Translate this page into:
Trace identification of sulfate anion in bottled and metropolitan water samples collected from various provinces of Saudi Arabia
⁎Corresponding author. mrkhan@ksu.edu.sa (Mohammad Rizwan Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Water quality especially drinking water is highly significant to human lives. To access the safe and secure drinking water, nowadays it has become an issue of global concerns. A novel method using an ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) was proposed to analyze sulfate level in bottled and metropolitan water collected from various provinces of Saudi Arabia. The chromatographic analysis was achieved within 1 min with reversed phase Waters Acquity® BEH C18 column and a triple quadrupole mass spectrometer. The performance of the optimized system was established, attaining linearity (r2 > 0.999) over a wide-ranging concentration from several below hundred mg/L to the detection limit of sulfate. In terms of relative standard deviation of the samples (RSD%), the excellent run-to-run (<2%, n = 5) and day-to-day (<4%, n = 5) precisions were achieved when examining a sulfate standard (0.01 mg/L) and non-spiked bottled water sample with the concentration of sulfate (4.76 mg/L). The developed method is helpful to identify sulfate in bottled and metropolitan water samples. Sulfate content in the bottled water ranged between 3.31 and 76.22 mg/L, while higher level was determined (36.78 to 268.42 mg/L) in metropolitan water samples. The excellent quality parameters and insignificant matrix effects achieved during analysis have made favorable to analyze sulfate in water samples, and offered advantages over conventional techniques and rigorous sample preparation procedures.
Keywords
Sulfate
Bottled water
Metropolitan water
Ultra-performance liquid chromatography-tandem mass spectrometry
1 Introduction
Water quality especially drinking water is highly significant to human lives. To access the safe and secure drinking water, nowadays it has become an issue of global anxieties. More than one billion people failed to access safe drinking water from developing countries (WHO, 2008; Frengstad et al., 2010; Alizadeh and Mahjoub, 2015). The excessive richness of nutrients level in the aquatic environment can be unsafe to human beings and can cause water eutrophication. Sulfate is known to be a major anion in aquatic environment, and can be present naturally or result of various point sources includes municipal sewage treatment plants, industrial discharges for instance plating industries, textile mills, tanneries, mining and pulp mills, and use of coal and petroleum products (Greenwood and Earnshaw, 1984; Moore, 2012; de Karla et al., 2018). Overspill from manure farming lands also adds sulfate to water reservoirs (Powell and Martens, 2005). In 1977, the US National Academy of Sciences has illustrated taste levels in drinking water ranged between 250 and 1000 mg/L for calcium sulfate, 250 to 500 mg/L for sodium sulfate and 400 to 600 mg/L for magnesium sulfate (National Research Council, 1977). Nevertheless, enhancing sulfate concentration in surface water offers a critical sign for water acidification in reservoirs that effect on quality of water and health concern (Shakirullah et al., 2005; Stambuk-Giljanovic, 2005; Luke et al., 2014).
Several analyses were carried out to identify the toxicity of sulfate in human beings (Cicchella et al., 2010). Cases report that the consumption of sulfate has shown catharsis especially in adult population (Morris and Levy, 1983). Cathartic effects are frequently observed by the individuals drink water containing sulfate in levels higher than 600 mg/L (USPHS, 1962). In addition, the high consumption of sulfate has also caused the dehydration in humans (Backer, 2000; Backer et al., 2001; Fingl, 1980). Many authors have also reported the gastrointestinal disease like diarrhea especially infants when they are exposed to drinking water having sulfate at amounts between 630 mg/L and 1150 mg/L (Chien et al., 1968). Owing to sulfate availability in water and its health benefits, it is require to formulate a sensitive, rapid, and selective technique for the identification of such kind of possibly toxic pollutants in drinking water.
Numerous analytical methods for instance flow-injection analysis (Fung et al., 2008), high performance liquid chromatography using ion-pair method (Zuo and Chen, 2003), laser Raman spectroscopy (Murata et al., 1997), spectrophotometer(de Oliveira and Korn, 2006), sequential injection analysis (van Staden and Taljaard, 1996), turbidimetry (Kolmert et al., 2000), sequential injection analysis-multivariate curve resolution(del Río et al., 2010), capillary electrophoresis (Kulka et al., 2006), ion exchange chromatography (Barry et al., 1978; Biesaga et al., 2004) and gravimetric method (Kolthoff et al., 1969) have been reported for sulfate determination from water samples. The drawbacks of these described traditional analytical methods are time consuming, low sensitive and selective, and require sample pre-treatment, high amount of samples and solvents. To overcome such limitations, the introduction of a fast analysis of sulfate is of great attention. Recently, we have also developed fast, sensitive and selective methods based on UPLC–MS/MS for the determination of inorganic compounds in drinking water and non-alcoholic beer samples. For example, UPLC–MS/MS based analysis was used for the determination of bromate in drinking water (Alsohaimi et al., 2012), UPLC-electrospray mass spectrometry (ESI/MS) method for bromate analysis in non-alcoholic beer (Khan et al., 2014). UPLC–ESI/MS method was proposed for the determination of nitrate, bromate and nitrite in drinking water (Khan et al., 2013; Khan et al., 2016). These optimized methods have been found very rapid, sensitive and selective during the analysis of inorganic compounds in such type of samples.
The main aim of present study was to develop quantitative and qualitative analytical method for the determination of sulfate in drinking water taking benefits of the reduction of analysis time and solvent consumption, and increase in selectivity and sensitivity. Taking these advantages into consideration, we therefore developed an analytical tool based on UPLC–MS/MS for the analysis of sulfate in drinking water. The outcomes of the present findings obtained with this novel method and outcomes achieved on various water samples approve that they are enough to offer it as a new standard method for the rapid and reliable analysis of sulfate in drinking water.
2 Materials and methods
2.1 Chemicals and reagents
All chemicals and solvents applied in the present study were of analytical grade (AR) or HPLC grade, purchased from Merck (Darmstadt, Germany). Sodium sulfate (purity ≥ 99%) was supplied from Merck (Munich, Germany). For sample preparation, Milli–Q water was used which was obtained from Milli–Q water purification system (Millipore Corporation, Bedford, USA). Sulfate stock standard solution (500 mg/L) was prepared in Milli-Q water (free from sulfate) and used for further analysis. To establish the linearity of the method and standard addition quantification procedures, sulfate standard at different concentrations were prepared by weight. Solutions including collected water samples were filtered using a PTFE syringe filter (0.22 μm) (Macherey-Nagel GmbH, Düren, Germany) before being analyzed by UPLC method.
2.2 Analysis of water samples
Bottled drinking water samples from various sources were obtained from superstores based in Saudi Arabia. In this study, water samples from Metropolitan source were obtained from various cities supplied by the indigenous National Company (Saline Water Conversion Corporation, Saudi Arabia). Water samples were stored in the containers at 4 °C and all experiments were performed within a week to avoid microbial growth. Besides, blank and quality control (QC) samples were also studied in every sample set to ensure sample quality. Standard method has been used for the determination of sulfate in both bottled and metropolitan waters.
To assess the efficiency of the procedure and avoiding the influence of any matrix effects on compound peak intensity, retention time and shape, the sulfate quantification was performed by means of standard addition method which consist two non-spiked samples (zero levels) and four spiked samples 50% (5 µg/mL, concentration demonstrating the rise of sulfate in the sample next to spiking), 100% (10 µg/mL), 500% (50 µg/mL) and 1000% (100 µg/mL). All samples were analyzed in triplicates. The recovery rates were determined from the slope achieved while establishing the correlation between the added and found concentration of sulfate, and the statistical analysis was carried out using ANOVA method.
2.3 UPLC-MS/MS conditions
Ultra-performance liquid chromatographic separation of sulfate in water samples was performed by Acquity® UPLC method using n Acquity® BEH C18reversed phase analytical column (Waters, Milford, USA). In this experiment, a guard-column (VanGuard™ BEH C18, 1.7 µm) was applied during the sample analysis. The optimum separation of sulfate was acquired by means of mobile phase containing water (75%) and methanol (25%) in isocratic mode of elution and the flow rate was maintained as 0.2 mL/min. The experiment was run only for 2 min at ambient temperature. The column was also washed with a mixture of methanol and water (50:50, v/v) for 5 min at every 20 sample applications. The sample injection volume was 1 µL.
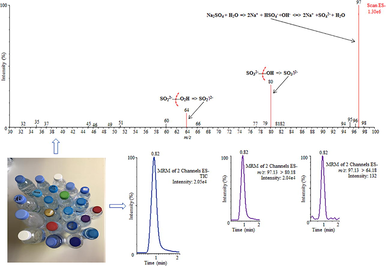
The triple quadrupole mass spectrometric detection of sulfate in water samples was carried out by Quattro Premier™ MS coupled with an electrospray ionization (ESI) source in the form of Z-spray. The source was operated in negative ionization method and the results acquisition was performed in Selected Reaction Monitoring (SRM) method. The ion transmission and fragmentation parameters were optimized by calibrating the equipment using sodium sulfate standard (10 mg/L). The target compound sulfate (SO42–, m/z: 97) was analyzed as a precursor ion, and the product ion transitions SO32– (m/z: 80) and SO22– (m/z: 64) were used for quantification and confirmation, respectively. The optimal working parameters of the system were: source temperature (120 °C); capillary voltage (2.4 kV); desolvation temperature (250 °C); cone voltage (44 V); cone gas (60 L/h) and desolvation gas (600 L/h). Nitrogen gas (cone gas) was obtained from a nitrogen generator (NM30LA, Inchinann, United Kingdom), whereas, Argon (collision gas) was obtained from Specialty Gas Centre (Jeddah, Saudi Arabia). The vacuum of mass spectrometer was created using a rotary pump, Oerlikon, model SOGEVAC SV40BI (Paris, France). Table 1 demonstrates the SRM conditions used with the triple quadrupole mass spectrometric system. The results were processed using MassLynx V4.1 software (Waters, Milford, USA).
Analyte
Precursor ion (m/z)
Quantification
Confirmationb
Product ion (m/z)
Collision energy (eV)
Product ion (m/z)
Collision energy (eV)
SO42–
97
80
30
64
35
3 Results and discussion
3.1 Analysis of water using an Ultra-performance liquid chromatography
In recent years, UP-LC method has been accepted as an innovative separation technique which permits for analysis and separation of small particles both speedily and efficiently (Swartz, 2005). The optimization of analytical parameters for water quality analysis using reversed phase chromatography is a significant task. In this method, small interactions between inorganic compound and a hydrophobic column were predictable. Indeed, sulfate content of water could not be eluted from the UP-LC system dead volume when only HPLC grade organic solvents was using as a mobile phase. Thus, the elution of sulfate was possible only with mobile phase containing either an aqueous or a mixture of both aqueous and organic phases. At first, the preliminary studies were performed on several hydrophobic columns containing stationary phases C18 and C8. Besides, the column based on Hydrophilic Interaction Chromatography (HILIC) containing amide group stationary phase were also studied. Various mobile phases such as water, methanol and acetonitrile were studied either alone or a mixture of varied proportions at flow rate of the sample ranged from 0.1 to 0.5 mL/min. Also, the effect of the formic acid concentration (0.5%–1%) with mobile phase and sample injection volume (1 µL–5 µL) were also studied. Among the studied columns, the hydrophobic C18has produced very nice symmetrical peak including low elution time of the target compound. Nevertheless, the hydrophilic HILIC and hydrophobic C8were showed very poor results for instance peak tailing, higher elution time and peak split. The addition of organic modifier (formic acid) was also not improve the quality of peak and produced the similar results obtained during columns studies. However, in the previous study the addition of formic acid in mobile phase play an important role and offered Gaussian peak for bromate in drinking water samples (Alsohaimi et al., 2012). The effect of sample injection volume (1 µL – 5 µL) was also studied and the found very distinct results. Firstly, the sample was injected 5 µL and result showed that the sulfate was splitting in two peaks (Fig. 1). Thereafter, we start to reduce the sample injection volume under similar chromatographic conditions and attained very nice Gaussian peak at 1 µL (Fig. 1).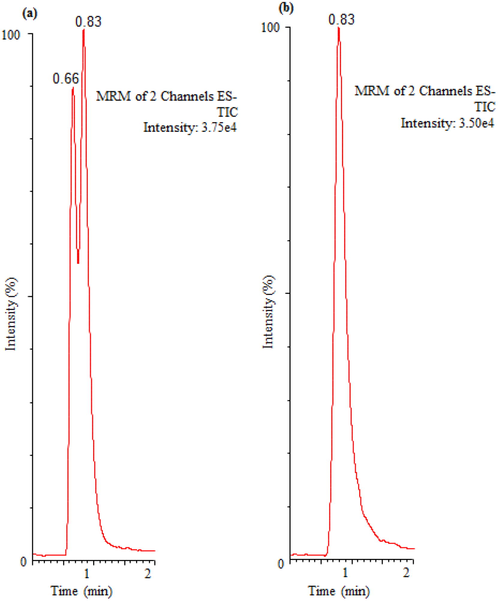
Effect of sample injection volume (a) 5 µL and (b) 1 µL.
The standard chromatographic separation of sulfate in water samples was attained on reversed phase Acquity® BEH C18 column using mobile phase containing water (75%) and methanol (25%) in isocratic elution mode at the rate of 0.2 mL/min and sample volume was 1 µL. Relatively low flow rate and sample injection volume were found to be most favorable parameters for the determination of sulfate which showed effective ionic evaporation and desolvation in the ESI source of MS system, and a symmetrical peak to be well-defined as a minimum of fifteen scan points in their identification. The dead volume of the column was attained in 0.1 min which approved that the little interaction between inorganic compounds and column stationary phase. Therefore, the sulfate peak was acquired in less than 1 min in spite of applying lower flow rate. One of the main benefits of using low sized particle Acquity® BEH C18column is that the column efficacy does not reduce while increasing the flow of the mobile phase.
3.2 Optimization of mass spectrometric conditions
In order to enhance the analyte ion response, effective desolvation of mobile phase and reduce ion fragmentation, the ESI source parameters were studied. Initially, these conditions were validated by calibrating sulfate standard (10 mg/L) into MS system. The optimized conditions have been demonstrated in section 2.3. The experiments point out that the influence of conditions, source temperature, desolvation temperature, capillary voltage and desolvation gas exceeded maximum values, were insignificant. However, the influence of the cone voltage was noticeable on the identification of sulfate. The full scan mode of mass spectrum analysis was applied to select the most abundant sulfate ion, which correspond to m/z 97. The intensity of the sulfate ion was used to optimize the conditions involved in the process of ionization and transmission. The collision energy ranged from 5 V to 50 V were investigated to obtain the most abundant product ions, which result the loss of oxygen group from parent ion (m/z: 97) to two product ions SO32– (m/z: 80) and SO22– (m/z: 64). The MS/MS conditions and fragmentation pattern of target analyte have been demonstrated in Table 1 and Fig. 2, respectively.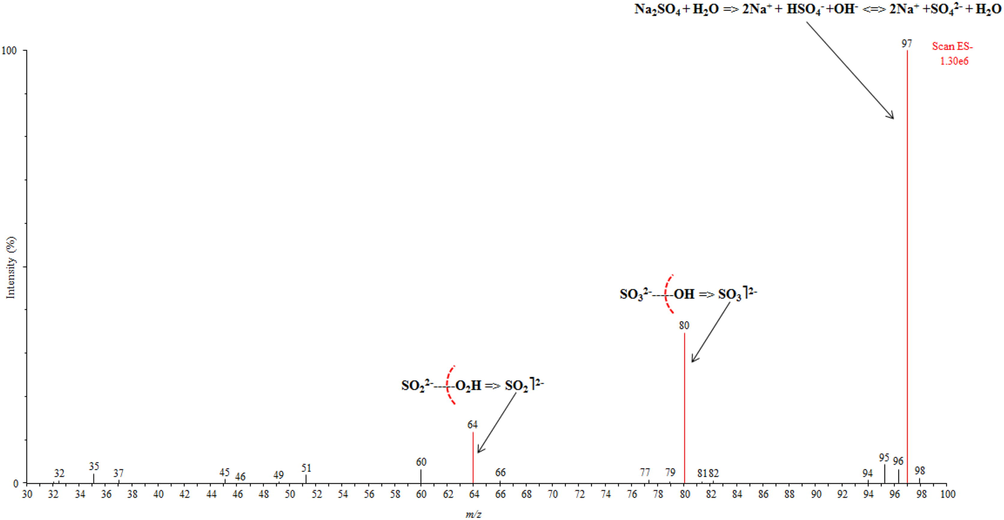
Mass spectrometric fragmentation pattern of sulfate with plausible mechanism.
3.3 Validation of UPLC-MS/MS method
In this study, the quality parameters such as, instance linearity range, detection limit, quantification limit, precisions and accuracy were investigated to analyze the performance of this method. Under the optimal instrumental parameters, linearity of the method was assessed between concentrations range1 mg/L and 100 mg/L. The standard curve was obtained by plotting the amounts of seven calibration standards and the peak area. The calibration curve was linear over higher concentrations with excellent r2 value (r2 = 0.999). The outcome has shown the excellent correlation between peak area and sulfate concentrations.
The detection limit and quantification limits were determined as the sulfate concentration that formed a signal-to-noise ratio of 3:1 and 10:1, respectively. Detection and quantification limits were calculated by using a blank spiked with a low amount of sulfate. Excellent detection and quantification limits values were obtained (0.01 mg/L and 0.04 mg/L). These outcomes are closer than those previously reported using the flow-injection analysis, ion chromatography with UV detection techniques.
To assess run-to-run precision, six replicates of sulfate (0.5 mg/L) were analyzed in the same day while day–to–day precision, six replicates of standard solution were analyzed for three successive days. The values were measured based on the determination of RSD (%) of the peak area of sulfate. The day–to–day and run–to–run precisions for sulfate were achieved (3.46% and 1.97%). The obtained RSD values have demonstrated that the precision of the proposed method was good enough for the detection of sulfate in drinking water.
To validate the accuracy of the offered method, recovery values of the target compound were estimated by standard addition procedure. Thirty water samples from bottled and metropolitan have been analyzed and the recovery values were obtained from 93% to 97%. The influence of sample matrix has been observed at lower level and this could be to some extent due to the shape of ion source (Z–configuration), which usually not allowed the neutral compounds admittance in chromatographic and mass spectrometric systems. The obtained results revealed that the sample matrix doesn’t alter the signal of analyzed compound in such kinds of samples and for quantitation purposes the external calibration method could also be used.
3.4 Sample analysis
The optimized UPLC-MS/MS method was used to analyze the sulfate concentration in bottled drinking water and metropolitan water samples. The sulfate content of the water samples was not affected by the sample matrix. Thus, the pre-treatment of water sample was not required prior to the UPLC-MS/MS analysis. Filtered water showed great benefit over conventional methods. In addition, the pre-treatment procedures for sample would greatly increase the materials and consumption of solvent, further losses of target analytes, sensitivity and longer analysis time.
Twenty-two bottled drinking water samples from different origins were obtained from Saudi Arabian markets, most of them were sterilized with ozone. Table 2 demonstrates the obtained sulfate concentration in bottled water samples. The sulfate concentrations were ranged from 3.31 mg/L to 76.22 mg/L. In most of the analyzed samples, the obtained sulfate concentrations were significantly different to the concentration claimed on label by the respective companies. The concentration of sulfate in samples 6, 7, 10, 12, 13, 15 and 22 was found to be similar with those claimed by the companies. However, in other samples the obtained concentration of sulfate was found to be nearly double than the concentration claimed by the companies. The concentration of sulfate in analyzed bottled water samples was found lower than the Secondary Maximum Contaminant Level (250 mg/L) (USEPA, 2009). The sulfate recovery rates in bottled water samples were obtained from 94% to 97%. The acquired UPLC-MS/MS chromatogram of sulfate in bottled drinking water (sample 22) has been displayed in Fig. 3. The peak is showing excellent symmetry, no tailing and no interfering ions with the target compound. To authenticate the cross contamination of the system, blank samples (Milli-Q water, free from sulfate) were analyzed after every real samples. The acquired UPLC-MS/MS chromatogram of Milli-Q water sample has been demonstrated in Fig. 4 which revealed that no any contamination occurs during the analysis. The metropolitan water samples from different regions were also studied, and total eight metropolitan samples were analyzed and all of them were treated with hypochlorite. The achieved outcomes have been shown in Table 3. The sulfate levels were ranged from 36.78 mg/L to 268.42 mg/L. In all of the analyzed samples, the concentrations were significantly different. The highest concentration of sulfate (268.42 mg/L) was obtained in sample 2 and this content was higher than that of prescribed limit for drinking water (250 mg/L) (USEPA, 2009). The sulfate recovery rates were obtained from 93% to 96%. In comparison to bottled drinking water, metropolitan water samples contained higher amounts of sulfate. The results obtained from this study are the source of data relating to the availability of sulfate in bottled and metropolitan water samples from Saudi Arabia. – not described; SD = standard deviation (n = 3); KSA = Kingdom of Saudi Arabia.
Bottled water
Water source
SO42– (mg/L) ± SD
SO42– claimed in the label (mg/L)
Disinfection process
Country of origin
Sample 1
–
23.76 ± 0.02
16
–
KSA
Sample 2
Well water
24.34 ± 0.02
30
Ozonation
KSA
Sample 3
–
28.75 ± 0.01
26
–
KSA
Sample 4
–
15.68 ± 0.02
30
–
KSA
Sample 5
–
24.13 ± 0.02
28
–
KSA
Sample 6
Well water
5.93 ± 0.03
5
Ozonation
KSA
Sample 7
–
13.35 ± 0.02
14
Ozonation
KSA
Sample 8
–
38.04 ± 0.01
51
–
KSA
Sample 9
–
3.31 ± 0.03
12
–
KSA
Sample 10
–
4.76 ± 0.03
5.40
–
Turkey
Sample 11
Well water
23.04 ± 0.02
32
Ozonation
KSA
Sample 12
–
21.40 ± 0.02
22
–
KSA
Sample 13
–
18.69 ± 0.02
18
Ozonation
KSA
Sample 14
Well water
19.17 ± 0.02
50
Ozonation
KSA
Sample 15
–
11.43 ± 0.03
12.60
–
France
Sample 16
–
36.21 ± 0.01
54
–
KSA
Sample 17
Well water
14.79 ± 0.02
22
–
KSA
Sample 18
Well water
72.09 ± 0.01
30
Ozonation
KSA
Sample 19
–
6.16 ± 0.03
10
–
KSA
Sample 20
–
12.07 ± 0.03
7
–
KSA
Sample 21
–
13.69 ± 0.03
20
–
KSA
Sample 22
–
76.22 ± 0.01
74.50
–
KSA
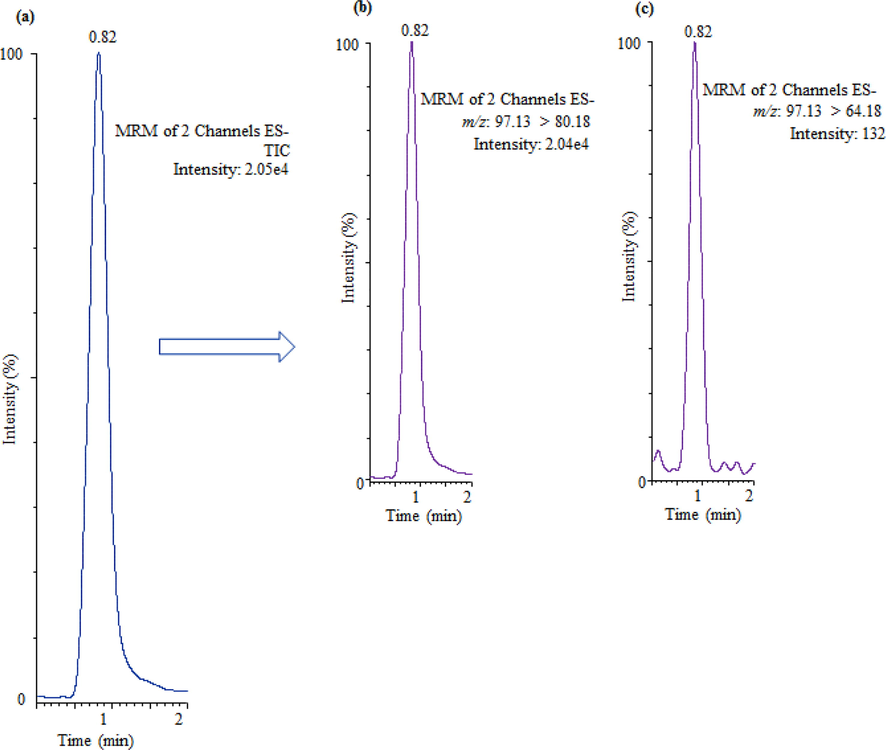
UPLC-MS/MS chromatogram of sulfate in bottled drinking water (sample 22).
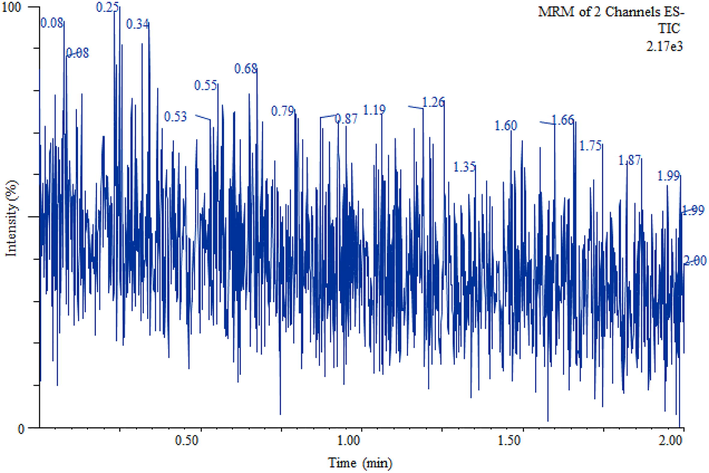
UPLC–MS/MS chromatogram of blank sample (Milli-Q water, free from sulfate).
Metropolitan watera
Water source
SO42– (mg/L) ± SD
Disinfection process
Sample 1
Desalinated + well water
82.91 ± 0.03
Chlorination
Sample 2
Desalinated + well water
268.42 ± 0.01
Chlorination
Sample 3
Desalinated + well water
151.31 ± 0.01
Chlorination
Sample 4
Desalinated water
102.78 ± 0.02
Chlorination
Sample 5
Desalinated water
96.85 ± 0.02
Chlorination
Sample 6
Desalinated water
82.68 ± 0.03
Chlorination
Sample 7
Desalinated water
142.23 ± 0.01
Chlorination
Sample 8
Desalinated water
36.78 ± 0.04
Chlorination
4 Conclusion
A novel technique based on UPLC−MS/MS was proposed to assess sulfate content in bottled and metropolitan water collected from various provinces of Saudi Arabia. The optimized procedure has illustrated to be faster with less than one-minute sample analysis time. Excellent detection and quantification limits values were achieved, and precise with excellent run–to–run precision and day–to–day precision values. The excellent quality parameter values and insignificant matrix effects achieved during analysis have made favorable to analyze sulfate in water samples, and offered advantages over conventional techniques and rigorous sample preparation. The performance of proposed method along with the achieved results from analyzed water samples make favorable to propose a novel method for the routine study of sulfate content in drinking water. The achieved data from this work could be applied to estimate the sulfate intake by individuals in Saudi Arabia, and therefore to advance the water quality and security.
Declaration of competing interest
The authors declare that they have no any conflict of interest.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project for funding this work through Research Number (RSP-2019/138), King Saud University, Riyadh, Saudi Arabia.
References
- A comparison between determination of trace amounts of sulfide in the presence and absence of micelle particles in natural waters (Qazvin, Iran): a kinetic spectrophotometric approach. Environ. Monit. Assess.. 2015;187:248.
- [Google Scholar]
- Determination of bromate in drinking water by ultraperformance liquid chromatography-tandem mass spectrometry. J. Sep. Sci.. 2012;35:2538-2543.
- [Google Scholar]
- Assessing the acute gastrointestinal effects of ingesting naturally occurring, high levels of sulfate in drinking water. Crit. Rev. Clin. Lab Sci.. 2000;37:389-400.
- [Google Scholar]
- Assessing acute diarrhea from sulfate in drinking water. J. Am. Water Works Assoc.. 2001;93:76-84.
- [Google Scholar]
- Analysis of fluoride, chloride, nitrate and sulphate in natural waters using ion chromatography. J. Geochem. Explor.. 1978;10(1978):245-258.
- [Google Scholar]
- Coupled ion chromatography for the determination of chloride, phosphate and sulphate in concentrated nitric acid. J. Chromatogr. A.. 2004;1026:195-200.
- [Google Scholar]
- Infantile gastroenteritis due to water with high sulfate content. Can. Med. Assoc. J.. 1968;99:102-104.
- [Google Scholar]
- Trace elements and ions in Italian bottled mineral waters: Identification of anomalous values and human health related effects. J. Geochem. Explor.. 2010;107:336-349.
- [Google Scholar]
- Drinking Water and Health. Vol Volume 1. Washington, DC: The National Academies Press; 1977.
- Spectrophotometric determination of sulphate in automotive fuel ethanol by sequential injection analysis using dimethylsulphonazo (III) reaction. Talanta. 2006;68:992-999.
- [Google Scholar]
- Determination of sulphate in water and biodiesel amples by a sequential injection analysis–multivariate curve resolution method. Anal. Chim. Acta. 2010;676:28-33.
- [Google Scholar]
- Laxatives and cathartics The Pharmacological Basis of Therapeutics. In: Gilman A.G., Goodman L.S., Gilman A., eds. 6th edn. York: Macmillan, New; 1980. p. :1002-1012.
- [Google Scholar]
- The chemistry of bottled mineral and spring waters from Norway, Sweden, Finland and Iceland. J. Geochem. Explor.. 2010;107:350-361.
- [Google Scholar]
- Determination of sulphate in water by flow-injection analysis with electrode-separated piezoelectric quartz crystal sensor. Sens. Actuators B. Chem.. 2008;130:551-560.
- [Google Scholar]
- Chemistry of the elements. Oxford, United Kingdom: Pergamon Press; 1984.
- Sulfate pollution: evidence for electrochemical production of persulfate by oxidizing sulfate released by the surfactant sodium dodecyl sulfate. Environ. Chem. Lett.. 2018;16:647-652.
- [Google Scholar]
- Quantitative analysis of bromate in non-alcoholic beer using ultra performance liquid chromatography-electrospray ionization mass spectrometry. Anal. Methods. 2014;6:4038-4045.
- [Google Scholar]
- An ultra performance liquid chromatography-electrospray ionization-mass spectrometry method for the rapid analysis of nitrate in drinking water. Anal. Methods. 2013;5:1225-1230.
- [Google Scholar]
- Method for the fast determination of bromate, nitrate and nitrite by ultra performance liquid chromatography–mass spectrometry and their monitoring in Saudi Arabian drinking water with chemometric data treatment. Talanta. 2016;152:513-520.
- [Google Scholar]
- A fast and simple turbidimetric method for the determination of sulfate in sulfate-reducing bacterial cultures. J. Microbiol. Methods. 2000;41:179-184.
- [Google Scholar]
- Quantitative Chemical Analysis. Vol vol. 826. New York: Macmillan; 1969.
- Automated sample preparation and analysis using a sequential-injection–capillary electrophoresis (SI–CE) interface. Analyst. 2006;131:739-744.
- [Google Scholar]
- Monitoring and assessment of surface water acidification following rewetting of oxidised acid sulfate soils. Environ. Monit. Assess.. 2014;186:1-18.
- [Google Scholar]
- Inorganic Contaminants of Surface Water: Research and Monitoring Priorities. New York: Springer-Verlag; 2012.
- Absorption of sulfate from orally administered magnesium sulfate in man. J. Toxicol. Clin. Toxicol.. 1983;20:107-114.
- [Google Scholar]
- Determination of sulfate in brackish waters by laser Raman spectroscopy. Anal. Chim. Acta. 1997;344:153-157.
- [Google Scholar]
- WHO, World Health Organization and United Nations children's fund joint monitoring programme for water supply and sanitation (JMP). Progress on drinking water and sanitation: special focus on sanitation, 2008.
- A review of acid sulfate soil impacts, actions and policies that impact on water quality in Great Barrier Reef catchments, including a case study on remediation at East Trinity. Marine Poll. Bull.. 2005;51:149-164.
- [Google Scholar]
- USPHS, 1962. Drinking Water Standards, 1962, Public Health Service Publication No. 956, US Govt Printing Office, Washinghton, 22-25.
- Physicochemical study of drinking water from Dir districts. J. Chem. Soc. Pak.. 2005;27:374-387.
- [Google Scholar]
- The quality of water resources in Dalmatia. Environ. Monit. Assess.. 2005;104:235-338.
- [Google Scholar]
- Ultra performance liquid chromatography (UPLC): An introduction Separation Science Re-Defined. LCGC Suppl.. 2005;8:8-14.
- [Google Scholar]
- USEPA, 2009. National primary drinking water regulations. EPA-816-F-09–004.
- Determination of sulphate in natural waters and industrial effluents by sequential injection analysis. Anal. Chim. Acta. 1996;331:271-280.
- [Google Scholar]
- Simultaneous determination of sulfite, sulfate, and hydroxymethanesulfonate in atmospheric waters by ion-pair HPLC technique. Talanta. 2003;59:875-881.
- [Google Scholar]







