Translate this page into:
Tolerance of Ziziphus and Acacia honeys to one year storage conditions and altitude
⁎Corresponding author. meaahmad@kku.edu.sa (Mohammed Elimam Ahamed Mohammed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives: This article investigated the effect of plant origin, storage and altitude on the triglycerides, cholesterol and some physicochemical properties of honey samples. Methods: Nineteen honey samples (9 Ziziphus and 10 Acacia) were involved in this study representing two floral origins and seasons and four altitudes. The plant origin of the honey samples was confirmed microscopically and their quality parameters were analyzed according to the International Honey Commission harmonized methods of honey analysis. The lipid parameters were analyzed using spectrophotometric- enzymatic methods. The SPSS version 20 was used for the statistical analysis of the obtained data. Results: The plant origin significantly affected the honey pH (P = 0.017), moisture (P = 0.009), fructose (P < 0.001) and the total of fructose and glucose (P = 0.001). Honey storage for one year significantly affected the Ziziphus honey pH (P = 0.005), moisture (P = 0.021), conductivity (P = 0.017), fructose (P = 0.036), glucose (P = 0.006) and the total of glucose and fructose (P = 0.013) while all the quality parameters of the stored Acacia honey were insignificantly different than those of the freshly harvested Acacia honey. The hydroxymethylfurfural (HMF) of the freshly harvested honey (Ziziphus of Acacia) was higher than that of the stored honey. The altitude had significant effect on the pH, acidity, moisture, conductivity, fructose, glucose, the total of fructose and glucose and the HMF of the Ziziphus honey while the altitude of the Acacia honey significantly affected the percentages of moisture and fructose only. Conclusions: Acacia honey was tolerant to one year storage at room temperature and to the high altitude climate conditions. Freshly harvested honey samples were with high HMF concentration compared to the stored honey samples.
Keywords
Triglycerides
Cholesterol
Acidity
Conductivity
Moisture
Sugars
- df
-
Degrees of freedom
- HMF
-
Hydroxy Methyl Furfural
- IHC
-
International Honey Commission
- M
-
Mean
- SD
-
Standard deviation
- SE
-
Standard Error mean
Abbreviations
1 Introduction
Many studies proved that the botanical origin of honey is the major force that determines the chemical composition and the quality parameters of honey (Mohammed, 2020; El Sohaimy et al., 2015; Ratiu et al., 2020). Some studies concluded that the botanical origin has insignificant effect on some of the physicochemical properties and chemical composition of honey (Majewska et al., 2019). However, the honeybee’s plant is the major determinant of honey quality because it is the major nutrient source for the honeybees (Adgaba et al., 2017).
The effect of storage on the physicochemical properties and chemical composition of honey depends on the storage temperature. Temperatures below 20 °C do not have extensive effects while above 35 °C has significant effects (Mouhoubi-Tafinine et al., 2018). Storing honey at room temperature is known to affect its acidity and to induce the microbial growth in it. The best way to store honey samples without affecting their quality and health characteristics is to store them at cooling temperatures (7–10 °C) (Martínez et al., 2018). It is proved that the honey tolerates temperature treatment and storage according to the honeybee species. Apis florea honey is more tolerant to heat dependent production of HMF compared to the Apis mellifera honey (Al-Ghamdi et al., 2019).
Because, high altitude areas are characterized by specific climate conditions such as low temperature, hypoxia, low barometric pressure and high UV radiation, the honeybees and the honey are affected by the bee farm altitude (Mohammed, 2020; Popov-Raljić et al., 2015; Mohammed et al. 2017; Al-Mosa et al., 2019; Mohammed et al., 2019). It is reported that the altitude do not support the production of the tutin containing toxic honey in New Zealand due to their climate characteristics. The toxic honey that contains the phytotoxin (tutin) is produced by the insect (Scolypopa australis) when feeding on tutu poisonus plant (Coriaria arborea) (Robertson et al., 2010).
Because of the climate conditions at high altitude, it is expected that the high altitude may be associated with some changes in the physicochemical properties of its honey. Furthermore, the effect of storage on the physicochemical properties of honey is mostly carried out through simulated conditions and not through storage at normal room conditions. It is expected that storage at normal room conditions may insignificantly affect the physicochemical properties of honey because of the gradual change in the climate conditions.
This article investigated the effect honey plant origin, bee farm altitude and one year storage of honey at room temperature on the concentration of triglycerides and cholesterol as well as their effect on some of the quality parameters; moisture, acidity and pH, conductivity, fructose, glucose, sucrose and HMF.
2 Material and methods
2.1 The honey samples
The honey samples were chosen form different floral origins, different altitudes and different harvesting seasons so as to meet the objective of the research. Nineteen honey samples were harvested from Asir region at the southwest part of Saudi Arabia in 2019 and 2020. The botanical origin of the samples was confirmed using an optical microscope with built in camera. Nine samples were Ziziphus honey samples harvested from three altitudes (sea level, 350 and 900 m above sea level) and two years (2019 and 2020) (Table 1). The Acacia honey samples were ten from two different altitudes (900 and 2000 m above sea level) and were harvested in two separate years (2019 and 2020) (Table 1). The temperature of the room temperature was ranging from 22 °C in January to 36 °C in June. The samples were harvested from Asir region at the southwestern part of Saudi Arabia.
Number
Year of production
Altitude (meters above sea level)
Location coordinates
Latitude
Longitude
Ziziphus-1
3
2020
Sea level
18° 00′ 39′' N
41° 41′ 43′' E
Ziziphus- 2
3
2019
350
18° 03′ 16′' N
42° 14′ 01′' E
Ziziphus-3
3
2020
900
17° 45′ 14′' N
43° 03′ 51′' E
Acacia-1
3
2020
900
17° 36′ 35′' N
42° 47′ 15′' E
Acacia-2
3
2019
900
17° 19′ 41′' N
43° 09′ 48′' E
Acacia −3
2
2020
2000
19° 41′ 52′' N
42° 30′ 57′' E
Acacia −4
2
2019
2000
19° 41′ 52′' N
42° 30′ 57′' E
2.2 Honey analysis
The floral origin of the honey samples was confirmed microscopically. 5 g of each honey sample were solubilized in 15 ml water in a falcon tube and two drops of safranin stain. The falcon tube and its contents were centrifuged at 6000 rpm for 20 min and the pellet was loaded on a microscope slide and investigated under the microscope (Louveaux et al., 1978). The percentage of the dominant pollen was calculated and the honey was considered as monofloral if its percentage was more than 50%. The samples of this study were all monofloral (Acacia and Ziziphus) (Song et al., 2012).
The triglycerides were measured by the usage of the spectrophotometric- enzymatic method. The triglycerides were catabolized by lipase enzymes to glycerol and fatty acids. The glycerol is converted to glycerol-3-phosphate by the action of the glycerol kinase. The glycerol-3-phosphate was oxidized to dihydroxyacetone phosphate and hydrogen peroxide with the activity of glycerol-3-phosphate oxidase. Finally, the hydrogen peroxide was reacted with 4-aminoantipyrene by the aid of the peroxidase to produce quinoneimine, HCl, water and 4-chlorophenol. The intensity of the red color produced by the quinoneimine is determined spectrophotometrically at 500 nm (de Souza et al., 2013).
The total cholesterol of the honey was determined using the spectrophotometric- enzymatic assay of human diagnostics company (kit code: 10017). The kit reaction involves conversion of cholesterol esters to cholesterol and fatty acids by the action of the cholesterol esterase enzyme. The free cholesterol from the first reaction and the already available free cholesterol in the samples are converted to chlestene-3-one and hydrogen peroxide by the cholesterol oxidase. Finally, the hydrogen peroxide is reacted with 4-amino-antipyrine and phenol to produce quinoneimine and water by the activity of the peroxidase enzyme. The quinoneimine has a red color which is measured spectrophotmetrically at the wavelength of 500 nm (Auerbach et al., 1990). This method is majorly used for the analysis of total blood cholesterol, but it is used for food samples (Dinh et al., 2011).
The quality parameters values were determined following the harmonized methods of the International honey commission (IHC methods). The moisture was determined using the refractometeric method and the pH and acidity were determined by titration to pH 8.3 (International Honey Commission, 2009).
The sugars were measured by the HPLC methods modified by Aglient Company of chromatography machines. Two hundred milligrams of each honey sample were dissolved in small amount of HPLC grade water and the volume was adjusted to 10 ml in a falcon tube. The treated honey samples were homogenized using vortex mixer and filtered through 0.22 µm filter. The used HPLC system was the Agilent 1260 Infinity II, Agilent Technologies, California, USA. The HPLC is equipped with pump, vial sampler, column for carbohydrate analysis (ZORBAX) and a refractive index detector. The mobile phase was composed of Acetonitrile: water (75:25, v/v) and the applied flow rate was 1.0 ml/min. Standard sugar solutions were run as reference so as to determine the honey sugars concentrations. The concentration of the standard fructose and glucose solutions was 2% (W/V) while the concentration of the sucrose was 1% (W/V). The injection volume of the samples and the standards was 10 µl. The samples and standards were analyzed in duplicates and the mean was considered as the final result. The peaks and the retention times of the samples were matched to those of the standards and the concentration of the sugars in the honey samples was determined accordingly by the software of the HPLC system.
The Agilent HPLC method for HMF measurement was used. HPLC system was (Agilent 1260 Infinity II, Agilent Technologies, California, USA). The pump attached to the HPLC system was (1260 Quat Pump VL, Agilent Technologies, California, USA) and the detector was diode array r (1260 DAD WR, Agilent Technologies, California, USA). Moreover, the HPLC is equipped with a C18 column (3.0 ×150 mm, 2.7 µm) and a vial sampler. The mobile phase was composed of water:acetic acid:methanol (89:1:10) and the flow rate was 0.3 ml/min. The standard HMF curve was developed by serial dilution starting from the highest standard (0.645 mg/ml). The injected volume of the standards and the samples was 5 µl and the analysis was done in duplicate. The concentration of the HMF in the honey was determined by the system software in mg/kg.
2.3 Statistical analysis
The obtained results were analyzed using the Statistical Package for Social Sciences (SPSS) version 20. The independent t-test, and the Fisher’s least significance difference (LSD) of Analysis of Variance (ANOVA) test were used. The independent t-test was applied to investigate the effects of floral origin and storage while the ANOVA test was applied for the investigation of the altitude effect.
3 Results
3.1 Effect of the storage and altitude on the appearance of the pollens
The altitude and one year storage affected the honey pollen physical appearance, integrity and the pattern safranin staining (Figs. 1 and 2). The pollens of the Ziziphus honey from sea level altitude were strongly stained by the safranin compared to the pollens of the 900 m altitude. The storage of honey for one year affected the integrity of the Ziziphus and Acacia honey, the parts of some of the Acacia pollens were separated from each other and similar to the effect of low altitude the pollens were deeply stained by safranin (Figs. 1 and 2).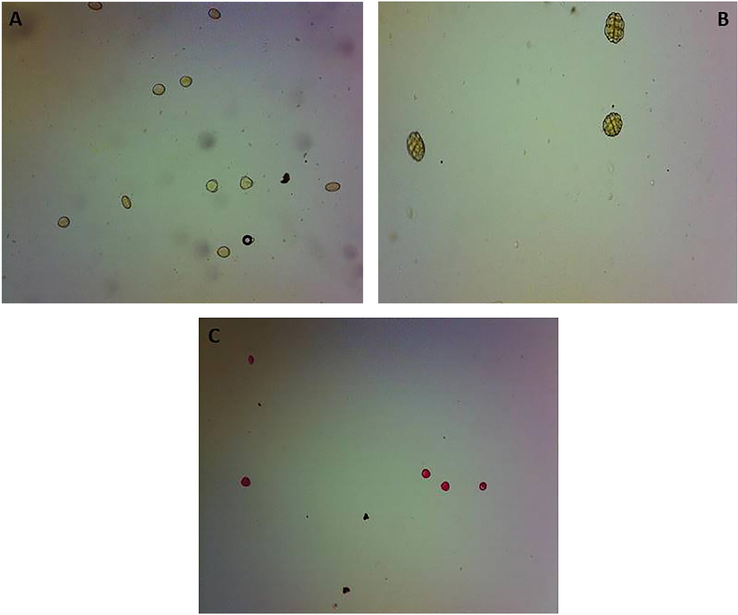
Effect of altitude on pollen shape. The pollens of the sea level Ziziphus honey are strongly pigmented with safranin stain compared to the pollen of the high altitude Ziziphus and Acacia honeys.
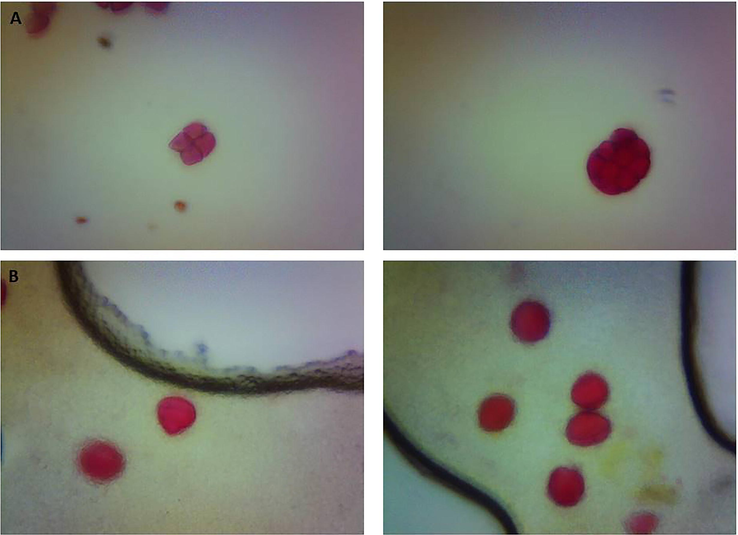
Effect of one year storage at room temperature on the pollen shape. Storage of Ziziphus and Acacia honeys caused the decomposition of the pollen grains.
3.2 Effect of floral origin on the studied parameters
There were insignificant variation between the Ziziphus and Acacia honeys with regard to their percentages of triglycerides and cholesterol (Figs. 2 and 3). The results of the triglycerides in the Ziziphus and Acacia honeys were (M = 0.43%, SD = 0.046, F = 5.11, df = 8.51, SE = 0.02) and (M = 0.4%, SD = 0.009, F = 5.11, df = 8.51, SE = 0.02), respectively. The t-test showed that there was insignificant difference between the triglycerides percentages of the Ziziphus and Acacia honeys (P = 0.127). Also, there was insignificant variation between the mean percentages of cholesterol in the Ziziphus honey (M = 0.12%, SD = 0.026, F = 4.41, df = 8.67, SE = 0.01) and in the Acacia honey (M = 0.12%, SD = 0.006, F = 4.41, df = 8.67, SE = 0.01) (P = 0.73) (see Fig. 4).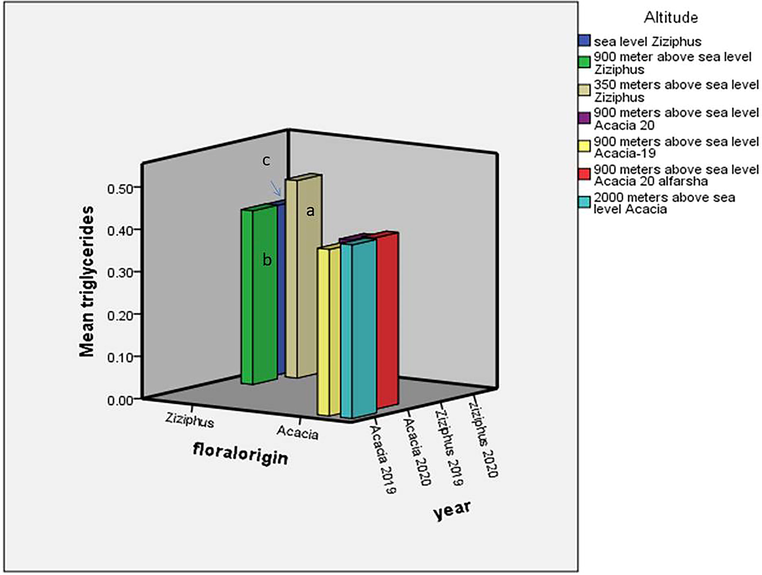
Effect of floral origin, storage and altitude on the triglyceride percentages of bee’s honey. The triglyceride percentage was not significantly affected by the floral origin or the storage. The Ziziphus honey from the 350 meters altitude was significantly with increased triglyceride percentage compared to the Ziziphus honey samples from the sea level and the 900 meters altitude. (a) Compared to (b) (p-value = 0.031); (a) compared to (c) (p-value = 0.014).
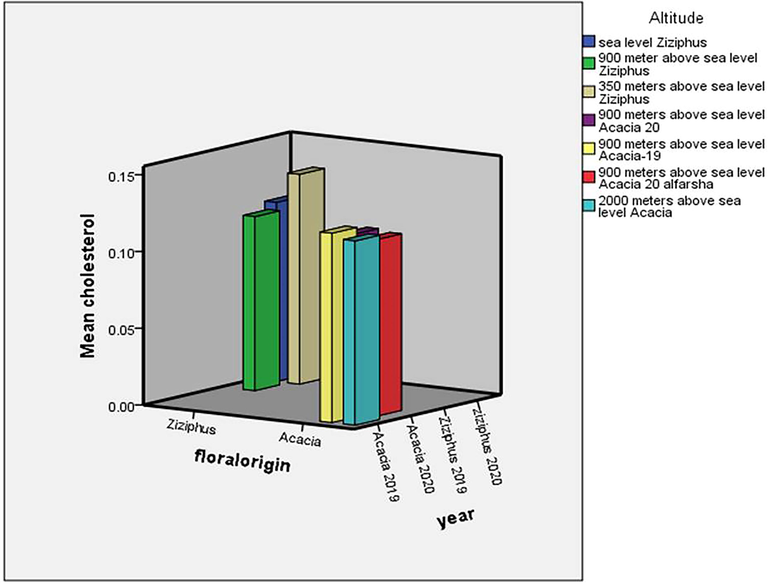
Effect of floral origin, storage and altitude on the cholesterol percentages of bee’s honey. The floral origin, one year storage at room temperature and the altitude had insignificant effects on the cholesterol percentage of honey.
Concerning the effect of floral origin on the quality parameters of honey, It significantly affected the pH, moisture percentage, fructose and the total of fructose and glucose (Table 2). The floral origin significantly affected the pH, moisture, fructose and sum of fructose and glucose.
pH
Acidity
meq acid/KgMoisture %
ConductivitymS/cm
Fructose %
Glucose%
Fructose + glucose%
Sucrose %
HMF
mg/kg
Ziziphus
Range
4.5–5.5
29–38
13–18
0.41–0.91
36–42
25.2–35.7
62.1–77.9
0.02–1.8
0.1–15
Mean
5.00
34.00
16.33
0.59
38.36
30.03
68.49
0.82
4.63
SD
0.36
3.54
1.95
0.18
2.33
4.31
6.74
0.65
6.57
F
1.43
5.98
2.01
4.72
0.83
0.82
1.03
3.40
8.88
df
14.08
11.77
14.15
11.88
16.59
14.86
15.34
12.28
12.15
SE
0.12
1.18
0.65
0.06
0.78
1.44
2.25
0.22
2.19
Acacia
Range
3.8–5.7
25–50
12–17
0.15–1.39
42–51
29–36.9
72.4–86.1
0.01–0.9
0.0–10
Mean
4.34
40.6
13.95
0.46
46.47
33.18
79.55
0.36
1.78
SD
0.66
9.32
1.36
0.48
3.06
3.26
5.39
0.37
3.62
F
1.43
5.98
2.01
4.72
0.83
0.82
1.03
3.40
8.88
df
14.08
11.77
14.15
11.88
16.59
14.86
15.34
12.28
12.15
SE
0.21
2.95
0.43
0.15
0.97
1.03
1.70
0.12
1.14
P
0.017
0.06
0.009
0.48
<0.001
0.09
0.001
0.09
0.27
3.3 Effect of storage on the studied parameters
The triglycerides and cholesterol percentages of the freshly harvested and the one year stored Ziziphus honeys were insignificantly different (P = 0.38 and 0.36, respectively). The results of the triglycerides in the recent Ziziphus honey was (M = 0.43%, SD = 0.06, F = 1.98, df = 6.51, SE = 0.03) while that of the stored Ziziphus honey was (M = 0.41%, SD = 0.017, F = 1.98, df = 6.51, SE = 0.03). The mean cholesterol percentage in the recently harvested and the stored Ziziphus honeys were (M = 0.13%, SD = 0.03, F = 2.03, df = 5.61, SE = 0.02) and (M = 0.11%, SD = 0.005, F = 2.03, df = 5.61, SE = 0.02), respectively (Figs. 2 and 3). Regarding the recent and stored Acacia honeys, there were insignificant variations between the triglycerides of recent Acacia honey (M = 0.39%, SD = 0.008, F = 0.33, df = 7.76, SE = 0.01) and stored Acacia honey (M = 0.4%, SD = 0.01, F = 0.33, df = 7.76, SE = 0.01) (P = 0.74). Similarly, the cholesterol of the recent Acacia honey (M = 0.12%, SD = 0.005, F = 1.52, df = 7.69, SE = 0.003) and that of the stored Acacia honey (M = 0.11%, SD = 0.004, F = 1.52, df = 7.69, SE = 0.003) were insignificantly different (P = 0.1) (Figs. 2 and 3).
The freshly harvested Ziziphus honey was characterized by significantly decreased pH, moisture and conductivity compared to the one year room temperature stored Ziziphus honey while it was characterized by significantly increased fructose, glucose and the total of them (Table 3). The HMF of the freshly harvested Ziziphus honey was more than that of the stored Ziziphus honey, mostly due to the sample of the sea level altitude. The pH, moisture, conductivity, fructose, glucose and the collective fructose and glucose were significantly different in the stored Ziziphus honey compared the recently produced Ziziphus honey.
pH
Acidity
meq acid/KgMoisture %
ConductivitymS/cm
Fructose %
Glucose%
Fructose + glucose%
Sucrose %
HMF
mg/kg
Freshly harvested
Range
4.5–5.1
32–39
13–18
0.4–0.6
37–42
28–35.7
65–77
0.03–1.8
0.36–15.0
Mean
4.8
33.5
15.5
0.49
39.27
32.2
71.6
0.93
6.86
SD
0.24
3.94
1.87
0.08
2.36
3.57
6.09
0.88
7.15
F
5.33
3.15
4.57
0.48
60.28
49.03
110.17
0.21
124.54
df
6.98
5.34
6.21
3.03
5.83
5.32
5.02
2.96
5.00
SE
0.10
1.61
0.76
0.03
0.96
1.46
3.12
0.25
2.92
Stored
Range
5.3–5.5
29–38
17.5–18.5
0.7–0.91
36–37
25.2–26.1
62.1–62.4
0.02–1.5
0.12–0.20
Mean
5.4
35.0
18.0
0.82
36.53
25.7
62.2
0.77
0.16
SD
0.10
3.0
0.5
0.11
0.5
0.46
0.17
0.60
0.04
F
5.33
3.15
4.57
0.48
60.28
49.03
110.17
0.21
124.54
df
6.98
5.34
6.21
3.03
5.83
5.32
5.02
2.96
5.00
SE
0.06
1.73
0.29
0.06
0.29
0.26
1.08
0.51
0.02
P
0.001
0.55
0.021
0.017
0.036
0.006
0.013
0.78
0.16
The freshly harvested Acacia honey and the one year stored Acacia honey at room temperature were with insignificantly different quality parameters. Similar to the Ziziphus honey, the freshly harvested honey was with high concentration of HMF compared to the stored Acacia honey. The Acacia honey tolerated the storage conditions (Table 4). There were no significant differences between freshly harvested and one year stored Acacia honey which reflected the tolerance of the Acacia honey to the storage conditions of this study.
pH
Acidity
meq acid/KgMoisture %
ConductivitymS/cm
Fructose %
Glucose%
Fructose + glucose%
Sucrose %
HMF
mg/kg
Freshly harvested
Range
3.8–5.7
25–50
13–15
0.15–1.39
42.8–49.9
29–36.2
72.4–86.1
0.01–0.83
0.00–10.0
Mean
4.48
40.0
13.86
0.63
45.60
32.10
77.46
0.35
3.4
SD
0.94
12.8
0.97
0.64
3.5
3.7
6.97
0.29
4.77
F
27.70
13.91
1.49
42.36
2.58
2.81
21.30
4.39
39.32
df
4.49
5.45
6.17
4.44
7.31
7.28
4.96
6.82
4.01
SE
0.42
5.73
0.44
0.29
1.58
1.65
3.12
0.13
2.14
Stored
Range
3.8–4.4
25–50
12–17
0.17–0.53
45–51.4
30.1–36.9
78.4–84.9
0.01–0.9
0.00–0.30
Mean
4.2
41.2
14.04
0.29
47.30
34.30
81.60
0.37
0.15
SD
0.23
5.50
1.79
0.15
2.6
2.7
2.43
0.46
0.14
F
27.70
13.91
1.49
42.36
2.58
2.81
21.30
4.39
39.32
df
4.49
5.45
6.17
4.44
7.31
7.28
4.96
6.82
4.01
SE
0.10
2.48
0.80
0.07
1.15
1.19
1.08
0.21
0.06
P
0.55
0.85
0.85
0.31
0.4
0.31
0.26
0.94
0.20
3.4 Effect of altitude on the studied parameters
With regard to the Ziziphus honey, the honey from the 350 m altitude was significantly with triglycerides percentage more than those from the sea level and from the 900 m (Fig. 1). However, the triglycerides percentages of the Ziziphus honey from the sea level, 350 and 900 m above sea level were (M = 0.4%, SD = 0.0, F = 4.05, df = 4.0, SE = 0.00), (M = 0.47%, SD = 0.07, F = 4.05, df = 4.0, SE = 0.003) and (M = 0.41%, SD = 0.02, F = 4.05, df = 4.0, SE = 0.01), respectively. The cholesterol percentages of the Ziziphus honeys from the sea level (M = 0.12%, SD = 0.01, F = 4.05, df = 4.0, SE = 0.007), 350 m (M = 0.14%, SD = 0.05, F = 4.05, df = 4.0, SE = 0.03) and 900 m (M = 0.11%, SD = 0.006, F = 4.05, df = 4.0, SE = 0.003) were insignificantly different (P ≥ 0.178) (Fig. 2). The Acacia honeys from the different altitudes were characterized by insignificant variations with regard to their triglycerides and cholesterol percentages (Figs. 1 and 2). The percentages of triglycerides in the Acacia honeys from the 900 m and 2000 m were (M = 0.39%, SD = 0.007, F = 4.05, df = 4.00, SE = 0.003) and (M = 0.41%, SD = 0.00, F = 4.05, df = 4.0, SE = 0.00), respectively. The values of the cholesterol of the Acacia honeys from the two altitudes were (M = 0.11%, SD = 0.006, F = 0.72, df = 4.0, SE = 0.002), and (M = 0.12%, SD = 0.00, F = 0.72, df = 4.0, SE = 0.00), respectively.
The pH of Ziziphus honey was significantly decreased in the 350 m altitude (P = 0.012) and significantly increased in the Ziziphus honey from 900 m altitude (p-value = 0.012) compared to the ziziphus honey of the sea level (Table 5). The acidity of the Ziziphus honey from the 350 m above sea level was significantly increased compared to the acidity of the sea level honey (P = 0.02). The moisture of the Ziziphus honey from the 350 m altitude was significantly lower than that of the Ziziphus honey from the sea level (P = 0.001) and from the 900 m altitude (P < 0.001). The conductivity of the 900 m Ziziphus honey was significantly more than the conductivity of the honey from the sea level and that from the 350 m (P < 0.001). The fructose, glucose and the total of fructose and glucose percentages of the 350 m altitude Ziziphus honey were significantly more than those of the Ziziphus honey from the sea level and from the 900 m (P < 0.001). The HMF concentration of the sea level altitude was significantly more than its concentration in the Ziziphus honeys from the 350 and 900 m altitudes (p-value < 0.001) (Table 5). There were insignificant variations in the percentage of sucrose in the Ziziphus honey samples from the three altitudes (P ≥ 0.62). The altitude significantly affected all the studied parameters (P ≤ 0.001) except the sucrose percentage. a sea level altitude; b 350 m above sea level; c 2000 m above sea level.
pH
Acidity
meq acid/KgMoisture %
ConductivitymS/cm
Fructose %
Glucose %
Fructose + glucose%
Sucrose %
HMF
mg/kg
Sea level
Range
4.9–5.1
29–31
16–18
0.48–0.6
37–37.3
28–30
65–67.3
0.02–1.02
12–15
Meana
5.0
30
17.0
0.55
37.1
29.0
66.1
0.68
13.3
SD
0.1
1.0
1.0
0.06
0.15
1.0
1.2
0.6
1.5
F
41.15
13.35
16.94
85.00
120.1
53.49
79.78
0.57
107.4
df
6.0
6.0
6.0
6.0
6.0
6.0
6.0
6.0
6.0
SE
0.06
0.58
0.58
0.04
0.09
0.58
0.66
0.33
0.88
350 m
Range
4.5–4.7
36–38
13–15
0.41–0.45
35–35.7
35–35.7
76.5–77.9
0.04–1.5
0.36–0.42
Meanb
4.6
37.0
14.0
0.43
41.4
35.4
77.1
0.85
0.39
SD
0.1
1.0
1.0
0.02
0.53
0.36
0.71
0.74
0.03
F
41.15
13.35
16.94
85.00
120.1
53.49
79.78
0.57
107.4
df
6.0
6.0
6.0
6.0
6.0
6.0
6.0
6.0
6.0
SE
0.06
0.58
0.58
0.01
0.31
0.21
0.41
0.43
0.02
900 m
Range
5.3–5.5
32–38
17–18.5
0.7–0.91
36–37
25.2–26.1
62.1–62.4
0.03–1.8
0.12–0.20
Meanc
5.4
35.0
18.0
0.82
36.5
25.7
62.1
0.93
0.16
SD
0.1
3.0
0.5
0.1
0.5
0.46
0.17
0.88
0.04
F
41.15
13.35
16.94
85.00
120.1
53.49
79.78
0.57
107.4
df
6.0
6.0
6.0
6.0
6.0
6.0
6.0
6.0
6.0
SE
0.06
1.73
0.29
0.06
0.29
0.26
61.8
0.51
0.02
P
ab = 0.01
ac = 0.01
bc<0.001
ab = 0.03
ac = 0.1
bc = 0.5
ab = 0.001
ac = 0.16
bc <0.001
ab = 0.05
ac< 0.001
bc< 0.001
ab = 0.36
ac<0.001
bc< 0.001
ab <0.001
ac = 0.001
bc <0.001
ab <0.001
ac = 0.01
bc < 0.001
ab = 0.75
ac = 0.62
bc = 0.86
ab <0.001
ac <0.001
bc <0.001
Regarding the effect of altitude on the studied Acacia honey, the studied quality parameters were insignificantly different in the Acacia honey from the 900 and 2000 m above sea level (Table 6) except the percentages of moisture and fructose which were significantly increased in the Acacia honey from the 2000 m compared to the Acacia honey from the 900 altitude (P = 0.01 and 0.02, respectively) (Table 6). The altitude significantly affected the moisture and fructose percentages of Acacia honey which reflects its tolerance for the altitude climate conditions compared to the Ziziphus honey.
pH
Acidity
meq acid/KgMoisture %
ConductivitymS/cm
Fructose %
Glucose%
Fructose + glucose%
Sucrose %
HMF
mg/kg
900 m
Range
4.9–5.1
25–50
12.8–15
0.15–1.39
42.8–49.9
29–36.9
72.4–86.1
0.01–0.9
0.0–10.0
Mean
4.4
40.1
13.5
0.47
45.6
33.5
79
0.34
2.22
SD
0.72
9.7
0.88
0.54
2.69
3.48
5.7
0.35
3.9
F
0.67
0.001
2.39
2.97
0.25
3.56
1.19
1.00
4.09
df
2.76
1.45
1.13
7.76
1.86
2.24
1.86
1.18
7.00
SE
0.25
3.44
0.31
0.19
0.95
1.23
2.02
0.12
1.4
2000 m
Range
3.8–5.7
35–50
14.5–17.0
0.35–0.53
48.3–51.4
30.1–33.5
78.4–84.9
0.02–0.85
0
Mean
4.1
42.5
15.8
0.44
49.9
31.8
81.65
0.44
0
SD
0.42
10.6
1.8
0.13
2.19
2.4
4.6
0.59
0
F
0.67
0.001
2.39
2.97
0.25
3.56
1.19
1.00
4.09
df
2.76
1.45
1.13
7.76
1.86
2.24
1.86
1.18
7.00
SE
0.3
7.5
1.25
0.09
1.55
1.7
3.25
0.42
0
P
0.48
0.69
0.01
0.93
0.02
0.41
0.45
0.84
0.34
4 Discussion
The floral origin of the honey had significant effect on the pH and acidity, moisture, fructose and glucose. The one year storage at room temperature affected the quality parameters of the Ziziphus honey without affecting the Acacia honey. The altitude had strong effect on the Ziziphus honey while it weakly affected the Acacia honey.
With regard to the effect of the altitude on the pollens of honey, it was reported that the altitude had affected the integrity and appearance of Acacia pollen shape and integrity (Mohammed et al., 2017). This study added that the staining pattern of the pollens is also affected by the altitude and storage.
One previous study published 1977 proved that the honey contains small amounts of triglycerides, monoglycerides, fatty acids, fatty alcohols and cholesterol (Kapoulas et al., 1977). This study proved the presence of trace amounts of triglycerides and cholesterol in honey samples of different origins and altitudes. Moreover, this study reported that the lipid parameters are not affected by the floral origin and the storage. However, the triglycerides are significantly affected by altitude of the Ziziphus honey.
It is well known that the floral origin is the major factor that determines the chemical composition and quality parameters of honey (Mohammed, 2020; El Sohaimy et al., 2015; Bogdanov et al., 2008; Moussa et al., 2012; Elenany, 2019; Bozbeyoglu et al., 2019; Hempattarasuwan et al., 2019; Majewska et al., 2019). In this study and in accordance with the previous studies, We have proved that the Ziziphus honey and Acacia honey are significantly different with regard to their pH and acidity and the percentages of moisture, fructose and the total of fructose and glucose.
This study reported significant effects of storage on the physicochemical properties of Ziziphus honey while the Acacia honey was not affected by the one year storage. Storage of honey is reported to increase the pH, acidity, conductivity and HMF and decrease the proline, diastase and invertase numbers (Mouhoubi-Tafinine et al., 2018; Qamer et al., 2013; Martínez et al., 2018; Juan-Borrás et al., 2015; Chou et al., 2020; Czipa et al., 2019). The storage conditions which have high impact on the honey are the temperature, packaging, and humidity (Martínez et al., 2018; mohammed, 2020; Singh and Singh, 2018)
Unlike the findings of this study, Czipa et al., (2019) showed that the storage of Acacia honey had significant effects on the physicochemical properties of Acacia honey. We have proved that the storage of Acacia honey at room temperature for one year had insignificant effects on its triglycerides, cholesterol and physicochemical properties. However, White et al., (1961) reported that some of the quality parameters honey samples are not affected by their storage at 26 ± 3° C.
This study reported that the sored honey at room temperature (22 °C–36 °C) was with decreased HMF concentration compared to the freshly harvested honey. This finding is against the findings of all of the previous studies, except the study of Fallico et al., (2008) who stated that the degradation rate of HMF in honey samples from different origins was higher than its formation rate at a temperature range of (25–50 °C). The conclusion of Fallico et al., (2008) is that stored honeys are characterized by low concentration of HMF due to the difference in the rate of production and degradation of HMF.
The altitude was reported to affect the physicochemical properties of honey either positively or negatively (Mohammed, 2020; Mohammed et al. 2017; Bouhala et al., 2020; Neupane et al., 2015; Popov-Raljić et al., 2015). In this report, the altitude affected significantly all the studied quality parameters of the Ziziphus honey except the sucrose while it affected significantly the moisture and fructose percentages of Acacia honey.
5 Conclusions
The floral origin and the altitude had significant effects on the quality parameters of the honey samples. The one year storage at room temperature significantly affected the most of the quality parameters of the Ziziphus honey while it did not affected the quality of the Acacia honey. This study supported the idea that the HMF is not associated with the honey storage conditions since all the recently produced honey were with high concentration of HMF compared to the stored honey samples.
Funding
This study is funded by the Scientific Research Deanship at King Khalid University and the Ministry of Education in KSA through the project number (KU/RCAMS/G001/21).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Nectar secretion dynamics and honey production potentials of some major honey plants in Saudi Arabia. Saudi J. Biol. Sci.. 2017;24:180-191.
- [CrossRef] [Google Scholar]
- Comparison of physicochemical properties and effects of heating regimes on stored Apis mellifera and Apis florea honey. Saudi J. Biol. Sci.. 2019;25:845-848.
- [CrossRef] [Google Scholar]
- Antioxidant vitamins in honey samples from different floral origins and altitudes in Asir Region at the South-Western Part of Saudi Arabia. Curr. Nutr. Food. Sci.. 2019;15:296-304.
- [CrossRef] [Google Scholar]
- A rapid and sensitive micro-assay for the enzymatic determination of plasma and lipoprotein cholesterol. Lipid Res.. 1990;30:738-742.
- [Google Scholar]
- Honey for nutrition and health: a review. J. Am. Coll. Nutr.. 2008;27:677-689.
- [CrossRef] [Google Scholar]
- Altitude effect on the properties of honeys from the region of Jijel (Algeria) Pol. J. Food Nutr. Sci.. 2020;70:169-178.
- [CrossRef] [Google Scholar]
- Bacteriological, physicochemical, and melissopalynologic properties of some Turkish Honeys. Akademik Gida. 2019;17:167-175.
- [CrossRef] [Google Scholar]
- Evaluation of honey quality with stored time and temperatures. J. Food Nutr. Res.. 2020;8:591-599.
- [CrossRef] [Google Scholar]
- Composition of acacia honeys following processing, storage and adulteration. J. Food Sci. Technol.. 2019;56:1245-1255.
- [CrossRef] [Google Scholar]
- Simple enzymatic methods for glycerol analysis in commercial beverages. CyTA J. Food. 2013;11:270-276.
- [CrossRef] [Google Scholar]
- Cholesterol content and methods for cholesterol determination in meat and poultry. Compr. Rev. Food Sci. Food Saf.. 2011;10:269-289.
- [CrossRef] [Google Scholar]
- Physiochemical parameters and rheological properties of citrus, clover, and marjoram Egyptian bee honeys. J. Entomol.. 2019;16:17-22.
- [CrossRef] [Google Scholar]
- Physicochemical characteristics of honey from different origins. Ann. Agric. Sci.. 2015;60:279-287.
- [CrossRef] [Google Scholar]
- Degradation of 5-hydroxymethylfurfural in honey. J. Food Sci.. 2008;73:C625-C631.
- [CrossRef] [Google Scholar]
- Impact of botanical source and processing conditions on physicochemical properties and antioxidant activity of honey in the Northern Part of Thailand. Int. J. Food Sci. Technol.. 2019;54:3185-3195.
- [CrossRef] [Google Scholar]
- International Honey Commission, 2009. Harmonized methods of the international honey commission. <https://www.ihc-platform.net/ihcmethods2009.pdf> (accessed 29 Jul 2021)
- Physicochemical quality parameters at the reception of the honey packaging process: influence of type of honey, year of harvest, and beekeeper. J. Chem. 2015 Article ID 929658
- [CrossRef] [Google Scholar]
- Identification of the lipid components of honey. Z. Lebensm. Unters.-Forsch.. 1977;163:96-99.
- [Google Scholar]
- International commission for bee botany of IUBS. Methods of Melissopalynology. Bee Word. 1978;59:139-157.
- [Google Scholar]
- Determination of the botanical origin of honeybee honeys based on the analysis of their selected physicochemical parameters coupled with chemometric assays. Food Sci. Biotechnol.. 2019;28:1307-1314.
- [CrossRef] [Google Scholar]
- Influence of temperature and packaging type on quality parameters and antimicrobial properties during Yateí honey storage. Food Sci. Technol. [online]. 2018;38:196-202.
- [CrossRef] [Google Scholar]
- Some physiochemical proprieties of Acacia honey from different altitudes in Asir region. Czech J. Food Sci.. 2017;35:321-327.
- [CrossRef] [Google Scholar]
- Hydrogen peroxide and dicarbonyl compounds concentration in honey samples from different botanical origins and altitudes in the South of Saudi Arabia. Curr. Res. Nutr. Food. Sci.. 2019;7:150-160.
- [CrossRef] [Google Scholar]
- Factors affecting the physicochemical properties and chemical composition of bee’s honey. Food Rev. Int. 2020
- [CrossRef] [Google Scholar]
- Effect of storage on hydroxymethylfurfural (HMF) and color of some Algerian honey. Int. Food Res. J.. 2018;25:1044-1050.
- [Google Scholar]
- The influence of botanical origin and physicochemical parameters on the antifungal activity of Algerian honey. J. Plant Pathol. Microb.. 2012;3:132.
- [CrossRef] [Google Scholar]
- Antioxidant properties of honey from different altitudes of Nepal Himalayas. Pol. J. Food Nutr. Sci.. 2015;65:87-91.
- [CrossRef] [Google Scholar]
- Evaluation of color, mineral substances and sensory uniqueness of meadow and acacia honey from Serbia. Rom. Biotechnol. Lett.. 2015;20:10784-10799.
- [Google Scholar]
- Effect of storage on various honey quality parameters of Apis dorsata honey from Nepal. Pakistan J. Zool.. 2013;45:741-747.
- [Google Scholar]
- Correlation study of honey regarding their physicochemical properties and sugars and Cyclitols Content. Molecules. 2020;25:34.
- [CrossRef] [Google Scholar]
- Investigating the importance of altitude and weather conditions for the production of toxic honey in New Zealand. N. Z. J. Crop Hortic. Sci.. 2010;38:87-100.
- [CrossRef] [Google Scholar]
- Honey moisture reduction and its quality. J. Food Sci. Technol.. 2018;55:3861-3871.
- [CrossRef] [Google Scholar]
- Pollen analysis of natural honeys from the central region of Shanxi, North China. PLoS One. 2012;7:e49545
- [CrossRef] [Google Scholar]
- Composition of honey. VI. The effect of storage on carbohydrates, acidity and diastase content. J. Food Sci.. 1961;26:63-71.
- [CrossRef] [Google Scholar]







