Translate this page into:
Toxicological effects of heavy metals on histological alterations in various organs in Nile tilapia (Oreochromis niloticus) from freshwater reservoir
⁎Corresponding author. mushahid@ksu.edu.sa (Shahid Mahboob)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The current study was aimed to evaluate the seasonal variation in bioaccumulation of metals (Pb, Cu and Ni) in the gills, kidney, liver and muscle of Oreochromis niloticus. Sixty fish samples (average wet body weight 460.77 ± 6.22 g) were collected from the freshwater reservoir Wadi Namar Riyadh, Saudi Arabia in winter, summer, spring and autumn seasons. The heavy metals were assessed through atomic absorption. The findings of this study revealed seasonal variation in bioaccumulation of metals. In winter season minimum concentration of Pb, Cu and Ni was recorded while the highest was recorded in summer season. The order of bioaccumulation of Pb and Ni in organs was: liver > kidney > muscle > gills in and liver > gills > kidney > muscle in winter and summer, respectively. The bioaccumulation order of Cu as kidney > liver > gills > muscle and gills > kidney > liver > muscle in winter and summer, respectively. The heavy metals concentration was crossing the permissible limits suggested by WHO. Histopathological examination in kidney exhibited congestion, dilation in bowman capsule space, necrosis. Liver of the fish exhibited cytoplasmic vacuolation, necrosis, sinusoid dilation. It has been concluded that this river is heavily contaminated due to the discharge of untreated waste from industry and domestic sewage from the city and there is a dire need to control this increasing contamination.
Keywords
River
Histopathology
Kidney
Liver
Heavy metals
Fish
1 Introduction
Aquatic pollution is a severe and growing problem for aquatic life (Alinnor and Obiji, 2010), which has been increased due to domestic, agricultural, commercial and industrial activities of man (Odoemelam, 2005). The contaminated water is the major cause of bioaccumulation of metals in the different vital organs of the freshwater fish (Afshan et al., 2014; Al-Ghanim and Ahmed, 2019). The discharge of toxic domestic wastes into freshwater reservoirs cause a decline in biological oxygen demand in the lethal level of decreasing oxygen from the water bodies. The discharge of untreated industrial wastewater increased the level which causes the death of aquatic life, even at very low concentration (Atli et al., 2015; Singla, 2015; Zaqoot et al., 2017). Higher level of heavy metals in waste water leading to detrimental histopathological changes in vital tissues of fish (Drishya et al., 2016). Among toxic heavy metals Pb is a non-essential metal. It is present everywhere, such as in water, soil, air and food. The source of Pb in aquatic environments is leaching and erosion of soil, lead-dust, fuel combustion, industrial, municipal and agricultural waste, streets and other surfaces run-off water deposits (Abdel-Khalek, 2015). “Lead is toxic and has adverse effects on human health. Absorption of ingested lead is a serious risk for health” (WHO, 2011; Hussain et al., 2018). Ni “is a potential hazard to the environment media (air, water and soil). It is a common by-product of electroplating industries, steel production, metal mining, smelting, refining, ceramic and processing along with fuel combustion, and waste ignition activities” (Atchison et al., 1987). Cu is an essential trace element required by human in very minute quantity (5–20 µg/g) and the main sources of Cu are mining and industrial effluents, waste from water treatment plants, and agriculture land runoff. Cu is one of the most toxic metals (that exceed 20 µg/g) to aquatic organisms and ecosystems (Ostrowski et al., 1999).
Fish is considered as a one of the vital sources of animal protein, especially low-income groups (Sayer and Cassman, 2013). Fish are relatively vulnerable to changes in their surrounding environment as well as pollution. Fish health is used as an indicator of water pollution in aquatic ecosystem (Mahboob et al., 2015; Al-Ghanim et al., 2016). The toxic effects of pollutants may be manifest at the cellular or tissue level before consequential changes in fish behavior or external appearances (Nikalje et al., 2012). Fish histological examination may be used as a one of the bio-monitoring tool of aquatic pollution (Muñoz et al., 2015). Histopathology may be as a biomarker to monitor the environment, investigating specific vital organs (Muñoz et al., 2015). Any damage to liver would eventually cause many physiological disruptions leading to subsequent mortality of fish (Pereira et al., 2017). In this study gills, liver and kidney were selected due to their metabolic importance. The study was aimed to assess the seasonal variation in heavy metals concentration in vital organs (gills, kidney, liver and muscle) of Oreochromis niloticus and interrelationship between toxicity of the metals through histological alterations in liver and kidney.
2 Materials & methods
2.1 Collection of fish
Sixty fish specimens of Oreochromis niloticus with an average total length 26.44 ± 2.19 cm and wet body weight 460.77 ± 6.22 g was collected from the Wadi Namar (WN), Riyadh, Saudi Arabia. WN is one of the subsidiary Wadis (valleys) of Wadi Haneefah draining the western area of Riyadh. Previously this water reservoir was used as a source of water, and currently for partial disposal of city’s wastewater. The dam lake is also used for bird and fish culture. The control fish specimens were obtained from the King Abdul Aziz City of Science and Technology. Riyadh. The collected fish specimen was transported alive in polyethylene bags to the Research Laboratory of the Department of Zoology for further analysis.
2.2 Analysis of heavy metals
The level of Pb, Cu and Ni were detected by following the methodology reported by Mahboob et al., (2014) by using an Atomic Absorption Spectrophotometer (Aurora A-I1200).
2.3 Histopathological studies
Tissue specimens were further processed for studying histopathological alterations by following the methodology of Sultana et al. (2016). Tissues were cut into pieces up to 3 µm with a microtome (Histo-line MR 2258). Paraffin wax containing tissue slice ribbons was placed in hot water for some time and then placed on the slide that contains adhesive material like albumen and glycerine. Slides containing tissues were placed in the oven at 37 °C for overnight. The slides were placed in clearing material xylene for three minutes each. Immerse slide in descending series of ethanol alcohol for 1–2 min each. Slides were stained with heamatoxylin for 3–5 min. Mounted with Canada balsam and cover slip and observed under a light microscope and photographs were captured and histological changes compared with control histological slides.
3 Results and discussion
The concentration of Pb, Ni and Cu heavy metals varied seasonally. The level of Pb in “gills, kidney, liver and muscle” recorded as 0.79 ± 0.04, 0.87 ± 0.04, 0.91 ± 0.01 and, 0.79 ± 0.03 mg/Kg, respectively in the winter. The order of accumulation of Pb in organs during winter was as liver > kidney > muscle > gills. The concentration of Pb during summer season in “gills, kidney, liver and muscle” was detected as 1.80 ± 0.12, 1.62 ± 0.09, 1.78 ± 0.09, and 1.60 ± 0.08 mg/kg and their order of accumulation was kidney > gills > liver > muscle (Table 1). The highest level of Pb was recorded in summer and minimum in winter season. A non-significant difference (P > 0.05) in summer, while the difference in winter and autumn seasons was significant (P < 0.05). The concentration of Cu in the “gills, kidney, liver and muscle” during the winter season was recorded as 1.71 ± 0.06, 1.92 ± 0.10, 1.78 ± 0.07 and 1.51 ± 0.06 mg/ Kg and the order of accumulation in organs was kidney > liver > gills > muscle. Whereas in summer the concentration of Cu in respective organs was 2.95 ± 0.11, 2.90 ± 0.11, 2.89 ± 0.10 and 2.79 ± 0.12 mg/kg and order of recorded concentration was gills > kidney > liver > muscle (Table 2). The highest quantity of Cu was detected in the summer, while the minimum was recorded in winter. The differences remained significant (P < 0.05) for the concentration of Cu in the kidney and in winter and non-significant (P < 0.05) during spring, summer and autumn. Liver and muscle exhibited significant difference (P < 0.05) between winter and spring, while exhibiting non-significant (P > 0.05) difference in summer and autumn. The concentration of Ni in the gills, kidney, liver and muscle during winter was recorded as 7.42 ± 0.19, 7.90 ± 0.16, 7.73 ± 0.20 and 7.69 ± 0.16 mg/L and order of concentration was kidney > liver > muscle > gills. The level of Ni in the summer in the gills, kidney, liver and muscle was detected as 8.69 ± 0.17, 8.79 ± 0.18, 8.61 ± 0.17 and 8.66 ± 0.20 mg/Kg, respectively. The minimum concentration of Ni was observed in winter, while maximum in summer (Table 3). The differences in the concentration of Ni were non-significant (P > 0.05) among all the organs and seasons (see Table 4). Means sharing similar letter in a row or in a column are statistically non-significant (P > 0.05). Small letters represent comparison among interaction means and capital letters are used for overall means. Means sharing similar letter in a row or in a column are statistically non-significant (P > 0.05). Small letters represent comparison among interaction means and capital letters are used for overall means. Means sharing similar letter in a row or in a column are statistically non-significant (P > 0.05). Small letters represent comparison among interaction means and capital letters are used for overall means. (–) Not Reported (+) Mild, (++) Moderate, (+++) Severe, (++++) Very Severe.
Organs
Seasons
Mean
Winter
Spring
Summer
Autumn
Gills
0.79 ± 0.04
1.22 ± 0.11
1.80 ± 0.12
0.92 ± 0.06
1.18 ± 0.07CD
Kidney
0.87 ± 0.04
1.19 ± 0.07
1.62 ± 0.09
1.02 ± 0.05
1.17 ± 0.05ABC
Liver
0.91 ± 0.05
1.26 ± 0.07
1.78 ± 0.09
1.05 ± 0.07
1.25 ± 0.06AB
Muscle
0.79 ± 0.03
1.16 ± 0.06
1.60 ± 0.08
0.92 ± 0.04
1.12 ± 0.05CDE
Organ
Seasons
Mean
Winter
Spring
Summer
Autumn
Gills
1.71 ± 0.06
2.22 ± 0.07
2.95 ± 0.12
2.26 ± 0.07
2.28 ± 0.08BCD
Kidney
1.92 ± 0.10
2.31 ± 0.08
2.90 ± 0.11
2.51 ± 0.09
2.41 ± 0.09B
Liver
1.78 ± 0.07
2.29 ± 0.06
2.89 ± 0.10
2.08 ± 0.09
2.26 ± 0.08CD
Muscle
1.51 ± 0.06
1.99 ± 0.09
2.79 ± 0.12
2.11 ± 0.08
2.10 ± 0.06EF
Organs
Seasons
Mean
Winter
Spring
Summer
Autumn
Gills
7.42 ± 0.19
7.68 ± 0.18
8.69 ± 0.17
8.19 ± 0.22
7.99 ± 0.19BC
Kidney
7.90 ± 0.16
8.11 ± 0.15
8.79 ± 0.18
8.24 ± 0.16
8.26 ± 0.16AB
Liver
7.73 ± 0.20
7.79 ± 0.12
8.61 ± 0.17
8.22 ± 0.16
8.09 ± 0.14BC
Muscle
7.69 ± 0.12
7.87 ± 0.18
8.66 ± 0.20
8.07 ± 0.22
8.07 ± 0.18BC
Parameters of kidney
O. niloticus damage
Parameters of liver
O. niloticus damage
Necrosis
+++
Cytoplasmic disorganization
+++
Tubular hemorrhage
++++
Cytoplasmic vacuolation
+++
Congestion
+++
Dilation of sinusoids
++++
Necrosis
++++
Necrosis
++++
The bioaccumulation of metals by the fish and vital organs is variable which the concentration of heavy metals in the water reservoir, sensitivity of fish, the age of the fish, the size of the fish, the physiological status of fish, their habitat preference, feeding behaviour, and rate of growth (Chapman et al., 1996). The results of this study showed that kidney and liver have maximum concentration of Pb, Cu and Ni. The maximum level of heavy metals was recorded in summer in all the organs, while lower values were observed winter seasons. The similar findings were reported by Iqbal and Shah (2014) who found that higher values of the metal levels were observed in summer and lower in winter in Cyprinus carpio. “The highest levels of heavy metals were found in the liver and kidney, while lowest levels were found in the muscle” (Sultana et al., 2016). “The liver and kidneys are involved in the detoxification and removal of toxic substances circulating in the blood stream. Moreover, liver and kidneys, being the major organs of metabolic activities including detoxification” (Amin et al., 2013; Al-Ghanim and Ahmed, 2019). In this study the higher accumulation of in the liver was probably because of its capacity to react with other metabolites (Cuevas et al., 2016). The higher accumulation of Pb, Cu and Ni during the summer season may be due to the rise in physiological activity of fish due to increase in water temperature (Canpolat and Çalta 2003). The contaminated fresh water has become a cause of great concern in the latest trends, not only because of responses to the water supply, but also from damages to aquatic life (Sultana et al., 2016; Jamdade and Gawande, 2017).
The histopathological changes were observed in the liver and kidney tissues of Oreochromis niloticus (Figs. 1–3). The investigated changes in the liver were cytoplasmic disorganization, cytoplasmic vacuolation, dilation of sinusoid, necrosis of hepatic cells, congestion of blood cells and Oedema. These findings are in accordance with findings in Labeo rohita by Bantu et al. (2017). The identical histopathological changes were also reported by Faheem et al. (2016) in the liver like inflammation, oedema, central vein congestion, degeneration and necrosis of hepatocytes. Bhuyan et al. (2014) reported similar types of abnormalities in the liver of O. mossambicus. These findings were also in accordance with findings of Cuevas et al. (2016) who observed necrosis, degradation of hepatocytes, degeneration of blood vessels, distended sinusoids with pyknotic nuclei and vacuolation of cells. In the present study kidney sections of experimental fish showed necrosis in renal tubular cells, tubular hemorrhage, Oedema, damaged glomerulus, collecting duct damage and blood congestion. Deore and Wagh (2012) reported abnormalities in kidney reflected the degeneration of the glomerulus tissue, necrosis and occlusion in tubular lumen.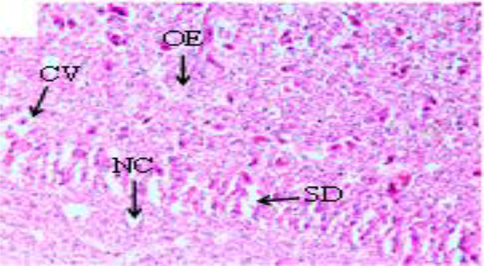
Photomicrographs of the liver Cirrhinus mrigala collected from River Chenab (H and E, X40) showed various histological abnormalities like Cytoplasmic vacuolation (CV), Necrosis (NC), Oedema (OE) and Sinusoid dilation (SD). Histological photomicrograph of C. mrigala sampled from the Chenab River polluted sites.
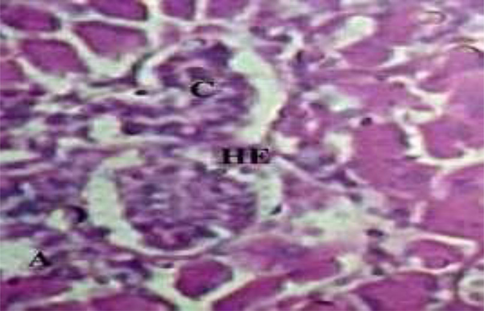
Photomicrograph of kidney of Cirrhinus mrigala collected from polluted site of the Chenab River indicating renal tubules myxospora and hyperemia (A) Glomerulonephritis (B) (40×10×3× magnification).
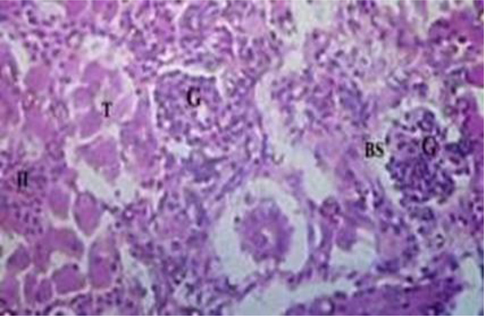
Photomicrograph of histopathology of fish Cirrhinus mrigala collected from a polluted site of the Chenab River indicating Necrosis, hyalinization of interstitium and clogging of tubules (40×10× magnification).
4 Conclusion
It has been concluded that bioaccumulation of Cu, Ni and Pb in Oreochromis niloticus has affected the changes in water temperature during different seasons. These metals from the fish muscles may ultimately affect the population in the vicinity of the river. Histopathological changes in Oreochromis niloticus are a useful tool to evaluate the impact of toxicity of xenobiotics in the vital functions of a living organism. The prominent alterations were observed in liver, kidney as compared to control group. Hence, the findings of this study are helpful for the evaluation of eco-toxicological impacts of pollutions reaching in aquatic fauna, particularly fish and indirectly to human populations. This study indicated that anthropogenic activities are the major cause of aquatic loss and imbalance food chain through the pollution of water bodies. Aquatic loss can be reduced through proper use of the advance technologies producing less heavy metal pollution to the environment.
Acknowledgements
“The authors (SM and KAAG) express their sincere appreciation to the Deanship of Scientific Research at the King Saud University for its funding of this research through the Research Group Project No. 1440-0138”.
Declaration of Competing Interest
Nothing to declare.
References
- Accumulation and histological transformation in the gills, liver, muscles, and skin in Oreochromis niloticus induced by mercury. Turk. J. Vet. Anim. Sci. 2019
- [CrossRef] [Google Scholar]
- Monitoring of trace metals in tissues of Wallago attu (lanchi) from the Indus River as an indicator of environmental pollution. Saudi J. Biol. Sci.. 2016;23:72-78.
- [CrossRef] [Google Scholar]
- Risk assessment: bioaccumulation of metals and histopathological alterations in Nile tilapia (Oreochromis niloticus) facing degraded aquatic conditions. Bull. Environ. Contam. Toxicol.. 2015;94:77-83.
- [Google Scholar]
- Assessment of trace metal composition in fish samples from Nworie River. Pak. J. Nutr.. 2010;9:81-85.
- [Google Scholar]
- Effect of cadmium chloride on the histoarchitecture of kidney of a freshwater Catfish, Channa punctatus. J. Chem. Biol. Phys. Sci.. 2013;3:1900-1905.
- [Google Scholar]
- Alterations in the serum biomarkers belonging to different metabolic systems of fish (Oreochromis niloticus) after Cd and Pb exposures. Environ. Toxicol. Pharmacol.. 2015;40:508-515.
- [Google Scholar]
- Qualitative and quantitative analysis of fish tissue of Oreochromis mossambicus collected from Kedilam river Cuddalore Tamil Nadu, India. Int. J. Appl. Sci. Biotech.. 2014;2(2):135-141.
- [Google Scholar]
- Heavy metals in some tissues and organs of Capoeta capoeta umbla (Heckel, 1843) fish species in relation to body size, age, sex and seasons. Fresen. Environ. Bull.. 2003;12(9):961-966.
- [Google Scholar]
- Policy analysis, peer reviewed: evaluation of bioaccumulation factors in regulating metals. Environ. Sci. Technol.. 1996;30(10):448-452.
- [Google Scholar]
- Multi-organ histopathology in gobies for estuarine environmental risk assessment: a case study in the Ibaizabal estuary (SE Bay of Biscay) Estuar. Coast. Shelf Sci.. 2016;179:145-154.
- [Google Scholar]
- Heavy metal induced histopathological alterations in liver of Channa gachua (Ham) J. Exp. Sci.. 2012;3:35-38.
- [Google Scholar]
- Histopathological changes in the gills of fresh water fish, Catla catla exposed to electroplating effluent. Int. J. Fish. Aquat.. 2016;4:13-16.
- [Google Scholar]
- M. Histopathological effects of bisphenol-A on liver, kidneys, and gills of Indiana major carps Catla catla (Hamilton, 1822) J. Anim. Plant Sci.. 2016;26:514-522.
- [Google Scholar]
- Study on impact of habitat degradation on proximate composition and amino acid profile of Indian major carps from different habitat. Saudi J. Biol. Sci.. 2018;25:755-759.
- [Google Scholar]
- Analysis of water quality parameters: a review. Int. J. Eng. Res.. 2017;6(3):145-148.
- [Google Scholar]
- Study of seasonal variations and health risk assessment of heavy metals in Cyprinus carpio from Rawal Lake, Pakistan. Environ. Monit. Assess.. 2014;186(4):2025-2037.
- [Google Scholar]
- Health risks associated with pesticide residues in water, sediments and the muscle tissues of Catla catla at Head Balloki on the River Ravi. Environ. Monit. Assess.. 2015;187:81.
- [CrossRef] [Google Scholar]
- A study on the accumulation of nine heavy metals in some important fish species from a natural reservoir in Riyadh, Saudi Arabia. Toxicol. Environ. Chem.. 2014;96:83-798.
- [CrossRef] [Google Scholar]
- Histopathological biomarkers in juvenile silver catfish (Rhamdia quelen) exposed to a sublethal lead concentration. Ecotoxicol. Environ. Saf.. 2015;113:241-247.
- [Google Scholar]
- Histopathological changes in liver of freshwater major carp, Labeo rohita after acute and chronic exposure to textile mill effluent. The Bioscan. 2012;7:215-220.
- [Google Scholar]
- Bioaccumulation of trace elements in fish from Oguta lake in Nigeria. J. Chem. Soc. Nig.. 2005;30:18-20.
- [Google Scholar]
- Agency for Toxic Substances and Disease Registry’s 1997 priority list of hazardous substances. Latent effects–carcinogenesis, neurotoxicology, and developmental deficits in humans and animals. Toxicol. Ind. Health. 1999;15(7):602-644.
- [Google Scholar]
- Hepatic microvascular dysfunction and increased advanced glycation end products are components of non-alcoholic fatty liver disease. PLoS One. 2017;12(6) e0179654
- [Google Scholar]
- Agricultural innovation to protect the environment. PNAS. 2013;110(21):8345-8348.
- [CrossRef] [Google Scholar]
- Histological changes induced by monocrotophos in the kidney of Ctenopharyngodon idellus (Cuvier and Valenciennes) Int. J. Fish. Aquac.. 2015;5:54-59.
- [Google Scholar]
- Histopathological changes in liver, gills and intestine of Labeo rohita inhabiting industrial waste contaminated water of river Ravi. Pak. J. Zool.. 2016;48:1171-1177.
- [Google Scholar]
- WHO, 2011. Guidelines for Drinking Water. 4th ed. 518p.
- Baseline concentration of heavy metals in fish collected from Gaza Fishing Harbor in the Mediterranean Sea along Gaza Coast, Palestine. Turk. J. Fish Aquat. Sci.. 2017;17:101-109.
- [Google Scholar]







