Translate this page into:
Total and structure colonization by arbuscular mycorrhizal fungi in native, perennial grasses of different forage quality exposed to defoliation
⁎Corresponding author. cebusso@criba.edu.ar (Carlos Alberto Busso),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Defoliation can compromise the quality and quantity of colonization by arbuscular mycorrhizal fungi (AMF), which contribute to vegetation persistence in semiarid rangelands. The effects of defoliation on total arbuscular mycorrhizal (AM) colonization percentage and on that of its structures (i.e., vesicles, arbuscules) were evaluated on three native, rangeland perennial grass species. These species (i.e., Poa ligularis, Nassella tenuis and Amelichloa ambigua) show different palatability to domestic livestock in Central Argentina. In August 2012, soil + roots (0–10 cm depth) were sampled below the foliage of 12 plants of each species. Half of the plants were then defoliated to 5 cm stubble leaving active meristems intact after defoliation. The other half remained undefoliated. In September, immediately after the differentiation of apical meristems from vegetative to reproductive, soil + roots samples were again obtained and thereafter plants were once again defoliated. The final soil + root sampling was conducted in October (6 plants/species/treatment). The study was repeated on a different plant set during 2013. The percentage of total AM colonization and that its structures were determined. Palatable species did not reach a greater total colonization by AMF in their roots than A. ambigua. Treatments affected the total colonization only at some sampling times (e.g., when it did affect at N. tenuis, the effect of defoliation was not consistent during the study years). At the last date, A. ambigua showed a greater percentage of arbuscules in both defoliation treatments in 2012 and on defoliated plants in 2013. In general, P. ligularis showed a greater vesicle percentage than the other species. Management practices which allow the recuperation of the perennial grasses after a moderate grazing, will not affect considerably their symbiotic relationships.
Keywords
Poa ligularis
Nassella tenuis
Amelichloa ambigua
Grasslands
Arbuscules
Vesicles
Arbuscular mycorrhizal fungi
1 Introduction
Plant persistence on unfavorable environments can be the result of multiple factors and strategies. One of these is the symbiosis between plants and arbuscular mycorrhizal fungi (AMF; Koltai and Kapulnik, 2010). In this association, plants provide photosynthates, a C source to the AMF (Smith and Read, 2008). Also, lipids have been recently shown to be transferred from host plants to the fungus (Jiang et al., 2017; Keymer et al., 2017; Luginbuehl et al., 2017). Intraradical mycelium produces characteristically branched structures within the cortical cells called arbuscules. Many AMF species also form large, globular intraradical cells called vesicles that have a reserve function. However, arbuscules are considered diagnostic structures of the arbuscular mycorrhizal symbiosis (AM; Smith and Read, 2008). The more important benefits that plants obtain from AMF are (1) a greater water and nutrient uptake, specially those which have poor mobility (e.g., P, Koltai and Kapulnik, 2010), (2) an increase in the tolerance to water stress (Pedersen and Sylvia, 1996), (3) a protection against pathogens (Pedersen and Sylvia, 1996) and (4) an important contribution of water and nutrients for the re-establishment of photosynthetic tissues after disturbances such as fire with or without defoliation (Ithurrart et al., 2015).
In general, anthropic practices that disturb vegetation are likely to modify the diversity of fungi in grasslands. Species composition of the AMF community could be altered by defoliation (Frank et al., 2003). Grazing alters root morphology, soil physico-chemical properties and the structure (and composition) of plant communities (Hiiesalu et al., 2014). These modifications alter root colonization by AMF to various degrees (Kojima et al., 2014). It has been shown that grazing or defoliation decreases (Eom et al., 2001), increases (Frank et al., 2003) or has no effect on AMF (Torres et al., 2011). In addition, defoliation can affect the quality of the root colonization by AMF. Defoliation increased the presence of arbuscules in the roots of the symbionts but had no effect on the amount of vesicles in the biennial herb, Gentianella amarella (Piippo et al., 2011). In another study, however, Parodi and Pezzani (2011) showed that grazing had a positive effect on the presence of vesicles in the roots of Coelorhachis selloana, a desirable, preferred grass species by livestock; these authors reported that it was a strategy that would allow the symbionts to face stress situations. When grazing is intensive, it can negatively affect the AMF in nutrient poor soils due to a reduced plant photosynthetic capacity (Harley and Smith, 1983). Under moderate grazing, the symbiosis with AMF increases grazing tolerance because of increasing nutrient availability, favoring the plant competitive ability (Hartnett and Wilson, 2002).
Palatable (i.e., preferred) perennial grasses produce short-life leaves, with a low protection against herbivory and of fast decomposition (Moretto and Distel, 2000). Species that produce low-quality litter have a low potential productiviy and tissue turnover (Aerts and Chapin, 2000). If grazing management is not adequate, preferred perennial grass species, of good forage quality (high N content, low C/N ratios and lignin) can be replaced by unpreferred species of low forage quality (Giorgetti et al., 1997).
Mycorrhiza can stimulate organic matter decomposition (Cheng et al., 2012) and soil nutrient dynamics (Nuccio et al., 2013). Defoliation can alter the competitive capacity of plants, the intensity and quality of colonization by AMF, nutrient cycling and the dynamics of the plant community (Grigera and Oesterheld, 2004).
The objective of this work was to compare the species specific and defoliation effects on the percentage of total colonization and on that of structures (i.e., arbuscules, vesicles) of AMF. Our hypotheses were that (1) the percentage of total colonization and of structures of AMF are greater on roots of palatable than unpalatable perennial forage grasses. This is because of the greater competitive ability and tissue turnover on palatable than unplatable perennial grass species, (2) total colonization of AMF is not affected by the study defoliation treatments, and (3) the presence of arbuscules and vesicles is greater on defoliated than undefoliated (i.e., control) plants.
2 Matrials and methods
2.1 Study site
The study was conducted during 2012 and 2013 within a 16-year-exclosure to domestic livestock in the Chacra Experimental Patagones, located at the south in the province of Buenos Aires (40° 39′S, 62° 54′W; 40 m.a.s.l.). This site is within the Phytogeographical Province of the ‘Monte’ (Cabrera, 1976). The climate is temperate, semiarid. The long-term (1981–2012) mean annual precipitation is 421 mm. The mean annual temperature is 14.1 °C (Ing. Montenegro, Chacra Experimental Patagones, Ministerio de Agroindustria de la provincia de Bs. As., personal communication).
The soil was classified as a typical Haplocalcid. Soil texture is loamy-clay-sandy in the first 20 cm from the soil surface. A compounded soil sample gave a pH of 8.26 ± 0.02, organic matter content of 2.19 ± 0.03%, total N of 0.12 ± 0.0008% and extractable P of 9.88 ± 0.06 ppm.
2.2 Study species
Poa ligularis Ness is a C3 perennial grass species, of late-successional stages (Correa, 1978); it is a cool-season grass [i.e., its growing cycle is in fall, winter and spring; flowers in mid-October, and fructifies at the end of spring-early summer]. It is a species preferred (i.e., palatable, desirable) by cattle. It produces aboveground litter of good quality (high N concentrations, low C/N ratio and low lignin concentrations: Moretto and Distel, 2003). It is dominant under rotational, low intensity grazing systems (Giorgetti et al., 2006). Under moderate grazing, this species is replaced by Nassella tenuis (Phil.) Barkworth, a forage species of good quality and high productivity (Giorgetti et al., 1997). It is also a C3 species, of intermediate successional stages. Its growing cycle includes fall, winter and spring; flowers and fructifies in November–December (Cabrera, 1970).
Amelichloa ambigua (Speg.) Arriaga and Barkworth is a cool-season, C3 perennial grass of early successional stages (Saint Pierre et al., 2004). Its growing cycle is during fall, winter and spring; flowers and fructifies in early summer. It produces low quality litter (low N concentrations, high C/N ratios and high lignin concentrations: Fernández et al., 2010). Its abundance indicates overgrazing (Busso and Fernández, 2018). This species is only cut off when a better forage is not available (Giorgetti et al., 1997).
2.3 Experimental design and treatments
During December 2011, thirty-six plants (n = 12) were marked at different sites dominated by P. ligularis, N. tenuis and A. ambigua. In January 2012, all study plants were cut to 5 cm stubble height with the purpose of eliminating all senescent plant material accumulated during the previous years. Thereafter, the initial sampling was conducted at the vegetative stage of developmental morphology (i.e., winter: August). It consisted of taking 36 samples of soil + roots (0–10 cm) underneath the canopy of the marked plants. In addition, we simulated a moderate grazing intensity (Quiroga et al., 2004) and a rotational grazing system, characteristic in the study region (Giorgetti et al., 2006). Because of this, half of the plants was defoliated (n = 6) to 5 cm stubble height, and the other half remained undefoliated (i.e., control). In September, when the vegetative growth apexes differentiate to reproductive, the above mentioned soil samplings were once again conducted, and previously defoliated plants were defoliated to 5 cm stubble (n = 6) by a second time. The final sampling was conducted in October, to evaluate the effects of the two defoliations made during the same growing season.
The study was repeated using a different plant set during 2013. Defoliation treatments were applied during July and September; samples were initially taken in July, and then in August (to determine the effects of the first defoliation) and October (to determine the effects of the second defoliation).
In both years, soils were sampled from 35 to 40 days following each defoliation event.
2.4 Determination of percentage of colonization by AMF
Roots were cut in segments of 1.5 cm length and introduced in glass flasks with KOH at 10% (w/v,) were then heated during 15 min to 90 °C. Afterwards, they were placed on containers with Tripan blue during 20 min at 90 °C to stain hyphae, vesicles and/or arbuscules of the mycorrhiza.
The percentage of total colonization and that of structures (arbuscules and vesicles) of AMF were determined following Giovannetti and Mosse (1980).
Finally, the percentage of total colonization by AMF was estimated according to McGonigle et al., (1990).
2.5 Statistical analysis
Data were analyzed using the software INFOSTAT (Di Rienzo et al., 2013). Previous to analysis, data were transformed to arcsine √x to comply with the assumptions of normality and homocedasticity. Variables were analyzed with multifactorial ANOVA taken as factors the (1) species, (2) defoliation treatments, (3) sampling dates and (4) years. We used mixed lineal models with independent errors and homoscedastic residual variances because of data correspond to repeated measures and two years of study. In those cases where a significant interaction was detected among the study factors, we proceeded to separate first by study year, and then by species and sampling dates. Comparison of means was conducted using the protected test of Fisher (i.e., LSD), with a significance level of 0.05.
3 Results
3.1 Total colonization of AMF
Data analysis gave significant interaction among species, defoliation treatments, and years; and among species, sampling dates, and years (Table 1).
df
Total colonization
Presence of arbuscules
Presence of vesicles
F
p value
F
p value
F
p value
Species
2
3.17
0.0445
4.68
0.0104
32.67
<0.0001
Defoliation
1
1.05
0.3061
0.64
0.4236
2.19
0.1405
Sampling dates
2
7.85
0.0005
5.4
0.0053
22.05
<0.0001
Years
1
846.16
<0.0001
260.46
<0.0001
1251.08
<0.0001
Species × Defoliation
2
2.18
0.1164
0.23
0.7982
1.91
0.1515
Species × Dates
4
4.45
0.0019
2.84
0.0256
0.97
0.4267
Species × Years
2
13
<0.0001
7.27
0.0009
9.71
<0.0001
Defoliation × Dates
2
1.98
0.1412
0.5
0.6089
1.36
0.2587
Defoliation × Years
1
1.1
0.2959
2.21
0.1388
0.0022
0.9622
Dates × Years
2
0.62
0.5389
8.5
0.0003
20.03
<0.0001
Species × Defoliation × Dates
4
2.02
0.0929
2.02
0.0936
1.73
0.1462
Species × Defoliation × Years
2
5.79
0.0036
3.21
0.0426
2.95
0.0546
Species × Dates × Years
4
4.68
0.0013
1.44
0.2232
3.29
0.0124
Defoliation × Dates × Years
2
0.57
0.565
1.21
0.3007
0.78
0.4587
Species × Defoliation × Dates × Years
4
1.67
0.1583
3.01
0.0195
0.47
0.7548
During 2012, plants of P. ligularis showed 28.99 ± 1.96% of total colonization by AMF, and were not affected neither by defoliation treatments nor by sampling dates. On average for the three sampling dates, defoliated plants of N. tenuis showed percentages significantly lower (21.91 ± 2.13%; F = 12.90, P = 0.0012) than control plants (30.74 ± 1.62%). Amelichloa ambigua plants were not affected by the defoliation treatments and showed greater total colonization in the October sampling (30.93 ± 2.63%; F = 6.55, P = 0.0043) than in the first two sampling dates (August = 22.59 ± 3.08%, September = 17 ± 2.48%). Significant differences among species were only detected in the September sampling, where defoliated plants of the palatable species showed a greater total colonization than A. ambigua (Fig. 1a; F = 9.66, P = 0.0020).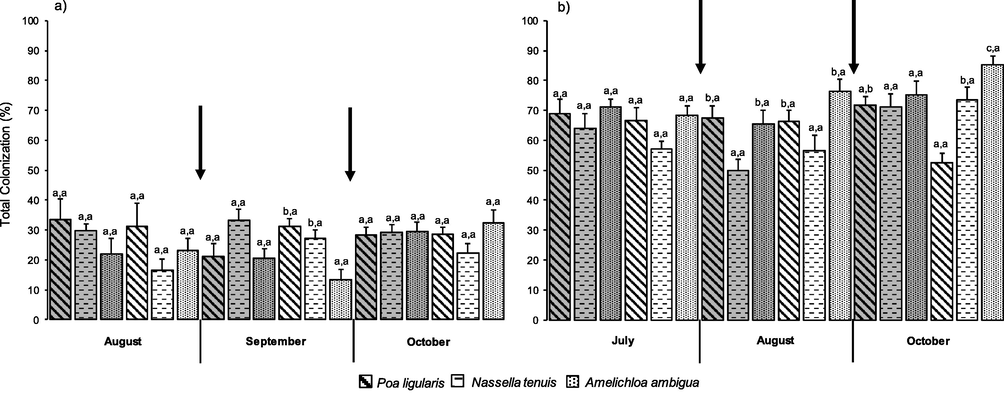
Interaction species x defoliation treatments on total colonization of arbuscular mycorrhiza (%) in 2012 (a) and 2013 (b). Each histogram is the mean ± 1 S.E. of n = 6. Unshaded histograms indicate plants before defoliation in August (a) or July (b), after one defoliation in September (a) or August (b), and after two defoliations in October (a and b); shaded histograms indicate undefoliated (i.e., control) plants. Within each sampling date, different letters before and after the comma indicate significant differences (p ≤ 0.05) among species and defoliation treatments, respectively. Arrows indicate the timing of defoliations.
In the second year (i.e., 2013), and October sampling, defoliated plants of P. ligularis showed percentages significantly lower than controls (defoliated = 52.41 ± 3.36%, control = 71.85 ± 2.72%; F = 20.48, P = 0.0011). In addition, values were greater in July (66.67 ± 4.32%) and August (66.3 ± 3.7%) than in October in this treatment (F = 4.47, P = 0.0301). Plants of N. tenuis were not affected by the defoliation treatments and showed a greater colonization at the third sampling date (July = 60.65 ± 2.77%, August = 53.33 ± 3.06%, October = 72.31 ± 2.94%; F = 10.59, P = 0.0003). In A. ambigua, defoliated plants (76.73 ± 2.46%) showed a greater colonization than controls (70.68 ± 2.37%) on average for the three sampling dates (F = 4.41, P = 0.0442). Also, the presence of AMF structures was greater towards the end of the study in both defoliation treatments (July = 69.81 ± 1.98%, August = 71.02 ± 3.31%, September = 80.28 ± 2.96%; F = 5.62, P = 0.0085). Plants of A. ambigua and P. ligularis showed a greater total colonization than N. tenuis at the second sampling date (Fig. 1b; F = 10.09, P = 0.0004). In October, defoliated plants of A. ambigua showed a greater total colonization than N. tenuis, and P. ligularis showed the lowest percentages (Fig. 1b; F = 20.21, P = 0.0001).
3.2 Presence of arbuscules
There was a significant interaction between the species, defoliation treatments, sampling dates and study years (Table 1).
In 2012, there was no effect of defoliation treatments on the species. Plants of P. ligularis and A. ambigua showed 2.93 ± 0.67 and 3.27 ± 0.65% of arbuscules in their roots, respectively. Significant differences were only detected among sampling dates in N. tenuis; the greatest value was in September (8.01 ± 2.00%) in comparison to the remaining sampling dates (August = 2.04 ± 0.54%, October = 2.41 ± 0.73%; F = 6.31, P = 0.0051). Species differed only in October, where A. ambigua showed a greater arbuscule percentage than P. ligularis in both defoliation treatments (Fig. 2a; F = 4.88, P = 0.0146), and N. tenuis did not show differences in comparison to the other species.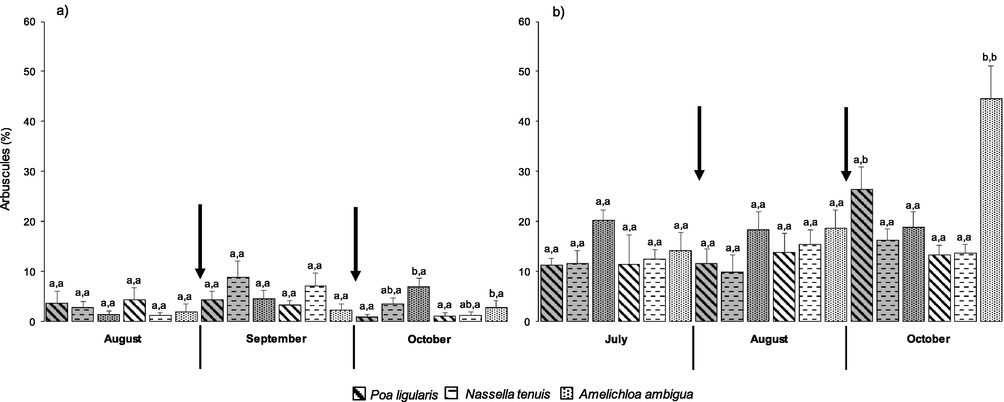
Interaction species x defoliation treatments on the presence of arbuscules (%) in 2012 (a) and 2013 (b). Each histogram is the mean ± 1 S.E. of n = 6. Unshaded histograms indicate plants before defoliation in August (a) and July (b), after one defoliation in September (a) or August (b), and after two defoliations in October (a and b); shaded histograms indicate undefoliated (i.e., control) plants. Within each sampling date, different letters before and after the comma indicate significant differences (p ≤ 0.05) among species and defoliation treatments, respectively. Arrows indicate the timing of defoliations.
During the second study year, the arbuscule percentage in plants of P. ligularis continues increasing towards the last sampling date in both defoliation treatments (July = 11.39 ± 2.84%, August = 12.78 ± 2.22%, October = 19.91 ± 3.05%; F = 3.24, P = 0.0531). Nassella tenuis was not affected neither by the defoliation treatments nor sampling dates and showed 13.21 ± 1.03% of arbuscules in its roots. Amelichloa ambigua showed significant differences between defoliation treatments at the last sampling date, when plants defoliated twice during the growing season showed greater arbuscule percentages than the control (F = 12.69, P = 0.0052) and the defoliated plants of P. ligularis and N. tenuis (Fig. 2b; F = 20.40, P = 0.0001). In addition, at this date, plants of P. ligularis defoliated twice showed lower values than controls (Fig. 2b; F = 7.37, P = 0.0218).
3.3 Presence of vesicles
Data analysis detected significant interactions between the species, defoliation treatments and years; and between the species, sampling dates and study years (Table 1).
In 2012, no interaction was detected between the study factors (Species, defoliation treatments and sampling dates); no effects were detected of neither sampling dates nor defoliation treataments (data not shown). Only differences between species were detected; plants of P. ligularis showed a greater vesicle percentage in their roots (1.42 ± 0.32%; F = 3.92, P = 0.0232;) than plants of N. tenuis and A. ambigua (0.68 ± 0.29 and 0.46 ± 0.14%, respectively).
In 2013, no interaction was detected between treatments and sampling dates within P. liguaris plants. On average for all sampling dates, vesicle percentages were greater in control (28.32 ± 2.27%) than defoliated (23.09 ± 2.33%; F = 4.96, P = 0.0337); also, on average for all defoliation treatment, the greatest vesicle percentages occurred in July (33.87 ± 1.96%) and the lowest ones in October (17.22 ± 2.21%; F = 16.29, P < 0.0001). Plants of N. tenuis were not affected neither by the defoliation treatments nor by the sampling dates and showed 15.06 ± 0.03% of vesicles in their roots. Amelichloa ambigua was not affected by the defoliation treatments; this species showed the greatest vesicle percentage in July (21.48 ± 1.82%) and the lowest in October (6.76 ± 1.2%; F = 24.68, P < 0.0001). At the first two sampling dates, plants of P. ligularis showed a greater vesicle percentage in their roots than the other two species, although they did not differ from N. tenuis in the October sampling (Fig. 3; July: F = 20.52, P < 0.0001, August: F = 11.77, P = 0.0002).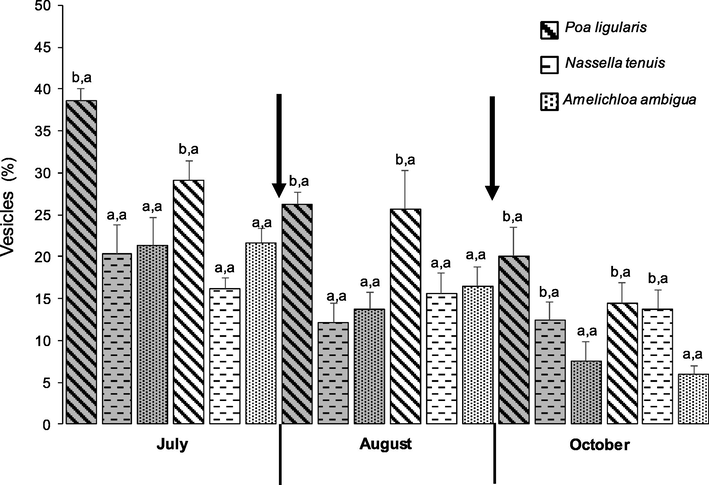
Interaction species x defoliation treatments on the presence of vesicles (%) in 2013. Each histogram is the mean ± 1 S.E. of n = 6. Unshaded histograms indicate plants before defoliation in July, and after one (August) or two defoliations (October); shaded histograms indicate control plants. Within each sampling date, different letters before and after the comma indicate significant differences (p ≤ 0.05) among species and defoliation treatments, respectively. Arrows indicate the timing of defoliations.
4 Discussion
This manuscript reports an analysis of the mycorrhization pattern in three different native perennial grass species (P. ligularis, N, tenuis and A. ambigua) of northeastern Patagonia Argentina, upon defoliation disturbance. The mean precipitation during the samplings dates was 16.2 mm in 2012 and 43.4 mm in 2013. This may be the explanation of the higher mycorrhization percentages observed in all parameters in the second year compared to the first year of study. Increased precipitation resulted in a greater root colonization and fungal biomass in a temperate steppe of northern China (Chen et al., 2017). Members of Glomeraceae, main family associated with the studied perennial grasses (Ambrosino et al., 2018), have been shown to respond positively to increased water availability by extending their mycelium into, and increasing colonization of, their host roots (Chen et al., 2017).
The variations on colonization by AMF can also be explained by the cost-benefit relationships experienced by the host plants (Koide and Schreiner, 1992). Compensatory growth can increase on plants after grazing if active meristems are left on them after such a disturbance (McNaughton, 1983). When this happens, the mycorrhizal association can help host nutrient uptake and increase photosynthetic rates (Allen, 1991), thus helping to overcome the effects of plant tissue removal (Parodi and Pezzani, 2011). In our study, in general, where defoliation treatments did not remove the plant active meristems, such treatments affected the total colonization by mycorrhiza only at some sampling times. When defoliation did affect total colonization percentage (e.g., in plants of N. tenuis), this effect of defoliation was not consistent during the study years. In addition, there was a greater colonization towards the October sampling on plants of A. ambigua in 2012 and 2013, and of N. tenuis in 2013. Growth is more rapid from apical and intercalary meristems than from axillary meristems (i.e., buds) (Briske and Richards, 1995). As a result, C might not have been a limiting factor on defoliated plants. This might have contributed to maintain or even increase (during the growing season) the association between the symbionts.
Increases of arbuscules in the roots of defoliated plants increase plant tolerance to defoliation (Piippo et al., 2011). Koziol and Bever (2015) demonstrated that roots of plant species of late successional stages have more arbuscules and hyphae colonization than those of early successional species. Contrarily to that expected, during the two study years, a greater presence of arbuscules in the roots of defoliated plants of the preferred species was not detected. In October, roots of A. ambigua showed greater arbuscule percentages in comparison to the other two species in both defoliation treatments in 2012, and on defoliated plants in 2013. A possible explanation for this result is the existent relationship between the root diameter and its degree of colonization by AMF. Amelichloa ambigua has a greater root diameter than the other two species and the mycorrhizal association can help to increase the volume of soil exploration, nutrient uptake (Koltai and Kapulnik, 2010; Ithurrart, 2015) and the reestablishment of a photosynthetic canopy after a disturbance (e.g., the defoliation in 2013; Walling and Zabinski, 2006). In addition, if we analyze it from the factors that influence growth and development of fungi, greater diameter roots might provide a more long-lived and stable habitat for their proliferation after their entrance into them (Reinhard and Miller, 1990).
Existing reports on the effects of defoliation on the presence of vesicles are diverse and contradictory. On the one hand, greater percentages have been found on grazed areas as a fungal strategy which allow them to face stress conditions (Parodi and Pezzani, 2011). However, other studies found that defoliation had no effects on these structures (Piippo et al., 2011). Grigera and Oesterheld (2004) demonstrated that the presence of vesicles was reduced by grazing in comparison to areas excluded to grazing during winter (dry season), but there was no effect in comparison to such areas in spring and summer (wet seasons). This demonstrates that environmental conditions can modulate the response of plant species and their symbionts in the face of a disturbance such as defoliation. In this work, precipitation in 2012 and 2013 were higher or similar to those of the long-term average and the soil moisture contents were the high values measured at sampling time (data not shown). This could be a reason why we do not detect effects of defoliation on the study mycorrhizal structures.
Previous studies conducted at the same site have reported the presence of spores pertaining to the families Acualosporaceae and Glomeraceae associated to roots of P. ligularis (Ambrosino et al., 2018). Such spores produce great quantities of vesicles in the roots of their symbionts (Lugo et al., 2003). This, would contribute to explain the greater presence of vesicles during the two study years, underneath plants of P. ligularis (a late-seral species) in relation to N. tenuis (an intermediate seral species) and A. ambigua (an early seral species; Distel and Boo, 1996). This is the first time that the presence of vesicles been related with the successional stage to which the species pertain to (Koziol and Bever. 2015). In addition, the proportion of roots colonized by vesicles is associated with the active nutrient uptake and root growth (Reinhard and Miller, 1990). This could be one of the reasons why in 2013 the percentage of vesicules was greater in July (active vegetative growth stage) and smaller in October in P. ligularis and A. ambigua.
5 Conclusion
Contrarily to the posted hypothesis, palatable species did not reach a greater total colonization in their roots than A. ambigua, and only plants of P. ligularis presented a greater presence of vesicles in both study years. In general, defoliation treatments did not affect total colonization by AMF. In relation to the quality of colonization, it was not possible to establish a clear pattern of the effects of the defoliation treatments on the study structures. Sustainable management practices produced by a moderate grazing, will allow the recuperation of the study plant species, without affecting their symbiotic relationships.
Acknowledgements
We thank finantial support from Universidad Nacional del Sur (UNS, PGI 24/A196) and the Consejo Nacional de Investigaciones Científicas y Técnicas de la República Argentina (CONICET, PIP 00211). We acknowledge Hugo Giorgetti and Gustavo Rodríguez, Chacra Experimental Patagones, Ministerio de Agroindustria, Province of Buenos Aires, who provided and helped us with the field facilities and fieldwork, respectively.
MLA, CAB and MNC designed the research work. MLA, DSC, YAT and LSI conducted field sampling. MLA and IRP conducted laboratory experiments. MLA, LSI and YAT analyzed data. MLA, CAB, MSV and MNC wrote the manuscript. CAB translated the manuscript from Spanish to English. All authors read and approved the manuscript.
References
- The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv. Ecol. Res.. 2000;30:1-67.
- [Google Scholar]
- The Ecology of Mycorrhizae. Cambridge: Cambridge University Press; 1991.
- Communities of arbuscular mycorrhizal fungi associated with perennial grasses of different forage quality exposed to defoliation. J. Arid Environ.. 2018;154:61-69.
- [Google Scholar]
- Plant response to defoliation: a physiological, morphological and demographic evaluation. In: Bedunah D.J., Sosebee R.E., eds. Wildland Plants: Physiological Ecology and Developmental Morphology. Denver, Colorado, USA: Society for Range Management; 1995. p. :635-710.
- [Google Scholar]
- Arid and semiarid rangelands of Argentina. In: Gaur M.K., Squires V.R., eds. Climate Variability Impacts on Land Use and Livelihoods in Drylands. U.S.A.: Springer, New York; 2018. p. :261-291.
- [Google Scholar]
- Flora de la Provincia de Buenos Aires Gramíneas. Colección Cientifica INTA. 1970;4:1-624.
- [Google Scholar]
- Regiones fitogeográficas argentinas. In: Ferreira Sobral E.F., ed. Enciclopedia Argentina de Agricultura y Jardinería. Buenos Aires, Argentina: ACME; 1976. p. :1-85.
- [Google Scholar]
- Nitrogen deposition and precipitation induced phylogenetic clustering of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem.. 2017;115:233-242.
- [Google Scholar]
- Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science. 2012;337:1084-1087.
- [Google Scholar]
- INFOSTAT. Grupo INFOSTAT, FCA, Universidad Nacional de Córdoba, Argentina; 2013.
- Vegetation states and transitions in temperate semi-arid rangelands of Argentina. In: West N., ed. Proceedings of the Vth International Rangeland Congress, Rangelands in a Sustainable Biosphere. Denver: Society for Range Management; 1996. p. :117-118.
- [Google Scholar]
- Effects of ungulate grazers on arbuscular mycorrhizal symbiosis and fungal community composition in tallgrass prairie. Mycologia. 2001;93:233-242.
- [Google Scholar]
- Fernández Mayer, A.E., Lauric, A., Tulesi, M., Gómez, D., Vázquez, L., 2010. Evaluación de la calidad nutricional del pasto puna (Stipa brachychaeta Godron) y la paja vizcachera (Stipa ambigua Spegazzini) a lo largo de todo un año. Sitio Argentino de Producción Animal. 10p. www.produccionanimal.com.ar.
- Soil community composition and the regulation of grazed temperate grassland. Oecologia. 2003;137:603-609.
- [Google Scholar]
- The comparative influence of past management and rainfall on range herbaceous standing crop in east-central Argentina: 14 years of observations. J. Arid Environ.. 1997;36:623-637.
- [Google Scholar]
- Cattle raising in central, semiarid rangelands of Argentina. Rangelands. 2006;28:32-36.
- [Google Scholar]
- An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol.. 1980;84:489-499.
- [Google Scholar]
- Mycorrhizal colonization patterns under contrasting grazing and topographic conditions in the flooding Pampa (Argentina) J. Range Manage.. 2004;57:601-605.
- [Google Scholar]
- Mycorrhizal Symbiosis. London: Academic Press; 1983.
- The role of mycorrhizas in plant community structure and dynamics: lessons from grasslands. Plant Soil. 2002;244:319-331.
- [Google Scholar]
- Species richness of arbuscular mycorrhizal fungi: associations with grassland plant richness and biomass. New Phytol.. 2014;203:233-244.
- [Google Scholar]
- Ithurrart, L., 2015. Efectos de la defoliación luego de la quema de gramíneas perennes nativas, palatables y no palatables, en el sudoeste bonaerense. Tesis de doctorado en Agronomía. Universidad Nacional del Sur. Bahía Blanca. 222 pp.
- Ithurrart, L., Busso, C., Torres, Y., Montenegro, O., Giorgetti, H., Rodriguez, G., Ponce, D., Ambrosino, M., Cardillo, D. y Montani, T., 2015. Micorrizas arbusculares en gramíneas perennes expuestas a defoliación, luego de una quema controlada. II Jornadas Patagonicas de Ciencias Ambientales, III Jornadas Patagonicas de Biologia, V Jornadas Estudiantiles de Ciencias Biologicas. Universidad Nacional de la Patagonia San Juan Bosco. Trelew, Chubut, Argentina.
- Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science. 2017;356:1172-1175.
- [Google Scholar]
- Regulation of vesicular-arbuscular mycorrhizal symbiosis. Annu. Rev. Plant Phys.. 1992;43:557-581.
- [Google Scholar]
- Arbuscular mycorrhizal diversity and function in grassland ecosystems. In: Solaiman Z., Abbott L., Varma A., eds. Mycorrhizal Fungi: Use in Sustainable Agriculture and Land RestorationSoil Biology. Berlin, Heidelberg: Springer; 2014. p. :149-169.
- [Google Scholar]
- Arbuscular Mycorrhizas: Physiology and Function. Dordrecht, Netherlands: Springer; 2010.
- Mycorrhizal response trades off with plant growth rate and increases with plant successional status. Ecology. 2015;96:1768-1774.
- [Google Scholar]
- Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science. 2017;356:1175-1178.
- [Google Scholar]
- Arbuscular mycorrhizal fungi in a mountain grassland: II Seasonal variation of colonization studied, along with its relation to grazing and metabolic host type. Mycologia. 2003;95:407-415.
- [Google Scholar]
- A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol.. 1990;115:495-501.
- [Google Scholar]
- Moretto, A.S., Distel, R.A., 2000. Nitrógeno disponible en suelos de sitios dominados por gramíneas de distinta relación C:N. XVII Congreso Argentino de la Ciencia del Suelo. Asociación Argentina de la Ciencia del Suelo, Mar del Plata (CD version).
- Decomposition of and nutrient dynamics in leaf litter and roots of Poa ligularis and Stipa gyneriodes. J. Arid Environ.. 2003;55:503-514.
- [Google Scholar]
- An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environ. Microbiol.. 2013;15:1870-1881.
- [Google Scholar]
- Micorrizas arbusculares en dos gramíneas nativas de Uruguay en áreas con y sin pastoreo. Agrociencia Uruguay. 2011;15:1-10.
- [Google Scholar]
- Mycorrhiza: ecological implications of plant interactions. In: Mukerji K.G., ed. Concepts in Mycorrhizal Research. Handbook of Vegetation Science. Dordrecht: Kluwer Academic Publishers; 1996.
- [Google Scholar]
- Do compensatory shoot growth and mycorrhizal symbionts act as competing above- and below-ground sinks after simulated grazing? Plant Ecol.. 2011;212:33-42.
- [Google Scholar]
- Quiroga, R.E., Blanco, L.J., Orionte, E.L., 2004. Efecto de la frecuencia e intensidad de defoliación sobre la productividad forrajera de Digitaria californica y Pappophorum caespitosum. Revista Argentina de Producción Animal 24. Disponible en CD.
- Size classes of root diameter and mycorrhizal fungal colonization in two temperate grassland communities. New Phytol.. 1990;116:129-136.
- [Google Scholar]
- Direct assessment of competitive ability and defoliation tolerance in perennial grasses. Can. J. Plant Sci.. 2004;84:195-204.
- [Google Scholar]
- Mycorrhizal Symbiosis (third ed.). London: Academic Press; 2008.
- Defoliation effects on the arbuscular mycorrhizas of ten perennial grass genotypes in arid Patagonia. Argentina. Appl. Soil Ecol.. 2011;49:208-214.
- [Google Scholar]
- Defoliation effects on arbuscular mycorrhizae and plant growth of two native bunchgrasses and an invasive forb. Appl. Soil Ecol.. 2006;32:111-117.
- [Google Scholar]







