Triazophos induced lethal, sub-lethal and transgenerational effects on biological parameters and demographic traits of Pectinophora gossypiella using two sex life table
⁎Corresponding authors. haiderynau170@yahoo.com (Nawaz Haider Bashir), chhuanhuan@163.com (Huanhuan Chen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Pectinophora gossypiella, which destroys cotton crops' brackets, squares, flowers, and bolls, has been identified as a severe pest in practically all the world's cotton-growing nations. Triazophos, a trans-laminar, broad spectrum organophosphate insecticide can manage Lepidopteran pests in cotton, rice, and maize. P. gossypiella has developed resistance against major insecticides including triazophos. To comprehend the function of insecticides in the environment, it is vital to grasp their lethal, low, and/or sublethal impacts as well as transgenerational consequences. In this study, triazohpos had lethal, sublethal, and transgenerational effects in two P. gossypiella generations (F0 and F1). Triazophos caused greater toxicity against P. gossypiella, according to the findings of the bioassay, with LC50 values of 2.728 mg/l for F0 and 1.852 mg/l for F1 after 48 h of exposure. Triazophos LC10 and LC25 concentrations caused considerable variations in biological parameters, which clearly indicated longer larval duration during F0 and F1 generations. Parameters related to reproduction showed significant differences with lower fecundity of 231.08 eggs/ female (LC10); 203.87 eggs/ female (LC25) and 220.95 eggs/ female (LC10) 209.21 eggs/ female (LC25) of F0 and F1 generations respectively and as compared to control which were 264.76 eggs/ female and 274.62 eggs/ female for F0 and F1 respectively. Demographic parameters for F0 and F1 population revealed net reproduction rate (R0); intrinsic rate of increase (r); mean generation time (T) and (λ) were having significant difference in triazophos treated populations as compared to control. From results it can be asserted that life table reproductive and demographic parameters affected by triazophos can be helpful in managing P. gossypiella in cotton crop and will result in more success against triazophos populations of P. gossypiella.
Keywords
P. gossypiella
Pink bollworm
Triazophos
Demographic parameters
Two-sex table
1 Introduction
Insects exposed to insecticides in field, lowers their densities for time being but insect population re-build depending upon its susceptibility to insecticides (Desneux et al., 2005). Determining the sub-lethal effects of insecticides is important clue to find the impact analysis of insecticides, because insecticides exposure may impair biological; reproductive or behavioral parameters of arthropods including bio-control agents (Liu et al., 2022, Gu et al., 2018, Desneux et al., 2007). Insecticides can affect arthropods in direct (resulting in mortality) or indirect way having sublethal impacts on life parameters; physiology and behavior (Idrees et al., 2022b; Liu et al., 2022). Insecticides may have a variety of sublethal effects, including disruption of enzymes, promoting and suppressing reproduction, impacts on behavior and physiology, and changes in sex ratio (Liu et al., 2022, Desneux et al., 2007). It can also cause habitat changes (Malaj and Morrissey 2022), induce sublethal and hormesis effects in key pests (Liang et al., 2021, Tamilselvan et al., 2021), lead to secondary pest outbreaks (Paula et al., 2021), modulate both direct and indirect interactions among species within the food web, and cause resistance development. All these things can have an effect on integrated pest management (IPM) (Shah et al., 2020).
Pectinophora gossypiella, sometimes known as the pink bollworm, was initially described by W.W. Saunders in 1843 under the name Deprassaria gossypiella (Reviewed by Naranjo et al., 2001). It was further recorded in Australia in 1911 (Wilson 1972) is serious cotton pest which decreases yield up to 67 % and causing 2.8 %–61.9 % losses in cotton seed yield. 2.1–47.1 % losses in oil contents, 10.7 %–59.2 % losses in boll opening (reviewed by Ali et al., 2020; Spielman et al., 2015; Majeed et al., 2016; Parmar and Patel 2016).
Researchers are trying to come up with ways to control agricultural pests that are safer for people, the environment, and natural enemies at the turn of the century (Ahmed et al., 2022; Idrees et al., 2016, 2017, 2021, 2022a,b). Triazophos is a broad-spectrum organophosphate insecticide that can be used on a wide range of crops to kill Lepidopteran pests like cotton bollworms (Liu, 1999) and pink bollworms instead of dangerous insecticides (Akhtar et al 2016). Work has been done on how triazophos affects insects that are pests and biological agents in a way that doesn't kill them. The sublethal effect of triazophos on the life table parameters of Sogatella furcifera has been investigated in detail (Liu et al 2016). When sublethal doses of triazophos are given to Nilaparvata lugens, the protein content of the male accessory glands changes (Wang et al., 2010). To study how predators act in cotton fields where triazophos has been sprayed, a two-sex life table of Orius sp. feeding on P. gossypiella larvae was investigated (Ali et al., 2020).
Life tables are often used in studies of population and community ecology (Chi et al., 2020). However, female-specific life tables don't take into account male members of the population, so their importance is limited (Chi et al., 2020). Age-stage two sex life table can be helpful in precise explanation of stage differentiation and can show the data analysis; description and interpretation; regarding both sexes in practical application (Chi et al., 2020). Two sex life table provides in-depth sights of both male and female changing behavior; impact of different diet regimens on biological performance on insect rearing (Ricupero et al., 2020); and adaptability to insecticide. Simulations of age-stage two sex life table provides the pest population accurate assessment at particular stage, so this tool can be used to select best control strategy (Chi, 1990). A sex table that shows how males and females age gives researchers valuable information on population stability, growth, and reproduction (Chi 1988; Chi and Liu 1985). According to the results of these studies, using a two-sex table, triazophos exhibits deadly and sublethal effects in P. gossypiella, and these effects may be inherited. In a life table for P. gossypiella, the female had a much longer cycle than the males. The demographic and reproductive factors of P. gossypiella throughout two generations were shown to be significantly different based on bioassay findings.
After observing the wide-spread resistance of P. gossypiella against broad-spectrum insecticides, this study was aimed to observe the developed resistance in perspective of two sex-table of this pest. Further, current study was aimed to know the lethal; sublethal and transgenerational effects of triazophos on P. gossypiella. Lethal concentration toxicity against P. gossypiella will help to know the efficacy for all larval instars and managing chemical control of this pest. While sublethal concentration research on P. gossypiella will be helpful to know impaired activity on development; reproduction; fecundity to devise plan for controlling pink bollworm in cotton.
In current research we sorted out lethal; sublethal and trans-generational effects of triazophos using the two-sex table for P. gossypiella in cotton. This research will be helpful for all cotton growing countries to enhance their cropping system by developing the management strategy of P. gossypiella. Which will be ultimately resulting in benefit for farmer and Pakistan’s agriculture sector which is dependent on cotton.
2 Materials and methods
2.1 Field insect collection; colony preparation; and insecticide
Pectinophora gossypiella adults and larvae were collected from cotton fields located at Adda Peer Murad, District Vehari fields for colony preparation. Insects after collection from the fields were kept in cages with cotton bolls were provided to them for feeding and plant terminals held in water for egg laying in the colonies. Cotton plants in green houses were also grown for continuous food supply of insects. For this purpose, plastic pots of 6x10x20 cm were used in green house. Bolls from plants were collected and were brought to the laboratory. Insecticide free cotton bolls were kept for colony preparation. Temperature and relative humidity were 27 ± 1 °C and 70 % respectively. Adults were fed with 10 % honey solution. While adults for their egg laying were provided with voiles having plant terminals held in water as substrate for egg laying. Insects were reared for 2 generations in the laboratory. For bioassay purpose, triazophos (Jaffer agro services) 40 % EC having active ingredients of 10 % per liter purchased from local market in package of 1000 ml.
2.2 Bioassay for lethal concentration of insecticide
This experiment was conducted to investigate the direct toxicity of triazophos on insect biology. Bioassay of newly hatched larvae (hatching within 12 h) was conducted by feeding them on insecticide treated bolls. Bolls was cut diagonal sections to make it convenient for larvae to feed on it. Pre-liminary range finding dilutions (0.050–10 mg/L) was already conducted to get a series of concentrations which provided mortality of 0–80 %. Dilutions of 0,1,2,3,4,5,6 mg/L were prepared and already diagonally cut bolls were dipped in insecticides and made it dry for 20 min on towels at room temperature. Same procedure as Gao et al. (2018) leaf-dip bioassay with modifications was conducted. Bioassay was conducted for each instar of larvae. One larva per vile was tested and 80 larvae per instar for each replicate was tested. For each treatment total 240 larvae in 3 replications were used at each dose. Each treatment was repeated 3 times and mortality was observed after 24 h till 7 days. Individuals not showing movement by touching the brush were considered as dead.
For F1 generation, bioassay was conducted with newly hatched larvae evolved from F0. These insects were separate from two sex table studies larvae. Same procedure of F0 was followed with each treatment repeated 3 times.
2.3 Evaluation of LC25 and sublethal LC10 sublethal effects of triazophos on life history traits of P. gossypiella at F0 and F1 generations
This experiment was aimed to know the direct impact of low or sublethal concentration on pink bollworm larvae on F0 generation and further impacts on F0 evolved generation F1 (without triazophos exposure). Different concentrations which were determined from baseline toxicity experiments were used to determine the LC25 and sublethal LC10sublethal effects of triazophos on life cycle parameters of P. gossypiella. 1500 adults were transferred to clean cage with tissue papers and cotton bolls inside to lay eggs on them. After 48 h they were removed from the cage and egg laying was transferred to other cage until eggs were hatched (within 12 h) and their first instar was used for F0 generation. 60 larvae were transferred to other cage for LC25 and LC10sublethal treatments in glass vials. Cotton bolls as diet was changed after one day interval till 2nd instar, while changed cotton bolls each day on becoming 3rd instar. For pairing the insects, newly hatched pupae were chosen and observed for male or female genital appearance (CABI 2010) and were kept in vile, they were paired and mated (30–32 per treatment). Fecundity and longevity were observed until mortality of pair. Oviposition period was observed for each female insect (Chi et al., 2018). Data was recorded after 12 h till death of individual insect. Each experiment was repeated 3 times. Honey-soaked cotton was given to adult moths.
2.4 Transgenerational effects of triazophos on F1 generations of P. gossypiella
Two eggs from each pair of each group of F0 (LC10; LC25; LC50; Control) were selected and kept in voiles for starting F1 generation. Hatched offspring from these eggs were used as F1 generation observations. Diagonally cut cotton bolls were placed in voiles for food. The transgenerational effects were studied at all stages of insect. At pupal stage, insects were paired and kept in voiles for egg laying. Data was recorded after 12 h till death of individual insect. Characteristics such as developmental time; adult longevity; and fecundity were observed. Temperature was kept at 27 ± 1 °C with relative humidity 75 %.
2.5 Statistical analysis
Lethal and sublethal concentrations were calculated using POLO-Plus and Probit analysis (LeOra Software 2006). ANOVA was used to examine mortality. TWO-SEX MS CHART assessed life table characteristics utilizing age-stage two-sex table (Chi 1988). (Chi 2015). Means and SEs were determined using bootstrap (n = 100,000) (Huang and Chi, 2012). Various life table metrics, including age-stage specific survival rate (sxj), life expectancy (exj), which tells us if a person will survive to an age appropriate for that stage, and age-stage specific net reproductive value, are available (vxj). As of the calculation, other population factors such as the intrinsic rate of rise (r), finite rate of increase (λ), net reproductive rate (R0), and mean generation time (T) (Chi and Liu 1985).
The Euler-Lotka equation was used to compute the intrinsic rate of rise (r) for the Age-stage, two sex life table, with age indexed as (Goodman 1982).
R0 (Net Reproductive Rate) which is total number of offsprings that an individual can produce during lifetime will be calculated as
Net Reproductive Rate (R0) and mean female fecundity (F) relationship was calculated as
N = total number of individuals, F = number of females adults in this study (Chi 1988).
The finite rate (λ) λ = er,
Mean generation time (T) = time span that population needs to increase R0 folds of its size. Value of T was calculated as.
Life expectancy (exj) was calculated as
fe probability (sxj) was calculated as
e-stage reproductive value (vxj) was calculated as
3 Results
3.1 Lethal effects of triazophos against F0 and F1 generations
The LC50 values for P. gossypiella at 0, 1, 2, and 4 instars were 2.72, 2.75, 3.34, and 3.27 mg/L of triazophos (Table 1). The LC10 for triazophos was 1.984, 1.859, 2.380, and 2.297 mg/L for the first, second, third, and fourth instars of the F0 generation, respectively (Table 1). Triazophos's LC25 for the F0 generation was 2.307 mg/L for the first instar, 2.241 mg/L for the second instar, 2.798 mg/L for the third instar, and 2.781 mg/L for the fourth instar (Table 1). The LC90 values of triazophos for first, second, third, and fourth instar P. gossypiella larvae of the F0 generation were 3.75, 4.09, 4.70, and 4.67 mg/L, respectively (Table 1).
| Gen. | LC10 (mg/L) | LC25 (mg/L) | LC50 (mg/L) | LC90 (mg/L) | Slope | X2 | P- value | df |
|---|---|---|---|---|---|---|---|---|
| 1st Instar | ||||||||
| F0 | 1.98 (1.87–2.07) |
2.30 (2.21–2.39) |
2.72 (2.64–2.80) |
3.752 (3.63–3.88) |
9.26 ± 0.55 | 10.73 | 0.71 | 79 |
| F1 | 0.98 (0.79–1.15) |
1.330 (1.13–1.50) |
1.85 (1.66–2.03) |
3.47 (3.12–3.97) |
4.68 ± 0.22 | 78.21 | 4.88 | 79 |
| 2nd Instar | ||||||||
| F0 | 1.85 (1.70–1.99) |
2.24 (2.10–2.36) |
2.75 (2.63–2.87) |
4.09 (3.89–4.34) |
7.47 ± 0.40 | 31.22 | 1.95 | 79 |
| F1 | 1.04 (0.83–1.22) |
1.42 (1.20–1.61) |
2.00 (1.79–2.21) |
3.86 (3.43–4.49) |
4.50 ± 0.19 | 95.85 | 5.99 | 79 |
| 3rd Instar | ||||||||
| F0 | 2.380 (1.98–2.65) |
2.79 (2.46–3.04) |
3.34 (3.08–3.57) |
4.70 (4.36–5.27) |
8.65 ± 0.52 | 15.66 | 1.60 | 79 |
| F1 | 1.55 (1.29–1.77) |
1.99 (1.74–2.20) |
2.62 (2.39–2.85) |
4.43 (4.01–5.06) |
5.62 ± 0.24 | 10.91 | 1.32 | 79 |
| 4th Instar | ||||||||
| F0 | 2.29 (1.94–2.55) |
2.71 (2.41–2.95) |
3.27 (3.03–3.49) |
4.67 (4.31–5.22) |
8.31 ± 0.45 | 15.60 | 1.59 | 79 |
| F1 | 1.77 (1.47–2.00) |
2.14 (1.87–2.34) |
2.63 (2.42–2.81) |
3.91 (3.62–4.36) |
7.46 ± 0.47 | 6.974 | 4.81 | 79 |
The values in parenthesis indicate the range of respective means.
For larvae, the LC50 values for the first, second, third, and fourth instars of the F1 generation (a different set of insects other than the two-sex table group) were 1.852, 2.008, 2.627, and 2.637 mg/L, respectively (Table 1). Mortality concentration response lines were used to calculate the LC10 and LC25. For the F1 generation, the LC10 values were 0.987, 1.042, 1.555, and 1.776 mg/L for the first, second, third, and fourth instar larvae, respectively (Table 1). The LC25 values in the first, second, third, and fourth instars of P. gossypiella larvae were 1.330, 1.422, 1.993, and 2.142 mg/L, respectively, for the F1 generation (Table 1). First generation P. gossypiella larvae had an LC90 value of 3.4763 mg/L for the first instar, 3.8673 mg/L for the second instar, 4.4382 mg/L for the third instar, and 3.915 % mg/L for the fourth instar when exposed to triazophos (Table 1).
3.2 LC25, LC10 and transgenerational effects of triazophos on biological and reproductive parameters of F0, F1 generations
Triazophos application to determine the LC25; sublethal LC10sublethal and transgenerational effects on biological and reproductive parameters on all instars and pupae of F0 and F1 (Tables 2 and 3) showed that significant differences occurred when compared to control. There was no significant difference in hatching duration of F0 (F = 4.20; df = 57; P = 0.013); F1 (F = 2.12; df = 57; P = 0.156) generations (Table 2). There was significant difference in 1st instar larval duration of F0 (F = 5.17; df = 57; P = 0.0001) at LC10 and LC25 showing prolonged duration as compared to control; F1 (F = 2.64; df = 57; P = 0.0002) generation (Table 2). For 2nd instars there was no significant difference in larval duration of F0 at LC10 and LC25 (F = 3.27; df = 57; P = 0.217); F1 (F = 1.94; df = 57; P = 0.143) generation (Table 2). For 3rd instars there were no significant difference in instar larval duration of F0 (F = 6.32; df = 57; P = 0.301); F1 (F = 2.29; df = 57; P = 0.244) generation (Table 2). For 4th instars there were no significant difference in instar larval duration of F0 (F = 3.31; df = 57; P = 0.0433); F1 (F = 1.87; df = 57; P = 0.0037) generation (Table 2). For pupa there were significant difference in duration of F0 (F = 2.24; df = 57; P = 0.0172); F1 (F = 3.76; df = 57; P = 0.0564) generations (Table 2).
| Conc. | Duration (Egg- larva) | 1st instar | 2nd instar | 3rd instar | 4th instar | Pupa |
|---|---|---|---|---|---|---|
| F0 | ||||||
| Control | 3.70 ± 0.10 | 2.39 ± 0.075 | 3.95 ± 0.075 | 3.97 ± 0.095 | 3.02 ± 0.074 | 7.74 ± 0.56 |
| LC10 | 3.18 ± 0.178 | 3.04 ± 0.075 | 3.95 ± 0.096 | 3.95 ± 0.095 | 3.60 ± 0.105 | 8.58 ± 0.065 |
| LC25 | 3.37 ± 0.017 | 3.01 ± 0.017 | 3.98 ± 0.017 | 3.98 ± 0.017 | 3.44 ± 0.065 | 8.13 ± 0.449 |
| P value | 0.013 | 0.0001 | 0.217 | 0.301 | 0.0433 | 0.0172 |
| F | 4.20 | 5.17 | 3.27 | 6.32 | 3.31 | 2.24 |
| df | 57 | 57 | 57 | 57 | 57 | 57 |
| F1 | ||||||
| Control | 3.7 ± 0.10 | 2.46 ± 0.098 | 3.80 ± 0.116 | 3.73 ± 0.119 | 2.73 ± 0.105 | 8.17 ± 0.363 |
| LC10 | 3.1 ± 0.10 | 3.21 ± 0.081 | 3.88 ± 0.073 | 3.70 ± 0.095 | 3.21 ± 0.131 | 7.42 ± 0.689 |
| LC25 | 3.33 ± 0.19 | 3.01 ± 0.10 | 3.68 ± 0.12 | 3.65 ± 0.12 | 3.23 ± 0.13 | 8.37 ± 0.22 |
| P value | 0.156 | 0.0002 | 0.143 | 0.244 | 0.0037 | 0.0564 |
| F | 2.12 | 2.64 | 1.94 | 2.29 | 1.87 | 3.76 |
| df | 57 | 57 | 57 | 57 | 57 | 57 |
| Conc. | Pre-oviposition period | Fecundity | Female adult longevity |
|---|---|---|---|
| F0 | |||
| Control | 2.52 ± 0.09 | 264.76 ± 3.94 | 13.48 ± 0.10 |
| LC10 | 3.086 ± 0.64 | 231.08 ± 1.65 | 12.43 ± 0.15 |
| LC25 | 3.130 ± 0.65 | 203.87 ± 3.38 | 11.65 ± 0.15 |
| P value | 0.0013 | 0.002 | 0.002 |
| F | 0.82 | 0.93 | 0.84 |
| F1 | |||
| Control | 2.34 ± 0.095 | 274.61 ± 4.031 | 13.50 ± 0.10 |
| LC10 | 3.13 ± 0.169 | 220.95 ± 2.289 | 12.78 ± 0.153 |
| LC25 | 3.65 ± 0.095 | 209.21 ± 3.531 | 12.69 ± 0.199 |
| P value | 0.0029 | 0.034 | 0.0001 |
| F | 1.24 | 0.88 | 1.13 |
Reproductive parameters for F0 and F1 when used LC10 and LC25 for P. gossypiella are shown in (Table 3). Pre-oviposition of F0 at LC10 and LC25 showed significant differences as compared to control (F = 0.82; P = 0.0013); significant difference occurred for F1 (F = 1.24; P = 0.0029). Fecundity for F0 at LC10 and LC25 showed significant differences as compared to control (F = 0.93; P = 0.002); F1 (F = 0.88; P = 0.034). Female adult longevity for F0 at LC10 and LC25 showed significant differences as compared to control (F = 0.84; P = 0.002); F1 (F = 1.13; P = 0.0001); (Table 3). Oviposition period for F0 at LC10 and LC25 showed significant differences with longer or shorter oviposition period as compared to control (F = 0.68; P = 0.021); F1 (F = 1.27; P = 0.002); (Table 3).
3.3 Effect of triazophos on demographic traits of F0 and F1 generations
Table 4 displays the results two-sex-stage-specific life table regarding the effects of triazophos on population characteristics (demographic attributes). The intrinsic rate of increase (r) for the F0 generation was negatively correlated with concentration and did not vary significantly from the control group (Table 4). There was no statistically significant difference between the mean finite rate of growth of insects treated with LC10 or LC25 and those in the control group (Table 4). Control had a greater net reproduction rate (R0), but LC10 and LC25 drastically reduced it. As shown in Table 4, the longer mean generation time (T) for insects treated with LC10 and LC25 compared to the control group would lead to fewer generations of insects in cotton fields. Comparing the control group to the LC10 and LC25 treated groups, the gross reproductive rate (GRR) of the latter was found to be considerably lower (Table 4).
| F0 | ||||
|---|---|---|---|---|
| LC10 | Control | LC25 | Control | |
| r | 0.147 ± 0.0054ab | 0.161 ± 0.0056a | 0.143 ± 0.0055ab | 0.161 ± 0.0056a |
| λ | 1.158 ± 0.0066a | 1.174 ± 0.0065a | 1.154 ± 0.0064a | 1.174 ± 0.0065a |
| R0 | 88.583 ± 14.53b | 110.33 ± 16.87a | 78.156 ± 12.82b | 110.33 ± 16.87a |
| T | 30.499 ± 0.177a | 29.168 ± 0.28a | 30.341 ± 0.18a | 29.168 ± 0.28a |
| GRR | 93.146 ± 14.86b | 115.71 ± 17.20a | 82.186 ± 13.11b | 115.71 ± 17.20a |
| F1 | ||||
| r | 0.145 ± 0.0057ab | 0.162 ± 0.0052a | 0.144 ± 0.005ab | 0.162 ± 0.0052a |
| λ | 1.157 ± 0.0065a | 1.176 ± 0.0061a | 1.155 ± 0.006a | 1.176 ± 0.0061a |
| R0 | 84.76 ± 13.92b | 119.03 ± 17.67a | 80.26 ± 13.19b | 119.03 ± 17.67a |
| T | 30.42 ± 0.212a | 29.47 ± 0.144a | 30.41 ± 0.17a | 29.47 ± 0.144a |
| GRR | 90.77 ± 14.37b | 126.76 ± 18.19a | 85.79 ± 13.63b | 126.76 ± 18.19a |
R = Intrinsic rate of increase; λ = Finite rate of increase; R0 = Net reproduction rate; T = Mean length of generation; GRR = Gross reproduction rate.
When comparing LC10 and LC25 to the control group, the F1 generation showed an intrinsic rate of rise (r) that was inversely proportional to concentration (Table 4). Both the LC10 and LC25 groups showed no statistically significant differences from the control group in terms of the mean finite rate of increase (Table 4). Insects treated with LC10 and LC25 had a lower net reproduction rate (R0) than controls, and this decline accelerated with increasing concentration (Table 4). Insects treated with LC10 and LC25 had a longer mean generation time (T) compared to controls (Table 4). Insects treated with LC10 and LC25 have dramatically reduced GRR compared to controls (Table 4).
Using bootstrap analysis, we were able to determine the age-stage specific survival rate (sxj). The sxj demonstrated the likelihood that a freshly placed egg would survive from age × to stage j when applied to the F0 generation. The LC10 and LC25 groups had an increase in total life span when compared to the control group (Fig. 1). The fact that the age-stage specific life expectancy exj was greater in LC10 and LC25 populations as compared to control (Fig. 2) demonstrated the likelihood that LC10 and LC25 treated insects would have a longer lifespan than control. Age-stage specific reproductive rate (vxj) indicates that the overall reproduction rate decreased in LC25 populations; further, LC10 populations also shown less reproductive rate as compared to control (Fig. 2), which demonstrated that although a population can survive longer, it will reproduce fewer offspring in the generations to come.
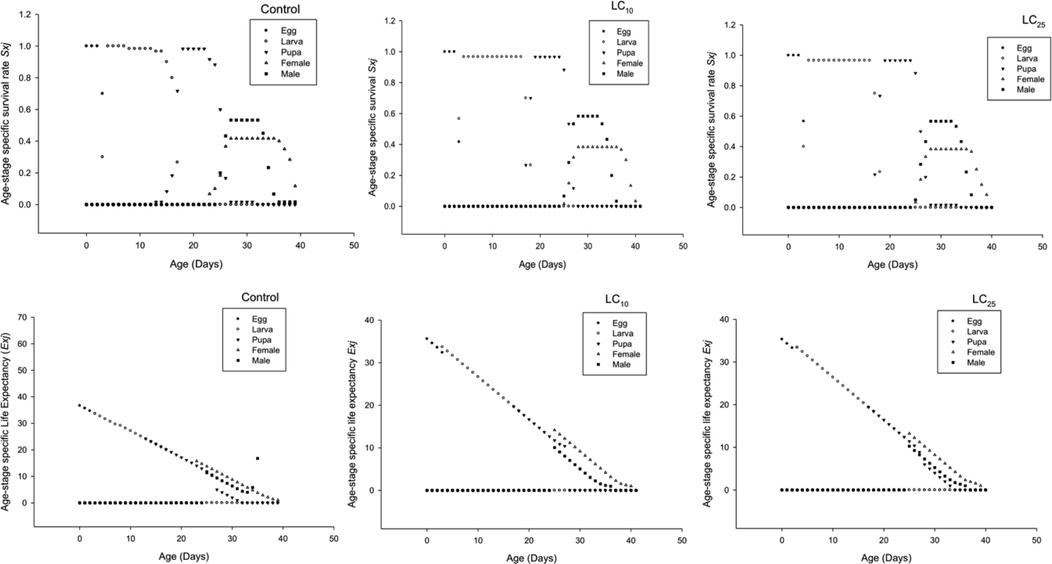
- Age Stage Specific Survival Rate (Sxj) and Age Stage Life Expectancy (Exj) of F0 generation P. gossypiella.
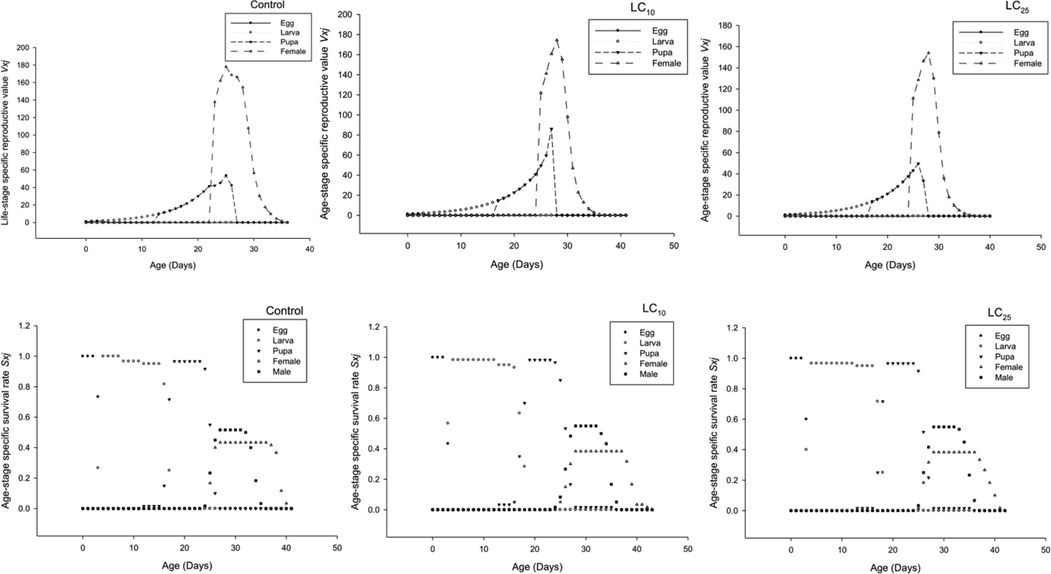
- Age Stage Reproductive Value (Vxj) of F0 generation and Age Stage Specific Survival Rate (Sxj) of F1 generation pink bollworm P. gossypiella.
To determine the survival rate (sxj) for each age group, a bootstrap sample was generated. For the F1 generation of a species, the survival probability from age x to age j was denoted by the symbol sxj. The LC10 and LC25 had significantly longer median ages than control (Fig. 3). Life expectancy at different ages exj was greater in the LC10 and LC25 populations than in the control group (Fig. 3), suggesting that treated insects lived longer than their untreated counterparts. Compared to controls, LC25 populations have a lower age-specific reproductive rate (vxj) of F0 (Fig. 3), indicating that longer-lived populations do not generate more offspring than those who perish earlier in their lives.
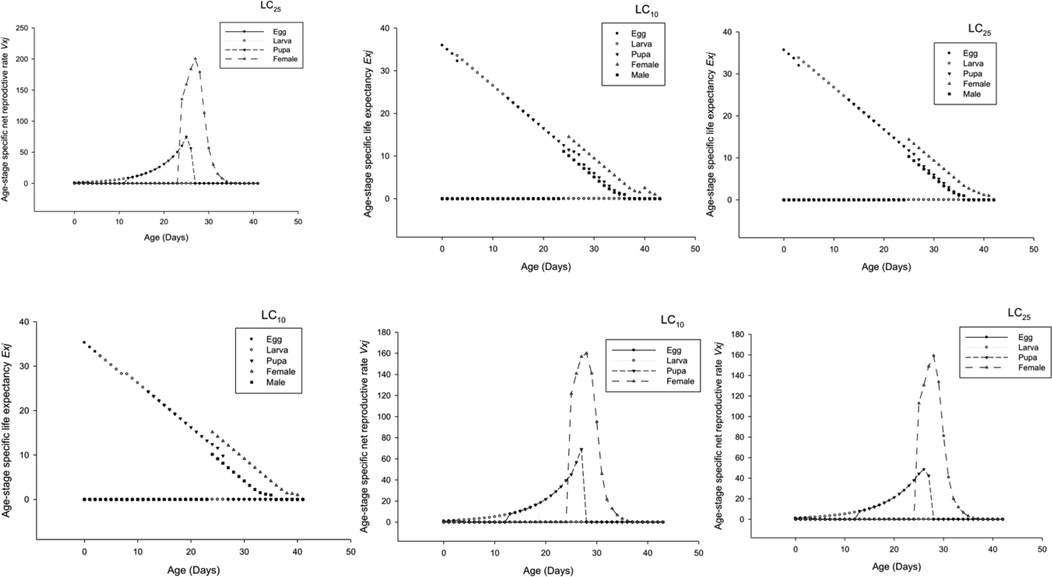
- Age Stage Life Expectancy (Exj) and Age Stage Reproductive Value (Vxj) of F1 generation P. gossypiella.
4 Discussion
A sex table broken down by age provides insight into the survival, growth, and reproduction of male and female populations (Chi 1988; Chi and Liu 1985). These investigations show that triazophos has both fatal and sublethal effects on P. gossypiella when utilizing a two-sex table, and that these effects may be passed down via subsequent generations. Two sex table on P. gossypiella life span showed female having prolonged life cycle as compared to males. Bioassay results found significant differences in the demographic and reproductive parameters of P. gossypiella for two generations.
Insecticides are only reliable method to control insect, frequent use of insecticide causes resistance problem in insects (Ghramh et al., 2019). Pectinophora gossypiella has also developed resistance against different group of insecticides (Akhtar et al., 2020). To use insecticides in rationale manner, sublethal effects of insecticides are important (Xiao et al., 2015; Liang et al., 2012). Triazophos being broad spectrum insecticide may cause sublethal effects, alterations of biology of P. gossypiella. Triazophos, in current studies, showed significant differences in fecundity and longer larval duration in P. gossypiella, because sublethal effects may change fecundity; hatchability; and longevity of arthropods (Desneux et al., 2007). Similarly, Liu et al. (2016) observed that triazophos caused significant prolonged nymphal duration of Sogetella frucifera, significantly depressed various life table parameters at sublethal concentrations of triazophos, it also stimulated reproduction in S. frucifera, triazophos also inhibited population growth of S. frucifera as compared to control (Liu et al., 2016). Our results were in agreement with Wang et al. (2010), in which triazophos was found to be affecting males and females and effects on fecundity of female N. lugens was observed.
In current studies, prolonged oviposition period in LC25 treated group was due to harmosis effects. Because pesticide induced homeostasis modulation which is term including both hormesis and stimulatory effects of pesticides on non-target arthropods (reviewed by Desneux et al., 2007). Hormesis includes both low-dose stimulatory and high dose inhibitory effects of insecticides. It is dose and time dependent phenomenon in which impacts are shown as result of exposure of pesticide sublethal dose rate (reviewed by Liu et al., 2016) so in current studies high dose inhibition (lower fecundity) and low dose stimulation (prolonged oviposition period) were in agreement with Desneux et al. (2007) and Calabrese (2008).
Demographic features from two sex table showed that r; λ; R0 were significantly affected in triazophos LC10 and LC25 treated insects as compared to control. Low value of relative fitness shows that more time and energy will be spent by insect to metabolize the insecticides triazophos. Our results were in agreement with (Zhang et al., 2018; Shi et al., 2022). Analysis curves of two generations regarding survival rate (sxj); female age specific rate (fx); age specific survival rate (lx); age specific fecundity (fx); age specific maternity (lxmx); age specific reproductive value (vx)explicitly revealed that LC10 and LC25 concentrations of triazophos negatively affected all these parameters (survival rate; fecundity) during F0 and F1 generations as compared to control. Triazophos also resulted in lower life expectancy (exj)Our results were in agreement with Shi et al. (2022) in which all these parameters were negatively affected at LC25 concentrations of nitenpyram against S. frucifera.
Trans-generational effects in these studies were important to determine the survival, growth and development of insect, so r is important demographic parameter (Bonte et al 2015; Chen et al., 2018). For population parameter “r” can be better explained using two sex table (Huang and Chi 2012). Using two sex table, reproduction of insects, Ro and GRR (reproductive parameters) were determined. Ro is significant indicator of population development which is directly related to number of eggs. In current studies r value was not significantly different in LC10 and LC25 treated insects as compared to control for both generations explicitly indicating that survival; growth and development were not significantly affected with sublethal doses of triazophos. In current studies, Ro and GRR were significantly lower in LC10 and LC25 treated groups as compared to control, which explicitly reveal that reproductive parameters were lower for triazophos treated insects which can be helpful in population reduction in the fields of cotton. Our results were in agreement with Chi (1988); Chi et al. (2020) for two sex table analysis, in which exact relationship between Ro and fecundity was found and, in our studies female produced offspring were equal to net reproductive rate. Intrinsic rate of increase (r) and finite rate of increase (λ) were not significantly affected as compared to control, these results were in agreement with Tang et al. (2019). Our results about longer oviposition period in F1 for LC25 as compared to control showed hormetic effect which was also in agreement with Tang et al. (2019), which we assert that un-intended longer or shorter oviposition duration at low concentration results in hormetic effects for pink bollworm.
Our research shows that triazophos, at both lethal and sublethal doses, may hinder the growth and reproduction of P. gossypiella based on its age. This is useful information for developing an Integrated Pest Management approach. Triazophos has sublethal effects, making it a viable alternative to DDT for managing pink bollworm. When it comes to controlling pink bollworms, a lower r value (population parameter) in the LC10 and LC25 treated groups will be very useful. Insect populations may be better managed in the future under field settings if they are targeted at both male and female stages of development, as shown by the two-sex life table. Current research shows that triazophos improves pink bollworm control in the field by causing death and reducing reproduction. Insecticides lose their effectiveness over time after being applied in the field, and their sublethal effects might lead to additional pest population reduction. Desneux et al. (2007) provide a technique for assessing the sublethal effects of triazophos on beneficial creatures, and these findings might one day be used to predators of Pectinophora gossypiella. Before these findings can be incorporated into regulatory systems, however, we need to create standardized methodologies for analyzing the impact of sublethal doses on the efficiency of beneficial species.
5 Conclusion
Our studies found that triazophos can impair P. gossypiella age-stage specific development; age-stage specific reproduction at lethal and sublethal concentrations which is helpful to prepare Integrated Pest Management strategy. These sublethal effects make triazophos as alternate insecticide to control pink bollworm. Lower r value (population parameter) in LC10 and LC25 treated groups will be helpful in managing pink bollworms in cotton fields. Two-sex life table will help to formulate a comprehensive strategy for controlling P. gossypiella during larvae and adult exposure to triazophos using male and female separate sexes for future control of insect population under field conditions. When applying these results in the field can be helpful in population management of pink bollworms on cotton bolls, brackets, and flowers. We conclude that a better understanding of the reproductive and demographic aspects of P. gossypiella influenced by triazophos would aid in the control of this pest in cotton crops.
Acknowledgments
Current study was funded by National Natural Science Foundation of China (Grant No. 31860620) and Agricultural Science and Technology Innovation Program(CAAS-ASTIP-2015-IAR). This work was supported by the King Khalid University through a grant KKU/RCAMS/22 under the Research Center for Advance Materials (RCAMS) at King Khalid University, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synergized toxicity of promising plant extracts and synthetic chemicals against fall armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) in Pakistan. Agronomy. 2022;12:1289.
- [Google Scholar]
- Genetic resistance in pink bollworms against transgenic cotton: an evidence from Pakistan. Pak. Entomol.. 2016;38(2):153-157.
- [Google Scholar]
- Akhtar ZR, Rasul A, Sagheer M, Ali A, Ashraf I, Saddiq B and Hasan M, 2020. Characterizing the mode of resistance inheritance and cross resistance in pink bollworm against Cry1AC toxin and organophosphate pesticides in Pakistan. Pak. J. Agri. Sci., Vol. 57(4), 1101–1106; 2020.
- Determination of fitness traits of Orius strigicollis Poppius (Hemiptera: Anthocoridae) on Pectinophora gossypiella (Lepidoptera: Gelechiidae) using two-sex life table analysis. PeerJ. 2020;8:e9594.
- [Google Scholar]
- Predation capacity, development and reproduction of the southern African flower bugs Orius thripoborus and Orius naivashae (Hemiptera: Anthocoridae) on various prey. Biol. Control.. 2015;86:52-59.
- [Google Scholar]
- Hormesis: why it is important to toxicology and toxicologists. Environ. Toxicol. Chem.. 2008;27:1451-1474.
- [Google Scholar]
- Demography and uncertainty of population growth of Conogethes punctiferalis (Lepidoptera: Crambidae) reared on five host plants with discussion on some life history statistics. J. Econ. Entomol.. 2018;111:2143-2152.
- [Google Scholar]
- Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol.. 1988;17:26-34.
- [Google Scholar]
- Age-stage, two-sex life table: an introduction to theory, data analysis, and application. Entomol Gen. 2020;40(2):103-124.
- [Google Scholar]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. 2015, volume 97, National Chung Hsing University, Taichung, Taiwan. Online http://140.120.197.173/Ecology/Download/Twosex-MSChart.rar (Assessed on 3rd of July, 2021).
- Diaeretiella rapae limits Myzus persicae populations after applications of deltamethrin in oilseed rape. J. Econ. Entomol.. 2005;98(1):9-17. PMID: 15765661
- [CrossRef] [Google Scholar]
- The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol.. 2007;52:81-106.
- [Google Scholar]
- Biogenic synthesis of silver nanoparticles using propolis extract, their characterization, and biological activities. Sci. Adv. Mater.. 2019;11:876-883.
- [Google Scholar]
- Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat.. 1982;119:803-823.
- [Google Scholar]
- The response of four braconid parasitoid species to methyl eugenol: optimization of a biocontrol tactic to suppress Bactrocera dorsalis. Biol. Control. 2018;122:101-108.
- [Google Scholar]
- Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci.. 2012;19:263-273.
- [Google Scholar]
- Acaricidal potential of some botanicals against the stored grain mites, Rhizoglyphus tritici. J Entomol. Zool. Stud.. 2016;4:611-617.
- [Google Scholar]
- Protein baits, volatile compounds and irradiation influence the expression profiles of odorant-binding protein genes in Bactrocera dorsalis (Diptera: Tephritidae) Appl. Ecol. Environ. Res.. 2017;15:1883-1899.
- [CrossRef] [Google Scholar]
- Effectiveness of entomopathogenic fungi on immature stages and feeding performance of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. Insects. 2021;12:1044.
- [CrossRef] [Google Scholar]
- Bioassays of Beauveria bassiana isolates against the fall armyworm, Spodoptera frugiperda. J. Fungi. 2022;8:717.
- [CrossRef] [Google Scholar]
- Laboratory efficacy of selected synthetic insecticides against second instar invasive fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. PLoS ONE. 2022;17(5)
- [Google Scholar]
- Short-term and transgenerational effects of the neonicotinoid nitenpyram on susceptibility to insecticides in two whitefly species. Ecotoxicology. 2012;21(7):1889-1898.
- [Google Scholar]
- Sublethal effect of spirotetramat on the life table and population growth of Frankliniella occidentalis (Thysanoptera: Thripidae) Entomol. Ge.n. 2021;41(3):219-231.
- [Google Scholar]
- Sublethal effects of triazophos on the life table parameters of Sogatella furcifera (Hemiptera: Delphacidae) Florida Entomol.. 2016;99(2):292-296.
- [Google Scholar]
- Shen J (2022) Sublethal effects of emamectin benzoate on fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) Agriculture. 2022;12:959.
- [CrossRef] [Google Scholar]
- Population dynamics of sucking pest complex on some advanced genotypes of cotton under unsprayed conditions. Pak. J. Zool.. 2016;48:475-480.
- [Google Scholar]
- Increased reliance on insecticide applications in Canada linked to simplified agricultural landscapes. Ecol. Appl.. 2022;32(3):e2533.
- [Google Scholar]
- A Bibliography of the Pink Bollworm, Pectinophora gossypiella (Saunders). U.S. Department of Agriculture, Agricultural Research Service, Bibliographies and Literature of Agriculture; 2001. p. :136.
- Pink bollworm: a notorious pest of cotton: a review. Agres Int. E-J.. 2016;5:88-97.
- [Google Scholar]
- Pires Paula, Débora; Lozano, Rosa E.; Menger, James P.; Andow, David A.; Koch, Robert L. 2021. Identification of point mutations related to pyrethroid resistance in voltage-gated sodium channel genes in Aphis glycines. Entomol Gen 41, 243-255.
- Action threshold development in cabbage pest management using synthetic and botanical insecticides. Entomol. Gen.. 2020;40(2):157-172.
- [Google Scholar]
- Shi Y, Chen HS, Wu S, Xia FJ, He MR, Yang L, Li RY, Liao X, Li M, 2022. Sublethal effects of nitenpyram on the biological traits and metabolic enzymes of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae), Crop Protection, 155, 105931, ISSN 0261-2194.
- Technological opportunity, regulatory uncertainty, and Bt cotton in Pakistan. AgBioForum. 2015;18:98-112.
- [Google Scholar]
- Sublethal and transgenerational effects of spinetoram on the biological traits of Plutella xylostella (L.) (Lepidoptera: Plutellidae) Ecotoxicology. 2021;30(4):667-677.
- [Google Scholar]
- Transgenerational hormetic effects of sublethal dose of flupyradifurone on the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae) PLoS ONE. 2019;14(1) e0208058
- [Google Scholar]
- Insecticide-induced increase in the protein content of male accessory glands and its effect on the fecundity of females in the brown planthopper Nilaparvata lugens Stål (Hemiptera: Delphacidae) Crop Prot.. 2010;29(11):1280-1285.
- [Google Scholar]
- Assessment of sublethal and transgenerational effects of pirimicarb on the wheat aphids Rhopalosiphum padi and Sitobion avenae. PLoS ONE. 2015;10(6) e0128936
- [Google Scholar]
- Fitness cost of nitenpyram resistance in the brown planthopper Nilaparvata lugens. J. Pest. Sci.. 2018;91:1145-1151.
- [Google Scholar]
Further reading
- Effectiveness of different soft acaricides against honeybee ectoparasitic mite Varroa destructor (Acari: Varroidae) Insects. 2021;12(11):1032.
- [Google Scholar]







