Translate this page into:
Toxic effect of polyethylene microplastic on testicles and ameliorative effect of luteolin in adult rats: Environmental challenge
⁎Corresponding authors. asmabinm@gmail.com (Asma Ashraf), shahidmahboob60@hotmail.com (Shahid Mahboob)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Polyethylene microplastic (PE-MP) is amongst the most manufactured plastics globally. However, emerging studies report its adverse effects on wildlife and humans. Luteolin (LUT) is a flavonoid compound known for its potent antioxidant properties. The present study was designed to estimate the toxic effect of PE-MP on testicles of adult male rats. The therapeutic potential of LUT was also determined in alleviating PE-MP instigated testicular dysfunctions. An experiment was carried on 48 adult male Sprague-Dawley rats, which were randomly classified into four groups: control, PE-MP (1.5 mg/kg), PE-MP + LUT (1.5 mg/kg + 50 mg/kg respectively), and LUT (50 mg/kg). The biochemical, spermatogenic, hormonal, and histopathological parameters were studied after 56 days of treatment. PE-MP exposure disrupted the biochemical profile by reducing catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione reductase (GSR) activities. In comparison, the concentration of reactive oxygen species (ROS) and malondialdehyde (MDA) levels were increased. PE-MP-intoxication also reduced the sperm motility, viability, the number of hypo-osmotic tails swelled spermatozoa, and epididymal sperm count; additionally, it increased the sperm morphological (head–tail and mid-piece) abnormalities. Furthermore, it lowered luteinizing hormone (LH), follicle-stimulating hormone (FSH), and plasma testosterone. PE-MP exposure led to histopathological damages in testicular tissues. However, LUT inverted all the illustrated impairments in testes. Our results demonstrate that PE-MP could cause testicular damage in male rats, effectively mitigated by LUT treatment.
Keywords
Polyethylene microplastic
Luteolin
Testicular damage
Oxidative stress
Antioxidant
1 Introduction
Many environmental pollutants can act as endocrine-disrupting chemicals (EDCs). Particular attention is being paid to contaminants of emerging concern (CECs), such as plasticizers, plastic debris, and plastic additives that are directly or indirectly discharged into the environment (EPA, 2021). As it is evident, plastic pollution is prevalent throughout the terrestrial, marine, and freshwater ecosystems to such an extent that we can say to live in a plastics world (Borrelle et al., 2020). According to global statistics, plastic production stretched to 368 million metric tons (Mt) in 2019, which is estimated to be doubled in the next 20 years (Plastics Europe, 2020). Once plastics enter the environment, they constantly break into frequently smaller particles called microplastics (MPs) (<5mm). Many scientists use MPs as a fourth core geological indicator of the Anthropocene era in parallel with ozone depletion, global climate change, and ocean acidification due to its abundant production, use, low degradation, and disposal (Galloway and Lewis, 2016).
Pathways of MPs exposure include ingestion, inhalation, and dermal contact due to their presence in food packaging (Sobhani et al. 2020), food (Conti et al., 2020), and even in the air, we breathe (Zhang et al. 2020). Humans could ingest mPs through the consumption of seafood and other food items such as table salt (7–681 items per kg), honey (40–60 items per kg), beer (12–109 items per L), and sugar (32 ± 7 items per kg) (Bouwmeester et al. 2015). MPs entering the human body through ingestion or inhalation can be transported through the circulatory system into different organs and affect human health via damaging cells or provoking immune and inflammatory reactions (Vethaak and Legler, 2021).
MPs particles mainly include polyethylene (PE), polystyrene (PE), polypropylene (PP), and polyester (Lu et al. 2018). Polyethylene (PE) is the most commonly identified MPs polymer in aquatic and terrestrial environments (Mde S. Machado et al., 2018). Due to its low cost, multiple uses, durability, and unique physical properties, PE has profound applications in different sectors, such as packaging, agricultural mulches, and composite materials (Beg et al., 2016). Moreover, based on a survey directed by Cosmetics Europe in 2012, PE estimated that 93 % of the MPs are used in skin cleaning products (Duis and Coors, 2016). In zebrafish, PE exposure led to neurotoxicity, disrupted oogenesis process, and aryl hydrocarbon receptor (AHR) pathway (Mak et al. 2019). Furthermore, PE-MPs exposure to Diptera larvae induced oxidative stress (OS) with potentially adverse effects on cellular metabolism and redox status (Silva et al., 2021). Thus, considering the PE-derived MP as a white pollutant and their harmful impacts on living organisms, research on remedies against PE-instigated toxicities is required.
Luteolin (3′,4′,5,7-tetrahydroxyflavone, LUT) is a natural dietary flavonoid that is commonly present in many edible plants such as flowers of Sophora viciifolia (Tai et al. 2011), Paeonia moutan, and Rumex nervosus (Desta et al. 2015), with reported antioxidant (Casierra-Posada and Jarma-Orozco, 2016), anti-inflammatory (Wang et al. 2020), antiangiogenic (Raffa et al. 2017), antidiabetic (Sherbet, 2017), immunomodulatory (Rangarajan et al. 2016), cytoprotective (Kempuraj et al., 2021), and anti-apoptotic activities (Xu et al. 2019). Furthermore, LUT can cross the blood–brain barrier and act as a neuroprotective agent (Theoharides et al., 2016). Moreover, Kempuraj et al. (2021) stated that LUT suppresses systemic and neuroinflammatory responses in coronavirus disease 2019 (COVID-19).
Limited literature is available on the effects of MPs on the male reproduction of mice. Therefore, the consequences of MPs and their potential toxicity mechanisms in the testicles of mammalian species require detailed research. Consequently, we conducted a thorough investigation to explore the potentially toxic effects of PE-MP on testicles, and promising curative potentials of LUT to ameliorate testicular toxicity instigated by PE-MP were also determined in adult male Sprague-Dawley rats.
2 Materials and methods
2.1 Chemicals
PE-MP and LUT were purchased from Sigma-Aldrich, Germany. All other chemicals used in this study were of analytical grade.
2.2 Animals
Adult male Sprague-Dawley rats (180 ± 20 g) were kept in steel cages at standard temperature (22–25 °C), humidity (45 ± 5%), and 12 h light/dark cycle in the animal house of the University. Tap water and food were given ad libitum to experimental animals. The European Union of animal care and experimentation approved study protocols (CEE Council 86/609).
2.3 Experimental design
Rats (n = 48) were randomly classified into four groups (n = 12/group). They were exposed to the following doses: Control group; PE-MP treated group (1.5 mg/kg. b. wt. of PE-MP orally); PE-MP + LUT treated group (1.5 mg/kg. b.wt. of PE-MP and 50 mg/kg. b.wt. of LUT), and LUT treated group (50 mg/kg. b.wt. of LUT orally). After the 56 days of the experimental trial, rats were slaughtered by decapitation, and testes were excised and stored at −80 °C till further analysis.
2.4 Antioxidant/Oxidant assay
The activities of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione reductase (GSR) were estimated by adopting methods of various researchers (Chance and Maehly, 1955; Kakkar et al. 1984; Lawrence and Burk, 1976; Carlberg and Mannervik, 1975). The concentration of reactive oxygen species (ROS), as well as malondialdehyde (MDA) level, was estimated, according to the protocols of Hayashi et al. (2007) and Placer et al. (1966), respectively. BR Biochem UV-5300 spectrophotometer was used to take the readings.
2.5 Semen analysis
Caudal piece of epididymis was isolated to take the semen samples studied by following the method described for motility (Kenjale et al. 2008), viability, epididymal count (Yokoi et al., 2003), and morphological anomalies (Cao et al., 2017). The integrity of the sperm plasma membrane was estimated by the hypo-osmotic swelling (HOS) test (Correa and Zavos’s, 1994). Nikon, 187842 microscopes were used for observation.
2.6 Hormonal assay
The levels of luteinizing hormone (LH) (BioCheck Inc., USA Catalog No. BC-1031), follicle-stimulating hormone (FSH) (BioCheck Inc., USA Catalog No. BC-1029), and plasma testosterone (BioCheck Inc., USA Catalog No. BC-115) were measured by enzyme-linked immunosorbent assay (ELISA) kits, according to the manufacturer's instructions. ELISA reader Apollo LB 913 was used to take the readings.
2.7 Histopathology
For the evaluation of histopathology, firstly, testicles were fixed for about 48 h in a 10% formaldehyde solution, later dehydrated in rising grades of alcohol, and encased in paraffin wax. Later these paraffin-inserted blocks (5 μm) were cut and stained with hematoxylin-eosin (H & E) and examined microscopically (Nikon, 187842, Japan). ImageJ2x software was used to analyze the images of the specimen.
2.8 Statistical analysis
The results were shown as Mean ± Standard error (SE). Tukey’s multiple comparison test was applied to interpret the entire data after using a one‐way analysis of variance (ANOVA) using GraphPad Prism 5.0. P < 0.05 was considered statistically significant.
3 Results
3.1 Effect of PE-MP and LUT on oxidant/antioxidant parameters
PE-MP exposure substantially (p < 0.05) reduced the activities of CAT, SOD, GPx, or GSR and augmented the concentration of ROS and MDA levels in contrast to the control group. Nevertheless, LUT supplementation in the PE-MP + LUT group provoked substantial (p < 0.05) altitude in CAT, SOD, GPx, and GSR activities and reduced ROS and MDA levels compared to the PE-induced group. Moreover, LUT alone administration showed a non-significant change in the antioxidant/oxidant parameters compared to the control group (Fig. 1).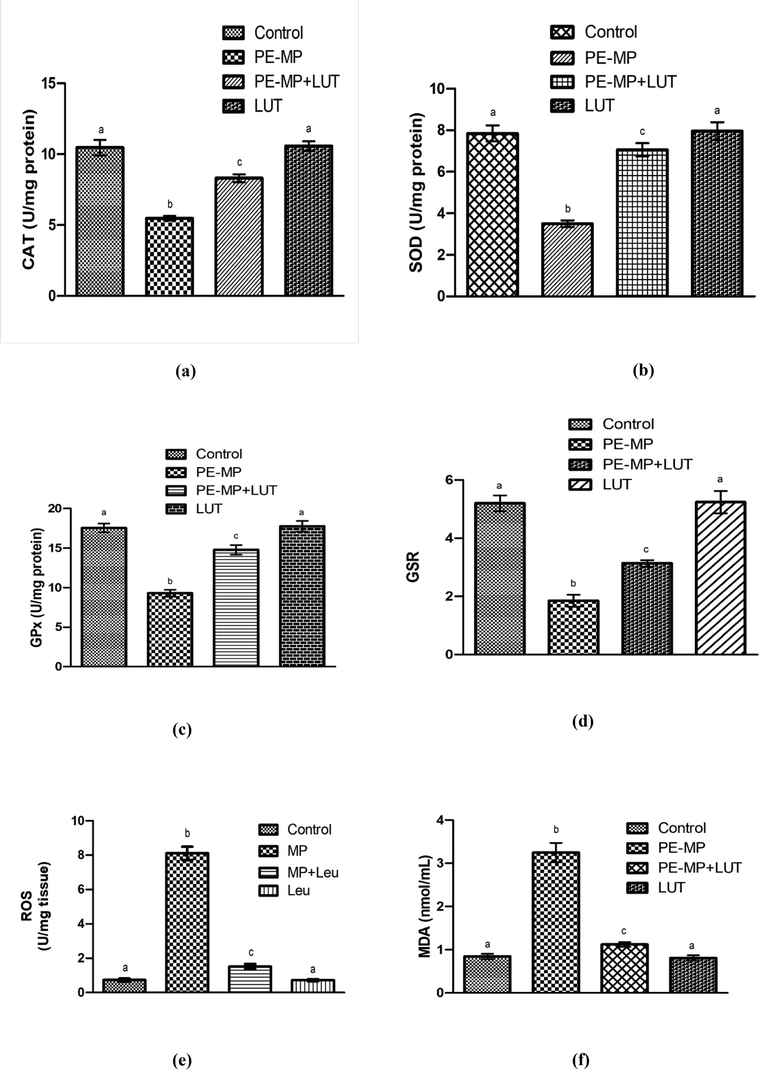
Antioxidant enzymes: (a) CAT, (b) SOD, (c) GPx (d) GSR activity, (e) concentration of ROS and (f) TBARS in the testicular tissues of control, PE-MP-treated, co-treated (PE-MP+LUT), and LUT groups. Based on Mean+SEM values, bar graphs are displayed. Various superscripts on the bar graphs show significant differences at p < 0.05.
3.2 Effect of PE-MP and LUT on sperm analysis
PE-MP exposure significantly (p < 0.05) lessened the sperm motility, viability, epididymal sperm count, and the number of HOS coil-tailed sperm, while morphological sperm abnormalities (head, mid-piece, and tail) were augmented in contrast to the control group. Nevertheless, LUT-treatment significantly (p < 0.05) inverted all these sperm indices to a normal state in the co-treated group compared to the PE-exposed group. Nonetheless, LUT alone administered group showed a regular semen profile and HOS coil-tailed sperm as in the control group (Table 1). Values having various superscripts are substantially variant from other groups.
Parameters
Groups
Control
PE-MP
PE-MP + LUT
LUT
Motility (%)
84.80 ± 1.82a
39.17 ± 1.38b
65.57 ± 1.79c
85.26 ± 2.50a
Dead Sperms (%)
12.83 ± 0.78a
81.37 ± 1.68b
29.05 ± 1.38c
12.75 ± 0.46a
Head Abnormality (U/mg protein)
4.97 ± 0.09a
16.01 ± 1.00b
7.44 ± 0.29c
4.82 ± 0.14a
Mid Sperm Abnormality (%)
0.84 ± 0.05a
8.49 ± 0.66b
2.36 ± 0.47c
0.75 ± 0.05a
Tail Abnormality (%)
1.60 ± 0.06a
12.10 ± 0.96b
3.11 ± 0.25c
1.53 ± 0.08a
Epididymal Sperm Count (million/mL)
26.19 ± 1.32a
14.27 ± 0.84b
21.34 ± 0.84c
27.34 ± 1.2 a
Hypo- osmotic swelled sperm count (HOS) (%)
81.21 ± 3.19a
28.57 ± 1.62b
58.39 ± 2.10c
82.62 ± 3.30a
3.3 Effect of PE-MP and LUT on hormonal assay
PE-MP exposure substantially (p < 0.05) decreased the level of LH, FSH, and plasma testosterone in PE-intoxicated rats compared to the control rats. Nonetheless, the LUT therapy significantly (p < 0.05) recovered the levels of all three above-stated hormones in the cotreated group, which are comparable to the PE-induced group. Additionally, LUT alone treatment showed normal levels of these hormonal markers as in the control group (Table 2). Values having different superscripts are significantly different from other groups.
Parameters
Groups
Control
PE-MP
PE-MP + LUT
LUT
LH (ng/ml)
2.54 ± 0.12a
0.83 ± 0.12b
2.11 ± 0.08a
2.61 ± 0.19a
FSH (ng/ml)
3.90 ± 0.13a
1.53 ± 0.08b
2.78 ± 0.09a
3.97 ± 0.21a
Plasma testosterone (ng/ml)
4.54 ± 0.09a
2.07 ± 0.16b
3.48 ± 0.15a
4.63 ± 0.13a
3.4 Effect of PE-MP and LUT on histopathology
The study results revealed that PE-MP exposure significantly (p < 0.05) lessened the diameter and the epithelial height of seminiferous tubules, besides the thickness of tunica propria. Moreover, it scaled up the luminal diameter of tubules. PE-treatment also reduced spermatogonia, primary and secondary spermatocytes, and spermatids compared to the control group. Nevertheless, LUT-supplementation in the cotreated group significantly (p < 0.05) recovered all these structural damages, and germ cell count in testicles compared with the PE exposed group. Besides, LUT alone treatment displayed a regular histopathological profile as matched with the control group (Table 3 and Fig. 2). Values having various superscripts are substantially variant from other groups.
Parameters units
Groups
Control
PE-MP
PE-MP + LUT
LUT
Interstitial Spaces (µm)
10.07 ± 0.92a
33.47 ± 1.34b
16.27 ± 1.56c
9.47 ± 0.69a
Tunica Propria (µm)
57.62 ± 2.22a
16.76 ± 0.60b
37.86 ± 3.42c
59.84 ± 2.64a
Seminiferous Tubules (µm)
323.29 ± 12.63a
122.09 ± 8.22b
275.47 ± 6.86c
369.02 ± 8.43a
Seminiferous Tubule Epithelial Height (µm)
85.70 ± 4.29a
33.79 ± 2.68b
68.78 ± 2.18c
89.56 ± 3.01a
Tubular Lumen (µm)
36.17 ± 1.58a
88.16 ± 3.76b
55.61 ± 2.53c
34.27 ± 1.87a
Spermatogonia (n)
61.66 ± 2.30a
22.10 ± 1.33b
46.14 ± 1.40c
61.90 ± 3.23a
Spermatids (n)
56.87 ± 1.02a
27.13 ± 0.83b
46. 01 ± 1.05c
58.11 ± 1.46a
Primary Spermatocytes (n)
46.45 ± 1.15a
19.86 ± 0.72b
33.5 ± 1.71c
46.55 ± 1.66a
Secondary Spermatocytes (n)
34.59 ± 1.35 a
12.82 ± 0.56b
27.18 ± 0.91c
34.92 ± 1.23 a
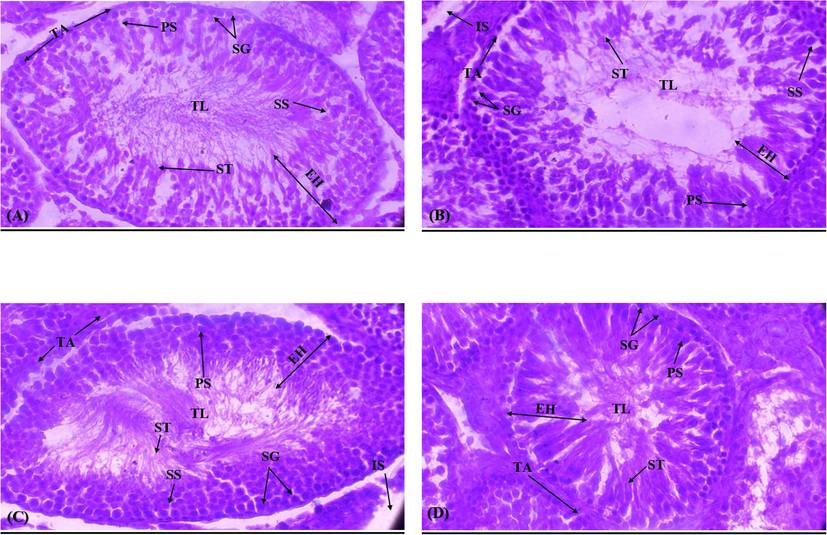
(A) Control group demonstrating thick germinal epithelium including different stages of germ cells and the slender luminal area carrying spermatozoa; (B) PE-MP group displaying sloughing of the epithelial layer, vacant lumen, and degeneration of IS; (C) PE-MP+ LUT group reduced sloughing of germinal epithelium, TL filled with ST and retrieved the degenerated IS; (D) LUT group representing compact ST with less IS. IS Interstitial spaces; TL: Tubular lumen; SHE: Seminiferous Epithelial height; ST: Seminiferous tubules; TP: Tunica propria; SG: Spermatogonia; ST: Spermatids; PS: Primary spermatocytes; SS: Secondary spermatocytes.
4 Discussion
In the current investigation, PE-MP exposure decreased the activities of antioxidant enzymes, such as CAT, SOD, GPx, and GSR, while increasing the concentration of ROS and MDA levels. The endogenous-antioxidant-enzymes such as CAT, SOD, GPx, and GSR are considered the first defense lines that protect biomolecules (proteins, lipids, and DNA) from OS by decreasing ROS generation. Nitric oxide (NO), hydroxyl radical (OH), superoxide anion (O-2), and hydrogen peroxide (H2O2) are the central reactive oxygen and nitrogen species (Mijatović et al., 2020). Thus, a reduction in the activities of antioxidant enzymes elevates the concentration of ROS, which attacks the polyunsaturated fatty acids (PUFA) in the sperm plasma membrane and triggers a cascade of chemical reactions, which is known as LP (Fois et al., 2018). In this study, MDA as a marker of LP increased the membrane permeability. However, this reduced activity of antioxidant enzymes in rat testicles was enhanced by the co-treatment of rats with LUT. LUT significantly elevated the activities of CAT, SOD, GPx, and GSR while lowering the ROS and MDA levels, probably due to its antioxidant property.
Secondly, PE-MP exposure led to a considerable reduction in epididymal sperm count, viability, and motility, a reduction in the number of HOS coiled-tail sperm, and a higher level of abnormality in the head mid-piece, and tail of sperm. OS performs a pivotal role in testicular impairments (Ijaz et al., 2021). The disproportion of the oxidants/antioxidants and ROS-prompts membrane damage due to the high level of PUFAs in sperm might be the reason behind the distorted integrity of sperm, as confirmed by the HOS test in the present study. However, LUT administration successfully resettled all the spermatogenic damages due to its potent ROS scavenging activity.
Similarly, PE-MP administration considerably reduced the LH, FSH, and plasma testosterone levels that play an essential role in spermatogenesis. FSH sustains the proliferation of immature Sertoli cells (SCs) and spermatogonia, whereas, on the other hand, LH accounts for the stimulation of Leydig cells (LCs) to produce testosterone (Ramaswamy and Weinbauer, 2014). Moreover, in combination with FSH, testosterone stimulates the growth of spermatids and the release of sperm (Oduwole et al., 2018). PE-MP exposure reduced the gonadotropins (LH and FSH) and androgen (testosterone) levels, possibly due to disturbing the hypothalamus-pituitary–gonadal axis (HPGA). Conversely, LUT treatment stabilizes the functions of the HPG axis that enhances the levels of gonadotropins and androgen, which eventually restores spermatogenic damages.
Lastly, PE-MP exposure caused severe histopathological alterations in the testes of the rats. We considered the vital role of hormones, dominantly gonadotropins and androgen, in sustaining spermatogenesis (Oduwole et al., 2018). The decline in the levels of these hormones in the PE-MP group might have caused spermatogenesis impairments such as germ cells apoptosis, the lessened seminiferous epithelial height, seminiferous tubular diameter, and tunica propria thickness. At the same time, interstitial space and tubular lumen diameter were escalated to support our hypothesized spermatogenesis damages. However, LUT treatment significantly alleviated the histopathological changes provoked by PE-MP. These histopathological improvements might be due to the antioxidant, androgenic, and anti-apoptotic nature of LUT.
5 Conclusion
PE-MP exposure prompted damages in rats' spermatogenic, hormonal, and structural profiles. Additionally, the activities of antioxidant enzymes, the concentration of ROS, and the MDA level exhibited a state of imbalance, thereby damaging the performance of the entire male reproductive system. However, LUT treatment alleviated PE-MP-induced damages in all the above-stated parameters due to its antioxidant androgenic potential.
Acknowledgements
The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP2022R436) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Preparation and characterization of low-density polyethylene/thermoplastic starch composites. Adv. Polym. Technol.. 2016;35:1-9.
- [Google Scholar]
- Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science. 2020;369:1515-1518.
- [Google Scholar]
- Potential health impact of environmentally released micro- and nanoplastics in the human food production chain: experiences from nanotoxicology. Environ. Sci. Technol.. 2015;49:8932-8947.
- [Google Scholar]
- Protective effect of selenium on aflatoxin B1-induced testicular toxicity in mice. Biol. Trace. Elem. Res.. 2017;180:233-238.
- [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250:5475-5480.
- [Google Scholar]
- Casierra-Posada, F., Jarma-Orozco, A., 2016. Nutritional composition of Passiflora species A2; In Nutritional Composition of Fruit Cultivars; Preedy, V. R., Ed, Academic Press: San Diego, 517−534.
- The hypoosmotic swelling test: its employment as an assay to evaluate the functional integrity of the frozen–thawed bovine sperm membrane. Theriogenology. 1994;42:351-360.
- [Google Scholar]
- Dietary-flavonoid-rich flowers of Rumex nervosus Vahl: Liquid chromatography with electrospray ionization tandem mass spectrometry profiling and in vitro anti-inflammatory effects. J. Sep. Sci.. 2015;38:3345-3353.
- [Google Scholar]
- Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur.. 2016;28:1-25.
- [Google Scholar]
- EPA. Contaminants of Emerging Concern including Pharmaceuticals and Personal Care Products. Available online: https://www.epa.gov/wqc/contaminants-emerging-concern-including-pharmaceuticals-and-personal-care-products (accessed on 29 January 2021).
- Evaluation of oxidative stress biomarkers in idiopathic pulmonary fibrosis and therapeutic applications: a systematic review. Respir. Res.. 2018;19:51.
- [Google Scholar]
- Marine microplastics spell big problems for future generations. Proc. Natl. Acad. Sci. U.S.A.. 2016;113(9):2331-2333.
- [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol Environ. Mutagen.. 2007;631:55-61.
- [Google Scholar]
- Protective effect of myricetin on nonylphenol-induced testicular toxicity: biochemical, steroidogenic, hormonal, spermatogenic, and histological-based evidences. Environ. Sci. Pollut. Res.. 2021;28:22742-22757.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. Biofactors. 2021;47(2):190-197.
- [Google Scholar]
- Effects of Chlorophytum borivilianum on sexual behaviour and sperm count in male rats. Phytothe. Res.. 2008;22:796-801.
- [Google Scholar]
- Glutathione peroxidase activity in selenium–deficient rat liver. Biochem. Biophys. Res. Commun.. 1976;71(4):952-958.
- [Google Scholar]
- Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ.. 2018;631–632:449-458.
- [Google Scholar]
- Acute toxic effects of polyethylene microplastic on adult zebrafish. Ecotoxicol. Environ. Saf.. 2019;182
- [Google Scholar]
- The double-faced role of nitric oxide and reactive oxygen species in solid tumors. Antioxidants. 2020;9:374.
- [Google Scholar]
- Role of follicle-stimulating hormone in spermatogenesis. Front. Endocrinol.. 2018;9:763.
- [Google Scholar]
- Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ. Res.. 2020;187
- [Google Scholar]
- Estimation of products of lipid peroxidation (malonyldialdehyde) in biological fluids. Anal. Biochem.. 1966;16:359-364.
- [Google Scholar]
- Plastics Europe: Plastics – the Facts 2020 an analysis of European plastics production, demand and waste data. Belgium: PlasticsEurope Brussels; 2020. https://www.plasticseurope.org/download_file/force/4261/181.
- Endocrine control of spermatogenesis: Role of FSH and LH/testosterone. Spermatogenesis. 2014;4(2)
- [Google Scholar]
- Role of dietary phenols in mitigating microglia-mediated neuroinflammation. Neuromol. Med.. 2016;18(3):453-464.
- [Google Scholar]
- The Anti-cancer Potential of Flavonoids. Molecular Approach to Cancer Management. Academic Press; 2017. p. :151-162.
- Immune response triggered by the ingestion of polyethylene microplastics in the dipteran larvae Chironomus riparius. J. Hazard. Mater.. 2021;414
- [Google Scholar]
- Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol.. 2018;24:1405-1416.
- [Google Scholar]
- Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chem.. 2011;126:1648-1654.
- [Google Scholar]
- Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl. Psychiatry. 2016;6(6)
- [Google Scholar]
- Luteolin alters macrophage polarization to inhibit inflammation. Inflammation. 2020;43(1):95-108.
- [Google Scholar]
- A review on the antioxidative and prooxidative properties of luteolin. React. Oxyg.. 2019;7:136-147.
- [Google Scholar]
- Nickel deficiency diminishes sperm quantity and movement in rats. Biol. Trace. Elem. Res.. 2003;93:141-154.
- [Google Scholar]
- Atmospheric microplastics: A review on current status and perspectives. Earth. Sci. Rev.. 2020;203
- [Google Scholar]







