Translate this page into:
Toxic effects of chlorpyrifos on the growth, hemocytes counts, and vital organ’s histopathology of freshwater mussel, Lamellidens marginalis
⁎Corresponding author. mamzad.fbg@sau.ac.bd (Mohammad Amzad Hossain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The Chlorpyrifos 20 EC (emulsifying concentration) is one of the extensively used agro-pesticide in Bangladesh, most of which are residual in nearby natural water reservoirs. The freshwater mussel, Lamellidens marginalis have been exposed to Chlorpyrifos 20 EC at varying concentrations of Tc 0 mgL-1, T1 2.53 mgL-1, T2 5.07 mgL-1 and T3 10.15 mgL-1 for 35 days to investigate its toxic effects on growth biometrics, hemocyte counts and histopathology of gill, muscle, and ovary. The 96-hour Chlorpyrifos 20 EC LC50 for L. marginalis has been computed as 25.37 mgL-1 from PROBIT analysis. Major water regulating parameters were recorded and analyzed for each treatment group. The specific growth rate (%) was reported statistically highest in TC (0.104 ± 0.412) and lowest in T3 (-0.52 ± 0.38). Similarly, the condition index (CI) and Fulton’s condition factor were decent at TC and downed at T3 (P < 0.05). A high mortality rate occurred in T3 and the lowest mortality in TC. The value of total hemocytes count were also reported to be highest for TC and gradually decreased as the dosage increased (P < 0.05). The histopathology of gill, muscle, and ovary also revealed moderate to severe pathological signs in treatment groups in comparison to control. Administration of Chlorpyrifos 20 EC results in detrimental growth, cytological and hematological alternation in L. marginalis.
Keywords
Chlorpyrifos 20 EC
Lamellidens marginalis
Hemocytes
Histopathology
Growth biometrics
1 Introduction
The pearl mussel, Lamellidens marginalis, is the most common and abundant freshwater bivalve species in the tropical climate of southeast Asia (Mishra et al., 2008; Siddique et al., 2020). They serve as excellent natural water filters and therefore, are used as biological agents in water quality monitoring (Hussain et al., 2022; Sicuro, 2015). Being a filter feeder, freshwater mussels are highly influenced by anthropogenic factors such as the internment of rivers, flow modification, pollution, and climatic change. (Bolotov et al., 2019). The freshwater mussel L. marginalis is the primary bivalve species used for commercial pearl production across the Indian subcontinent (Sicuro, 2015). They also contributed to ecological importance by providing a source of food, and shelter for a wide range of terrestrial and aquatic life (Carboni et al., 2019).
Organophosphorus has been among the most widely applied group of insect repellents in the global pest control industries (Cacciatore et al., 2013) and it has successfully replaced the old organochlorine derivatives in Agri farm practices (Zahran et al., 2018). Chlorpyrifos is labeled as a priority element following the directive of the European Commission aquatic ecosystems protection policy (Rouillard et al., 2018). This group represents low environmental persistence with a moderately little half-life in aquatic habitats ranging from 29 to 74 days (Bondarenko et al., 2004). Histopathology, hematology, and growth evaluation of mollusks could be evidence of their overall health status (Zahran et al., 2018). The changes in the abundance of mussels in a particular water body would be a truthful indicator of its physicochemical properties (Traiger et al., 2022). The histological assays are more widely used methods in evaluating the impact of toxicants at the cellular or tissue level (Ait-Ayad et al., 2011; Hussain et al, 2022). The present study is a baseline effort to expand information concerning the risks of existing pesticides usages to native freshwater mussels in Bangladesh. Very little data exist pertaining to the toxicity of pesticides in invertebrates and other aquatic biotas in Bangladesh.
2 Material and methods
2.1 Collection of animals and rearing
Live samples of freshwater mussel, L. marginalis were collected from the nearby river water areas and transported in a large, aerated plastic drum to the wet laboratory facilities of Sylhet Agricultural University, Bangladesh. The collected mussels were initially checked for health status and acclimatized in separate aquariums with proper temperature and aeration. There was no provision for feeding during acclimatization. After acclimatizing, 20 healthy and uniformed-sized mussels were stocked into each of the 50-liter treatment aquariums to rear for up to 35 days. A control and three different treatment groups were assigned following the 96-hour LC50 value of chlorpyrifos 20 EC. The mussels were fed with dried and formulated green algae three times a day at 1.5–1.25 % of their body weight and the amount of food was sequentially decreased after two weeks to prevent over-primary productivity in the aquarium system. Uneaten feed and the feces materials were siphoned out once per two days and partially water was added to prevent loss via evaporation.
2.2 LC50 test and planning the dose of exposure
A 96-hour LC50 test has been done with 20 animals in each of 0, 3.0, 6.0, 9.0, 12, 18, 24, 36, 48, 72, and 96 mgL-1 of chlorpyrifos 20 EC treatment units. Each aquarium contained 25 L of water, a well aeration system and 20 animals were employed in each unit following the standard OECD guidelines (OECD, 2009). The resulting mortality rates were put in the SPSS for the PROBIT regression model to analyze LC50 (Finney, 1971) (Fig. 1). The different doses of pesticide used in different treatments unit were assigned as TC 0 mgL-1 (0 % of LC50), T1 2.53 mgL-1 (10 % of LC50), T2 5.07 mgL-1 (20 % of LC50) and T3 10.15 mgL-1 (40 % of LC50) following the LC50 values.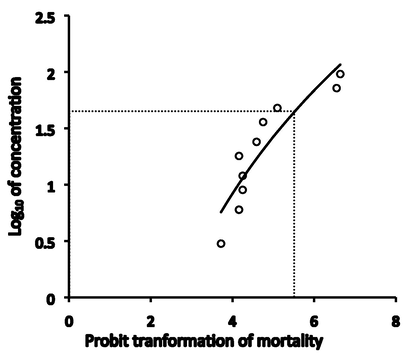
A 96-h LC50 logarithmic curve for L. marginalis exposed to Chlorpyrifos 20EC.
2.3 Monitoring hydrological parameters and sampling
Various hydrological parameters such as temperature, dissolved oxygen, salinity, TDS, and pH were measured each 7 days interval by using YSI Professional Plus Handheld Multiparameter Meter (Model 6050000), and ammonia levels were detected by employing a commercially manufactured ammonia test kit (Model HI 3824, Japan). At the same interval, three animals from each replication unit were sampled randomly for all the treatment groups, and length, weight, and body depth data were acquired immediately. At the end of the trial, all animals were sacrificed to measure their soft tissue wet weight, and a section from muscle tissue, gonad, and gill was preserved in neutral buffered formalin for histological analysis.
2.4 Tools for growth biometrics
Different growth biometrics were calculated by using the below formulae.
Percentage of weight gain = (Wf − WI /WI) × 100, Where Wf-Mean final weight and WI-Mean initial weight.
Specific growth rate SGR (%) = (lnW2 − lnW1 /T2 − T1) × 100, Where W1 = the initial weight (g) at a time, W2 = the final weight (g) at a time, T2-T1 = Duration in days.
Percentages of length gain = (Lf − LI /LI) × 100; Where, Lf-Mean final length and LI-Mean initial length.
The Fulton’s condition factors were computed from the length-weight equation of Htun-Han, (1978). The condition index (CI) has been calculated by using following the methodology adopted from Uddin et al., (2010). The shells were sundried for 2.5 h before taking their weight in the ratio between wet tissue wet and dry weight of the shell.
2.5 Collection of hemolymphs and counting total hemocytes
At the end of 25 days of rearing, the hemolymph samples were collected from fleshy abductor muscle by using a 0.8 mm needle and 1 ml syringe. About 300–500 µl of samples were collected from each animal and placed in the centrifuge tube. The samples were then mixed as a 1:2 ratio of Alsevier solution (MP Biomedicals, Ohio) prepared with the same volume of 3 % formaldehyde solution following the description of Juhel et al., (2015). The concentration of the cell was counted by using a conventional hemocytometer process.
2.6 Histology of gill, muscle, and gonad tissues
Previously NBF preserved samples were washed under tap water and underwent repeated alcoholic dehydration and xylene cleaning process before infiltrating them on paraffin. Paraffin-embedded tissue was sliced at 3–5 µm and stained with Hematoxylin-Eosin stain to visualize the cell under the light microscope. Photographs are taken by using Zeiss software version 2.3 on Windows 10 installation connected to Carl ZEISS Primostar 3 Microscope.
2.7 Statistical analysis and visual constructions
A comparison of the mean between different parameters was conducted by using IBM SPSS v27 and Duncan's Multiple Range test (DMRT) at p < 0.05. Graph and visualization were performed in office 365 tools by using data from SPSS analysis.
3 Results
3.1 Toxicity tests
The 96-hour LC50 of Chlorpyrifos 20 EC for L. marginalis in the current trial was computed as 25.37 mgL-1 from the PROBIT regression model (P < 0.05) (Fig. 1). A reference table has been constructed based on previous trials in 96-hour LC50 of different formulations of organophosphate pesticides in bivalves (Table 1). A moderately high estimate of LC50 had already been reported for Dimethoate, Malathion, Atrazine, and Glyphosate in several bivalves.
Species
Value of 96-hour LC50 (mgL-1)
Formulation of Pesticides
References
Mytilus galloprovincialis
7700
Neonicotinoid Calypso 480 SC (CAL)
(Stara et al., 2020)
Unio tigridis
324
Chlorpyrifos 48 EC
(Al-Fanharawi et al., 2019)
L. marginalis
36.36
Dimethoate
(Kumar et al., 2012)
Utterbackia imbecillis
7.9
Carbaryl
(Conners and Black, 2004)
19.4
Atrazine
18.3
Glyphosate
241.3
Diazinon
215
Malathion
(Keller and Ruessler, 1997)
Elliptio icterina
32
Villosa lienosa
111
Villosa villosa
180
3.2 Hydrological parameters
The environmental data obtained in Table 2 demonstrates that the temperature value was stable for all treatments and fluctuation took place as the duration of rearing increased. It occurred due to seasonal changes in Bangladesh and entering the winter season in the last 15 days of the trial. The salinity contents remained relatively the same for different time slots and treatment units and no statistically significant changes were reported (P < 0.05). The ammonia and pH levels were not significantly varied throughout the whole experiment period. However, a significant increase in dissolved O2 values was reported in all the experimental units from day 15 to the end of the trial (P < 0.05) (Table 2). The raw with different superscripts indicate significant differences at P < 0.05; values are means ± SD.
Parameter
Treatments
DAY-0
DAY-15
DAY-35
Temperature (°C)
TC
25.22 ± 0.60b
24.2 ± 0.6b
20.5 ± 1.29a
T1
24.87 ± 0.51b
23.25 ± 0.369b
20.12 ± 1.31a
T2
25.02 ± 0.61b
23.62 ± 0.59b
20.87 ± 0.62a
T3
25.02 ± 0.67b
23.95 ± 0.64b
21.12 ± 0.25a
pH
TC
7.29 ± 0.45a
7.87 ± 0.10a
7.65 ± 0.23a
T1
7.47 ± 0.12a
7.60 ± 0.12a
7.55 ± 0.05a
T2
7.64 ± 0.10a
7.66 ± 0.08a
7.61 ± 0.06a
T3
7.49 ± 0.17a
7.71 ± 0.08a
7.6 ± 0.18a
Dissolved O2
TC
6.59 ± 0.10a
7.27 ± 0.15b
6.97 ± 0.33b
T1
6.48 ± 0.25a
7.06 ± 0.48b
7.22 ± 0.12b
T2
6.62 ± 0.32a
6.91 ± 0.44b
7.32 ± 0.35b
T3
6.83 ± 0.24a
7.21 ± 0.23b
7.15 ± 0.12b
NH3 (mg/L)
TC
0.34 ± 0.21a
0.71 ± 0.35a
0.30 ± 0.22a
T1
0.40 ± 0.149a
0.48 ± 0.15a
0.35 ± 0.06a
T2
0.57 ± 0.17a
0.54 ± 0.14a
0.37 ± 0.09a
T3
0.46 ± 0.17a
0.54 ± 0.08a
0.40 ± 0.15a
Salinity (mg/L)
TC
0.08 ± 0.017a
0.08 ± 0.01a
0.08 ± 0.01a
T1
0.08 ± 0.00a
0.08 ± 0.00a
0.07 ± 0.01a
T2
0.08 ± 0.00a
0.08 ± 0.01a
0.07 ± 0.01a
T3
0.08 ± 0.01a
0.09 ± 0.01a
0.08 ± 0.01a
Total dissolved solids (mg/L)
TC
109.12 ± 22.06a
101.87 ± 16.12a
107 ± 8.86a
T1
100.12 ± 15.45a
93.00 ± 6.58a
93.0 ± 4.69a
T2
109.75 ± 18.30a
103.12 ± 1.65a
101.25 ± 2.5a
T3
109.00 ± 17.59a
102.25 ± 6.65a
97.50 ± 4.12a
3.3 Growth biometrics, survival rate, and condition indices
The obtained data in Table 3 confirmed that the value of final total body weight differed statistically between the different treatment groups (P < 0.05). The highest value of the final total body weight was observed in TC as 49.24 ± 6.7 g and the lowest was 36.67 ± 4.37 g in T3. The least value of weight gain was reported in the T3 (-7.37 ± 5.0 g), while maximized at Tc (49.24 ± 6.7 g) (P < 0.05). Specific growth rate (SGR %) among the treatment varied statistically, and the lowest SGR was −0.52 ± 0.38 at T3, while the highest was 0.104 ± 0.412 in the control group. Similarly, the soft tissue wet weight was highest in Tc (12.20 ± 2.12 g) and lowest in T3 (8.82 ± 1.40 g). The value of Fulton’s condition factor and condition index (CI) follows a similar course of declination after being in peak at the control unit. The lowest values for both indices were allocated for the T3 treatment group, while the highest was for the control Tc group (Fig. 3). There were no significant changes over other parameters such as initial shell length, initial total body weight initial, maximum shell depth, final shell length final, dry shell weight, and length gain. The mortality rate was statistically highest in T3 and lowest in Tc (P < 0.05) (Fig. 2). The column with different superscripts indicates significant differences at P < 0.05; values are means ± SD.
Parameters
TC
T1
T2
T3
Shell length initial (cm)
7.85 ± 0.56a
7.93 ± 0.52a
7.53 ± 0.49a
7.93 ± 0.42a
Total body weight initial (g)
47.79 ± 9.44a
49.05 ± 10.04a
45.32 ± 14.97a
44.04 ± 5.61a
Maximum shell depth (cm)
3.87 ± 0.20a
4.05 ± 0.48a
3.85 ± 0.48a
3.98 ± 0.18a
Shell length Final (cm)
7.86 ± 0.70a
7.95 ± 0.37a
7.57 ± 0.61a
7.94 ± 0.41a
Total body weight Final (g)
49.24 ± 6.7b
46.08 ± 10.37b
38.82 ± 9.67a
36.67 ± 4.37a
Maximum shell depth Final
3.83 ± 0.38a
3.91 ± 0.22a
3.72 ± 0.27a
3.78 ± 0.23a
Soft tissue wet weight (g)
12.20 ± 2.12b
11.53 ± 1.83b
8.97 ± 1.47a
8.82 ± 1.40a
Dry shell weight (g)
18.09 ± 5.39b
18.62 ± 5.89b
13.95 ± 2.44a
16.75 ± 3.104ab
Length gain (cm)
0.008 ± 1.06a
0.025 ± 0.36a
0.041 ± 0.60a
0.0083 ± 0.45a
Weight gain (g)
1.44 ± 8.21b
−2.97 ± 7.30ab
−6.5 ± 13.30a
−7.37 ± 5.0a
Length gain (%)
0.83 ± 106.46a
2.5 ± 36.46a
4.16 ± 60.06a
0.83 ± 45.41a
Weight gain (%)
144.91 ± 821.72b
−297.33 ± 730.7ab
−650 ± 1330.07a
−737.25 ± 500.47a
Specific growth rate SGR% (weight)
0.104 ± 0.412b
−0.19 ± 0.48ab
−0.40 ± 0.83a
−0.52 ± 0.38a
Average daily length gain (cm)
0.0002 ± 0.030a
0.0007 ± 0.01a
0.0012 ± 0.01a
0.00023 ± 0.01a
Average daily weight gain (g)
0.041 ± 0.23b
−0.085 ± 0.20ab
−0.186 ± 0.38a
−0.21 ± 0.14a
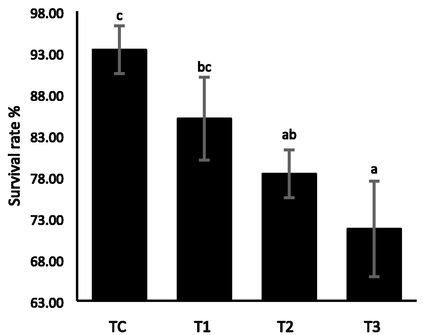
Survival rate and mortality rate of freshwater mussel L. marginalis exposed to Chlorpyrifos 20 EC for 35 days.
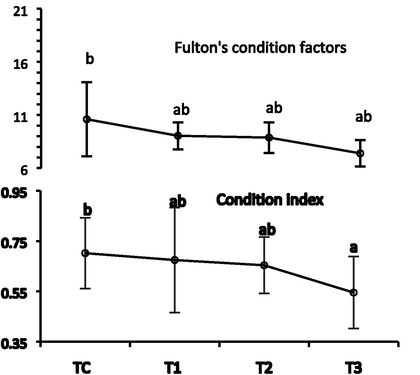
Fulton’s condition factors and condition index of freshwater mussel L. marginalis exposed to Chlorpyrifos 20 EC for 35 days.
3.4 Total hemocytes count
The highest hemocytes count has been noted for the control group Tc (3.47 × 106 cells/ml) while the lowest was for the T3 (2.3 × 106 cells/ml) group (P < 0.05) (Fig. 4). The hemocytes counts between T1 (3.2 × 106 cells/ml) and T2 (2.7 × 106 cells/ml) also confirmed moderate statistical variation (P < 0.05) (Fig. 4).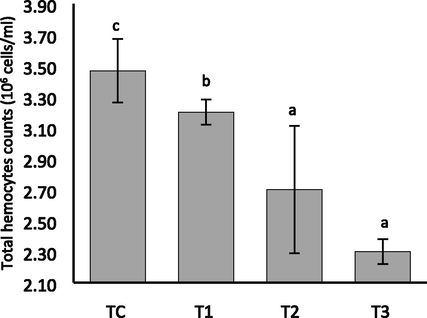
Total hemocytes counts of freshwater mussel L. marginalis exposed to Chlorpyrifos 20 EC for 35 days.
3.5 Histopathology of gill, muscle, and ovary
The bivalves exposed to higher doses of Chlorpyrifos 20 EC endured more damage to their gill architecture. Gill lamellae in bivalves kept as controls are made up of a large number of closely spaced, thin, vertical gill filaments that have a porous structure punctured by minute openings and bound filaments (Fig. 5A). Degeneration of epithelial cells, swelling, and the vacuolated and necrotic epithelium were seen in all treatment groups (Fig. 5B-D). The secondary gill lamellae were fused together, and the gill filament bases widened. Following the exposure of Chlorpyrifos 20 EC, increased the intercellular junction, necrosis occurred in the cell, and tissue rupture and hyperplasia were observed (Fig. 5B-D). The epithelium of the gill filaments and all forms of the cilia were disrupted, and the epithelium showed considerable oedema formation and necrotic bodies. Epithelial separation, lamellar distortion, epithelia loss, and necrosis were increased at the gill with the intensified concentration of chlorpyrifos 20 EC. The histopathology of pesticide-exposed bivalves' gills indicated clear signs of damage not seen in the control group. The histo-micrograph of adductor muscles of L. marginalis from different treatment groups are shown in Fig. 6. The control group showed well-organized muscular fibers that were connected perfectly (Fig. 6A). In the case of T1, T2, and T3 treatment groups, degeneration of muscle fiber (DMF), vacuoles (V), degenerative waving pattern, and necrosis were common pathologies was seen after pesticide exposure (Fig. 6B-D). The T3 treatment group showed a higher level of deformities in all cases. Fig. 7 A displays the normal structure of the mussel's ovary at vitellogenic stage. After pesticide administration, the normal structure of gonad cells was disorganized, follicle degradation occurred, and yolk globules started to disrupt at different intensities as observed in Fig. 7B-D.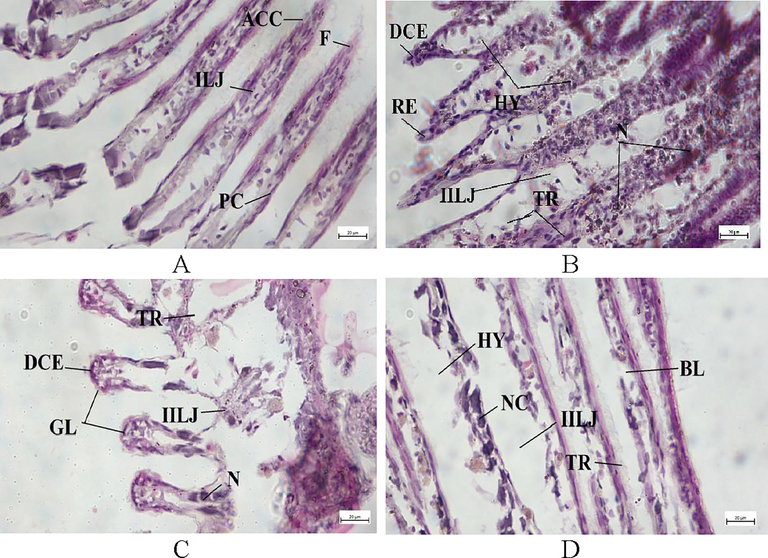
Gill transverse section photomicrograph of L. marginalis. A. TC, B.T1, C. T2 D. T3. (GL-gill lamellae, ACC-abfrontal ciliated cell, F-frontal, PC-Pillar cells, ILJ-interlamellar junction, DCE-damaged cilia epithelium, TR-tissue rupture, HY-hyperplasia, BL-breakdown of lamellae, NC-necrosis, NL-necrotic lamellae, IILJ-Increased interlamellar junction).
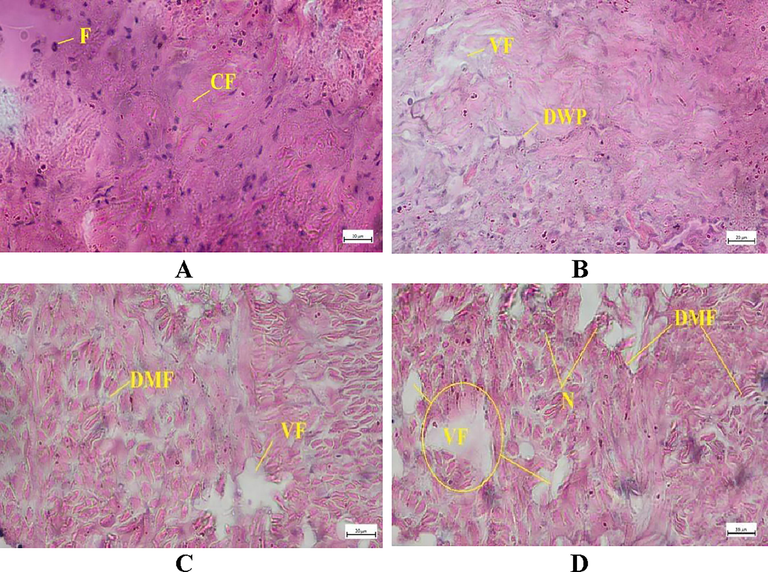
Muscle transverse section photomicrograph of L. marginalis. A. TC, B.T1, C. T2, D. T3. (DMF-degeneration of muscle fiber; VF-vacuole formation; DWP-disruption of waving pattern, N-necrosis; F-healthy fiber, CF-collagen fiber).
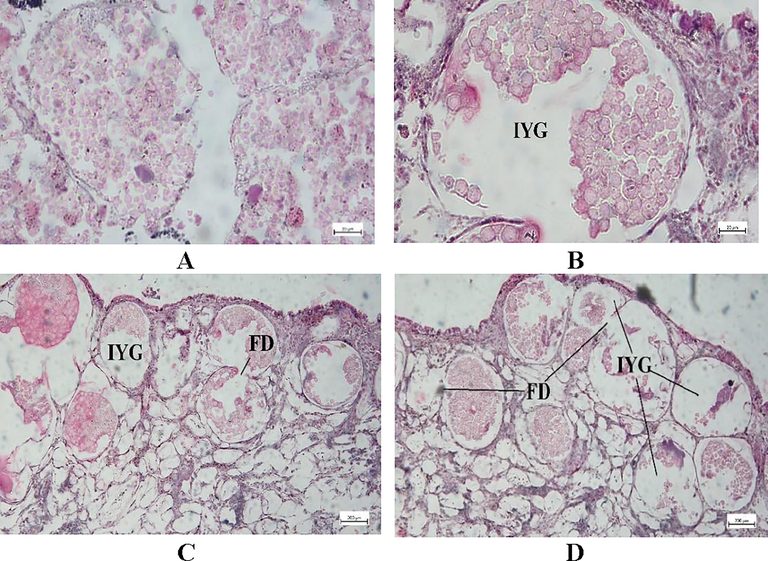
Ovarian transverse section photomicrograph of L. marginalis. A. TC, B.T1, C. T2, D. T3. (FD-follicle degradation, GD-gonadal degeneration, IYD-yolk globule disruption).
4 Discussions
The growth, development, and propagation of aquatic animals are solely associated with water quality parameters of surroundings (Ahmed et al., 2020). The thermal constitution varied between 20℃ to 36℃ and seems to be optimal for supporting life in tropical aquatic systems (Kua et al., 2020). A value of DO > 5 ppm is vital to sustaining aquatic animals’ existence and development (Bhatnagar and Singh, 2010). Water quality features acquired in current research remained quite optimal for the growth and reproduction of aquatic animals following the hydrology assessment criteria of Bhatnagar & Singh, (2010) and Chapman et al., (2016).
The LC50 data from previous studies exemplifies that aquatic mollusks are moderate to highly tolerant to acute toxicity of different organophosphate formulations (Becker et al., 2020; Conners and Black, 2004). The LC50 values for different mollusks ranged from least 7.9 mgL-1 Carbaryl (Kumar et al., 2012) to 7700 mgL-1 Neonicotinoid Calypso 480 SC (CAL) (Stara et al., 2020). The animals in the control group of the current trial demonstrate 100 % survival rates, which fall within the regulation and description code of APHA, (1999) for testing rapid and acute toxicity trials in aquatic animals.
The substantial biochemical and cellular changes in animals are reported to be initiated by organophosphate toxicants (Stalin et al., 2017). Intensification of toxicant in living water cause behavioral, physiological, and cellular changes in the animal which eventually lead to death (Kumar et al., 2012). Cypermethrin was reported to disrupt normal valve activity and survival rate of marine mussel Mytilus galloprovincialis (Ait-Ayad et al., 2011). Similar fixation has been recounted in current research and mortality rates were higher as the concentration of Chlorpyrifos 20 EC increased. The intrusion of the municipal effluent causes reduced growth in Elliptio complanate (Gagné et al., 2007). The accumulation of Pb was addressed to alter soft tissue weight, filtration behavior, and mortality of Dreissena polymorpha (Rahnama et al., 2010). Chlorpyrifos had been registered to prevent growth, reproduction, and development in Unio tigridis and Viviparous benglensis (Al-Fanharawi et al., 2019).
The Fulton’s condition factor and condition index are widely used morphometric tools in assessing the animal’s fitness, nutritive status, and associated ecological condition (Siddique et al., 2020). The alternation in CIs and Fulton’s condition was described to be correlated with food availability, parasitic loads, and water-born stressors in several invertebrates (Marwaha et al., 2019). Intensifying the pesticide toxicant in current research also revealed reduced values for both condition factors in the treatment group in comparison with the control group. This would happen due to decreased food intake in treatment groups and earning poor soft tissue weight as well. Hemocytes are treated as a vital regulatory component of the molluscan immune system (Uddin et al., 2010) and their concentrations are related to being impacted by different forms of aquatic toxicants (Lu et al., 2021). The reduced concentration of hemocytes was noted by 21 days of exposure to neonicotinoid Calypso 480 SC in Mytilus galloprovincialis (Stara et al., 2020) and with polystyrene microplastics treatment in oyster Crassostrea gigas (Sussarellu et al., 2016). The current trial also resulted in reduced hemocytes counts for highly concentrated treatment groups in comparison to the control.
The gill is a vital organ in bivalve mollusk, which is engaged in the organism's respiration and food-sieving activities (Andreyeva et al., 2021). Analysis of histological malformations is a widely used method to assess the effects of toxicants and the environment (Hussain et al., 2022). Increased concentration of toxicants like pesticides, metals, etc. inhibits the metabolic processes of cells inside the tissues and leads to cell death, hyperplasia, or cellular inequality (Joshy et al., 2022). Swollen gill filament, lamellar fusing, necrosis, and structural modification were noted in L. marginalis exposed to 5 ppm 30 days chlorpyrifos toxicant (Stalin et al., 2017). Similar histological alterations in gill were documented by Katalay et al., (2016). The epithelium oedema formation and necrotic gill were observed in mussels associated with agro-pesticides (Rane et al., 2019). Histological alterations in muscle tissue due to bisphenol-A exposure were reported in soft tissues of Corbicula fluminea (Benjamin et al., 2019). Increased vacuole formation and necrotic cell death are prominent pathologies of muscle tissue in response to toxicants (Alonso et al., 2019). The current research also acts as a supportive trail to the previous finding and extensive histopathology was photographed for the animals in a higher concentration of Chlorpyrifos 20 EC treatment.
Current results concluded that exposure to sublethal concentrations of the Chlorpyrifos 20 EC leads to the significant manifestation of growth, fitness, and survival of bivalve mollusks. The study also noticed major alterations in cellular architecture. Chlorpyrifos 20 EC is also documented to impact the reproduction and growth of mussels as indicated by condition indices and histopathology of gonads.
Funding
This research has been funded by UGC (University Grant Commission), Bangladesh through Sylhet Agricultural University Research System (SAURES), Bangladesh (budget reference code 3632104). The research student stipend was supported by the National Science and Technology Fellowship scheme (NST) from the Ministry of Science and Technology, Bangladesh.
CRediT authorship contribution statement
Mohammad Amzad Hossain: Methodology, Software, Visualization, Validation, Writing – review & editing, Funding acquisition. Tumpa Rani Sarker: Methodology, Software, Visualization, Validation, Writing – review & editing, Funding acquisition. Lipi Sutradhar: Writing – original draft. Monayem Hussain: Writing – review & editing, Supervision. Mohammed Mahbub Iqbal: Conceptualization, Methodology, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The influence of the endogenous and exogenous factors on hematological parameters in different fish species: a review. Aquac. Int.. 2020;28(3):869-899.
- [CrossRef] [Google Scholar]
- Effects of cypermethrin (pyrethroid insecticide) on the valve activity behavior, byssal thread formation, and survival in air of the marine mussel Mytilus galloprovincialis. Arch. Environ. Contam. Toxicol.. 2011;60(3):462-470.
- [CrossRef] [Google Scholar]
- Multi-biomarker responses after exposure to organophosphates chlorpyrifos in the freshwater mussels Unio tigridis and snails Viviparous benglensis. Hum. Ecol. Risk Assess. An Int. J.. 2019;25(5):1137-1156.
- [CrossRef] [Google Scholar]
- Gonadal histopathological disorders in Mytilus galloprovincialis male exposed to tars used in mussel farms. Front. Mar. Sci.. 2019;6:1-20.
- [CrossRef] [Google Scholar]
- Acute hypoxic exposure: effect on hemocyte functional parameters and antioxidant potential in gills of the pacific oyster, Crassostrea gigas. Mar. Environ. Res.. 2021;169:105389
- [CrossRef] [Google Scholar]
- American Public Health Association, 1999. Standard methods for examination of water and wastewater, twentieth ed. American Public Health Association, American Water Works Association, Water Environment Federation, Washington DC, USA.
- Pesticide pollution in freshwater paves the way for schistosomiasis transmission. Sci. Rep.. 2020;10(1):3650.
- [CrossRef] [Google Scholar]
- Histopathological effects of bisphenol a on soft tissues of Corbicula fluminea Mull. Toxicol. Environ. Health Sci.. 2019;11(1):36-44.
- [CrossRef] [Google Scholar]
- Culture fisheries in village ponds: a multi-location study in Haryana. India. Agric. Biol. J. North Am.. 2010;1(5):961-968.
- [CrossRef] [Google Scholar]
- Freshwater mussels house a diverse mussel-associated leech assemblage. Sci. Rep.. 2019;9(1):16449.
- [CrossRef] [Google Scholar]
- Persistence of selected organophosphate and carbamate insecticides in waters from a coastal watershed. Environ. Toxicol. Chem.. 2004;23(11):2649.
- [CrossRef] [Google Scholar]
- Cholinesterase and carboxylesterase inhibition in Planorbarius corneus exposed to binary mixtures of azinphos-methyl and chlorpyrifos. Aquat. Toxicol.. 2013;128–129:124-134.
- [CrossRef] [Google Scholar]
- Mussel consumption as a “food first” approach to improve Omega-3 status. Nutrients. 2019;11(6):1381.
- [CrossRef] [Google Scholar]
- Developments in water quality monitoring and management in large river catchments using the Danube River as an example. Environ. Sci. Policy. 2016;64:141-154.
- [CrossRef] [Google Scholar]
- Evaluation of lethality and genotoxicity in the freshwater Mussel Utterbackia imbecillis (Bivalvia: Unionidae) exposed singly and in combination to chemicals used in lawn care. Arch. Environ. Contam. Toxicol.. 2004;46(3):362-371.
- [CrossRef] [Google Scholar]
- Finney, D.J., 1971. Probit Analysis, third ed. Cambridge University Press, 32 E. 57th St., New York, Ny 10022. https://doi.org/10.1002/jps.2600600940.
- Changes in metallothionein levels in freshwater mussels exposed to urban wastewaters: effects from exposure to heavy metals? Biomark Insights. 2007;2:107-116.
- [CrossRef] [Google Scholar]
- The reproductive biology of the dab Limanda limanda (L.) in the North Sea: Seasonal changes in the ovary. J. Fish Biol.. 1978;13(3):351-359.
- [CrossRef] [Google Scholar]
- Freshwater mussel (Lamelliedens marginalis) to reduce the lead (Pb) bioaccumulation in aquaculture of stinging catfish, Heteropneustes fossilis. J. Appl. Aquac.. 2022;1–17
- [CrossRef] [Google Scholar]
- Histopathological evaluation of bivalves from the southwest coast of India as an indicator of environmental quality. Aquat. Toxicol.. 2022;243:106076
- [CrossRef] [Google Scholar]
- Effect of the microcystin-producing cyanobacterium, microcystis aeruginosa, on immune functions of the zebra mussel Dreissena polymorpha. J. Shellfish Res.. 2015;34(2):433-442.
- [CrossRef] [Google Scholar]
- Histological Effects of pollution on gill and hepatopancreas tissues of black mussels (M. Galloprovincialis L.) from izmir Bay of Turkey. Fresenius Environ. Bull.. 2016;25(5):1461-1467.
- [Google Scholar]
- The toxicity of malathion to unionid mussels: relationship to expected environmental concentrations. Environ. Toxicol. Chem.. 1997;16(5):1028-1033.
- [CrossRef] [Google Scholar]
- Water warming increases aggression in a tropical fish. Sci. Rep.. 2020;10(1):20107.
- [CrossRef] [Google Scholar]
- Acute toxicity and behavioural responses of a freshwater mussel Lamellidens marginalis (Lamarck) to dimethoate exposure. Recent Res. Sci. Technol.. 2012;4(11):39-45.
- [Google Scholar]
- Gender differences in hemocyte immune parameters of hong kong oyster Crassostrea hongkongensis during immune stress. Front. Immunol.. 2021;12:1-12.
- [CrossRef] [Google Scholar]
- Host (Salmo trutta) age influences resistance to infestation by freshwater pearl mussel (Margaritifera margaritifera) glochidia. Parasitol. Res.. 2019;118(3):1519-1532.
- [CrossRef] [Google Scholar]
- Differential growth of the freshwater mussel, Lamellidens marginalis in relation to certain drugs. Environ. Toxicol.. 2008;23(3):379-386.
- [CrossRef] [Google Scholar]
- OECD, 2009. Test No. 403: Acute inhalation toxicity, OECD guidelines for the testing of chemicals, section 4. OECD. https://doi.org/10.1787/9789264070608-en.
- The effects of lead bioaccumulation on filtration rate of zebra mussel (Dreissena polymorpha) from Anzali wetland – Caspian Sea. Toxicol. Environ. Chem.. 2010;92(1):107-114.
- [CrossRef] [Google Scholar]
- Histopathological alterations in gills of freshwater bivalve, Lamellidens marginalis (Lamarck) after acute exposure to thiamethoxam and triazophos. Int. J. Life Sci. 2019:23-28.
- [Google Scholar]
- Protecting and restoring biodiversity across the freshwater, coastal and marine realms: is the existing EU policy framework fit for purpose? Environ. Policy Gov.. 2018;28(2):114-128.
- [CrossRef] [Google Scholar]
- Freshwater bivalves rearing: a brief overview. Int. Aquat. Res.. 2015;7(2):93-100.
- [CrossRef] [Google Scholar]
- Annual gametogenic cycle of the freshwater pearl mussel, Lamellidens marginalis (Lamarck, 1819) collected from a perennial lentic habitat of Bangladesh. Molluscan Res.. 2020;40(2):36-43.
- [CrossRef] [Google Scholar]
- Effect of chlorpyrifos on biochemical changes in freshwater mussel Lamellidens marginalis. Int. J. App. Res.. 2017;3(8):157-159.
- [Google Scholar]
- Assessing the effects of neonicotinoid insecticide on the bivalve mollusc Mytilus galloprovincialis. Sci. Total Environ.. 2020;700:134914
- [CrossRef] [Google Scholar]
- Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci.. 2016;113(9):2430-2435.
- [CrossRef] [Google Scholar]
- Evidence of increased mussel abundance related to the Pacific marine heatwave and sea star wasting. Mar. Ecol.. 2022;43(4):e12715.
- [Google Scholar]
- Seasonal changes in Perkinsus olseni infection and gametogenesis in manila clam, Ruditapes philippinarum, from Seonjaedo Island in Incheon, off the West Coast of Korea. J. World Aquac. Soc.. 2010;41:93-101.
- [CrossRef] [Google Scholar]
- Acute exposure to chlorpyrifos induces reversible changes in health parameters of Nile tilapia (Oreochromis niloticus) Aquat. Toxicol.. 2018;197:47-59.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102482.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







