Translate this page into:
To extinguish or not to extinguish: The role of forest fire in nature and soil resilience
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Forest fire is a commonly occurring phenomenon in all ecosystems around the world. It has numerous short- and long-term effects on the ecosystem. Intensive research has made it easy for one to analyze the changes made in the soil system. However, these results remain complex, ranging from altering biomass to reducing or eliminating the underground physical, chemical, and microbial processes. The soil is a significant contributor to the ecosystem, and its recovery is fundamental for the sustainability of the ecosystem. The effects of forest fire are either beneficial or disastrous, depending on the fire severity. Unlike high-impact burning, low-impact burning can stimulate more beneficial herbaceous flora and increase the nutrients available to plants. This article analyzes the effect of fire on the different aspects of the soil depending on the various analysis and experiments conducted on the impact of fire on different principals. The paper is a general review of the effects of fire on the belowground systems, emphasizing the changes in physical, biogeochemical, and biological properties of the soils and the subsequent consequences for ecosystem sustainability. Understanding this process is of much concern and essence to the ecologists, land managers, and other relative professionals.
Keywords
Forest fire
Soil biological properties
Forest fire phase
Lignin
Cellulose
Amino acid
1 Introduction
Forest Fire is a natural forest disruption that occurs in most terrestrial ecosystems. The forest fire, to any ecosystem, ends up affecting both the vegetation and the soil. However, the general effect of forest fire on soil remains very complex; up to some extent, it also helps maintain the diversity and the overall stability of the ecosystems (Thompson et al., 2009). Organic matter present in the soil is susceptible to catch fire and combust rapidly. Change in the soil organic matter due to fire, in turn, changes many soil properties. Most affected factors include macro and micro-nutrients and soil properties like texture, color, pH, and bulk density (Verma and Jayakumar, 2012).
It is well known that fire can change various physical, chemical, mineralogical, and biological soil properties (Certini, 2005). The most severe impact of forest fire on the environment is greenhouse gases produced by biomass burning. The extent of the harmful effect of fire on the soil depends on different factors, such as the intensity of the fire, type of fuel present, and amount of soil moisture(Parson et al., 2010)
Soil is the biggest source of carbon present on the earth; out of total carbon present, 70% share goes to soil organic carbon, and the rest is present in the form of salt(Batjes, 1996). The impact of fire on wildlife and vegetation is more vivid than its impact on the soil. Therefore, it is challenging to assume what happens to the soil life and nutrients in a forest fire. The effects range from altering the above-ground biomass to the extended belowground properties involving chemical, physical, and microbial mediated processes (Kraus and Goldammer, 2007). Fire burn severity and its impact on soil are shown in Fig. 1.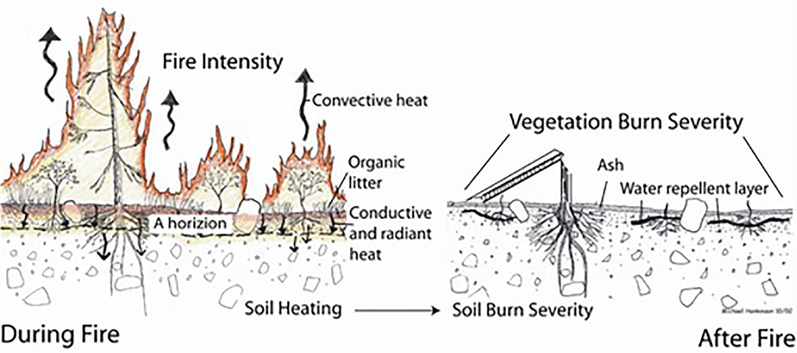
Burns severity above and below the soil surface (Parson et al., 2010).
Prescribed and controlled fires are the two main types of forest fire (HYVÄRINEN et al., 2009). The effects of fire vary from short-term, long-term, and permanent fire (Ribeiro-Kumara et al., 2020). The kind of fire depends on many elements that involve different fire severity measures, property, and frequency of the fire (Neary and Leonard, 2020). Not much is known about how forest fire affects the soil's carbon pool or carbon stabilization (Zhao et al., 2012). This paper aims to review the different forest fire phases and describe how the soil’s physical, chemical, and biological properties are affected by fire-related changes.
2 Phases of forest fire
Combustion is a physio-chemical process caused by fire. The complete combustion process involves a source of chemical energy, thermal energy as an ignition, and oxygen that supports the combustion process (Griffiths, 2019). Since it is a chain reaction, there are three phases: incipient, growth, and fully developed. In the first phase, oxygen, heat, and fuel combine to ignite the fire. This clears the first stage of the process and is followed by glowering, burning, and extinction (DeBano et al., 1998). Next, it involves heating the fuel, which may be either solid, liquid, or gas. Different stages of fire are shown in Fig. 2.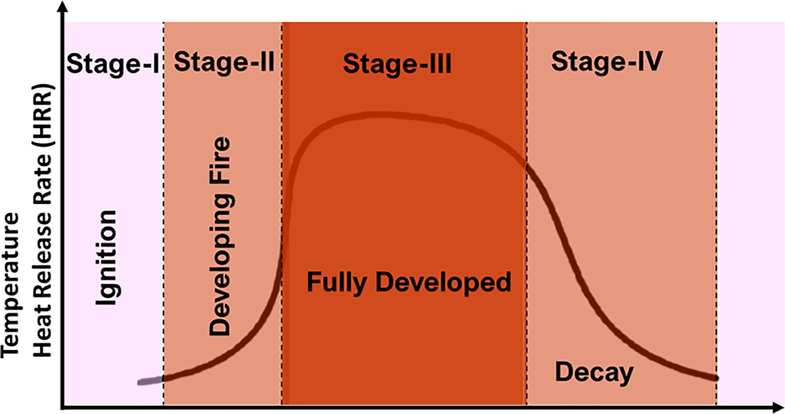
Various Stages of Fire.
The second phase is the growth phase that involves releasing the solar energy usually stored in the fuel as heat, various gases, and other particulate matter (DeBano et al., 1998). Sometimes, the combustion process is called rapid oxidation because of its similarity to the rusting process. Rusting involves the addition of oxygen, except that this process speeds up quickly using fire and oxygen. Until the process reaches the smoldering phase, the combustion process does not slow down (Simard, 1991). The smoldering phase is a flameless form of combustion and is identified with the decrease in temperature and results in altogether low heat flux.
The final stage of the combustion process is the extremely slow oxidation of the residue. The slow process makes the final stage long-lasting in the soil (Simard, 1991). However, according to various research, what makes the phase more effective is its ability to emit temperatures ranging from 400 to 760 degrees for a lengthy time. Table 1 illustrates the features of various stages in the combustion process.
Stage Of Combustion
Process
Features of various stages in the combustion process
Flaming Stage
Solid Phase
Drying or Distilling
Different soil volatile components evaporate or permeate into the deeper layer under the soil's surface.
Pyrolysis Process
Begins at temperature 400 K as an endothermic process and above 450 K becomes exothermic at this temperature decomposition of Dehydrocellulose takes place
Glowing Combustion
Process starts in the presence of oxygen at 800 K.
Gas Phase
The Flame
low-molecular-weight products are formed from the emitted volatiles.
Smoldering Stage
Smoldering Process
This process takes place at a low percentage of oxygen (5%). In addition, the presence of moisture makes the process too long to complete.
3 Heat transfer process
The heat generated during the combustion process spreads through different methods such as conduction, convection, radiation, and mass transport (Chandler et al., 1983). Radiation is a method of heat transfer through a wave that sends heat energy to an object. It does not require any contact between the heat sources. This is the transfer of heat through space via infrared radiation, a type of electromagnetic radiation (DeBano et al., 1998). Neither mass nor medium is required. Heat transfer by radiation can dry out the surrounding fuels or sometimes ignite the fuel as well. An excellent example of this manner of heat transfer is heat transfer from the sun. Convection heat transfer occurs as a mass movement of the heated fluid, and it is made to shift to the side of the heat and thus carries energy with it (Bejan, 2013). When a liquid is heated, it becomes less dense and rises according to the Gas Law. Air is a classic example of this medium. Despite the difference, these two forms of heat transfer are responsible for the heat transfer from light fuels to the soil. Convection heat transfer is the reason behind the aggressive and explosive fire behavior due to removing the heat energy from one area to another through air mixing. Fig. 3 shows the heat transfer process refers to a tree.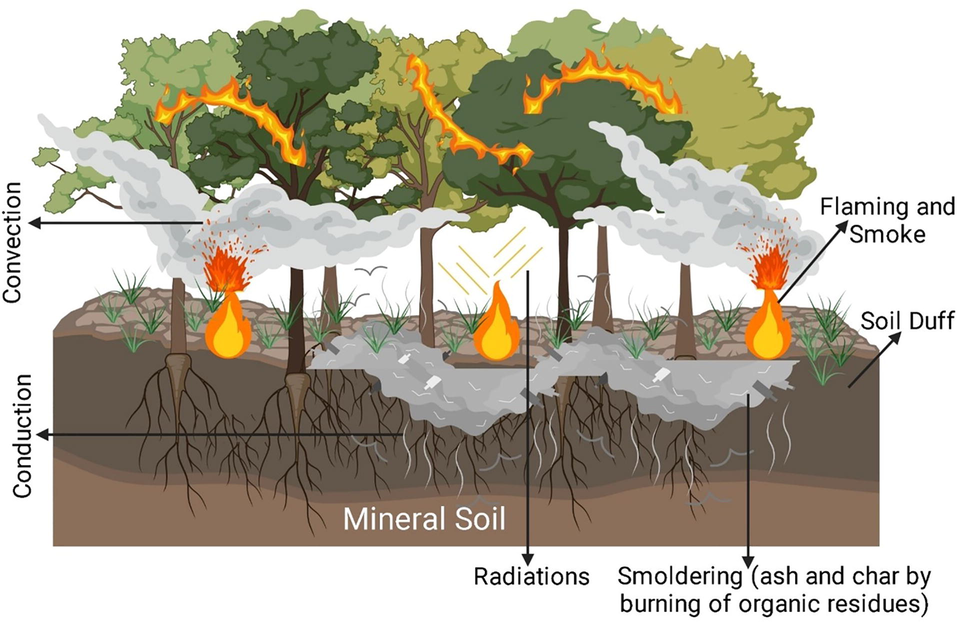
Heat transfer process during a forest fire.
The transfer of heat by conduction involves molecular agitation that is held within a particular material. This happens without any movement of the element(Kakaç et al., 2018). It is an efficient way of heat transfer, especially in heavy fuels. Furthermore, vaporization and condensation are other modes of transmission of heat into the soil but at a faster rate. When the soil water is heated, it shifts quickly through the soil pores as a vapor, and when it is forced to condense, it releases the same amount of heat.
3.1 Temperatures
Temperature is the intensity of the heat present in a substance that is exposed to fire. It can be expressed according to a comparative scale. In soil, this is referred to as both diurnal and seasonal variations of the created climate(Simard, 1991). The heated biomass transports the temperature in the shrubs and grasses into the soil. The quantity and the period of exposure determine the severity of the impact caused by forest fire, both positively and negatively. The effect of temperatures on the soil impacts soil's physical, chemical, and biological components. The physiological impact begins to be established at the 40–70 degrees range, and this is due to the quick protein degradation that causes plant tissue death. Roots become dehydrated at soil temperatures of 48 to 54 degrees. (Chandler et al., 1983). The different elements in soil have different threshold temperatures of volatilizations. However, the by-product of all the phases of fire has an impact on belowground sustainability. Heat transfer to the organic and mineral horizons of the soil cause intense effect on the soil biomolecules, physical, chemical, and biological processes essential in maintaining soil sustainability (DeBano et al., 1998). The time is taken for the combustion process, and the transfer of heat is highly varied. Thus, the extent of the effects of the fire is equally variable (Simard, 1991). The research has indicated that the low impact combustion on the different terrains worldwide can promote herbaceous flora, essential in maintaining the belowground soil sustainability (DeBano et al., 1998).
4 The effect of fire on different biomolecule present in soil
4.1 Cellulose and pectin
Cellulose and pectin together form 50% of dry plant mass. Most of the plant char for measuring forest fire is derived from the cellulose and pectin components. On high temperature exposed, significant observable structural changes occur with a weight loss of approximately 10% (Díaz-Raviña et al., 1996). A small amount of glucose in the cellulose is hydrolyzable from the char that is formed after combustion. However, when cellulose is exposed to 270 degrees, pyrolysis reaction starts, and substantial physical and chemical changes occur (Leisted, 2014). New molecular structural changes are observed at much higher temperatures like phenol, aromatic, aliphatic, and aryl carbon. At 250 degrees, the crystalline structure of sugar is still observed; also, glucans start to appear. Above 300-degree temperature, aromatic hydrocarbon and furan are the main product of pyrolysis. Fig. 4 shows pyrolysis reactions of the cellulose molecule.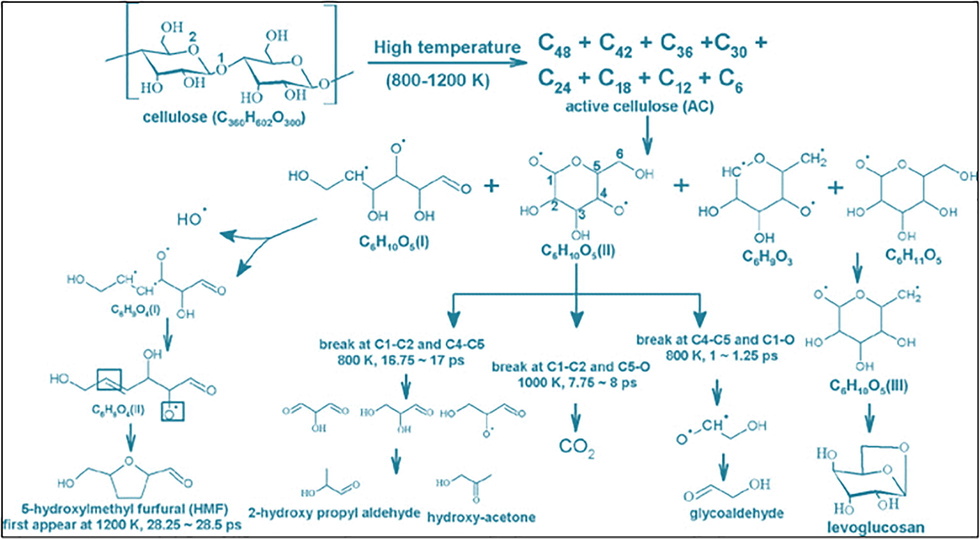
Pyrolysis of cellulose (Zheng et al., 2016).
The changes at high temperatures result from nonvolatile anhydroglucose cores that serve as aldol-condensation sites for organic compounds emitted inside the char during combustion. They end up working as molecular attachments that hold tightly together larger nonvolatile residues. Eventually, a polymer is formed that establishes itself gradually by trapping down small reactive pyrolysis products. The further inference made by Boon (1994) is that at a temperature greater than 250 degrees, polyaromatic structures were formed, and it contained furanoid, hydroxy aromatics, different types of carbonyl, and carboxyl groups, and also a significant amount of unsaturated hydrocarbon side chains (Boon et al., 1994). Further, at more severe heating, the furan polymer is observed to disproportionation through the loss of CO and CO2 that eventually led to the creation of aromatic polymer. In another similar experiment conducted by McGrath, he applied low temperature (30–300 degrees) to D-glucose and sucrose instead of using a higher temperature. He obtained pyrolyzed product furans, pyranones, anhydrosugars, and 5-hydroxymethylfurfural along with cellulose [24]. However, only at a higher temperature of 300 to 600 degrees, polycyclic aromatic hydrocarbons were detected (Fig. 5). The addition of oxygen to the combustion process reduced the number of polycyclic aromatic hydrocarbons emitted from the char, even at higher 400 to 600 degrees temperature. More complex condensed polycyclic aromatic hydrocarbons structures are formed through what McGrath indicates as a Diels-Alder reaction (McGrath et al., 2003).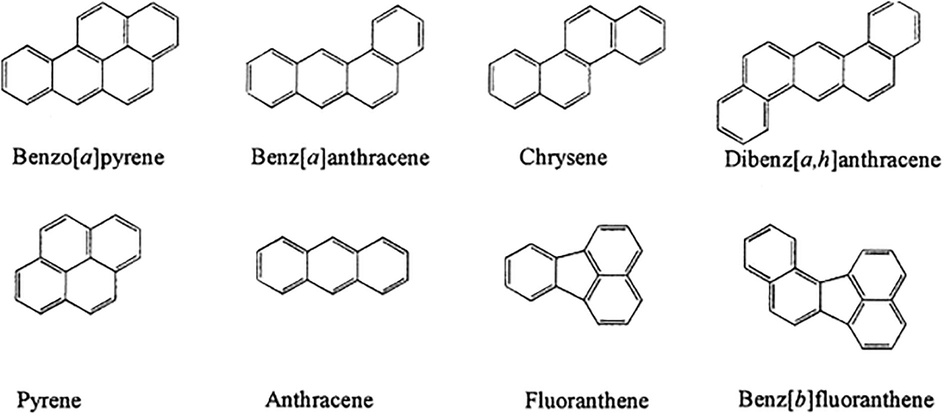
Examples of polycyclic aromatic hydrocarbons (PAHs).
On the other hand, pectin contains approximately 10 to 15% plant dry weight and is a water-soluble galacturonic-glycan. However, a gradual drop is observed in the amount of both the oxidative and non-oxidative pectin. At temperatures higher than 350 degrees, there is a significant drop by 20% of the initial note oxidative pectin substrate. Furthermore, when oxygen is introduced to the pectin, they were fully oxidized at higher 550 degrees (Waymack et al., 2004). NMR image shows that oxidized and non-oxidized char look virtually identical (Wang et al., 2016).
4.2 Lignin
Lignin is a complex natural noncarbohydrate polymer composed of phenylpropanoid subunits present in the plant vascular tissues and algae. Lignin provides strength and rigidity to wood and barks (Ekielski and Mishra, 2020; Prasad et al., 2021); it is recalcitrant, making it difficult to degrade. The lignin percentage in wood differs from 20% to 40%, whereas it goes down to 4% in grasses (Mishra et al., 2016). They make most of the wood and barks for lending stability to the plants, and fundamentally they do not get easily degraded. Phenylpropanol is a common polymer linked together by ether bonds and C–C bonds (Lüdemann and Nimz, 1973). Many volatile organic compounds are present in wood (lignocellulosic biomass). During a forest fire, these volatile organic compounds get exposed to heat and cause the emission of various gases. Lignin gets exposed to high temperatures that produce volatile gases. Exposing lignin to heat produces char and volatile products that are majorly substituted with methoxyphenyl.
According to Sharma et al. (2004), the highest char is produced at 500 to 600 degrees. However, the pyrolyzing of the pure lignin under the anoxic conditions at 450 degrees and 750 degrees yield 40% to 60%char, respectively (Sharma et al., 2004). Coke is formed when a volatile compound undergoes a condensation reaction. Such responses are absent under anoxic pyrolysis. Pyrolysis at 350C and 550C produce the same amount of char. However, at 550 degrees, only 20% could be collected as char, commonly observed through both procedures. Infrared and Solid-state nuclear magnetic resonance spectroscopy observation revealed that chemical alteration of lignin takes place due to dehydration at low temperatures. Despite there being some minimal decarboxylation occur at the high temperatures, the aromatic ring remains intact. The observation by Sharma et al. (2004) indicated that sodium and potassium in the reactions led to the enhancement of the de-volatilization but, on the other end, reduces the overall yield of char(Sharma et al., 2004).
4.3 Amino acid
Amino acids polymerize to form a protein molecule. When proteins are exposed to high temperatures, it decomposes through a reaction referred to as systematic and random depolymerization reaction. Thermal cyclization of amino acids occurs at high temperatures, and cyclic dipeptides are formed, scientifically referred to as diketopiperazines (Reeves Iii and Francis, 1998). The pyrolysis of amino acids leads to the formation of cyclic oligomers, and water is lost due to the nucleophilic attack of the N-terminal amino group on the C-terminal carbonyl group. The pyrolysis of polypeptides results in decarboxylation, deamination, lower dipeptides, and subsequent dehydration to give 2,5-diketopiperazine molecules. Eventually, in the reaction, ammonium is lost through the aliphatic side chains' homolysis to end up with a cyclic product (Chiavari and Galletti, 1992) (Fig. 6).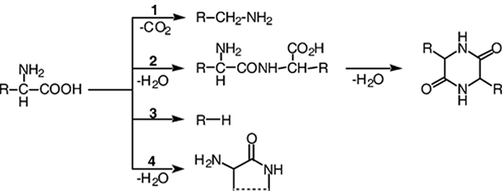
The primary mechanisms for amino acid thermal breakdown (Britt et al., 2004).
Cyclic products are formed at a higher temperature (840 degrees) due to the pyrolysis of aromatic hydrocarbons containing proteins, phenols, and their N-containing analogs (nitriles and anilines) (Knicker, 2007).
4.3.1 Maillard reaction
The Maillard reaction entails a complex set of chemical reactions between amino acids and reducing sugars, usually requiring heat. This reaction is responsible for the long-term stabilization of organic nitrogen into the soil.
Carbinolamine is formed because of a nucleophilic attack on the glucose molecule. Carbinolamine then dehydrates to give Schiff bases. Schiff base forms Amadori product after a series of slow rearrangement reactions. A mixture of many isoforms of Amadori products is formed during the Millard reaction Fig. 7.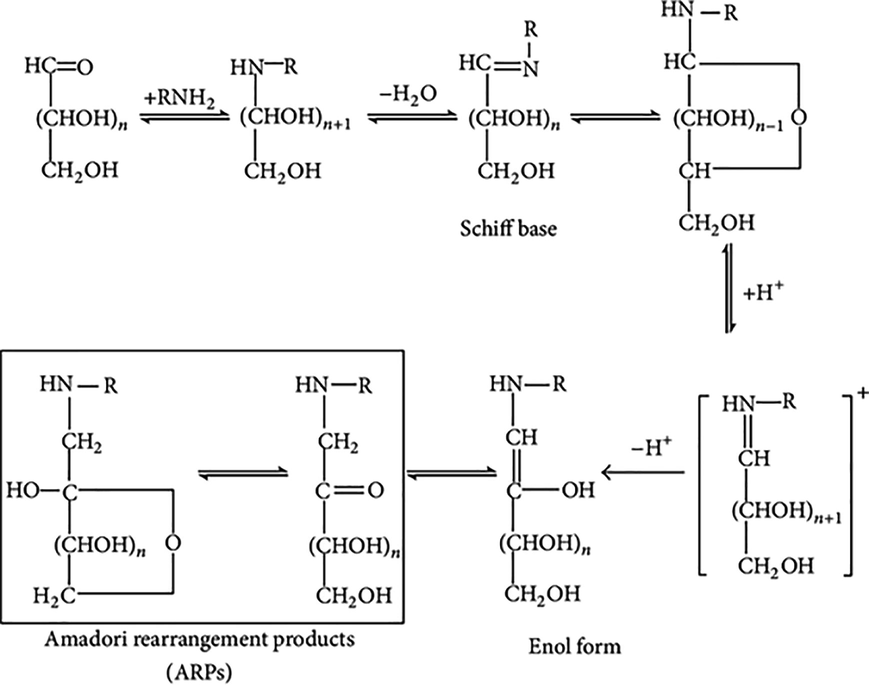
Chemistry of Maillard Reaction and Amadori rearrangement (Li et al., 2014).
4.4 Free Lipids
The lipid component of soil contains a large and diversified collection of hydrophobic compounds like fatty acids and more complex compounds such as terpenes, polynuclear hydrocarbons, chlorophyll, fats, waxes, sterols, and resins (Braids and Miller, 1975). Together with the waxes, Lipids are the significant contributors to soil water repellency (Stevenson, 1994). Lipids present in the soil undergo major structural alteration due to fire, lipid polymerase into the kerogen-like matter. This could be the main reason for the water repellant nature of burn soil. Fire-affected soil shows a lower percentage of lipid.
In contrast, burn soil has six times more lipid fraction than unburn soil, most likely due to the release of organic compounds from burning biomass. The main difference in the molecular arrangement of lipid before and after the fire is the arrangement of alkyl compounds. The lipid composition of burn soil shows a higher percentage of low molecular weight homologs units. The resin acid shows the dominance of dehydroabietic and secodehydroabietic acids and less presence of pimaric acid. Extensive study is required to understand the chemical composition of soil organic matter up to the molecular level(Schmidt and Noack, 2000).
4.5 Organic matter content
Most of the soil nutrients reside on the top surface of the soil, i.e., O or A horizon (Harrison et al., 2011). Soil organic matter is the main source of Nitrogen phosphorous Sulphur and an essential source of NH4, potassium, Calcium, Magnesium, Copper, iron, Manganese, and Zinc in soil (Lehmann et al., 2003). However, soil organic matter is most affected by fire (DeBano et al., 1998). The combustion process is often incomplete; it gives rise to pyrogenic organic matter and many high heterogeneous aromatic materials. The fire level happens to be highly variable due to different accumulative factors (Ye et al., 2017). These factors include the fire type: an underground or above-ground fire, how intense the fire was, and the slope. The observed effects will range directly from the extreme destruction of the organic matter to the addition of a surface layer that contributes to approximately 30% of surface input. It does not take after the forest fire for a sharp decrease in the organic matter of some soil to be observed. Gradually, it is accelerated by the notable change in the physic-chemical properties, such as water repellency and the temporal removal of the herbaceous insulating layer (Chandler et al., 1983). However, researchers have indicated that carbon–nitrogen ratio in the soil after a fire is way lower than the original fire, depending on the intensity of the cause fire. After a fire, the soil texture becomes coarser due to stable aggregates (Ulery and Graham, 1993).
4.6 Humic acids
Humic acid is usually an extract of the humic substances that form major organic constituents of soil. It is created by the microbial degradation of the matter contained in decaying dead plants such as charcoal. Heating this soil component will lead to an overall increase in the abundance of the humus fraction (Almendros et al., 2003). The fractions with a more thermolabile character have a more advanced effect. If this fraction possesses free organic matter and non-humified matter, it may contain a weak association with the mineral fraction. Humic acid has two polar functional groups, a phenolic group, and a carboxylic group. It cannot be dissolved in water in its acidic state. Thus, it is used as a soil supplement with agriculture experts(Liu et al., 2018). If the soils are consistently affected by a fire, the humus in the soil will correspond to stuffing up the black carbon.
4.7 Black carbon
The term black carbon is the term that is preferred by most literature personnel to refer to charcoal and other compounds of carbon such as elemental carbon and pyrogenic carbon(Doerr et al., 2018). In common, it is a black material that is usually emitted from different sources that burn fossil fuel. The combustion process of these fossil fuels and vegetation must be incomplete to allow carbon creation. The intensity of studies and analysis done for the black carbon has increased notably in the geochemical and biological studies on the soil (Wagner et al., 2018). This is because of the realization of the magnitude of its importance on different types of biogeochemical processes. Black charcoal is a ubiquitous material with varying forms of organizations that would range from low-rank coal and the likes to that of anthracite and coal resembling material (Kuhlbusch, 1998). The black carbon mainly formed in forest fire consists of randomly oriented layers of graphitic material pushed tightly together (Schmidt and Noack, 2000). However, in any case, this material, made of graphite, is rather an entirely amorphous, substantial alkyl domain and a sustainable oxygen content.
5 Impact of fire on the physical properties of soil
The physical structure of soil reflects the nature of its component. For example, pore-size distribution is determined by soil physical structure, which influences water flow and erosion and potential, microbiological, and faunal behavior. Properties of the soil that can be described in physical terms or equations are an outcome of physical forces. Fire in the forest may alter several physical soil properties, such as soil structure, porosity, wet ability, Ph, Color, texture, infiltration rates, and water holding capacity. The extent of fire effects is attributed to the fire severity.
5.1 Soil color and texture
The effects of fire on the color and texture are differently noticed in different conditions. For instance, the soil that gets burnt severely under concentrated fuel is highly noticeable as opposed to that soil moderately burnt. When the temperatures get to higher degrees, the soil changes to red due to the Fe-oxides transformation and completely eradicates organic matter(Ulery and Graham, 1993). However, in low temperatures or when the soil is exposed to moderate fire, its color changes to black or grey due to the ash content (Neary et al., 2005). However, at low temperatures that range from 300 to 600 degrees, the Munsell hues change to yellower. This is due to the drastic decrease in values and chromas (Ngole-Jeme, 2019). Spatially accumulating thermally produced iron oxides can be detected easily in the areas where the fire has affected a massive area. In the severely burned soil, the entire underlying layer gets blackened and thick up to 15 cm and causes an overall reduction of the Munsell value.
The impact of fire on soil differs depending on the kind of soil. Some soils such as sand, silt, and clay have a higher heat tolerance and usually are not affected by combustion unless directly exposed to temperatures higher than 400 degrees at the mineral soil surface. On the other end, clay holds minimal resistance to temperatures, and change is observable starting from 300 degrees. This is because the clay hydration and clay lattice structure break down (Beyers et al., 2005).
5.2 Soil pH and bulk density
Another physical aspect that gets affected is soil pH. Research indicated a general increase in the soil pH after the forest fire (Aref et al., 2011). At considerably high temperatures, there is rather more increase in the levels of pH. This can be attributed to the increased ash level, which holds higher acidity levels (Schafer, 2010). The other aspect affected is the bulk density, which involves the amount of dry soil per unit bulk volume and is expressed on the S.I unit of g/cm3. It is crucial concerning porosity. Porosity in the soil is pore volume in a soil sample divided by the total volume of the bulk sample. The collapsing of aggregates and blockage of spaces by ash and scattered clay particles increases the soil bulk density after a forest fire. The relation between the increase in soil bulk density and the combusted soil is that the bulk density increases alongside the ash depth (Cerda, 2009).
5.3 Soil aggregation
Soil aggregate stability refers to the flexibility of soil structure in response to applied external mechanical forces. It plays a critical role in re-establishing soil structure and function. Soil burning causes a significant decrease in the clay content of the soil. As a result, increase in sand-size course particles this initiates aggregation of finer particles into coarse particle. Some studies also showed no sign of an increase in the soil particle size (García-Corona et al., 2004). Soil aggregation is mainly affected by high-intensity fire; generally, low-intensity fires do not produce many notable changes. After a high-intensity fire, partial or complete soil combustion changes soil aggregation and has a different fluvial behavior than unburn soil (Mataix-Solera et al., 2011). The study by Katrin Burri suggests that Revegetation improves soil aggregate stability by speeding up vegetation growth and boosting soil formation processes (Burri et al., 2009). There is no regular pattern in soil aggregate stability of burnt soil. Stability may decrease, increase or remain the same. Table 2 summarizes some past studies showing the aggregate response to wildfire. Soil aggregation response to wildfire from different studies is summarized in Table 2.
Soil type
Fire severity
Time between burning and Measurement
Aggregate stability (AS) Methods
AS: burnt vs. unburntMore, Less, Same
References
Andosol
High
7–15 days
Number of Drops test
less
(Jordán et al., 2011)
Andosol
Low
7–15 days
Number of Drops test
same
(Jordán et al., 2011)
Haploxerolls
Low
7 days
Rainfall simulator
same
(Arcenegui et al., 2008)
Lithic Xerochrept
Low-High
0 day
Number of Drops test
more
(Ubeda and Bernia, 2005)
Lithic Xerochrept
Low-High
240 days
Number of Drops test
less
(Ubeda and Bernia, 2005)
Lithic Xerochrept
Low-High
976 days
Number of Drops test
more
(Ubeda and Bernia, 2005)
Orthents
No data
2–8 year
Wet Sieving
less
(Kavdır et al., 2005)
Orthents
No data
15 days
Wet Sieving
same
(Kavdır et al., 2005)
Typic Calcixeroll
Low
180–540 days
Rainfall simulator
same
(Mataix-Solera et al., 2002)
Typic Calcixeroll
High
180–540 days
Rainfall simulator
same
(Mataix-Solera et al., 2002)
Typic Haplumbrept
High
180 days
Wet Sieving
same
(Providoli et al., 2002)
Typic Xerorthent
Low
180–540 day
Rainfall simulator
more
(Mataix-Solera et al., 2002)
Xerorthents
Medium
7 days
Rainfall simulator
more
(Arcenegui et al., 2008)
6 Effect of fire on the chemical properties
Different chemical components of the soil are affected by a forest fire. Organic matter is one of them, and it holds the third most abundant terrestrial carbon pool (Lal, 2004). In the oceanic pool, organic matter is an essential component of ecosystem sustainability as this is the insulation against erosion and high temperatures. Furthermore, it holds the habitat to a significant number of biota (Craswell and Lefroy, 2001; González-Pérez et al., 2004).
The amount of organic matter post-fire declines immediately; however, it surpasses the pre-fire quantity in the long run. The organic matter undergoes a remarkable change after the fire, with a relative fraction of it becoming biochemically resistant. This could result from a combination of selective burning of fresh leftovers (leaves, twigs) and the generation of new (humic-like) aromatic compounds. (Almendros et al., 1992). Charring because of incomplete combustion can present for decades or centuries. Nutrient supply grows, often significantly, but only for a short time. Parts of organic nitrogen volatilize (Fisher and Binkley, 2000) and mineralize to ammonium, which adsorbs negative charges on the mineral and organic surfaces (Mroz et al., 1980). Eventually, Ammonium is biochemically converted into nitrate, which can be easily leached if not absorbed by cells (Covington and Sackett, 1992).
In a few years, nitrogen supply returns to pre-fire levels(Weston and Attiwill, 1990). Post-fire, organic P mineralizes to orthophosphate(Cade-Menun et al., 2000) due to volatilization; orthophosphate is not easily leached, but it precipitates as minimally accessible mineral forms. After a fire, magnesium, calcium, and potassium levels rise, but only for a short time. The depletion of organic matter lowers the exchange capacity proportionally(Badía and Martí, 2003). Base saturation rises due to the continual release of combusting organic materials (Macadam, 1987). However, on the other end, a fire has been attributed to the addition of nitrogen into the soil (Marion et al., 1991). As a result of burning the nutrients in the small twigs, they are volatized and lost into the atmosphere in a highly soluble form that eventually ends up in the soil. This adds up nutrients in the soil, which is further boosted by the indirectly altering of the soil environment. Nitrogen is the macronutrient profoundly affected by the forest fire as the other magnesium, calcium, and manganese have a much higher temperature threshold of 1107 to 1962 degrees (DeBano et al., 1998).
7 Biological properties
The soil microbes are the most affected soil components during forest fire as they are concentrated on the surface and remain 2.5 cm deep. Previous studies indicate that the immediate victims of the fire were the fungus than bacteria (Dumontet et al., 1996). However, the impact was so severe that the only remnants of the organism were usually left inactive and left only the produced spores as the residual for recovery after the fire. Microbial Carbon and Nitrogen content get dramatically affected at 600-celsius temperatures (Díaz-Raviña et al., 1996). The soil moisture on its own reduces the mortality rate of the microbial drastically due to fastened heat transfer. In addition, many toxic compounds are present in the soil; they get redistributed after the forest fire cause more mortality to soil microorganisms. An experiment to affirm this was conducted by Grasso et al.(1996) that ten days after the combustion, it was evident that the aerobic microbial population increased faster by 2.7 times compared to the controlled soil(Grasso et al., 1996). This is attributed to the deposition of ash to the soil after combustion that flushes nutrients, increasing the respiration rate after the forest fire. However, the leaching of the ash to the lower horizons leads to drop in the bacterial population after 30 days. Amazingly other studies have indicated that some microbial and fungi biomass took about ten years to recover from the fire (Fritze et al., 1993).
8 Water repellency
Soil-water repellency can be affected severely during forest fires, and its extent can depend on the burn severity, vegetation, type, soil moisture, soil texture, and time since burning (DeBano, 2000). During forest fires, soil water repellency increases when exposed to high temperatures. Fire-induced water repellency which remains highest at the soil surface gets affected by fire severity. Water repellency decreases with increasing soil depth and decreasing temperature. Fire-induced water repellency that remains highest at the soil surface is affected by high and moderate severity and decreases in strength with dropping temperatures and increased depth. The burnt hydrophobic organic soil matter gets volatized. This vapor eventually ends up moving downwards due to the temperature gradient and condenses on cooler soil particles at or below the soil surface, making them water repellent (Fig. 8). This involves forest fire eradicating the capacity of the soil organic horizon of storing water. The formation of hydrophobic layers on the soil particles and the presence of occasional hydrophobic organic matter results in a substantial reduction in wetting and infiltration, which may increase runoff and soil erosion (Robichaud and Hungerford, 2000). Water repellency in soils can lead to a loss of plant-available water, decreasing agricultural crop yield, and turf degradation on sports fields.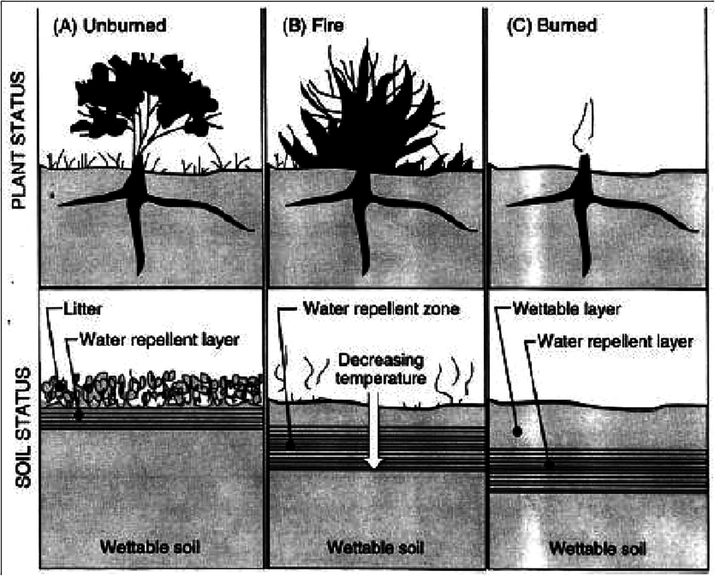
Alteration of Soil-water repellency by fire: (A) before the fire, most of the hydrophobic substances were just beneath the shrub (B) during the fire, hydrophobic substances move downward; (C) After the fire, a water repellent layer gets parallel to the soil surface (DeBano, 2000).
9 Conclusion
With changing climate, forest fires are likely to become more common. All type of fire (wild or prescribed) has a significant effect on forests. These fires make forest soil more water repellent, resulting in more runoff and soil erosion. Soil color, pH, bulk density, texture, and other variables are all compromised by fires. After burning, the shift in soil microbial population has a significant role in the reforestation of burned areas. The impact of fire on the soil organic matter is determined by various factors, including soil type, fire severity, and soil moisture. Consequently, the impact of fire on soil processes and their intensity is highly variable, and no generalized patterns can be established. After a forest fire, chemical changes in the soil are more significant. Soil biological properties are also greatly affected, as soil microorganisms and invertebrates are sensitive to high temperatures. Thus, it concludes that the belowground systems' overall response and the consequent process influence to the above-ground system are complex and highly variable. The impact of fire might end up as beneficial or hazardous to the ecosystem, depending on its severity. The effect of the short-term and the long-term will vary intensively. More research is needed to determine the consequences and changes in aggregates caused by fire on the functioning of the soil system. In conclusion, the overall effect of forest fire below and above ground is highly variable and cannot be easily predicted; sometimes, it might benefit the ecosystem if fire severity is not high. The range of effects of forest fire varies from short- to long-term impacts.
References
- Solid state NMR studies of fire-induced changes in the structure of humic substances. Science of the Total Environment. 1992;117-118:63-74.
- [Google Scholar]
- Rearrangement of carbon and nitrogen forms in peat after progressive thermal oxidation as determined by solid-state 13C-and 15N-NMR spectroscopy. Organic Geochemistry. 2003;34(11):1559-1568.
- [Google Scholar]
- Immediate effects of wildfires on water repellency and aggregate stability in Mediterranean calcareous soils. Catena. 2008;74(3):219-226.
- [Google Scholar]
- Effect of Forest Fires on Tree Diversity and some Soil Properties. International Journal of Agriculture & Biology. 2011;13
- [Google Scholar]
- Plant ash and heat intensity effects on chemicaland physical properties of two contrasting soils. Arid Land Research and Management. 2003;17(1):23-41.
- [Google Scholar]
- Total carbon and nitrogen in the soils of the world. European journal of soil science. 1996;47(2):151-163.
- [Google Scholar]
- Convection heat transfer. John wiley & sons; 2013.
- Beyers, J.L., Brown, J.K., Busse, M.D., DeBano, L.F., Elliot, W.J., Ffolliott, P., Jacoby, G.R., Knoepp, J.D., Landsberg, J.D., Neary, D.G., 2005. Wildland fire in ecosystems: Effects of fire on soil and water. Rocky Mountain Research Station Technical Report.
- Structural studies on cellulose pyrolysis and cellulose chars by PYMS, PYGCMS, FTIR, NMR and by wet chemical techniques. Biomass and Bioenergy. 1994;7(1-6):25-32.
- [Google Scholar]
- Does glucose enhance the formation of nitrogen containing polycyclic aromatic compounds and polycyclic aromatic hydrocarbons in the pyrolysis of proline? Fuel. 2004;83(11-12):1417-1432.
- [Google Scholar]
- Revegetation measures improve soil aggregate stability: a case study of a landslide area in Central Switzerland. Forest Snow and Landscape Research. 2009;82:45-60.
- [Google Scholar]
- Phosphorus forms and related soil chemistry of Podzolic soils on northern Vancouver Island. II. The effects of clear-cutting and burning. Canadian Journal of Forest Research. 2000;30(11):1726-1741.
- [Google Scholar]
- Fire effects on soils and restoration strategies. CRC Press; 2009.
- Certini, G., 2005. Effects of fire on properties of forest soils: a review. Oecologia 143, 1–10. https://doi.org/10.1007/s00442-004-1788-8
- Chan, C.-Y., Engling, G., Sang, X., Zhang, T., 2011. Biofuel Combustion Emissions-Chemical and Physical Smoke Properties, in: Environmental Impact of Biofuels. InTech.
- Fire in forestry. Volume 1. Forest fire behavior and effects. Forest fire management and organization. 1983;Volume 2
- [Google Scholar]
- Pyrolysis—gas chromatography/mass spectrometry of amino acids. Journal of Analytical and Applied Pyrolysis. 1992;24(2):123-137.
- [Google Scholar]
- Soil mineral nitrogen changes following prescribed burning in ponderosa pine. Forest Ecology and Management. 1992;54(1-4):175-191.
- [Google Scholar]
- Managing Organic Matter in Tropical Soils: Scope and Limitations. Dordrecht: Springer Netherlands; 2001. p. :7-18.
- [CrossRef]
- The role of fire and soil heating on water repellency in wildland environments: a review. Journal of hydrology. 2000;231-232:195-206.
- [Google Scholar]
- Fire effects on ecosystems. John Wiley & Sons; 1998.
- Bacterial activity in a forest soil after soil heating and organic amendments measured by the thymidine and leucine incorporation techniques. Soil Biology and Biochemistry. 1996;28(3):419-426.
- [Google Scholar]
- Fire as a removal mechanism of pyrogenic carbon from the environment: effects of fire and pyrogenic carbon characteristics. Frontiers in Earth Science. 2018;6:127.
- [Google Scholar]
- Post-fire soil microbial biomass and nutrient content of a pine forest soil from a dunal Mediterranean environment. Soil Biology and Biochemistry. 1996;28(10-11):1467-1475.
- [Google Scholar]
- Lignin for Bioeconomy: The Present and Future Role of Technical Lignin. Int J Mol Sci. 2020;22:63.
- [CrossRef] [Google Scholar]
- Ecology and Management of Forest Soils. New York, USA: John Willey & Sons. Inc.; 2000.
- Recovery of soil microbial biomass and activity from prescribed burning. Canadian Journal of Forest Research. 1993;23(7):1286-1290.
- [Google Scholar]
- Effects of heating on some soil physical properties related to its hydrological behaviour in two north-western Spanish soils. International Journal of Wildland Fire. 2004;13(2):195.
- [CrossRef] [Google Scholar]
- The effect of fire on soil organic matter—a review. Environment international. 2004;30(6):855-870.
- [Google Scholar]
- Effects of heating on the microbial populations of a grassland soil. International Journal of Wildland Fire. 1996;6(2):67.
- [CrossRef] [Google Scholar]
- Griffiths, J.F., 2019. Flame and combustion. Routledge.
- Deep soil horizons: contribution and importance to soil carbon pools and in assessing whole-ecosystem response to management and global change. Forest Science. 2011;57:67-76.
- [Google Scholar]
- HYVÄRINEN, E., Kouki, J., Martikainen, P., 2009. Prescribed fires and retention trees help to conserve beetle diversity in managed boreal forests despite their transient negative effects on some beetle groups. Insect Conservation and Diversity 2, 93–105.
- Effect of fire severity on water repellency and aggregate stability on Mexican volcanic soils. Catena. 2011;84(3):136-147.
- [Google Scholar]
- Heat conduction. CRC Press; 2018.
- Soil aggregate stability and 13C CP/MAS-NMR assessment of organic matter in soils influenced by forest wildfires in Canakkale, Turkey. Geoderma. 2005;129:219-229.
- [Google Scholar]
- How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry. 2007;85(1):91-118.
- [Google Scholar]
- Fire regimes and ecosystems: An overview of fire ecology in tropical ecosystems. In: Proceedings of the Forest Fires in India. 2007.
- [Google Scholar]
- Soil carbon sequestration impacts on global climate change and food security. science. 2004;304(5677):1623-1627.
- [Google Scholar]
- Lehmann, J., Kern, D., German, L., Mccann, J., Martins, G.C., Moreira, A., 2003. Soil fertility and production potential, in: Amazonian Dark Earths. Springer, pp. 105–124.
- Leisted, R., 2014. Design Fire for Building Content in Arson Scenarios. LUTVDG/TVBB.
- Li, C., Wang, H., Juárez, M., Ruan, E.D., 2014. Structural Characterization of Amadori Rearrangement Product of Glucosylated Nα-Acetyl-Lysine by Nuclear Magnetic Resonance Spectroscopy. International Journal of Spectroscopy 2014.
- Assessing the influence of humic acids on the weathering of galena and its environmental implications. Ecotoxicology and environmental safety. 2018;158:230-238.
- [Google Scholar]
- Carbon-13 nuclear magnetic resonance spectra of lignins. Biochemical and biophysical research communications. 1973;52(4):1162-1169.
- [Google Scholar]
- Effects of broadcast slash burning on fuels and soil chemical properties in the sub-boreal spruce zone of central British Columbia. Canadian Journal of Forest Research. 1987;17(12):1577-1584.
- [Google Scholar]
- Fire severity, ash deposition, and clipping effects on soil nutrients in chaparral. Soil Science Society of America Journal. 1991;55(1):235-240.
- [Google Scholar]
- Fire effects on soil aggregation: a review. Earth-Science Reviews. 2011;109(1-2):44-60.
- [Google Scholar]
- Soil organic matter and aggregates affected by wildfire in a Pinus halepensis forest in a Mediterranean environment. International Journal of Wildland Fire. 2002;11(2):107.
- [CrossRef] [Google Scholar]
- Low temperature mechanism for the formation of polycyclic aromatic hydrocarbons from the pyrolysis of cellulose. Journal of Analytical and Applied Pyrolysis. 2003;66(1-2):51-70.
- [Google Scholar]
- Mishra, P.K., Gregor, T., Wimmer, R., 2016. Utilising Brewer’s Spent Grain as a Source of Cellulose Nanofibres Following Separation of Protein-based Biomass. BioResources 12. https://doi.org/10.15376/biores.12.1.107-116
- Effects of fire on nitrogen in forest floor horizons. Soil Science Society of America Journal. 1980;44(2):395-400.
- [Google Scholar]
- Neary, D.G., Leonard, J.M., 2020. Effects of fire on grassland soils and water: A review. In: Kindomihou, Valentin Missiako, ed. Grasses and grassland aspects. IntechOpen. Online: https://www. intechopen. com/books/grasses-and-grassland-aspects/effects-of-fire-on-grassland-soils-and-water-a-review.
- Neary, D.G., Ryan, K.C., DeBano, L.F., 2005. Wildland fire in ecosystems: effects of fire on soils and water. Gen. Tech. Rep. RMRS-GTR-42-vol. 4. Ogden, UT: US Department of Agriculture, Forest Service, Rocky Mountain Research Station. 250 p. 42.
- Fire-Induced Changes in Soil and Implications on Soil Sorption Capacity and Remediation Methods. Applied Sciences. 2019;9(17):3447.
- [CrossRef] [Google Scholar]
- Parson, A., Robichaud, P.R., Lewis, S.A., Napper, C., Clark, J.T., 2010. Field guide for mapping post-fire soil burn severity. Gen. Tech. Rep. RMRS-GTR-243. Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station. 49 p. 243.
- Recent advancements in lignin valorization and biomedical applications: A patent review. Recent Patents on Nanotechnology. 2021;15
- [CrossRef] [Google Scholar]
- Post-fire management and splash erosion in a chestnut coppice in southern Switzerland. Forest Ecology and Management. 2002;162(2-3):219-229.
- [Google Scholar]
- PYROLYSIS-GAS-CHROMATOGRAPHY FOR THE ANALYSIS OF PROTEINS: WITH EMPHASIS ONFORAGES. Book Chapter. 1998
- [Google Scholar]
- Long-term effects of forest fires on soil greenhouse gas emissions and extracellular enzyme activities in a hemiboreal forest. Science of the Total Environment. 2020;718:135291.
- [CrossRef] [Google Scholar]
- Water repellency by laboratory burning of four northern Rocky Mountain forest soils. Journal of Hydrology. 2000;231–232:207-219.
- [CrossRef] [Google Scholar]
- Effects of fire on nutrient availability and limitation in Florida scrub ecosystems. University of Florida; 2010.
- Black carbon in soils and sediments: analysis, distribution, implications, and current challenges. Global biogeochemical cycles. 2000;14(3):777-793.
- [Google Scholar]
- Characterization of chars from pyrolysis of lignin. Fuel. 2004;83(11-12):1469-1482.
- [Google Scholar]
- Fire severity, changing scales, and how things hang together. International Journal of Wildland Fire. 1991;1(1):23.
- [CrossRef] [Google Scholar]
- Humus chemistry: genesis, composition, reactions. John Wiley & Sons; 1994.
- Forest resilience, biodiversity, and climate change. Presented at the Secretariat of the Convention on Biological Diversity, Montreal. Technical Series. 2009;no. 43. 1–67:1-67.
- [Google Scholar]
- The effect of wildfire intensity on soil aggregate stability in the Cadiretes Massif. NE Spain. IAHS PUBLICATION. 2005;299:37.
- [Google Scholar]
- Forest fire effects on soil color and texture. Soil Science Society of America Journal. 1993;57(1):135-140.
- [Google Scholar]
- Verma, S., Jayakumar, S., 2012. Impact of forest fire on physical, chemical and biological properties of soil: A review. proceedings of the International Academy of Ecology and Environmental Sciences 2, 168.
- Dissolved black carbon in aquatic ecosystems. Limnology and Oceanography Letters. 2018;3(3):168-185.
- [Google Scholar]
- Multidimensional solid-state NMR spectroscopy of plant cell walls. Solid state nuclear magnetic resonance. 2016;78:56-63.
- [Google Scholar]
- Effects of metal salts on char oxidation in pectins/uronic acids and other acid derivative carbohydrates. Fuel. 2004;83(11-12):1505-1518.
- [Google Scholar]
- Effects of fire and harvesting on nitrogen transformations and ionic mobility in soils of Eucalyptus regnans forests of south-eastern Australia. Oecologia. 1990;83(1):20-26.
- [Google Scholar]
- Factor contribution to fire occurrence, size, and burn probability in a subtropical coniferous forest in East China. PloS one. 2017;12(2):e0172110.
- [CrossRef] [Google Scholar]
- Effect of fires on soil organic carbon pool and mineralization in a Northeastern China wetland. Geoderma. 2012;189-190:532-539.
- [Google Scholar]
- Initial reaction mechanisms of cellulose pyrolysis revealed by ReaxFF molecular dynamics. Fuel. 2016;177:130-141.
- [Google Scholar]







