLP15; (C) Gel image of 16S rDNA amplicon [Lane 1: DNA markers and Lane 2: 16S rDNA amplicon of bacterial isolate KLP15]; and (D) Phylogenetic tree of B. subtilis (mentioned as isolate KLP15) generated by CLC drug discovery work bench software showed prominent similarity of the investigated amplified DNA (for rRNA) to 9 nearest neighbours.](/content/185/2020/32/1/img/10.1016_j.jksus.2018.05.025-fig1.png)
Identification of bacterial isolate. (A) Gram’s staining image; (B) Endospore staining image of the Bacterial isolate KLP15; (C) Gel image of 16S rDNA amplicon [Lane 1: DNA markers and Lane 2: 16S rDNA amplicon of bacterial isolate KLP15]; and (D) Phylogenetic tree of B. subtilis (mentioned as isolate KLP15) generated by CLC drug discovery work bench software showed prominent similarity of the investigated amplified DNA (for rRNA) to 9 nearest neighbours.
3.2 Choice of nitrogen source, production time, temperature and pH for production of lipopeptides by B. subtilis KLP2015
The recorded results indicated that beef extract was the best nitrogen source as far as the yield of lipopeptides (200 mg/l) was concerned (Fig. 2A). After 72 h fermentation, the maximum amount of lipopeptide was produced (530 mg/l; Fig. 2B) by B. subtilis KLP2015 that was considered as the best incubation duration. Among the different fermentation temperatures, the maximum lipopeptide production by B. subtilis KLP2015 was observed at temperature 30 °C (545 mg/l; Fig. 2C). The optimal yield of 547 mg/l by B. subtilis KLP2015 was however, observed at pH 7.0 (Fig. 2D).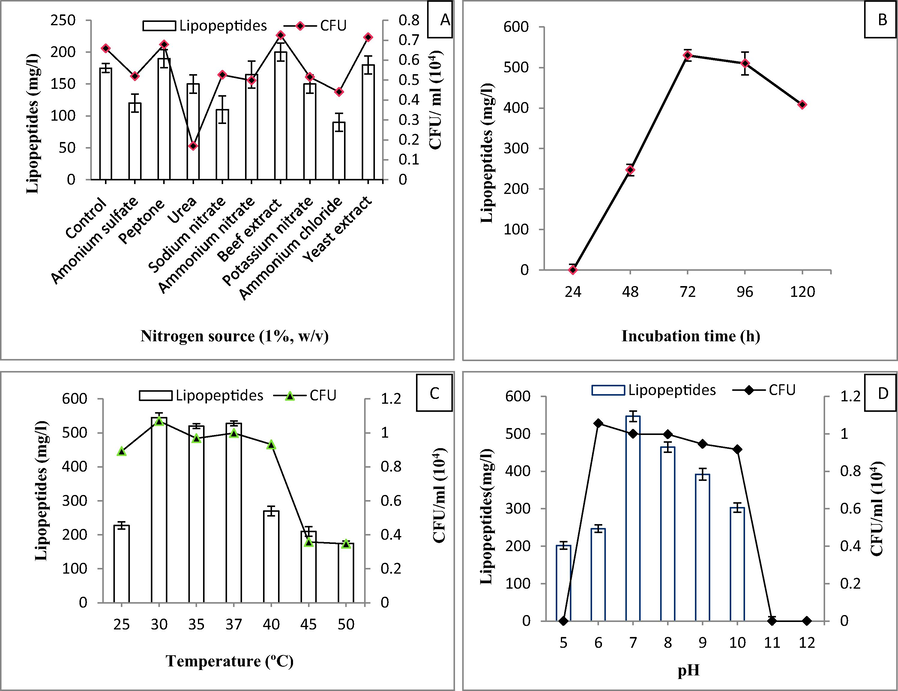
(A) Optimization of different nitrogen source; (B) production time; (C) temperature; and (D) pH for the for optimal yield of LP in a batch culture.
3.3 Response surface methodology for optimal synthesis of lipopeptide by B. subtilis KLP2015
Among 12 experiments executed in the present study, the LPs yield in the fermentation from seeded with B. subtilis KLP2015 varied between 612 to 930 mg/l and the ‘experiment protocol/ set-up 9′ consisting of beef extract (30 g/l), production time (84 h) and KH2PO4 (15 mM) was found to be most appropriate that gave a yield 930 mg/l of lipopeptide in the fermentation broth, which was the maximum one obtained following the Plackett-Burman design (Table 1). To select the positive significant factors from the Plackett-Burman design which might have influenced the yield of extracellular LPs produced by B. subtilis KLP2015, the Pareto chart (Fig. 3A) was analyzed. The production time and KH2PO4 were further selected for CCD analysis. The highest LP (985 mg/l) production by B. subtilis KLP2015 was recorded in the 9th experimental set-up of CCD conducted with an extended incubation time (96 h), KH2PO4 (10 mM) and pH set to 7.0 (Table 2). Different models were generated by the Design expert version 7.0, and out of these a linear model was suggested wherein a p value of <0.0001 was recorded which was highly significant (Table 3). #: These observed values were significant when tested under a Linear-model.
Experiment set-up
Beef extract (g/l)
Production time (h)
KH2PO4 (mM)
Lipopeptide yield (mg/l)
1
30.0
60.0
5.0
612.0
2
10.0
84.0
5.0
928.0
3
10.0
60.0
5.0
613.0
4
10.0
84.0
15.0
629.0
5
10.0
84.0
15.0
927.0
6
10.0
60.0
5.0
614.0
7
30.0
60.0
15.0
618.0
8
30.0
84.0
5.0
925.0
9
30.0
84.0
15.0
930.0
10
10.0
60.0
15.0
626.0
11
30.0
84.0
5.0
926.0
12
30.0
60.0
15.0
616.0
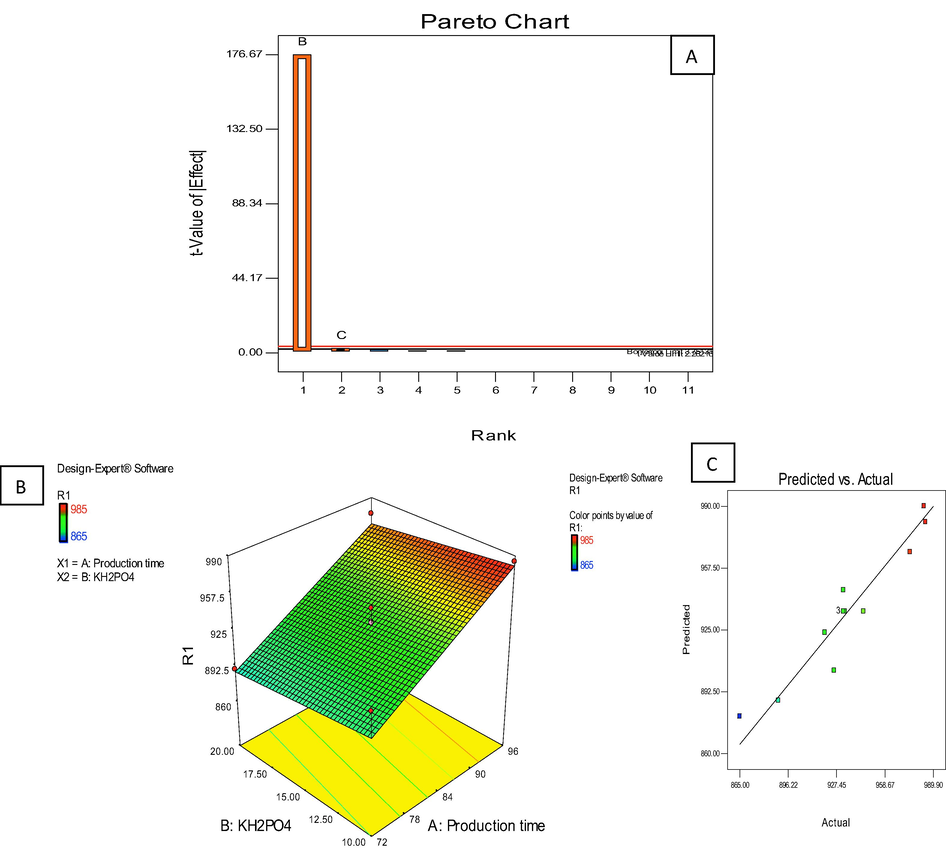
(A) Plackett-Burman design indicating the effect of different factors on the LPs production by B. subtilis KLP2015; (B) 3 D contour plot showed the effect of two significant factors (production time and KH2PO4; and (C) The predicted and actual values of the all runs generated by the CCD model.
Experiment set-up
Production time (h)
KH2PO4 (mM)
Lipopeptide yield (mg/l)
1
84.0
15.0
932.0
2
67.0
15.0
865.0
3
72.0
10.0
926.0
4
96.0
20.0
975.0
5
84.0
15.0
933.0
6
84.0
15.0
945.0
7
72.0
20.0
890.0
8
84.0
7.9
932.0
9
96.0
10.0
985.0
10
84.0
22.1
920.0
11
84.0
15.0
932.0
12
84.0
15.0
932.0
13
100.97
15.0
984.0
Component(s) under consideration
Sum of squares
df
Mean square
p-Value
B - Production time
12190.74
1
12190.74
<0.0001#
C - KH2PO4
495. 66
1
495.66
0.0580#
Lack of Fit
1053.57
6
175.59
0.0629
Linear vs Mean
12686.40
2
6343.20
<0.0001#
2FI vs Linear
169.00
1
169.00
0.2521
Quadratic vs 2FI
2.34
2
1.17
0.9919
Cubic vs Quadratic
179.10
2
89.55
0.6149
3.4 ANOVA analysis for selected factorial model to improve the yield of LPs by B. subtilis KLP2015
A p value of <0.0500 indicated that model terms were significant. In this case the factor B (production time) and C (KH2PO), were significant factors that exhibited strong influence on the LPs production by B. subtilis KLP2015. The 3D contour plot and graph of predicted and actual values generated by the CCD model were presented (Fig. 3).
3.5 Purification and characterization of lipopeptide of B. subtilis KLP2015
3.5.1 HPLC analysis of the extracted LP of B. subtilis KLP2015
The retention time (RT) of the Surfactin during HPLC was 1.270 min (Fig. 4A), Iturin A was 4.234 min (Fig. 4B) and that of the purified LP of B. subtilis KLP2015 was 1.088 min (Fig. 4C). Thus the recorded RT of the LP preparation of B. subtilis KLP2015 was similar to RT of Surfactin which indicated the presence of ‘Surfactin molecule(s)’ in the acid precipitated and purified lipopeptide fraction. The HPLC analysis showed ∼9.952 mg yield of the lipopeptide from 500 ml of optimized fermentation broth inoculated with B. subtilis KLP2015.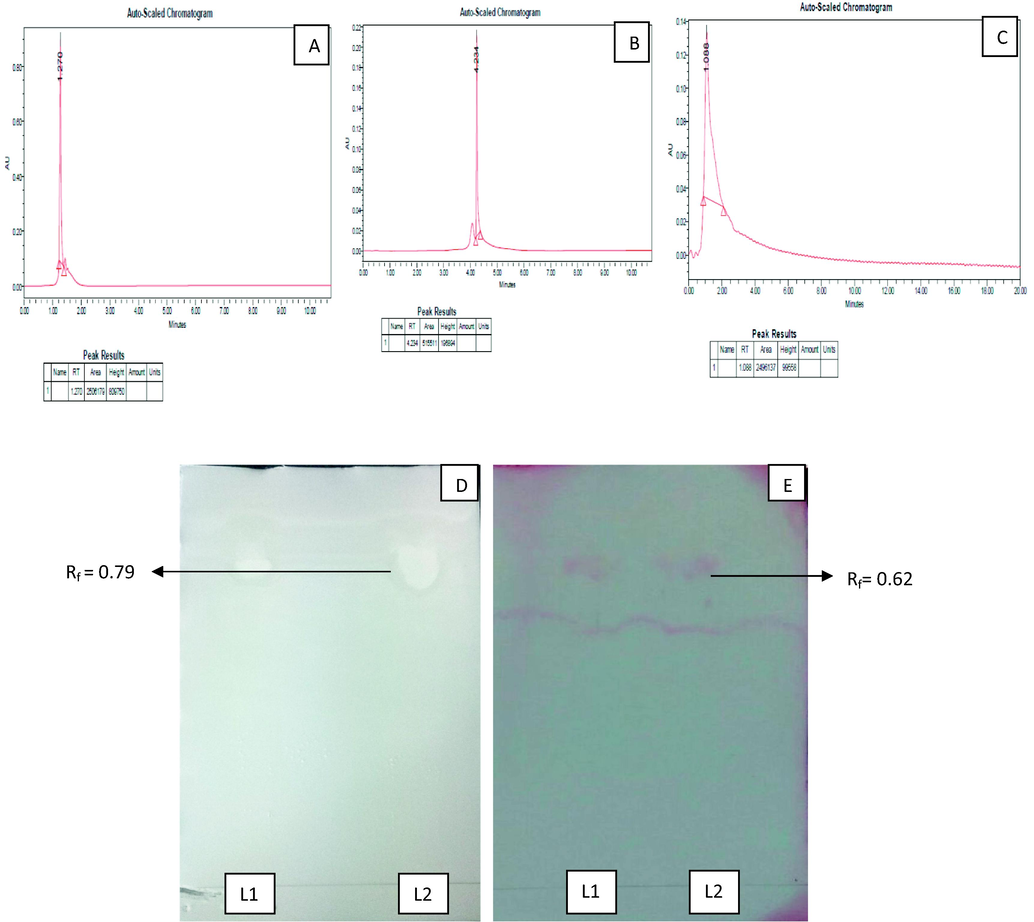
(A) HPLC analysis of the authentic Surfactin; (B) Iturin A; (C) purified lipopeptide of B. subtilis KLP2015; (D) Visualization of white spots on TLC plate indicated the presence of lipidic moiety in the lipopeptide (L1) of B. subtilis KLP2015 as well as in “Surfactin” (L2); and (D) Blue violets spots on the TLC plate indicated the presence of amino acids in the lipopeptide preparation (L1) of B. subtilis KLP2015 as well as in the authentic ‘Surfactin’ (L2).
3.5.2 TLC analysis of LP of B. subtilis KLP2015
The TLC plate containing purified lipopeptide of B. subtilis KLP2015 was sprayed with ninhydrin for the amino acid detection and water for the lipid moiety detection (Fig. 4D & E). The Rf value of 0.79 and 0.62 were recorded when TLC plate was sprayed with the water and ninhydrin, respectively. White spots indicated existence of lipid moiety while blue-violet spots denoted the presence of amino acids in the lipopeptide preparation of B. subtilis KLP2015.
3.5.3 UV-VIS spectra of the purified lipopeptide of B. subtilis KLP2015
Purified LP fraction of B. subtilis KLP2015 (2.0 mg ml) was dissolved in methanol and was used for UV-vis spectral analysis. The UV-vis spectrum of authentic Iturin A (1.0 mg/ml) molecule showed absorbance maxima at 382, 362, 277 and 201 nm while the authentic ‘Surfactin’ (10 mg/ml) showed absorbance at 275, 220 and 208 nm. In contrast, the purified lipopeptide of B. subtilis KLP2015 showed absorbance maxima at 273, 222 and 216 nm (Fig. 5C).![UV-vis spectra of the Iturin A [A]; Surfactin [B] and purified LP of B. subtilis KLP2015 [C] where Iturin A and Surfactin were used as reference molecules.](/content/185/2020/32/1/img/10.1016_j.jksus.2018.05.025-fig5.png)
UV-vis spectra of the Iturin A [A]; Surfactin [B] and purified LP of B. subtilis KLP2015 [C] where Iturin A and Surfactin were used as reference molecules.
3.5.4 FTIR analysis of the purified LP of B. subtilis KLP2015
The FTIR spectrum of the Iturin A (Fig. 6A) and Surfactin (Fig. 6B) were taken as reference profiles/ spectra. In the FTIR spectra of the purified lipopeptide (Fig. 6C), a peak at 3286.6 cm−1 depicted the carbon-containing compound with the amino group (N-H stretching) that indicated the LP nature of this molecule. Peak at wave number 3063.6 cm−1 and 2957.6 cm−1 showed the presence of aliphatic chain and C-CH3 bonding, respectively. Absorbance peak recorded at 2871.6 cm−1 showed the C-H stretching in the long alkyl chain. Peak at 1736.7 cm−1 showed the presence of a lactone ring in the purified lipopeptide of B. subtilis KLP2015. A high intensity peak at 1543.6 cm−1 indicated the presence of C=O bond in the purified LP, which might have been caused due to the C=O stretching vibrations. Peak at 1399.7 cm−1 was due to the presence of aliphatic chain in the purified lipopeptide while the peak at 1274 cm−1 showed the C-O deformation vibrations. The FTIR spectra confirmed ‘Surfactin’ nature of the purified LP of B. subtilis. Thus the purified LP showed maximum similarity to the Surfactin used as a reference molecule.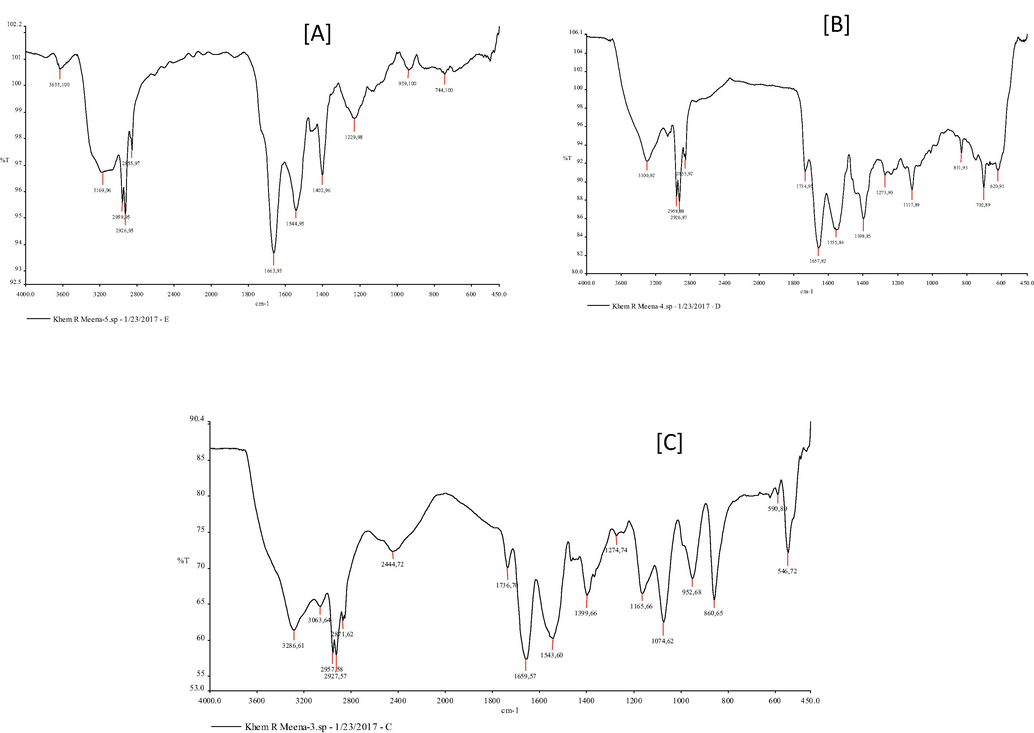
FTIR analysis of the lipopeptide(s). (A) FTIR spectra of the Iturin A; (B) Surfactin; and (C) purified lipopeptide of B. subtilis KLP2015.
3.5.5 MALDI-TOF-MS of the purified lipopeptide of B. subtilis KLP2015
Results of the MALDI-TOF clearly showed that the authentic Surfactin and lipopeptide of B. subtilis KLP2015 showed more or less the similar pattern, which confirmed the ‘Surfactin’ nature of the purified lipopeptide of B. subtilis KLP2015. The Surfactin showed peak at 1058.523 and 701.358 (m/z) while the purified lipopeptide of B. subtilis KLP2015 presented peak at (m/z) 1057.430 (Fig. 7).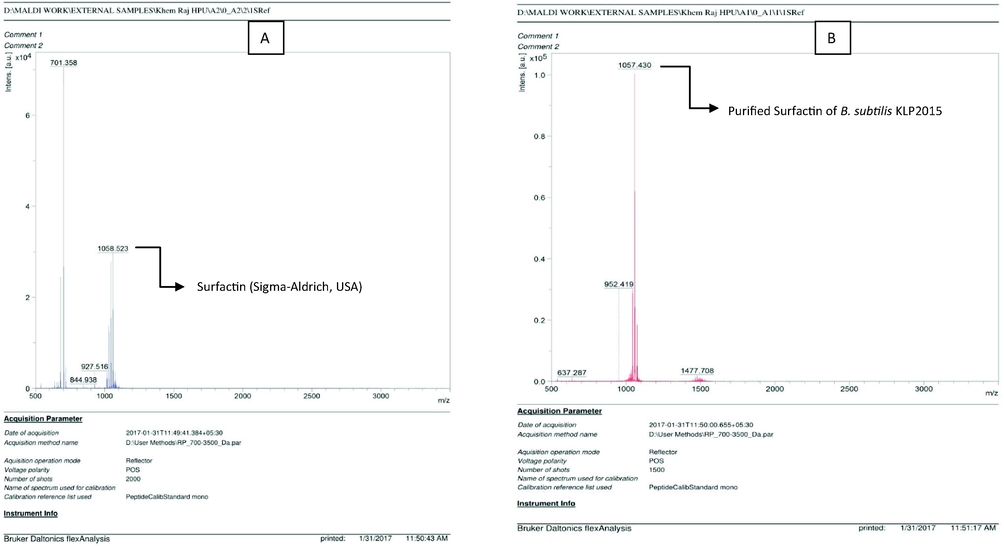
MALDI-TOF analysis of the purified lipopeptide of B. subtilis KLP2015 and Surfactin standard. (A) Surfactin standard; and (B) purified lipopeptide of B. subtilis KLP2015.
3.5.6 Antifungal activity of lipopeptide by Dual-culture test
Antifungal activity of the purified lipopeptide was tested against selected fungal strains (Mucor sp, and A. niger). Maximum mycelial growth inhibition (%) was obtained against the fungus Mucor sp. (Fig. 8A). Lipopeptide of B. subtilis KLP2015 showed the antifungal activity 75.1% and 41.9% for Mucor sp and A. niger, respectively.![Antifungal activity of lipopeptide of B. subtilis KLP2015 against fungal sp. [A] Mucor sp; [B] A. niger; [C] Effect of purified lipopeptide preparation on the C. annuum plants challenged with the Mucor sp. In the photoframes, [1–3] were of plants treated with placebo; [4–6], control plants treated only with Mucor sp. after 10, 20 and 30 days of post inoculation, respectively; while Test plants inoculated with both fungal and LP suspension. Curling of leaves of the control plants was the most prominent effect of fungal infection. The test plants treated with purified LP of B. subtilis KLP2015 remained healthy and disease free.](/content/185/2020/32/1/img/10.1016_j.jksus.2018.05.025-fig8.png)
Antifungal activity of lipopeptide of B. subtilis KLP2015 against fungal sp. [A] Mucor sp; [B] A. niger; [C] Effect of purified lipopeptide preparation on the C. annuum plants challenged with the Mucor sp. In the photoframes, [1–3] were of plants treated with placebo; [4–6], control plants treated only with Mucor sp. after 10, 20 and 30 days of post inoculation, respectively; while Test plants inoculated with both fungal and LP suspension. Curling of leaves of the control plants was the most prominent effect of fungal infection. The test plants treated with purified LP of B. subtilis KLP2015 remained healthy and disease free.
3.5.7 MIC of LP of B. subtilis KLP2015 and lactophenol staining of tested fungal strains
The purified lipopeptide preparation of B. subtilis KLP2015 was most effective against the growth of the Mucor sp. as reflected by the recorded MIC values of 6.25 µg/ml while MIC for the A. niger was 12.5 µg/ml. Lactophenol staining of the Mucor sp. treated with purified LP of B. subtilis KLP2015 prevented the curling of Mucor sp. filaments thus indicating LP’s strong antifungal activity (Fig. 9).![[A] Lactophenol staining of the Mucor sp. without LP treatment; and [B] Lactophenol staining of the Mucor sp. with LP treatment. Lipopeptide causes the curling of Mucor filaments after the treatment, showed strong antifungal activity.](/content/185/2020/32/1/img/10.1016_j.jksus.2018.05.025-fig9.png)
[A] Lactophenol staining of the Mucor sp. without LP treatment; and [B] Lactophenol staining of the Mucor sp. with LP treatment. Lipopeptide causes the curling of Mucor filaments after the treatment, showed strong antifungal activity.
4 Discussion
Present study highlights the optimization of bioprocess parameters to enhance the lipopeptide production by a Bacillus subtilis KLP2015 strain in the fermentation broth. Interestingly, the LP of Bacillus subtilis KLP2015 showed strong antifungal activity against selected the phytopathogenic fungal strains namely Mucor sp. (75.1%) and A. niger (41.9%). This activity might be due to the amphiphilic nature of the Surfactin LP which binds with the fungal membrane by hydrophobic interaction(s). In the previous studies, although the bacterial LPs have been purified but little antifungal activities were found to be associated with these LPs. According to previous literatures, Surfactin has been reported only to show antibacterial activity but not the antifungal activity (Ongena and Jacques, 2008; Gordillo and Maldonado, 2012). Mucor sp. causes a disease in the Capsicum annuum plants that can be effectively treated by using this Surfactin lipopeptide of B. subtilis KLP2015. Acid precipitation method was selected as a better method due to higher antifungal activity of the isolated LPs. Environmental factors and growth conditions such as agitation and oxygen availability also affect biosurfactant production through their effect on cellular growth or activity (Cameotra and Makkar, 1998).
In the present study, the beef extract 1% (w/v) as a complex organic nitrogen source was found to be the best ingredient for LP production by B. subtilis KLP2015 while ammonium chloride as an inorganic nitrogen source was least useful. When beef extract was used at concentration of 1% (w/v) that provided 250 mg/l of LP in fermentation broth, the maximum CFUs/ ml (0.98 × 104) were observed in the B. subtilis KLP2015 inoculated flask containing beef extract. Beef extract is generally considered as a rich source of proteins which might have enhanced the B. subtilis growth as well as LPs production in the fermentation broth. The maximum synthesis of Surfactin has been reported from Bacillus licheniformis R2 in a mineral medium containing NH4NO3 (1 g/l) as a nitrogen source (Joshi et al., 2008). A medium containing glucose (10.0 g/l) and ammonium nitrate (4.0 g/l) could lead to the highest quantity of Surfactins (439.0 mg/l) produced by B. subtilis ATCC 21332 (Fonseca et al., 2007).
In the previous reports, the maximum production of lipopeptides has been noticed at 72 h post inoculation. In our study, the maximum LP yield (530 mg/l) in the fermentation broth seeded with B. subtilis KLP2015 was also obtained at 72 of incubation at 30 °C (545 mg/ l) under shaking. Above 30 °C temperature, LP yield as well as bacterial growth decreased. At temperature 40 °C and 45 °C, little growth was observed that showed that B. subtilis KLP2015 was not a thermostable strain. The LP produced by the B. subtilis KLP2015 was however, heat stable as observed after the heat stability test. At optimum temperature, the bacterial genes of the operon system might be activated causing synthesis of lipopeptides by the non-ribosomal peptide synthetase. In an earlier study, the maximum production of Iturin A by B. subtilis RB14-CS was recorded in the pH range of 5.9–6.3 at 25 °C (Mizumoto and Shoda, 2007). In the present study, however, a pH 7.0 of fermentation broth appeared to be best suited for both the cell growth as well as production of LPs by B. subtilis KLP2015 after 72 h post inoculation under shaking. Little growth and LP production were noticed at pH 5 and pH > 10. These results indicated that B. subtilis KLP2015 efficiently produced the LP at a neutral pH of the fermentation medium. A differential synthesis of LPs has been previously reported under the influence of selected pH of the culture broth (Mizumoto and Shoda, 2007). On the basis of broth pH, bacterial strains are generally classified into three groups such as acidophiles, neutrophiles and alkaliphiles. In our study, the B. subtilis KLP2015 was recognized as a neutrophile bacause it grew optimally at pH 7. The fermentation process parameters [nitrogen source, production time, temperature and pH] were optimized with OFAT that increased the lipopeptides yield from 200.0 mg to ∼547.0 mg/ l of fermentation broth.
In the present study, the RSM involving ’TFAT’ approach interestingly increased the LPs yield of B. subtilis KLP2015 in the fermentation broth up to 985 mg/l. The optimal production/ incubation time increased from an earlier value of 72 h to 96 h when optimized concentration of KH2PO4 (10 mM) was kept in the fermentation broth. Thus RSM increased the yield of LPs of B. subtilis KLP2015 by 6.37% relative to that achieved by ‘OFAT’ approach. The extracted lipopeptide of B. subtilis KLP2015 was purified and characterized by HPLC, TLC, UV-vis, FTIR and MALDI-TOF analyses. Sivapathasekaran et al., 2009 purified the LP after its co-crystallization with 2, 5-dihydroxybenzoic acid. This crystallized LP was subjected to MALDI-TOF for its molecular mass determination. However, in the present study, MALDI-TOF analysis was performed using the cyno-4 hydroxy cinnamic acid as a matrix. The reference/ authentic molecule (Surfactin) and the purified LP of B. subtilis both showed a Mr. of ∼1.0 kDa, which confirmed that the LP of B. subtilis KLP2015 was a member of Surfactin family. The robust antifungal activity of this lipopeptide against Mucor sp. which is a phytopathogen of C. annum makes it a useful and potent biopesticide/ antibiotic to prevent the fungal growth and subsequent damage to these plants. According to previous literatures, Iturins and Fengycins interfere with some intracellular processes in the fungal plant pathogens leading to prevention of toxin formation by the fungal cells (Hu et al., 2009). However, the most common effect of these LPs was the disruption of integrity of the cell membrane, leading to lysis of fungal life stages i.e. mycelium, conidia/ zoospores for oomycete pathogens (Raaijmakers et al., 2010). The efficacy of cell disruption depends on the lipid composition of the fungal/ target membrane as well illustrated in the case of Surfactin, which is poorly fungitoxic. In our study, Surfactin might cause the inhibition of fungal (Mucor sp.) growth by interacting with fungal cell membrane by hydrophobic interactions followed by disruption of integrity leading to cell death.
5 Conclusion
Lipopeptide(s) production from B. subtilis KLP2015 increased from 200.0 to 985.0 mg/l after the TFAT-RSM approach. The extracted lipopeptide was purified and characterized by HPLC, TLC, UV-Visible spectrophotometry, FTIR and MALDI-TOF alalyses. The FTIR and MALDI-TOF analyses showed that the purified lipopeptide of B. subtilis KLP2015 was similar to an authentic Surfactin molecule. The purified LP exhibited potent antifungal activity of 75.1% and 41.9% against Mucor sp. and Aspergillus niger, respectively on the basis of zone of inhibition of growth of fungal strains.
Acknowledgements
This work has been funded by Department of Biotechnology, Ministry of Science and Technology, New Delhi under a DBT-JRF Fellowship grant awarded to one of the authors (KRM) vide a Letter No. DBT-JRF/2011-12/270. The authors are thankful to Sub-DIC Facility, and Department of Biotechnology, Ministry of Science &Technology, New Delhi for the financial support for this work.
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
References
- Characterization of surfactin produced by Bacillus subtilis isolates BS5. Appl. Biochem. Biotechnol.. 2008;150:289-303.
- [Google Scholar]
- Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol.. 2010;87:427-444.
- [Google Scholar]
- Isolation and partial chemical characterization of an antimicrobial peptide produced by a strain of Bacillus subtilis. J. Agric. Food. Chem.. 1998;46:5355-5361.
- [Google Scholar]
- Synthesis of biosurfactants in extreme conditions. Appl. Microbiol. Biotechnol.. 1998;50:520-529.
- [Google Scholar]
- Recent applications of biosurfactants as biological and immunological molecules. Curr. Opin. Microbiol.. 2004;7:262-266.
- [Google Scholar]
- Antimicrobial potential of a lipopeptide biosurfactant derived from a marine Bacillus circulans. J. Appl. Microbiol.. 2008;104:1675-1684.
- [Google Scholar]
- Optimizing carbon/nitrogen ratio for biosurfactant production by a Bacillus subtilis strain. Appl. Biochem. Biotechnol.. 2007;137:471-486.
- [Google Scholar]
- Purification of peptides from Bacillus strains with biological activity. Crotia: In. Chromatography and its Applications. INTECH; 2012. p. :201-225.
- Molecular and biochemical approaches for characterization of antifungal trait of potent bio-control agent Bacillus subtilis RP24. Curr. Microbiol.. 2010;60:99-106.
- [Google Scholar]
- Evaluation of primers and PCR conditions for the analysis of 16S rRNA genes from a natural environment. FEMS Microbiol. Lett.. 2003;221:299-304.
- [Google Scholar]
- Comparing methods for identifying Bacillus strains capable of producing the antifungal lipopeptide iturin A. Curr. Microbiol.. 2008;56:1-5.
- [Google Scholar]
- Inhibition of fengycins on the production of fumonisin B1 from Fusarium verticillioides. Lett. Appl. Microbiol.. 2009;48:84-89.
- [Google Scholar]
- Application of response surface methodology to evaluate the optimum components for the enhance production of Lichenysin by Bacillus licheniformis R2. Biochem. Eng. J.. 2008;41:122-127.
- [Google Scholar]
- Production of biosurfactant lipopeptides iturin A, fengycin, and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J. Microbiol. Biotechnol.. 2010;20:138-145.
- [Google Scholar]
- Rapid quantification of Bacillus subtilis antibiotics in the rhizosphere. Soil. Biol. Biochem.. 2009;41:374-379.
- [Google Scholar]
- Production and characterization of lipopeptide biosurfactant from Bacillus subtilis A8–8. J. Microbiol. Biotechnol.. 2006;16:716-723.
- [Google Scholar]
- Lipopeptides as the Antifungal and Antibacterial Agents: Applications in food safety and therapeutics. BioMed. Res. Int.. 2015;2015:1-9.
- [Google Scholar]
- Isolation and partial characterization of iturin like lipopeptides (a bio-control agent) from a Bacillus subtilis strain. Int. J. Curr. Microbiol. Appl. Sci.. 2014;3:121-126.
- [Google Scholar]
- Lipopeptides: A distinct class of antibiotics with diverse applications. Adv. Biotechnol. Microbiol.. 2017;7:1-7.
- [Google Scholar]
- Lipopeptide antibiotic production by Bacillus velezensis KLP2016. J. Appl. Pharm. Sci.. 2018;8:091-098.
- [Google Scholar]
- A broad-spectrum bactericidal lipopeptide with anti-biofilm properties. Sci. Rep.. 2017;7:2198.
- [Google Scholar]
- Medium optimization of antifungal lipopeptide, iturin A, production by Bacillus subtilis in solid state fermentation by response surface methodology. Appl. Microbiol. Biotechnol.. 2007;76:101-108.
- [Google Scholar]
- Recent advances in the environmental applications of bio surfactants. Curr. Opin. Colloid. Interface. Sci. 2009;14:372-378.
- [Google Scholar]
- Mass spectrometric analysis of lipopeptides from Bacillus strains isolated from diverse geographical locations. FEMS Microbiol Lett. 2007;272:83-89.
- [Google Scholar]
- Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends in Microbiol.. 2008;16:115-124.
- [Google Scholar]
- The bacterial endospore stain on schaeffer fulton using variation of methylene blue solution. J. Phys. Conf. Ser.. 2017;812:1-5.
- [Google Scholar]
- Inheritance of resistance to anthracnose caused by Colletotrichum capsici in Capsicum. Plant Breed. 2005;124:206-208.
- [Google Scholar]
- Poonpolgul, S., Kumphai, S., 2007. Chilli Pepper Anthracnose in Thailand. Country Report. In: Oh, D.G., Kim, K.T. (Eds.), Abstracts of the first international symposium on chilli anthracnose. National Horticultural Research Institute, Rural Development of Administration, Republic of Korea, p. 23.
- Poulos, J.M., 1992. Problems and Progress of Chilli Pepper Production in the Tropics. In: Hock, C.B., Hong, L.W., Rejab, M., Syed, A.R. (Eds.), Proceedings of the Conference on Chilli Pepper Production in the Tropics. Kuala Lumpur, Malaysia, pp. 98–129.
- Comparative study for biosurfactant production by using Bacillus subtilis and Pseudomonas aeruginosa. Int. J. Botany. Res.. 2009;2:284-287.
- [Google Scholar]
- Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS. Microbiol. Rev.. 2010;34:1037-1062.
- [Google Scholar]
- The neighbor-joining method: A new method for reconstructin phylogenetic trees. Mol. Biol. Evol.. 1987;4:406-425.
- [Google Scholar]
- Microbial biosurfactants: Methods for their isolation and characterization. J. Microbiol. Biotechnol. Food. Sci.. 2016;6:641-648.
- [Google Scholar]
- Empirical testing of 16S rRNA gene PCR primer pairs reveals variance in target specificity and efficacy not suggested by in silico analysis. Appl. Environ. Microbiol.. 2009;75:2677-2683.
- [Google Scholar]
- Molecular characterization and bioinformatics studies of a lipase from Bacillus thermoamylovorans BHK67. Int. J. Biol. Macromol.. 2017;107:2131-2140.
- [Google Scholar]
- Pathogenecity of Aspergillus niger in plants. Cibtech. J. Microbiol.. 2012;1:47-51.
- [Google Scholar]
- High-performance liquid chromatography purification of biosurfactant isoforms produced by a marine bacterium. Anal. Bioanal. Chem. 2009;396:845-854.
- [Google Scholar]
- Lipopeptide production by Bacillus subtilis R1 and its possible applications. Braz. J. Microbiol.. 2016;47:955-964.
- [Google Scholar]
- MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol.. 2007;24:1596-1599.
- [Google Scholar]
- Purification and antitumour activity of a lipopeptide biosurfactant produced by Bacillus natto TK-1. Biotechnol. Appl. Biochem.. 2009;52:97-106.
- [Google Scholar]







