Translate this page into:
Trimetazidine with an adjuvant therapy to normalize the circulating visfatin concentration: Future perspective and mechanistic strategies
⁎Corresponding authors. kanchana39@gmail.com (Kanchana M. Karuppiah), kanchank1@srmist.edu.in (Kanchana M. Karuppiah), nicholaszam1990@gmail.com (Nicholas Daniel Amalorpavanaden) nicholasdaniel@mukuba.edu.zm (Nicholas Daniel Amalorpavanaden)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Visfatin is an adipocytokine that exists in two forms, intracellular and extracellular. Circulating visfatin, which lacks the nicotinamide phosphoribosyltransferase (NAMPT) enzyme activity, functions as adipocytokine. Increased concentration of circulating visfatin is associated with several diseases, including cardiovascular disease. However, therapeutic strategies to normalize the circulating visfatin concentration are less understood. In heart failure (HF) patients with obesity and insulin resistance (IR), the routine HF therapy, trimetazidine (TMZ), which is a sirtuin1 (sirt1) activator, is known to normalize the circulating visfatin concentration. Besides this preferred effect of TMZ, an adjuvant therapy including N-acetylcysteine (NAC, antioxidant and anti-inflammatory molecule), niacin (vitamin B3, NAD + booster) and magnesium (sirt1 activator and anti-inflammatory molecule) can be considered to address the underlying molecular mechanisms that are associated with the pathogenesis of HF. Such mechanisms include excess oxidative stress, increased circulating visfatin concentration, NAD + deficiency, sirt1 down regulation and elevated systemic and cardiac inflammation. Together, the proposition is that TMZ and the suggested adjuvant therapy could improve the clinical symptoms and normalize the circulating visfatin concentration by addressing the underlying mechanisms associated with HF.
Keywords
Heart failure
Trimetazidine
Magnesium
N-acetylcysteine
Oxidative stress
Visfatin
- HF
-
Heart Failure
- HFrEF
-
Heart failure with reduced ejection fraction
- HFpEF
-
Heart failure with preserved ejection fraction
- TMZ
-
Trimetazidine
- PBEF
-
pre-B cell colony-enhancing factor
- NAMPT
-
Nicotinamide phosphoribosyl transferase
- NAD+
-
Nicotinamide adenine dinucleotide
- NMN
-
Nicotinamide mononucleotide
- NMNAT
-
Nicotinic acid mononucleotide adenyl transferase
- NADPH
-
nicotinamide adenine dinucleotide phosphate
- eNOS
-
endothelial nitric oxide synthase
- iNOS
-
inducible nitric oxide synthase
- IR
-
Insulin Resistance
- WAT
-
White adipose tissue
- T2D
-
Type 2 Diabetes
- JNK
-
c-Jun N-terminal kinase
- ERK
-
extracellular signal-regulated kinases
- P13K
-
Phosphoinositide 3-kinases
- NFkB
-
Nuclear factor kappa B
- STAT 3
-
Signal transducer and activator of transcription 3
- MMP
-
Matrix metalloproteinases
- VEGF
-
Vascular endothelial growth factor
- FGF
-
Fibroblast growth factor
- MPC1
-
Mitochondrial pyruvate carrier 1
- IL6
-
Interleukin 6
- ONOO
-
Peroxynitrite
- Mg
-
Magnesium
- NAC
-
N-acetyl cysteine
Abbreviations
1 Introduction
Visfatin is an adipokine that elicits paracrine and autocrine effects on the cardiovascular system. Previously, visfatin has been considered to be identical to pre-B cell colony-enhancing factor (PBEF) that promotes the maturation of early B-lineage precursor cells (Adeghate 2008). Further, visfatin has intrinsic enzyme activity as nicotinamide phosphoribosyl transferase (NAMPT) (Rongvaux et al. 2002). NAMPT catalyses the rate-limiting step in NAD+ (nicotinamide adenine dinucleotide) biosynthetic salvage pathway, wherein NAD + is an essential cofactor in several redox reactions (Revollo, Grimm, and Imai 2004). As shown in Fig. 1, NAMPT converts nicotinamide to nicotinamide mononucleotide (NMN); then nicotinamide/nicotinic acid mononucleotide adenyl transferase (NMNAT) transforms NMN to NAD+ (Formentini, Moroni, and Chiarugi 2009).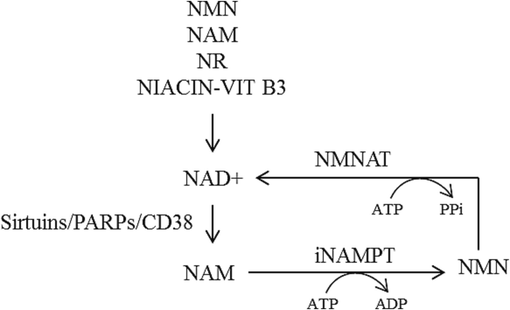
NAD + biosynthesis by salvage pathway. CD38-cyclic ADP ribose hydrolase; NAD + -Nicotinamide adenine dinucleotide; NAM-nicotinamide; NR-nicotinamide riboside; NMN-nicotinamide mononucleotide’; iNAMPT-intracellular nicotinamide phosphoribosyltransferase (visfatin); NMNAT-nicotinate mononucleotide adenylyltransferase’ PARP-poly-ADP ribose polymerase; PPi-pyrophosphate; Vit B3-vitamin B3.
Visfatin exists in two forms, intracellular and extracellular. Intracellular form exhibits NAMPT enzyme activity so as to maintain the enzyme activities of NAD + -dependent enzymes, hence, the intracellular visfatin regulates cellular metabolism and energy homeostasis (Ho et al. 2009). Whereas the extracellular form, which is synthesised and secreted by adipocytes and several other cell types (Romacho, Sánchez-Ferrer, and Peiró 2013) (including cardiac fibroblasts, cardiomyocytes, vascular smooth muscle cells, endothelial cells, cells in the atherosclerotic plaque, activated immune cells, circulating blood cells), is an adipocytokine which is associated with hormone-like signalling pathways and intracellular signalling cascades (Verdin 2015). As circulating visfatin has been considered as a biomarker of inflammation and endothelial dysfunction (Romacho et al. 2013), here the focus is on the extracellular circulating visfatin and its association with the pathogenesis of heart failure (HF). Interestingly, a recent report has shown that extracellular circulating visfatin does not elicit enzymatic activity (for NMN biosynthesis) due to the insufficient concentration of ATP (the activator of enzymatic activity of visfatin/NAMPT) in the extracellular spaces (Hara et al. 2011), which shows that circulating visfatin functions as an adipocytokine rather than an enzyme (NAMPT).
The physiological relevance and function of circulating visfatin remains controversial. However, enhanced circulating visfatin concentration has been reported in several pathologies (Romacho et al. 2013) including, obesity, type 2 diabetes (T2D), hypoxia, chronic kidney disease, preeclampsia, acute coronary syndromes, cerebrovascular diseases and non-metabolic chronic inflammatory diseases, among the others. Further, strategies to normalize the elevated circulating visfatin concentration are less explored. TMZ also exhibited antiinflammatory activities (Liang et al., 2020; Engin etal., 2022).
Trimetazidine (TMZ), a cytoprotective and an anti-ischemic agent, is a pharmacological drug that was previously approved for angina pectoris (Milinković et al. 2016). Eventually, the direct influence of TMZ in improving the myocardial metabolism via beta oxidation was established (Kantor et al. 2000), besides the other benefits of TMZ. Hence, the European Society of Cardiology (ESC, 2016 guidelines) has included TMZ for the treatment of angina pectoris with HF (Milinković et al. 2016). Since then, experimental and clinical studies have reported on the efficacy of TMZ in HF (Brottier et al. 1990). Different mechanisms by which TMZ exerts its cardioprotective effect includes sirt1 activation (Brottier et al. 1990), energy metabolism (Heggermont et al. 2016), apoptosis of cardiomyocytes, myocardial autophagy (Yang et al. 2019), myocardial interstitial fibrosis, myocardial inflammation, expression of atrial natriuretic peptide (Morgan et al. 2006), modifying the phosphate levels in left ventricle (Fragasso et al. 2006) and electrophysiological influence (Cera et al. 2010). Nevertheless, whether or not TMZ can influence the circulating visfatin concentration is yet to be understood.
2 Mechanisms associated with circulating visfatin and heart failure
Abdominal obesity occurs due to an imbalance between the energy intake and energy expenditure. Further, obesity is associated with T2D and insulin resistance (IR) (LinPark et al. 2017). The two major cellular events that occur in abdominal obesity are white adipose tissue (WAT) inflammation and hypoxia, as shown in Fig. 2. In cardiovascular disease, classically excessive oxidative stress occurs as an underlying molecular mechanism. Such elevated oxidative stress leads to more tissue inflammation and reduced ejection fraction, which cumulatively leads to HF development. Majority of the reports (Erten 2021; Hara et al. 2011; Peiró et al. 2010) show that visfatin concentration is up-regulated in HF. Besides these oxidative stress induced effects, excess reactive oxygen species down-regulates the sirt1 enzyme activity and expression which in-turn leads to increase in circulating visfatin concentration (Vargas-Ortiz, Pérez-Vázquez, and Macías-Cervantes 2019). Therefore, it is possible that excess circulating visfatin contributes to HF development.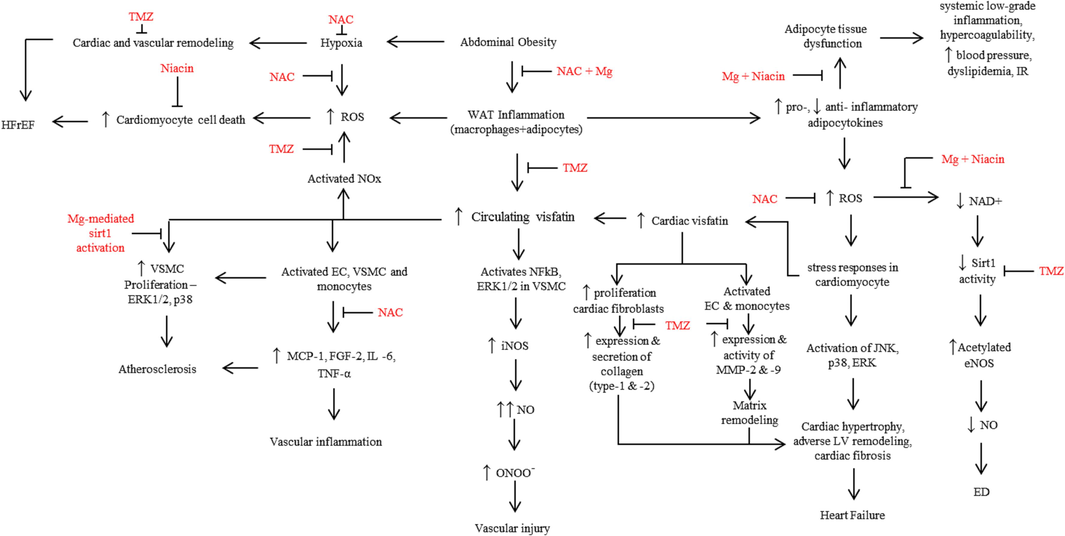
Flowchart summarizes the reported effects of circulating visfatin in the cardiovascular system and the potential cardioprotective effects of trimetazidine (TMZ) and adjuvant therapy. EC-endothelial cells; ED-endothelial dysfunction; eNOS-endothelial nitric oxide synthase; FGF2-fibroblast growth factor-2; HFrEF-heart failure with reduced ejection fraction; IL-6-interleukin-6; iNOS-inducible nitric oxide synthase; LV-left ventricle; MCP-1-Monocyte chemoattractant protein-1; Mg-magnesium; MMP-matrix metalloproteinase; NAC-N-acetylcysteine; NAD+ - Nicotinamide adenine dinucleotide; NO-nitric oxide; NOx-NADPH oxidase; ONOO-peroxynitrite; ROS-reactive oxygen species; Sirt1-siruin-1; TNFα-tumor necrosis factor-alpha; VSMC-vascular smooth muscle cells; WAT-white adipose tissue; ↑-upregulation; ↓-downregulation.
2.1 White adipose tissue inflammation and adipocyte dysfunction
The excess energy, which is stored in the adipose tissue, leads to adipocyte enlargement. Eventually, these hypertrophic adipocytes produce chemotactic adipocytokines, such as leptin, adiponectin, resistin, and visfatin, among the others. The secreted adipocytokines, in turn, attract the macrophages into the adipose tissue, to trigger inflammation (WAT inflammation) in the adipose tissue (Fig. 2). In WAT inflammation, lipolysis of the hypertrophic adipocytes causes leakage of free fatty acids. These free fatty acids directly contribute to apoptosis of non-adipose tissue, microvascular inflammation and altered adipose tissue perfusion to result in hypoxia and necrosis. These cellular events in turn promote several pro-inflammatory signalling pathways in adipocytes, fibroblasts and immune cells (Wang, Wood, and Trayhurn 2007). Besides these events, an imbalance in the biosynthesis and secretion of pro- and anti-inflammatory adipocytokines is created, which causes adipocyte dysfunction. Adipose tissue dysfunction then becomes an underlying factor for several systemic and metabolic consequences such as, IR, systemic low grade inflammation, hyperlipidemia and hypercoagulability which cumulatively furthers the pathogenesis of cardiovascular disease and T2D (Schrover et al. 2016).
2.2 White adipose tissue inflammation, reactive oxygen species and circulating visfatin
Excess circulating visfatin stimulates the pathogenesis of atherosclerosis and HF (Peiró et al. 2010) through multiple mechanisms, including, cell proliferation, cell survival, extracellular matrix, vascular reactivity, inflammation and myocardial fibrosis. However, pre-treatment with visfatin exhibits cardioprotective effect under hypoxia-reperfusion (Lim et al. 2008). Hence, visfatin may have potential therapeutic benefits in the pathologies associated with ischemia.
Elevated concentration of circulating visfatin activates NADPH (nicotinamide adenine dinucleotide phosphate, Fig. 2) oxidase in endothelial cells to contribute to endothelial dysfunction in the coronary vessels (Romacho et al. 2013). Activated NADPH oxidase generates excessive reactive oxygen species. Excess oxidative stress successively influences several critical molecules and cellular events including the following i) aggravated cell death of cardiomyocytes. Such an excessive cardiomyocyte cell death is one of the fundamental reasons for the pathogenesis of HF with reduced ejection fraction (HFrEF) (Simmonds, Cuijpers, and Heymans 2020); ii) degradation of the circulating NAD + level (Braidy et al. 2011). It has been reported that, in HF patients, NAD + deficiency is prevalent (Ziobrowski, Hannah N., Sonneville, Kendrin R. Eddy, Kamryn T., Crosby, Ross D., Micali, Nadia, Horton, Nicholas J., Field 2019), whose restoration reverses HF (Ziobrowski, Hannah N., Sonneville, Kendrin R. Eddy, Kamryn T., Crosby, Ross D., Micali, Nadia, Horton, Nicholas J., Field 2019). As NAD + is an essential co-factor for the deacetylase enzyme, sirt1, the deacetylase enzyme activity gets compromised in NAD + deficiency, which leads to accumulation of acetylated proteins in HF (Ziobrowski, Hannah N., Sonneville, Kendrin R. Eddy, Kamryn T., Crosby, Ross D., Micali, Nadia, Horton, Nicholas J., Field 2019). On the other hand, acetylation of endothelial nitric oxide synthase (eNOS) resulted in impaired enzyme activity, thereby contributing to nitric oxide (the primary vasodilator) deficiency, endothelial dysfunction and atherosclerosis (Heiss and Dirsch 2014); and iii) in response to excess oxidative stress, activation of JNK, p38 and ERK pathways occurs in cardiomyocytes. Such cumulative activation of different pathways, eventually, results in cardiac hypertrophy, adverse left ventricle remodeling, cardiac fibrosis and HF (Romacho et al. 2013). Many of the visfatin-elicited effects (proliferative, proinflammatory and proangiogenic), on the cardiovascular system are through activation of several signalling pathways, such as PI3K, NFkB, STAT3 and ERKs (Lin et al. 2019).
Besides the production and release of visfatin from the primary source (adipocytes and activated immune cells), apical epicardial adipose tissue, periadventitial adipose tissue, cardiac fibroblasts, myocytes and the cells in the vascular walls contribute to the up-regulated local cardiac visfatin concentration (Pillai et al. 2013), which has been reported to have an autocrine effect in the cardiovascular system (Romacho et al. 2013). In support, experimental studies have shown that in addition to the influence of circulating visfatin, visfatin that is secreted from rat cardiac cells becomes a local source of the adipocytokine that leads to cardiac fibrosis (Erten 2021).
Circulating visfatin, besides eliciting its proliferative effects on the cells in the vascular wall, it mediates the proliferation of cardiac fibroblasts. Proliferating fibroblasts synthesise and release more collagen (type-1 and −2), which eventually promotes cardiac fibrosis (Erten 2021). Moreover, visfatin up-regulates the mRNA and protein levels as well as enzyme activity of matrix metalloproteinases (MMP-2 and −9) in monocytes and endothelial cells. MMPs promote angiogenesis by two simultaneous cellular events, degradation of the extracellular matrix and reduction in the concentrations of the tissue inhibitors of MMPs (TIMP-1 and −2) (Romacho et al. 2013). Importantly, as these MMPs degrade the matrix, this degradation furthers the plaque vulnerability (Oviedo-Orta et al. 2008). Besides, the presence of more cardiac fibroblasts, excess collagen and accumulation of extracellular matrix promotes myocardial fibrosis and remodeling (Yu et al. 2010). In endothelial cells, visfatin up-regulates the biosynthesis of pro-angiogenic soluble factors including VEGF, FGF-2, MPC-1 and IL-6 (Adya et al. 2009), which play a crucial role in the initiation of atherosclerosis.
Experimental and clinical studies have reported on the pro-inflammatory role of visfatin. Exposure of human vascular smooth muscle cells to exogenous visfatin activated ERK1/2 and NFkB, which up-regulated the expression of a pro-inflammatory molecule, inducible nitric oxide synthase (iNOS). Consequent to iNOS induction, the biosynthesis of nitric oxide and peroxynitrite (ONOO–) were up-regulated. The potent oxidant, ONOO– then expedites the occurrence of endothelial dysfunction, vascular injury and vascular inflammation (Pacher et al. 2012).
Based on these observations, it is clear that, in patients with obesity, IR and HF, circulating concentrations of visfatin, biomarkers of inflammation and oxidative stress are elevated; and that the concentration of NAD + is down-regulated. However, therapeutic strategies to cumulatively normalize these factors are few. Yet, one study (Haider et al. 2006) has reported that exercise training lowers circulating visfatin concentrations.
2.3 Abdominal obesity, hypoxia and heart failure
In obesity-mediated WAT inflammation, excessive circulatory visfatin from the adipocytes and activated immune cells becomes the underlying factor for the occurrence of hypoxia (Berezin, Berezin, and Lichtenauer 2020). Hypoxia occurs due to enhanced utilization of oxygen or attenuated perfusion of the hypertrophic adipocytes. Hypoxic environment further leads to the over-expression of pro-inflammatory genes (including hypoxia-inducible-factor-1), excessive oxidative stress, lipotoxicity in adipose tissue and altered adipocytokines secretion. These cellular events cumulatively facilitate the self-propagative vicious cycle as well as WAT inflammation to foster the development of IR, skeletal muscle wasting, cardiac and vascular remodelling and eventually to HF development (Murdolo et al. 2013).
3 Potential strategies to alter the concentration of circulating visfatin for ameliorative effect in cardiovascular disease
In obesity, adipose tissue dysfunction and hypoxia, among the other cellular events, are crucial factors in creating imbalances in the ratio of oxidants/antioxidants and pro-/anti-inflammatory molecules. It is evident that TMZ, one of the established conventional drugs in HF therapy, normalizes the circulating visfatin concentration [11]. Further, the effect of selected nutritional supplements, such as niacin (as NAD + booster) (Pirinen et al. 2020), magnesium (as sirt1 activator and anti-inflammatory molecule) (Martins 2016; Veronese et al. 2022) and NAC (as anti-oxidant and anti-inflammatory) (Tenório et al. 2021) are well established. In this context, our proposition is that along with TMZ, inclusion of an adjuvant therapy which comprises of a multi-ingredient nutritional supplement [such as niacin (vitamin B3 form; an NAD + booster), magnesium (sirt1 activator and anti-inflammatory) and N-acetylcysteine (anti-inflammatory and anti-oxidant)] could improve the adverse outcomes in patients with HF, obesity and IR, than TMZ alone. Possibly, TMZ + multi-ingredient nutritional supplement could address the unfavourably altered biochemical parameters (circulating concentrations of visfatin, NAD + and biomarkers of oxidative stress and inflammation) and cardiac structure and function (Fig. 2).
In support of the inclusion of nutritional supplements, one study has reported that Quercetin, a sirt1 activator, reduces visfatin secretion (Vargas-Ortiz et al. 2019). Hence it is possible that TMZ as a sirt1 activator could directly reduce the circulating concentration of visfatin. Interestingly, exercise is known to activate sirt1 (Shu et al. 2021) and TMZ has been reported to improve HF symptoms by sirt1 activation (Milinković et al. 2016). Therefore, based on these reports, it is only logical to consider sirt1 activators as adjuvants for therapeutic effect in HF.
Deficiencies of magnesium and NAD + are prevalent in HF (Zhu et al. 2016). In this regard, based on our hypothesis, supplementation of NAC and magnesium could address the elevated oxidative stress and pro-inflammatory milieu. Parallelly, as a consequence of elevated circulating visfatin and locally generated visfatin in the cardiovascular system, cardiomyocyte cell death could be triggered. Such an apoptosis-induced augmented in vitro cell death has been reported to be abrogated by exogenous NAD + treatment (Zhu et al. 2016). Thus, therapeutically, NAD + booster could potentially mitigate the excess visfatin-induced cell death. Besides this in vitro cytoprotective effect of NAD+, repletion of NAD + in mice fed with nicotinamide riboside (NAD + precursor and direct activator of NAD + biosynthesis) resulted in the reversal of HF with preserved ejection fraction (HFpEF) (Ziobrowski, Hannah N., Sonneville, Kendrin R. Eddy, Kamryn T., Crosby, Ross D., Micali, Nadia, Horton, Nicholas J., Field 2019). For these HF-associated cellular events, supplementation of magnesium for anti-inflammatory effect and niacin as NAD + booster could potentially mitigate the onset or pathogenic progression in HF.
In HF, oxidative stress induces a reduction in the expression and activity of sirt1 (the deacetylase enzyme). This sirt1-deficiency then leads to hyper-acetylated proteins. In addition to the sirt1-deficiency-induced hyper-acetylated proteins, myocardial hyper-acetylated proteins accumulate due to impaired NAD + biosynthesis pathway (causing NAD + deficiency) and compromised sirt3 expression (Ziobrowski, Hannah N., Sonneville, Kendrin R. Eddy, Kamryn T., Crosby, Ross D., Micali, Nadia, Horton, Nicholas J., Field 2019). Besides the NAD + deficiency-mediated hyperacetylation, elevated oxidative stress compromises the sirt1 enzyme activity (Salminen, Kaarniranta, and Kauppinen 2013) which consequently results in accumulation of hyperacetylated proteins. To counter the hyperacetylation milieu, magnesium as sirt1 activator has been reported to be effective. Thus, magnesium supplementation could restore the physiological status of acetylated/de-acetylated proteins in the myocardium.
Together, TMZ and the nutritional supplements including sirt1 activators, antioxidants, anti-inflammatory molecules and NAD + booster could potentially normalize the concentrations of visfatin, NAD+, free radicals and pro-inflammatory molecules, to eventually improve the adverse symptoms of HF. Besides the influences of nutrient supplements, it is possible that TMZ could reduce the excess circulating visfatin level via potential sirt1 activation.
4 Conclusion
To sum up, while the routine HF therapy with TMZ supports the improvement of clinical manifestations of HF and cardiac function, in terms of ejection fraction(Brottier et al. 1990) and the potential reduction of circulating visfatin concentrations, the adjuvant therapy with multiple nutritional supplements could have an additive effect to TMZ, as the individual supplement(s) is/are known for their effect to normalize the adverse cellular events associated with oxidative stress, inflammation, mitigated sirt1 activity and modified circulating concentrations of analytes (visfatin and NAD + ) that leads to the development and progression of HF (Fig. 2). Hence, TMZ with the said nutritional supplements may have therapeutic and preventive effects in HF.
Compliance with ethical standards
Funding None.
Ethical Approval Not applicable.
Research involving human participants and/or animals Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Visfatin: Structure, Function and Relation to Diabetes Mellitus and Other Dysfunctions. Curr. Med. Chem.. 2008;15(18):1851-1862.
- [CrossRef] [Google Scholar]
- Pre-B Cell Colony Enhancing Factor (PBEF)/Visfatin Induces Secretion of MCP-1 in Human Endothelial Cells: Role in Visfatin-Induced Angiogenesis. Atherosclerosis. 2009;205(1):113-119.
- [CrossRef] [Google Scholar]
- Emerging Role of Adipocyte Dysfunction in Inducing Heart Failure Among Obese Patients With Prediabetes and Known Diabetes Mellitus. Frontiers in Cardiovascular Medicine. 2020;7(November):1-20.
- [CrossRef] [Google Scholar]
- Age Related Changes in NAD+ Metabolism Oxidative Stress and Sirt1 Activity in Wistar Rats. PLoS One. 2011;6(4):1-18.
- [CrossRef] [Google Scholar]
- Therapeutic Value of a Cardioprotective Agent in Patients with Severe Ischaemic Cardiomyopathy. Eur. Heart J.. 1990;11(3):207-212.
- [CrossRef] [Google Scholar]
- Beneficial Electrophysiological Effects of Trimetazidine in Patients with Postischemic Chronic Heart Failure. J. Cardiovasc. Pharmacol. Ther.. 2010;15(1):24-30.
- [CrossRef] [Google Scholar]
- Visfatin as a Promising Marker of Cardiometabolic Risk. Acta Cardiologica Sinica. 2021;37(5):464-472.
- [CrossRef] [Google Scholar]
- Detection and Pharmacological Modulation of Nicotinamide Mononucleotide (NMN) in Vitro and in Vivo. Biochem. Pharmacol.. 2009;77(10):1612-1620.
- [CrossRef] [Google Scholar]
- Effects of Metabolic Modulation by Trimetazidine on Left Ventricular Function and Phosphocreatine/Adenosine Triphosphate Ratio in Patients with Heart Failure. Eur. Heart J.. 2006;27(8):942-998.
- [CrossRef] [Google Scholar]
- Exercise Training Lowers Plasma Visfatin Concentrations in Patients with Type 1 Diabetes. J. Clin. Endocrinol. Metab.. 2006;91(11):4702-4704.
- [CrossRef] [Google Scholar]
- Nicotinamide Phosphoribosyltransferase/Visfatin Does Not Catalyze Nicotinamide Mononucleotide Formation in Blood Plasma. PLoS One. 2011;6(8)
- [CrossRef] [Google Scholar]
- Metabolic Support for the Heart: Complementary Therapy for Heart Failure? Eur. J. Heart Fail.. 2016;18(12):1420-2149.
- [CrossRef] [Google Scholar]
- Regulation of ENOS Enzyme Activity by Posttranslational Modification. Curr. Pharm. Des.. 2014;20(22):3503-3513.
- [CrossRef] [Google Scholar]
- SIRT1 Markedly Extends Replicative Lifespan If the NAD+ Salvage Pathway Is Enhanced. FEBS Lett.. 2009;583(18):3081-3305.
- [CrossRef] [Google Scholar]
- The Antianginal Drug Trimetazidine Shifts Cardiac Energy Metabolism from Fatty Acid Oxidation to Glucose Oxidation by Inhibiting Mitochondrial Long- Chain 3-Ketoacyl Coenzyme A Thiolase. Circ. Res.. 2000;86(5):580-658.
- [CrossRef] [Google Scholar]
- Trimetazidine attenuates diabetic inflammation via Nrf2 activation. Int. J. Cardiol.. 2020;15(307):153.
- [CrossRef] [Google Scholar]
- The Novel Adipocytokine Visfatin Exerts Direct Cardioprotective Effects. J. Cell Mol. Med.. 2008;12(4):1395-1403.
- [CrossRef] [Google Scholar]
- King Resistin, Inflammation, and Cardiometabolic Diseases. Korean J. Intern. Med.. 2017;32(2):239-247.
- [CrossRef] [Google Scholar]
- Magnesium Therapy Prevents Senescence with the Reversal of Diabetes and Alzheimer’s Disease. Health. 2016;08(07):694-710.
- [CrossRef] [Google Scholar]
- The Role of Ivabradine and Trimetazidine in the New ESC HF Guidelins. Card. Fail. Rev.. 2016;123–29
- [CrossRef] [Google Scholar]
- Chronic Treatment with Trimetazidine Reduces the Upregulation of Atrial Natriuretic Peptide in Heart Failure. Fundam. Clin. Pharmacol.. 2006;20(5):503-505.
- [CrossRef] [Google Scholar]
- Oxidative Stress and Lipid Peroxidation By-Products at the Crossroad between Adipose Organ Dysregulation and Obesity-Linked Insulin Resistance. Biochimie. 2013;95(3):585-594.
- [CrossRef] [Google Scholar]
- Comparison of MMP-2 and MMP-9 Secretion from T Helper 0, 1 and 2 Lymphocytes Alone and in Coculture with Macrophages. Immunology. 2008;124(1):42-50.
- [CrossRef] [Google Scholar]
- Role of Nitrosative Stress and Peroxynitrite in the Pathogenesis of Diabetic Complications. Emerging New Therapeutical Strategies. Curr. Med. Chem.. 2012;12(3):267-275.
- [CrossRef] [Google Scholar]
- Peiró, Concepción, Tania Romacho, Raffaele Carraro, and Carlos F. Sánchez-Ferrer. 2010. “Visfatin/PBEF/Nampt: A New Cardiovascular Target?” Frontiers in Pharmacology 1 NOV(November):1–7. doi: 10.3389/fphar.2010.00135.
- Nampt Secreted from Cardiomyocytes Promotes Development of Cardiac Hypertrophy and Adverse Ventricular Remodeling. Am. J. Physiol. Heart Circ. Physiol.. 2013;304(3):415-426.
- [CrossRef] [Google Scholar]
- Erratum: Niacin Cures Systemic NAD+ Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy (Cell Metabolism (2020) 31(6) (1078–1090.E5), (S155041312030190X), (10.1016/j.Cmet.2020.04.008)) Cell Metab.. 2020;32(1):144.
- [CrossRef] [Google Scholar]
- The NAD Biosynthesis Pathway Mediated by Nicotinamide Phosphoribosyltransferase Regulates Sir2 Activity in Mammalian Cells. J. Biol. Chem.. 2004;279(49):50754-50763.
- [CrossRef] [Google Scholar]
- Romacho, Tania, Carlos F. Sánchez-Ferrer, and Concepción Peiró. 2013. “Visfatin/Nampt: An Adipokine with Cardiovascular Impact.” Mediators of Inflammation 2013(Cv). doi: 10.1155/2013/946427.
- Pre-B-Cell Colony-Enhancing Factor, Whose Expression Is up-Regulated in Activated Lymphocytes, Is a Nicotinamide Phosphoribosyltransferase, a Cytosolic Enzyme Involved in NAF Biosynthesis. Eur. J. Immunol.. 2002;32(11):3225-3234.
- [CrossRef] [Google Scholar]
- Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process. Int. J. Mol. Sci.. 2013;14(2):3834-3859.
- [CrossRef] [Google Scholar]
- Adipose Tissue Dysfunction: Clinical Relevance and Diagnostic Possibilities. Horm. Metab. Res.. 2016;48(4):213-225.
- [CrossRef] [Google Scholar]
- Trimetazidine in Heart Failure. Front. Pharmacol.. 2021;11(January):1-10.
- [CrossRef] [Google Scholar]
- Cellular and Molecular Di Ff Erences between HFpEF and HFrEF : A Step Ahead in an Improved. Cells. 2020;9(242):1-22.
- [Google Scholar]
- N-Acetylcysteine (Nac): Impacts on Human Health. Antioxidants. 2021;10(6)
- [CrossRef] [Google Scholar]
- Exercise and Sirtuins: A Way to Mitochondrial Health in Skeletal Muscle. Int. J. Mol. Sci.. 2019;20(11):1-11.
- [CrossRef] [Google Scholar]
- NAD+ in Aging, Metabolism, and Neurodegeneration. Science. 2015;350(6265):1208-1213.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102950.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







