Translate this page into:
Toxicity of neem seed extract and different insecticides on Trichogramma chilonis (Hymenoptera: Trichogrammatidae)
⁎Corresponding authors. asrar@gcuf.edu.pk (Muhammad Asrar), shahbaz@kfueit.edu.pk (Shahbaz Ali), liyunzhou2007@126.com (Yunzhou Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background and objectives
Trichogramma chilonis is an important biological control agent (parasitoid) that kill the host species before they damage the crops. However, insecticide sprayed after T. chilonis release significantly alter its efficacy. The acute toxicity risks of neem seed extract and commonly used insecticides for the control of lepidopteran insects are unknown for T. chilonis. The main objective of this study was to assess the toxicity of four insecticides, i.e., buprofezin, lufenuron, methoxyfenozide, pyriproxyfin and neem seed extract to T. chilonis.
Methods
Egg card and dipped surface bioassays were used to test the toxicity insecticides, i.e., lufenuron (2 ml/liter), methoxyfenozide (2 ml/liter), buprofezin (5 g/liter), pyriproxyfin (5 ml/liter) and neem seed extract (2 ml/liter). Egg cardboards prepared from the parasitized eggs of Sitotroga cerealella collected at 1st, 3rd, 5th, 7th, and 8th day after oviposition were dipped in the respective concentrations of inleticides and neem seed extract. The dipped cardboards were dried and emergence of T. chilonis was recorded. Similarly, dipped surface bioassay was conducted to observe the mortality of T. chilonis at 4 and 24-h after exposure.
Results
The applied insecticides exhibited varying degree of toxicity against T. chilonis. Lufenuron and neem seed extract proved least toxic, methoxyfenozide and buprofezin were moderately toxic and pyripoxyfen exhibited the highest toxicity during the study. Similarly, lufenuron and neem seed extract proved less toxic for the adults, buprofezin was moderately toxic, and methoxyfenozide and pyripoxyfen exhibited the highest toxicity in the dipped surface assay after 4 h of exposure. All insecticides proved highly toxic for the adults 24 h after exposure. The results reveled that lufenuron and neem oil are relatively safe for T. chilonis.
Conclusions
The tested insecticides and neem seed oil exhibited varying degree of toxicity against T. chilonis. The overall toxicity was classified as; pyriproxyfen > methoxyfenozide > buprofezin > lufenuron > neem seed extract. Therefore, neem seed extract and lufenuron are safer for T. chilonis compared to the rest of the insecticides included in the study.
Keywords
Toxicity assay
Neem seed extract
Egg card assay
Dipped surface assay
1 Introduction
Trichogramma (Hymenoptera: Trichogrammatidae) are polyphagous parasitoid wasps, distributed globally and play an important role in the management of several lepidopterous pests (Cadapan, 1986; Khan, 2020; Mahankuda and Sawai, 2020). They target the eggs of host species; thus, emerging adults are parasitized (Khan, 2022, 2020; Sont et al., 1999). Trichogrammatids are excessively used for the management of Helicoverpa armigera Hübner, Scirpophaga incertulas Walker, and Plutella xylostella Linnaeus in rice, vegetables, and cotton crops (Jalali et al., 2014; Kurtuluş and Kornoşor, 2015; Lei et al., 2021). In Pakistan Trichogramma is effectively used for the management of sugarcane borer (Hamid et al., 1998; Hussain et al., 2012). Trichogramma are used as egg parasitoids in the biological control programs (Preetha et al., 2018; Singhamuni et al., 2015). These species are easy to rear, and their production is economically profitable (Preetha et al., 2010, 2018). Trichogramma is frequently in the Indian sub-continent for effective management of H. armigera (Lingathurai et al., 2015).
Trichogrammatids are beneficial biological control agents in the egg parasitoid group (Sarwar and Salman, 2015). They have been successfully used for biological control in > 50 countries of the world. Trichogramma are released on ∼ 32 million ha annually; however, the insecticides used for the management of harmful pests significantly reduce their efficacy (King and Dunkin, 1986; Mahankuda and Sawai, 2020; Thangavel et al., 2018). Biological control with Trichogramma coupled with chemical control is not an attractive option due to harmful effects linked with the pesticides (Saber, 2011).
Insect growth regulators (IGRs) attack specific biochemical systems that are unique to insects and safe to non-target organisms, i.e., other arthropod species and mammals (Cadapan, 1986; Jalali et al., 2016; Zengxin et al., 2021). Bio-rational approaches would play a key role in reducing the risks associated with pest management tactics, including pesticides (Horowitz et al., 2020). The IGRs are effective at lethal and sublethal concentrations and used for the effective results on physiological parameters of exposed insect species (Sadeghi et al., 2021). The IGRs significantly influence pupil and larval weight (Yin et al., 2008), fecundity, developmental time (Mahmoudvand et al., 2011), egg size, hatching (Guo et al., 2013), adult endurance (Mahmoudvand et al., 2011), pupal ratio, adult development (Sial and Brunner, 2010) and other biological parameters (Ahmad et al., 2012).
Over time, excessive use of synthetic insecticides has resulted in serious problems, including evolution of insecticide resistance, insecticide-induced resurgence of insect pests, adverse effects on non-target organisms, i.e., parasitoids, predators, honeybees, pollinators, birds, cattle, and human (Arif et al., 2020; Naeem et al., 2022, 2021c, 2021a, 2021b). In addition, phytotoxicity, environmental pollution, and excessive increase in the cost of pesticides have necessitated alternative, effective and biodegradable pest control materials with greater selectivity (Cherif et al., 2021; Mohapatra and Shinde, 2021; Subandi et al., 2017). For these reasons, natural pesticides are often preferred over synthetic ones. Among several options, neem (Azadirachta indica) has evoked a great deal of interest because of its bio-efficacy and biodegradability (Ahmad et al., 2012; Preetha et al., 2018). However, the safety of neem-based pesticides to non-target organisms has been the subject of some controversy. Although sufficient data are available on the bioactivity of neem against insect pests, there have been few detailed studies on the effects of neem on parasitoids.
This study assessed the effect of four insecticides and neem seed extracts on T. chilonis. Insecticides were selected based on their potential use in the management of lepidopteron insects. The results of the study would help to devise integrated pest management practice for lepidopteron insects.
2 Materials and methods
2.1 Experimental site and details
This study was carried out in the Eco-toxicological laboratory, Ayyub Agricultural Research Institute (AARI) Faisalabad, Pakistan. Comparative toxicity of four insecticides and neem seed extract was tested against T. chilonis under laboratory conditions. Four insecticides, i.e., lufenuron, methoxyfenozide, buprofezin, pyriproxyfin and neem seed extract were included in the study. The experiment Research work was started in March 2019.
The experiment was conducted at 27 ± 1 °C temperature and 65 ± 5% relative humidity. The experiment was arranged according to completely randomized design and each treatment had three replications. The concentrations of the insecticides and neem extract were selected based on their recommended doses for the registered crops. The concentrations were lufenuron (2 ml/liter), methoxyfenozide (2 ml/liter), buprofezin (5 g/liter), pyriproxyfin (5 ml/liter) and neem seed extract (2 ml/liter). These insecticides are usually used to manage the pests of a lot of crops in the landscape.
Trichogramma chilonis culture was maintained on the eggs of Sitotroga cerealella under laboratory conditions at 25–27 °C and 70–80% relative humidity. The toxicity of different insecticides and neem seed extract on different life stages of T. chilonis was tested by egg card and dipped surface residue bioassays described below.
2.2 Egg card bioassay
Egg cards were prepared from parasitized Sitotroga cerealella eggs during various phases of immature T. chilonis and used in the egg card bioassays. Sitotroga cerealella eggs were exposed to T. chilonis for likely parasitism for 24 h and then egg cards were prepared from the exposed eggs (Khan, 2022, 2020). Five randomly chosen egg cards (each having 40 eggs) were dipped into the solutions of insecticides and neem seed extract for one second at 1st, 3rd, 5th, 7th, and 8th day after oviposition. Untreated cards were regarded as control. These days correspond to the life stages of Trichogramma, i.e., eggs (1d), larvae (3d), early pupae (5d), pupae (7d) and late pupae or one day before adult emergence (8d). Dipped egg cards were dried at ambient room temperature. Once dried, the egg cards were placed in a small glass Petri dish (5 cm diameter and 5 cm deep) and kept at 25 ± 2 °C temperature and of 60 ± 5% relative humidity until all healthy parasitoids emerged. Afterwards, the remaining eggs were examined under microscope. The percentage emergence of T. chilonis was computed for each treatment and used in the interpretation of the results.
2.3 Dipped surface residue bioassay
The insecticide residue bioassay was conducted in ventilate glass bioassay chambers (15 cm length and 4 cm diameter). Whatman no. 1 filter paper was dipped in the respective solutions of each treatment. The filter papers were then dried and put into the glass bioassays tube. The control tubes contained filter papers moistened with distilled water and then dried. Afterwards, the newly emerging adults were released in the tubes. The mortality in the bioassay tubes was recorded 4 and 24 h after the release of the adults. Percent mortality was computed and interpreted. Cardboard and dipped surface residue experiments were arranged according to completely randomized design and each treatment had three replications.
2.4 Data analysis
The collected data on emergence and mortality were analyzed by one way analysis of variance (ANOVA) (Steel et al., 1997). The normality in the data was tested which indicated that data had normal distribution (Shapiro and Wilk, 1965). The means were compared by Tukey’s post hoc test at 95% probability level where ANOVA indicated significant differences. Based on means, toxicity was interpreted. The treatment means having higher emergence and low mortality was considered less toxic and vice versa. The statistical computations were done on SPSS statistical software version 20.0 (IBM Inc., 2012).
3 Results
The T. achilonis emergence from different aged-parasitized eggs was significantly altered by different insecticides and neem seed extract included in the study. Lufenuron proved least toxic for the 1-day old eggs with 25 % emergence. Overall, the emergence was 25, 23, 21, 23 and 19% the 1-day old egg cardboards dipped in lufenuron, methoxifenazide, pyriproxyfen, beprofezone and neem seed extract, respectively. The control treatment resulted in 94.5% emergence on 1st observation (Fig. 1).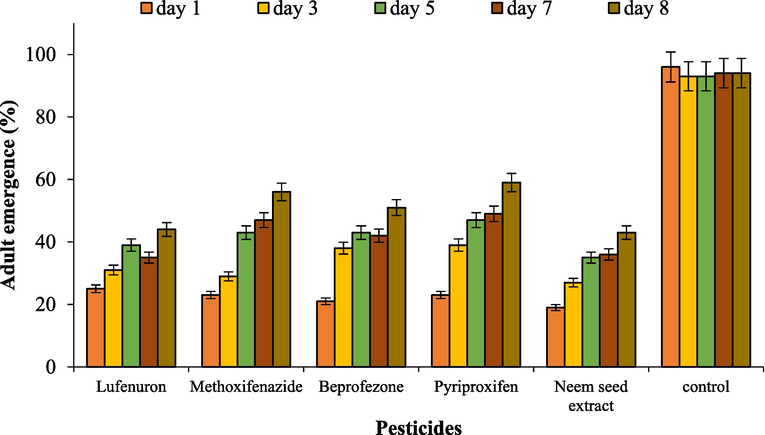
The effectiveness of different insecticides and neem seed extract on Trichogramma chilonis emergence at 1, 3, 5, 7 & 8 days after post parasitism.
Pyripoxifen was least toxic against T. chilonis with 39% emergence for 3-day old parasitoid egg. Overall emergence was 31, 29, 38, 39 and 27% for the eggs treated with pyriproxyfen, beprofezone, lufenuron, methoxifenazide and neem seed extract, respectively at 2nd observation (Table 1). The values represent the means ± SE (n = 3). The means followed by different letters are significantly different form each other (P < 0.01).
Parasitoid emergence (%)
Treatments
1-day old eggs
3-day old eggs
5-day old eggs
7-day old eggs
8-day old eggs
Lufenuron
25.0 ± 1.06C
31.0 ± 1.31 AB
39.0 ± 1.02 BC
35.0 ± 2.83 D
44.0 ± 1.04B
Methoxifenazide
23.0 ± 0.45 AB
29.0 ± 2.12B
43.0 ± 0.91 AB
47.0 ± 1.04 AB
56.0 ± 0.41A
Beprofezone
21.0 ± 0.71 A
38.0 ± 1.28 A
43.0 ± 1.02 AB
42.0 ± 2.32 BC
51.0 ± 1.02 AB
Pyriproxifen
23.0 ± 1.31AB
39.0 ± 0.62 A
47.0 ± 0.62 A
49.0 ± 0.52 A
59.0 ± 0.58 A
Neem seed extract
19.0 ± 0.93 A
27.0 ± 2.01B
35.0 ± 2.07C
36.0 ± 2.91CD
43.0 ± 1.05B
Pyripoxifen was comparatively less toxic against 5-day old parasitoid eggs of T. chilonis as it gave 47% emergence. The emergence from other insecticides, i.e., lufenuron, methoxyfenozide, buprofezin, pyripoxifen and neem seed extract was recorded 39, 43, 43, 47 and 35%, respectively.
Pyriproxifen proved the least toxic against 7-day old parasitoids egg with 49% emergence. The recorded emergence from methoxifenazide, beprofezone, neem seed extract and lufenuron was 47, 42, 36 and 35%, respectively.
Similarly, pyriproxifen proved the least toxic against 8-day old parasitoids egg with 59% emergence. The recorded emergence from methoxifenazide, beprofezone, lufenuron and neem seed extract was 56, 51, 44 and 43%, respectively.
The percentage mortality of T. chilonis adults after 4-hour exposure to lufenuron, methoxyfenozide, buprofezin, pyripoxifen and neem seed extract was recorded 48, 76, 52, 76 and 44% (Fig. 2). The mortality reached to 100, 100, 80, 80 and 96% after 24 h exposure to lufenuron, methoxyfenozide, buprofezin, pyripoxifen and neem seed extract, respectively (Table 2). The values represent the means ± SE (n = 3). The means followed by different letters are significantly different form each other (P < 0.01).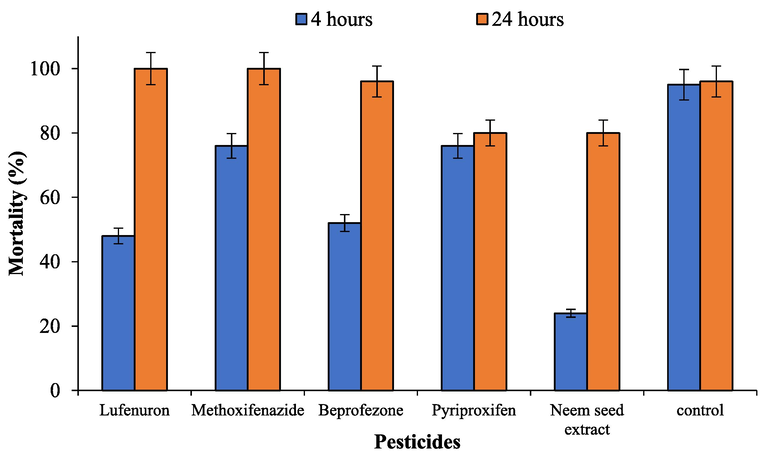
Mortality of Trichogramma chilonis adults after 4 h-old parasitized eggs 24 h after exposure to insecticides residues.
Exposure time (h)
Lufenuron
Methoxifenazide
Beprofezone
Pyriproxifen
Neem seed extract
Mortality/%
4
48.0 ± 1.02 AB
76.0 ± 0.32A
52.0 ± 1.26 AB
76.0 ± 0.39 A
24.0 ± 1.21B
24
100.0 ± 0.82A
100.0 ± 0.41 A
80.0 ± 0.62 A
80.0 ± 0.72 A
96.0 ± 0.62 A
4 Discussion
Trichogramma has been used as important biological control agents in >50 countries of the world (Khan, 2022, 2020). These are released on ∼32 million ha; however, excessive use of pesticides significantly lowers their efficacy (King and Dunkin, 1986). Biological and chemical control combination in IMP is unfit due to harmful effects of insecticides on Trichogramma (Saber, 2011). Therefore, this study assessed whether the frequently used insecticides exert negative impacts on different immature stages of Trichogramma. The tested insecticides significantly differed in their toxic effects indicating that the insecticides with lower toxicity could be combined with Trichogramma release in IPM.
Trichogramma eggs are used biological control program which increases their population and suppress harmful pests (Lei et al., 2021; Mahankuda and Sawai, 2020; Preetha et al., 2018; Singhamuni et al., 2015). Trichogramma speices can be reared easily and effectively control harmful pests (Khan, 2022, 2020; Preetha et al., 2010). Trichogramma are frequently used Indian sub-continent for the management of American bollworm (Lingathurai et al., 2015).
Combination of T. chilonis with various insecticides successfully controlled sucking pests (Sant et al., 2019; Thangavel et al., 2018; Wahengbam et al., 2018). The T. chilonis integrated with insecticides in Bathinda, India, reduced bollworm damage by 70.30% and improved cotton yields up to 44.50% compared to sole application of insecticides (Brar et al., 2002).
Trichogramma chilonis are affected by insecticides’ spray which normally lower their efficacy. The oviposition of this species is significantly altered by the insecticides (Suzuki and Waller, 1984). Trichogramma wasps are tiny in size, belong to friendly insect fauna and used against lepidopterous insects in IPM for pest management in vegetables and field crops (Nagarkatti and Nagaraja, 1977). It is very destructive parasitoid (Subandi et al., 2017).
Trichogramma species usually attack on the eggs of ∼200 species (Sattar et al., 2011). There and reared as the biological control agents and released in the target fields (Tarès et al., 2000). Their egg phases start in March and end during September. Trichogramma chilonis regulate their host eggs in July.
When their hosts complete their three generations then they continue to egg parasitized at the end of October, but its occurrence started from July onward, so their discharge adjusted as early as their oviposition started in the area and they depend on their native main temperature(Ullah et al., 2012). The applied insecticides exerted differential effects on the emergence and adult mortality of T. chilonis. Although plant extracts are regarded safe for the beneficial organisms, some stages of T. chilonis were significantly affected by the neem seed extract. However, it was least toxic to T. chilonis compared to the rest of the insecticides included in the study. The differences among insecticides can be explained with their chemical composition and use. Nevertheless, the applied concentration of the insecticides could be the other major reason for the differences among insecticides.
Khan (2020) reported that acetamiprid, spinetoram, fipronil and abamectin proved highly toxic against T. chilonis, while the remaining pesticides were less toxic and can be integrated with the parasitoid in agroecosystem.
The results of the current study warrant that neem seed extract could be incorporated in IMP due to its less toxicity to T. chilonis. However, the toxicity of neem seed extract should be determined for the target harmful pests before the inclusion of neem seed extract in IMP.
5 Conclusion
The results revealed that lufenuron and neem seed extract proved least toxic for Trichogramma chilonis; therefore, these could be used for the management of harmful pests and their use will had lower toxic effects on T. chilonis compared to the rest of the insecticides included in the study. Methoxyfenozide and buprofezin showed moderately toxicity, whereas pyripoxifen proved highly toxic for the emergence of T. chilonis. Lufenuron and neem oil were comparatively less toxic to adults after 4 h of exposure, buprofezin was moderately toxic, while methoxyfenozide and pyripoxifen were highly toxic to adults of T. chilonis. It is therefore recommended that lufenuron and neem seed extract could be used for the management of lepidopteran pests in various field crops.
Acknowledgements
The current study was supported by Science and Technology Project of Guizhou Province (Qiankehe Foundation-ZK[2022]General 071), the National Natural Science Foundation of China (32060679), the Guizhou University Cultivation Project (Guizhou University Cultivation [2019]No.52). The authors extend their appreciation to the Researchers supporting project number (RSP-2021/173), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effects of neem based insecticides on Plutella xylostella (Linn.) Crop. Prot.. 2012;34:18-24.
- [CrossRef] [Google Scholar]
- Molecular characterization and RSV Co-infection of Nicotiana benthamiana with three distinct begomoviruses. Methods. 2020;183:43-49.
- [Google Scholar]
- Biocontrol based management of cotton bollworms in the Punjab. J. Biol. Control. 2002;16:121-124.
- [Google Scholar]
- Cadapan, E.P., 1986. Trichogramma parasitoid production and utilization in the Philippines, in: Diamond Jubilee Professorial Chair Lecture, College, Laguna (Philippines), 24 Oct 1986.
- The egg parasitoids Trichogramma: from laboratory mass rearing to biological control of lepidopteran pests. Biocontrol Sci. Technol.. 2021;31:661-693.
- [CrossRef] [Google Scholar]
- Sublethal and transgenerational effects of chlorantraniliprole on biological traits of the diamondback moth, Plutella xylostella L. Crop Prot.. 2013;48:29-34.
- [CrossRef] [Google Scholar]
- Hamid, F., Qureshi, M.A., Mohyuddin, A.I., 1998. Use of Trichogramma chilonis (Ishii) as biological control agent for Gurdaspur borer, Acigona steniella (Hampson) at Tandlianwala Sugar Mills Ltd., Kannwani. Faisalabad. Proc. 33rd Annu. Conv. Pak. Soc. Sugar Tech., Lahore 63–70.
- Insecticide resistance and its management in Bemisia tabaci species. J. Pest. Sci.. 2020;93:893-910.
- [Google Scholar]
- Developmental and phenological responses of wheat to sowing dates. Pakistan J. Agric. Sci.. 2012;49:459-468.
- [Google Scholar]
- IBM, C., IBM SPSS Inc., 2012. SPSS Statistics for Windows. IBM Corp. Released 2012 Version 20, 1–8.
- Temperature-dependent development of the five psyllophagous ladybird predators of Agonoscena pistaciae (Hemiptera: Psyllidae) Ann. Entomol. Soc. Am.. 2014;107:445-452.
- [Google Scholar]
- Trichogrammatids. In: Ecofriendly Pest Management for Food Security. Elsevier; 2016. p. :139-181.
- [Google Scholar]
- Lethal and parasitism effects of selected novel pesticides on the immature stages of Trichogramma chilonis (Trichogrammatidae: Hymenoptera) Int. J. Trop. Insect Sci.. 2022;42:1077-1093.
- [Google Scholar]
- Lethal and parasitism effects of selected novel pesticides on adult Trichogramma chilonis (Hymenoptera: Trichogrammatidae) J. Plant Dis. Prot.. 2020;127:81-90.
- [Google Scholar]
- The effect of nutrition on the reproductive performance of first-litter sows 4. The relative effects of energy and protein intakes during lactation on the performance of sows and their piglets. Anim. Sci.. 1986;43:319-325.
- [Google Scholar]
- Effects of some insecticides used in maize (Zea mays L.) on preimaginal stages of Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae). Turkish. J. Entomol.. 2015;39
- [CrossRef] [Google Scholar]
- Evaluation of toxicity of nine commonly used pesticides to Trichogramma chilonis. 农药学学报. 2021;23:716-723.
- [Google Scholar]
- Ecotoxicological performances and biochemical effect of selected pesticides on Trichogramma chilonis(Ishii)(Hymenoptera: Trichogrammatidae) J. Entomol. Zool. Stud. 2015;3:109-114.
- [Google Scholar]
- Effect of insecticides on adult emergence from different developmental stages of Trichogramma chilonis (Ishii) (Hymenoptera: Trichogrammatidae) under laboratory condition. J. Entomol. Zool. Stud.. 2020;8:1861-1864.
- [Google Scholar]
- Fumigant toxicity of some essential oils on adults of some stored-product pests. Chil. J. Agric. Res.. 2011;71:83-89.
- [CrossRef] [Google Scholar]
- Contact toxicity of different insecticides against egg parasitoid, Trichogramma japonicum Ashmead under laboratory condition. J. Entomol. Zool. Stud.. 2021;9:134-139.
- [Google Scholar]
- The impact of different crop sequences on weed infestation and productivity of barley (Hordeum vulgare L.) under different tillage systems. Crop Prot.. 2021;149:105759
- [CrossRef] [Google Scholar]
- The impact of different weed management systems on weed flora and dry biomass production of barley grown under various barley-based cropping systems. Plants. 2022;11:718.
- [CrossRef] [Google Scholar]
- Weed flora composition of different barley-based cropping systems under conventional and conservation tillage practices. Phytoparasitica. 2021;49:751-769.
- [CrossRef] [Google Scholar]
- Impact of different barley-based cropping systems on soil physicochemical properties and barley growth under conventional and conservation tillage systems. Agronomy. 2021;11:8.
- [CrossRef] [Google Scholar]
- Biosystematics of Trichogramma and Trichogrammatoidea species. Annu. Rev. Entomol.. 1977;22:157-176.
- [Google Scholar]
- Impact of chloronicotinyl insecticide, imidacloprid on egg, egg-larval and larval parasitoids under laboratory conditons. J. Plant Prot. Res.. 2010;50:535-540.
- [Google Scholar]
- Effect of neem oil based nanoemulsion on egg parasitoid, Trichogramma chilonis (Ishii) (Hymenoptera: Trichogrammatidae) J. Biol. Control. 2018;32:103-107.
- [Google Scholar]
- Acute and population level toxicity of imidacloprid and fenpyroximate on an important egg parasitoid, Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae) Ecotoxicology. 2011;20:1476-1484.
- [Google Scholar]
- Insecticidal activity of the essential oil of Perovskia artemisioides Boiss. Nat. Prod. Res.. 2021;35:5929-5933.
- [CrossRef] [Google Scholar]
- Residual toxicity of some newer insecticides against Trichogramma chilonis Ishii under laboratory conditions. J. Pharmacogn. Phytochem.. 2019;8:886-888.
- [Google Scholar]
- Toxicity of oils formulation as a new useful tool in crop protection for insect pests control. Int. J. Chem. Biomol. Sci.. 2015;1:297-302.
- [Google Scholar]
- Toxicity of some new insecticides against Trichogramma chilonis (Hymenoptera: Trichogrammatidae) under laboratory and extended laboratory conditions. Pak. J. Zool.. 2011;43:1117-1125.
- [Google Scholar]
- An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591-611.
- [Google Scholar]
- Lethal and sublethal effects of an insect growth regulator, pyriproxyfen, on obliquebanded leafroller (Lepidoptera: Tortricidae) J. Econ. Entomol.. 2010;103:340-347.
- [CrossRef] [Google Scholar]
- Evaluation of the potential of Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae) as a bio-control agent for Trichoplusia ni, cabbage semi-looper. Trop. Agric. Res.. 2015;26:223-236.
- [Google Scholar]
- Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long-term treatment. Am. J. Respir. Crit. Care Med.. 1999;159:1043-1051.
- [Google Scholar]
- Steel, R.., Torrei, J., Dickey, D., 1997. Principles and Procedures of Statistics A Biometrical Approach, A Biometrical Approach.
- Suitability of Corcyra cephalonica eggs parasitized with Trichogramma japonicum as intermediate host against sugarcane borer Chilo auricilius. Bulg. J. Agric. Sci.. 2017;23:779-786.
- [Google Scholar]
- Biosynthesis and biodegradation of caffeine, theobromine, and theophylline in Coffea arabica L. fruits. J. Agric. Food Chem.. 1984;32:845-848.
- [Google Scholar]
- Cloning and expression of cytochrome P450 genes belonging to the CYP4 family and to a novel family, CYP48, in two hymenopteran insects, Trichogramma cacoeciae and Apis mellifera. Biochem. Biophys. Res. Commun.. 2000;268:677-682.
- [Google Scholar]
- Evaluation of toxicity of Emamectin benzoate 5 WG on immature stages of egg parasitoid, Trichogramma chilonis. Ann. Plant Prot. Sci.. 2018;26:217-218.
- [Google Scholar]
- Efficacy of trichogramma chilonis ishii in comparison with two commonly used insecticides against sugarcane stem borer chilo Infuscatellus snellen (lepidoptera: pyralidae) J. Anim. Plant Sci.. 2012;22:463-466.
- [Google Scholar]
- Efficacy of new generation insecticides against Trichogramma chilonis Ishii and Trichogramma pretiosum Riley. Mortality. 2018;10:100.
- [Google Scholar]
- Sublethal effects of spinosad on Plutella xylostella (Lepidoptera: Yponomeutidae) Crop Prot.. 2008;27:1385-1391.
- [CrossRef] [Google Scholar]
- Toxicity and safety evaluation of four new insecticides on Trichogramma chilonis. 热带生物学报. 2021;12:83-87.
- [Google Scholar]







