Translate this page into:
Transcriptional alterations associated with overexpression of a chlorogenic acid pathway gene in eggplant fruit
⁎Corresponding author. arpithashnkr94@gmail.com (Bandi Arpitha Shankar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The fruits of eggplants have different shapes and sizes, which render them ideal for metabolic engineering. They can aid in increasing eggplant chlorogenic acid content, a critical nutrient. Among the phenolic acids found in eggplants, chlorogenic acid is the most important and highly bioactive. This phenolic acid is essential because it promotes good health in humans, and for its production in the fruit flesh, the hydroxyquinone CoA transferase (SmHQT) enzyme is crucial. Therefore, we explored this further by using the agroinfiltration protocol, thereby comparing transgenic and wild type expression via RNA-seq analysis. Given the SmHQT overexpression of 415, the characteristics of the phenylpropanoid pathway are regulated in transgenic eggplants. Furthermore, agroinfiltrated fruit showed around twofold (3.98 g/mg of FW) chlorogenic acid content as compared to the wild type (2.02 g/mg of FW). As a result, the findings shed new light on how to increase eggplant chlorogenic acid content at the molecular level.
Keywords
Eggplant
Agroinfiltration
Chlorogenic acid
Phenyl-propanoid pathway
SmHQT
Transcriptome analysis
1 Introduction
Eggplants are the same plant family members as tomatoes and peppers, i.e., Solanaceae and the cultural practices and production techniques are almost like those plants (Welbaum, 2015; Knapp and Peralta, 2016). Eggplants originated in India and continued to grow as a wild type, but thanks to breeding techniques that improved these wild varieties' yield, appearance, taste, and color to be consumed and cooked easily (Page et al., 2019). Eggplants have a high amount, i.e., 95 % of chlorogenic acid, a phenolic compound that has proven beneficial for humans and the plant itself (Docimo et al., 2016). Phenolic acids benefit human health significantly because they protect against diseases like diabetes, cancer, and rheumatoid arthritis. Similarly, these phenolic acids are valuable for plants because they help protect the plant against insects and pathogen infestations (Kaushik et al., 2015). Moreover, a high percentage of chlorogenic acid is present in Eggplant compared to other vegetables of Solanaceae.
As a result, increasing the amount of chlorogenic acid in eggplant is a significant breeding objective currently being pursued (Caruso et al., 2017). Hydroxycinnamoyl CoA:quinate hydroxycinnamoyl transferase (SmHQT) plays a critical role in catalyzing chlorogenic acid synthesis (Kaushik and Saini, 2019). While cultivated eggplants contain fewer phenolic acids than wild ones, using wild species in the eggplant breeding program requires additional time to eliminate undesirable genes resulting from the severed connection. Also, planting wild species is difficult because of the duration of the plants and their adaptability (Kaushik et al., 2017). Agrofiltration is the most common technique that allows the expression of transient genes in plant networks (Ahmad et al., 2012). The agroinfiltration approach for gene expression is regularly performed in fleshy fruits such as tomatoes, strawberries, melons, and cucumber and succeed (Guidarelli and Baraldi, 2015). Therefore, changes in the expression of SmHQT gene via agroinfiltration in the eggplant fruits will open new insights.
Several breeding techniques have been adopted for enhancing the effect of chlorogenic acid, but they gave fewer results (Garg et al., 2018; Lu et al., 2020). Certain unwanted genes also interfere with chlorogenic acid production in wild relatives of eggplants, and certain techniques were unable to eradicate these undesirable genes. Recently, certain molecular approaches have enhanced certain genes' qualitative effects by genome editing and transgenic technology (Docimo et al., 2016). With transgenic technology, it is now possible to give sequence-specific traits. Thus, the aim of this study is to enhance SmHQT gene expression in eggplant fruit flesh (Sinha et al., 2019). This P19 gene is well-known for its ability to prevent post-transcriptional gene silence from occurring. As a result, it was also included in the vector (Chu et al., 2000).
The transcripts in the transgenic plant can be contrasted by RNA-seq, as this approach may provide valuable insight into the expression of a transgene (Mahdavi Mashaki et al., 2018; Kaundal et al., 2021). Therefore, here RNA Sequencing (RNA Seq) dependent gene expression of agroinfiltrated fruits were compared. This is because the agroinfiltration method is straightforward, cheap and has maximum transformation efficiency than any other method. With this technique's help, we can efficiently study the function of genes involved in different pathways. Thus, with the help of a low-cost system, we can overexpress the gene of interest, i.e., the one related to chlorogenic acid for its production.
2 Materials and methods
2.1 Plant material, SmHQT gene construct and agroinfiltration
The green, long, and barrel-shaped ordinary bearing eggplant course of action Arka Shrish was picked. The seeds of this assortment were filled in a blend of turf soil and perlite in a 2:1 degree under a nursery with steady light at 20–25 degreesC and 65–70 % of relative sponginess. The genomic DNA was taken out from the eggplant verdant food hotspots for the SmHQT quality, and it's close by the advertiser. Two areas were gotten for cloning: one contains marketing expert and exon 1; piece two contains exon 2. These pieces are utilized for cloning in UC based cloning vector, for example (pUC57 vector; Addgene, Spain) at HindIII/BamHI regions. After digesting the pUC57 + p19 clone with HindIII/BamHI, the liberated quality was cloned into pBIN19 (HindIII/BamHI). A 2 ml needle was used to permeate the agro culture in Eggplant at a rate of about 10–15 spots per minute. Beginning there ahead, the plants were left to observe for 3 to 10 days after infiltration (DAI). Customary thing tests were collected from the invaded plants at 3 DAI. Typical things were gathered and dealt with in − 80⁰C for additional evaluations from now into the foreseeable future.
2.2 Endorsement and quantification of transgene expression
X-Gluc staining based comparison of normal and agro-infiltrated fruits was performed using a light microscope (LYZER LT-1610X) as described previously by Kaushik et al. (2020). Further, chlorogenic content was also determined with the help of High-execution liquid chromatography using a 1220 Infinity LC System (Agilent Technologies, Santa Clara, CA, USA) as described in detail elsewhere (Kaushik et al., 2017). Whereas the precise estimations of peaks were made using the OpenLAB CDS ChemStation Edition software programme (Agilent Technologies).
2.3 RNA-sequencing and data analysis
Three agroinfiltrated fruits were grown together with a control fruit for RNA seclusion, and the fruits' skins were removed. The RNA Qiagen RNAeasy Plant Mini Kit (Qiagen, Valencia, CA) is used for extraction. The quality of the RNA extracted from the samples was determined using a bioanalyzer (Agilent Technologies 2100), and samples having an RNA Integrity Number (RIN) greater than or equal to seven were processed for further use.RNA tests with the necessary RIN esteem are pooled independently, relying on their equimolar fixations for library arrangement. The Illumina® RNA Library Prep Kit v2 (Illumina Inc., San Diego, CA, USA) was used to create the two distinct libraries; the libraries were then measured using a Qubit Fluorometer (QubitTM, Carlsbad, California). The libraries were then sequenced using the Illumina HiSeq 2500 (2 150 bp) stage (Illumina, Dedham, MA, USA). The raw data from RNA-seq is uploaded to BioProject under the project ID PRJNA531188.
2.4 Quality control and transcriptome assembly
The reading quality is filtered using the Cutadap (version 1.8.1). A low-quality base is removed with trimmomatic software (version 0.39) under traditional settings. Read from each sample is combined for assembly transcripts de novo using Trinity assembler version (v2.11.0) and subsequently grouped with CD-HIT-EST application. In this study, we use this program with criteria for identity sequences 0.9 thresholds plus the length of 0.9 lengths to get a non-redundant transcript set (Unigene).
2.5 Differential gene expression analysis
Unigene was analyzed further for annotations and profiling expressions. Uniggenes expression is estimated by RSEM and Bowtie software already configured by Trinity Program (Li and Dewey, 2011; Langmead and Salzberg, 2012). Trinity program is a tool for assembly transcript de novo from RNA-SEQ data. After that, the count of unigenes was sent to the Deseq software, which is used to analyze data from high-throughput sequencing experiments such as RNA-SEQ and differential expression experiments. Significant transcripts were chosen based on a P 0.05 significance threshold (Love et al., 2014).
2.6 Functional characterization
In the Function of the Annotator Platform, unigene functions are linked by beating blastx against databases like NCBI NR, Priam, and PFAM (E-Value 1e-5) (Chen et al., 2017). Gene Ontology (Go) was assigned to unigenes by blast2go lawsuit included in the function of the Annotator platform, which is a multipurpose and efficient web tool for organism annotation. Pathway analysis was performed using the KAAS Annotation (Conesa et al., 2005); plant pathways are used as a reference. We used the R package(Gu et al., 2016), which is a collection of functions and data sets developed by the scientific community to build the primary gene heat map.
3 Results
3.1 Agroinfiltration assay and HPLC
Similarly, temporary expressions are also carried out with original promoters. GMO plants are obtained by transforming fruits mediated from Agrobacterium tumefaciens to the greenhouse, 3 DAI. Among fruits that expressed SMHQT too much, some fruits appeared phenotypic variations compared to normal plants. Next, the chlorogenic acid content in agroinfiltrated fruit is more than double the normal fruit (Fig. 1).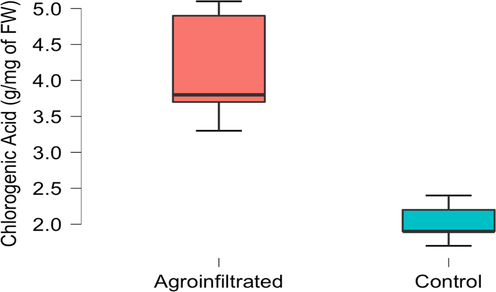
Comparison of chlorogenic acid concentration (g/mg of FW) of agroinfiltrated plant SmHQT and control fruit samples.A.
3.2 De novo assembly
After sequencing, 21.93 million reads for the transgenic plant and 44.34 million reads were obtained for the control sample (Table 1). After filtering, this number reduced to 20.86 million and 41.71 million for control and treatment, respectively (Table 1).
Raw Data Quality Summary
Treatment
Control
Total No. of reads
21,927,240
44,340,368
Total HQ reads
20,861,372
41,709,294
Total Low quality reads
1,065,868
2,631,074
A total of 1372 short scrutinizes were settled from cDNA libraries of OE SmHQT in natural items. The reads were assembled in 45,919 sequences using a trinity assembler comprising 34.13 million nucleotides (Table 2). The most extended sequence length was 10,902 nt, with an N50 of 1151 (Table 2). In contrast, the GC content of the assembly was 42.03 % (Table 2). The instances of SmHQT OE characteristics in eggplant were picked after an agroinfiltration season of 3 days of tainting to choose differentially imparted characteristics (DEGs). Table S1 contains the list of transcript annotations.
Parameter
Value
Number of sequences
45,919
Total length (nt)
34,132,170
Longest sequence (nt)
10,902
Shortest sequence (nt)
201
Mean sequence length (nt)
743
Median sequence length (nt)
467
N50 sequence length (nt)
1151
L50 sequence count
9291
GC-content (%)
42.03 %
3.3 Degs analysis and enrichment of gene ontology (GO)
As a result, the difference in DEGs between the OE and control genotypes is determined (Fig. 2). Both conditions generated 3166 and 134 DEGs; however, the DEGs expressed in only one condition were 3 1238 and 1794, respectively, and were significantly expressed in agroinfiltrated and control plants (Fig. 2). The differential expression of each transcript in control vs agroinfiltrated fruits is detailed in Table S1, as are the transcripts themselves. This resulted in discovering about 694 DEGs that were downregulated in response to OE; however, it is unknown if any genes in the control group were silenced. Additionally, it was determined that OE inhibits the synthesis of 169 DEGs, indicating that OE serves as a negative regulator of the chlorogenic biosynthetic pathway. Furthermore, the GO analysis identified a large number of functional categories (Fig. 3), including catalytic activity, transporter activity, and transcription regulatory activity (Fig. 3). As with the cellular component category, when the OE genotype is compared to the control fruit sample, a significant number of DEGs are selected in the OE genotype (Fig. 3).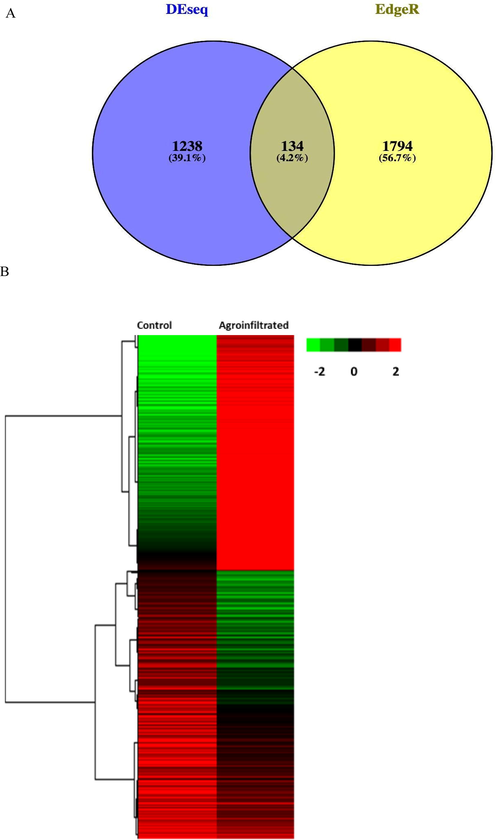
Venn diagrams illustrating the expression of DEG genes in and among control and agroinfiltrated fruits. DEG values were calculated using NGS sequencing data (A). Transcriptome heat map of control vs agroinfiltrated eggplant fruit (B).
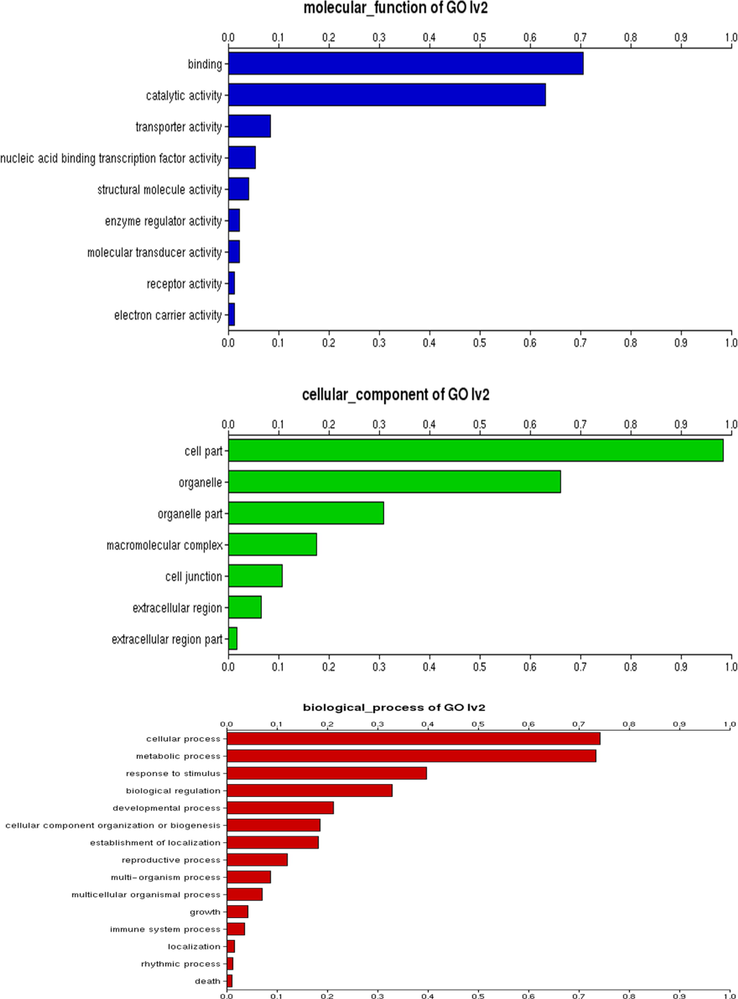
Gene Ontology (GO) terms as classified based on molecular function, cellular component and biological process that were discovered in the eggplant fruits overexpressing the SmHQT gene.
4 Discussion
Eggplant is an essential vegetable in India it is preferred because of its added nutrition advantages and ease of cooking (Taher et al., 2017). Chlorogenic acid is the most common phenolic acid discovered in this eggplant. It is also the most critical and valuable phenolic acid for both the plant and people since it nourishes humans while also protecting the plant from certain pests and viruses (Manach et al., 2004). So far, the trials conducted regarding the enhancement of chlorogenic acid were not very successful and up to the mark. Our study has chosen the agroinfiltration technique to improve the chlorogenic acid content in eggplant fruits (Šilarová et al., 2019). The standardized protocol for the agroinfiltration method was selected. Based on this standardized protocol, we have incorporated the gene for chlorogenic acid synthesis and its enhancement in the fruits of eggplant. By using the agroinfiltration technique, which is very cheap, easy and less laborious to induce the gene of interest into the plant parts and with the help of this, the expression of the gene SmHQT is enhanced because it is the key factor for the synthesis of chlorogenic acid (Yang et al., 2000; Lee and Yang, 2006).
Agroinfiltration is a compatible genetic expression or gene silencing process. It could be used as an alternative to Agrobacterium-mediated traditional approach to producing enzymes used for industrial use and very beneficial in detecting fruit tissue gene functions and protein development (Gleba et al., 2013; Krenek et al., 2015). This approach quickly offers detailed knowledge about gene function. In recent agro-infiltration research, valuable insights into several essential processes, such as promoting activity, gene expression, subcellular protein location, and metabolism, have been given. Agroinfiltration is a flexible process related to RNA-seq that can be used for plant crops, which already have standardized agroinfiltrated protocols (strawberries, tomatoes and grapes) (Rigano et al., 2013; Chialva et al., 2016). Many plants, including the transgenic development of eggplant with healthy gene expression, can take time and can be difficult. For the first time, Eggplant was transformed by Guri and Sink (1988) with a transformation of genes mediated by agrobacterium. Numerous fruitful attempts have been made to refine this technique and produce those transgenic eggplants with different important characteristics such as tolerance to Colorado potato beetle (Arpaia et al., 1997), root-knot nematode (Papolu et al., 2016), and shot and fruit borer (Rai et al., 2013). Although the technology for integrating foreign genes into eggplant has advanced significantly, studies have been conducted to optimize the transformation protocol to produce transgenic plants resistant to abiotic stress like tolerance to salt, drought, and chilling, as well as osmotic stress (Saini and Kaushik, 2019; Ahmad et al., 2019; Kohli et al., 2019; Mansoor et al., 2022). Additionally, several beneficial agronomic traits have been transferred to eggplant through transgenic technologies (Singh et al., 2010).
Processed functional food additives have gained appeal in part due to their incorporation into well-known food products. The protocol for agroinfiltration of SmHQT genes to increase the chlorogenic acid content of eggplant is described here. Eggplant output has risen, as has their appeal. Additionally, bioactive chemicals may be added “naturally” to food products. This effective functionalization results in extra health advantages, which may be achieved via the intentional addition of bioactive substances to dietary items (Sonker et al., 2017). Biotechnology is a parallel advancement that does not confine farmers and clinicians to a small, precarious natural development (Winickoff et al., 2005; Bertheau, 2015). Vegetable genetic modifications to produce a higher concentration of bioactive compounds and incorporate functional foods have unmatched reliability and characteristics. It remains to be seen how this innovation can respond to its participants' varied needs, including the realistic food industry (Pinela et al., 2016; Kamiloglu et al., 2021). Nonetheless, the opportunities for a thriving functional food market and a balanced community are undoubtedly worth pursuing.
5 Conclusion
Eggplants are immense vegetables that are from the Solanaceae family. They are high in valuable phenolic compounds, including chlorogenic acids. In this study, high-quality verbalization research was used to extend the spectrum of eggplant's chlorogenic dangerous component. This agroinfiltration method has gained popularity because of its superiority in fast quality exchange by skipping the typical replication cycle. In a utilitarian assessment of SmHQT, it was concluded that overexpression of SmHQT increased chlorogenic acid content, making it ineffective as a phenolic acid controller. Other interaction partner features discovered using our RNAseq data may restrict the eggplant during overexpression, but this is not a guarantee of success. Using agroinfiltration, we were able to increase the content of chlorogenic acid in eggplants. To evaluate and analyse its efficacy, the SmHQT gene may be overexpressed in the flesh of an agroinfiltrated eggplant fruit. Our findings suggest that agroinfiltration increases the quantity of a key phenolic acid, chlorogenic acid, leading to the overexpression of the SmHQT gene and therefore enhancing the nutritional quality of eggplant fruits.
6 Consent to participate
All authors consent to participate in this manuscript.
7 Consent for publication
All authors consent to publish this manuscript in Journal of King Saud University - Science.
8 Availability of data and material
Data will be available on request to corresponding or first author.
Author contributions
AS drafted the experimental design, performed the experiments. PK, MNA, SA worked in data collection, data analysis and initial draft of manuscript text. PYY revised the manuscript. All authors read the manuscript before communication.
Acknowledgement
The authors extend their appreciation to Researchers Supporting Project Number (RSP2023R180), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Role of transgenic plants in agriculture and biopharming. Biotechnol. Adv.. 2012;30:524-540.
- [Google Scholar]
- Revisiting the role of ROS and RNS in plants under changing environment. Environ Exp Bot. 161: 1–3.Arpaia, S., Mennella, G., Onofaro, V., Perri, E., Sunseri, F., Rotino, G., 1997. Production of transgenic eggplant (Solanum melongena L.) resistant to Colorado potato beetle (Leptinotarsa decemlineata Say) Theor. Appl. Genet.. 2019;95:329-334.
- [Google Scholar]
- Bertheau, Y., 2015. Feeding the World: Are Biotechnologies the Solution? Advances in Food Biotechnology, 71.
- Agricultural practices, biology and quality of eggplant cultivated in Central Europe. a review. Horticult. Sci.. 2017;44:201-212.
- [Google Scholar]
- FunctionAnnotator, a versatile and efficient web tool for non-model organism annotation. Sci. Rep.. 2017;7:1-9.
- [Google Scholar]
- Development and use of biotechnology tools for grape functional analysis. Grape Wine Biotechnol. 2016:1052-1426.
- [Google Scholar]
- Genetic dissection of tomato bushy stunt virus p19-protein-mediated host-dependent symptom induction and systemic invasion. Virology. 2000;266:79-87.
- [Google Scholar]
- Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674-3676.
- [Google Scholar]
- Phenylpropanoids accumulation in eggplant fruit: characterization of biosynthetic genes and regulation by a MYB transcription factor. Front. Plant Sci.. 2016;6:1233.
- [Google Scholar]
- Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front. Nutr.. 2018;5:12.
- [Google Scholar]
- Plant viral vectors for delivery by Agrobacterium. Plant Viral Vectors 2013:155-192.
- [Google Scholar]
- Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32:2847-2849.
- [Google Scholar]
- Transient transformation meets gene function discovery: the strawberry fruit case. Front. Plant Sci.. 2015;6:444.
- [Google Scholar]
- Kamiloglu, S., Tomas, M., Ozdal, T., Yolci-Omeroglu, P., Capanoglu, E., 2021. Bioactive component analysis. In: Innovative Food Analysis, Elsevier, pp: 41-65.
- Transcriptional profiling of two contrasting genotypes uncovers molecular mechanisms underlying salt tolerance in alfalfa. Sci. Rep.. 2021;11:1-15.
- [Google Scholar]
- Breeding vegetables with increased content in bioactive phenolic acids. Molecules. 2015;20:18464-18481.
- [Google Scholar]
- Phenolics content, fruit flesh colour and browning in cultivated eggplant, wild relatives and interspecific hybrids and implications for fruit quality breeding. Food Res. Int.. 2017;102:392-401.
- [Google Scholar]
- Enhancement of chlorogenic content of the eggplant fruit with eggplant hydroxycinnamoyl CoA-quinate transferase gene via novel agroinfiltration protocol. Pharmacogn. Mag.. 2020;16:450.
- [Google Scholar]
- Sequence analysis and homology modelling of SmHQT protein, a key player in chlorogenic acid pathway of eggplant. bioRxiv 2019599282
- [Google Scholar]
- Knapp, S., Peralta, I.E., 2016. The tomato (Solanum lycopersicum L., Solanaceae) and its botanical relatives. In: The tomato genome, Springer, pp: 7-21.
- Kohli, S.K., Khanna, K., Bhardwaj, R., Abde_Allaha, E.F., Ahmad, P., Corpas, F.J., 2019. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants. 8(12):641.
- Transient plant transformation mediated by Agrobacterium tumefaciens: principles, methods and applications. Biotechnol. Adv.. 2015;33:1024-1042.
- [Google Scholar]
- Lee, M.W., Yang, Y., 2006. Transient expression assay by agroinfiltration of leaves. In: Arabidopsis Protocols, Springer, pp: 225-229.
- RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf.. 2011;12:1-16.
- [Google Scholar]
- Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol.. 2014;15:1-21.
- [Google Scholar]
- Application of non-invasive low-intensity pulsed electric field with thermal cycling-hyperthermia for synergistically enhanced anticancer effect of chlorogenic acid on PANC-1 cells. PLoS One. 2020;15:e0222126.
- [Google Scholar]
- RNA-Seq analysis revealed genes associated with drought stress response in kabuli chickpea (Cicer arietinum L.) PLoS One. 2018;13:e0199774.
- [Google Scholar]
- Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr.. 2004;79:727-747.
- [Google Scholar]
- Reactive oxygen species in plants: from source to sink. Antioxidants (Basel). 2022;11(2):225.
- [Google Scholar]
- Eggplant domestication: pervasive gene flow, feralization, and transcriptomic divergence. Mol. Biol. Evol.. 2019;36:1359-1372.
- [Google Scholar]
- Expression of a cystatin transgene in eggplant provides resistance to root-knot nematode, Meloidogyne incognita. Front. Plant Sci.. 2016;7:1122.
- [Google Scholar]
- Pinela, J., Carocho, M., Dias, M.I., Caleja, C., Barros, L., Ferreira, I., 2016. Wild plant-based functional foods, drugs, and nutraceuticals. Wild Plants, Mushrooms and Nuts: Functional Food prOperties and Applications; Ferreira, ICFR, Morales, P., Barros, L., Eds, 315-352.
- Shoot and fruit borer resistant transgenic eggplant (Solanum melongena L.) expressing cry1Aa3 gene: development and bioassay. Crop Prot.. 2013;53:37-45.
- [Google Scholar]
- Production of pharmaceutical proteins in solanaceae food crops. Int. J. Mol. Sci.. 2013;14:2753-2773.
- [Google Scholar]
- Visiting eggplant from a biotechnological perspective: a review. Sci. Hortic.. 2019;253:327-340.
- [Google Scholar]
- Monitoring of chlorogenic acid and antioxidant capacity of Solanum melongena L. (eggplant) under different heat and storage treatments. Antioxidants. 2019;8:234.
- [Google Scholar]
- Plastid transformation in eggplant (Solanum melongena L.) Transgenic Res.. 2010;19:113-119.
- [Google Scholar]
- Ascertaining the paradigm of secondary metabolism enhancement through gene level modification in therapeutic plants. J. Young Pharm.. 2019;11:337.
- [Google Scholar]
- Bionanotechnology: past, present and future. Nova Science Pub Inc, New York: New approaches in biological research; 2017.
- World vegetable center eggplant collection: origin, composition, seed dissemination and utilization in breeding. Front. Plant Sci.. 2017;8:1484.
- [Google Scholar]
- Welbaum, G.E., 2015. Vegetable production and practices, CABI.
- Adjudicating the GM food wars: science, risk, and democracy in world trade law. Yale J. Int'l L.. 2005;30:81.
- [Google Scholar]
- In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J.. 2000;22:543-551.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102577.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







