Time dependent inhibition of Morinda citrifolia essential oils against multi drug resistant bacteria and A549 lung cancer cells
⁎Corresponding author: Wen-Jun Li, State Key Laboratory of Biocontrol, Guangdong Provincial Key Laboratory of Plant Resources and Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), School of Life Sciences, Sun Yat-Sen University, Guangzhou 510275, PR China. liwenjun3@mail.sysu.edu.cn (Wen-Jun Li)
⁎⁎Corresponding author: Dr. G. Ramachandran, Department of Marine Science, Bharathidasan University, Tiruchirappalli, Tamil Nadu 620024, India. ramachandhiranmicro@gmail.com (Govindan Ramachandran),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Recent years, multi-drug resistant bacteria (MDRs) and lung cancer cells are threatening to human worldwide due to failure of sufficient drugs. It was suggested that the new sources needed to discover new varieties of drugs, antibiotics and other format to eradicate these infections. As like, the medicinal plant of Morinda citrifolia (M. citrifolia) was used in this study to inhibit both the bacterial infection and cancer cells. First, the LC-MS measurement was evidently reported the available essential oils from medicinal plant of M. citrifolia. It has excellent anti-bacterial properties against P. mirabilis, E. coli, P. aeruginosa and S. aureus with 24, 22, 26 and 18 zone of inhibition respectively by agar well diffusion. Subsequently, the liquid interference assay was exhibited 97 %, 96 %, 94 % and 90 % inhibition at 1000 µg/mL concentration for all the tested pathogens. In addition, M. citrifolia EOs have excellent anti-bacterial activity, and it arrested the bacterial growth completely at 1000 µg/mL concentration for 24 h after compared with other time intervals. Subsequently, the intracellular membranes and more death cells with condensed chromosome like structure were effectively observed by confocal laser scanning electron microscope. Then, invitro cytotoxicity result of M. citrifolia EOs treated A549 lung cancer cells was clearly observed. The cytotoxicity result confirmed that the 500 µg/mL of M. citrifolia EOs against A549 lung cancer cells as fixed as IC50 dose. Hence, current study conclude, M. citrifolia is the excellent medicinal plant to inactivate bacterial growth and cancer cells efficiently.
Keywords
Medicinal plant
EOs
Biological properties
Intracellular inactivation, cytotoxicity assay
Anti-cancer activity
1 Introduction
In traditional culture plant and plant-derived medicines are used all over the world. Plants produces numerous chemicals which are not necessary for the plant growth and most of them are from same origin background or arisen from same chemical scaffolds like terpenoids, ketides, etc., So scientist interested in its widespread applications and less toxicity(Yun et al., 2012). Likewise, the Rubiaceae family of M. citrifolia is an important folk medicine having significant biological properties. It is initially discovered and used for medicinal purpose in Tropical and sub-tropical region of Asia and Hawaii. Polynesians migrated 2000 years ago from South East Asia, during that time they brought many plants with them. In that M. citrifolia is the second most common medicinal plant among twelve (Wang et al., 2002). Previously, excellent pharmacological properties of the M. citrifolia were more effective against diabetics, tuberculosis, jaundice, liver infections and cancer diseases (Chan-Blanco et al., 2006). All the parts of leaves, roots, seeds of M. citrifolia was very effective and it shown excellent anti-microbial (Zhang et al., 2016), anti-oxidant (Xue et al., 2023), anti-cancer (Rajivgandhi et al., 2020) and some other infectious diseases (Karuppaiah et al., 2020).
All the organic solvents of ethanol, ethyl acetate, alcohol, dichloromethane and petroleum ether are utilized to extract the bioactive compounds from M. citrifolia leaves, roots, barks and seeds (De La Cruz-Sánchez et al., 2019). The report of Zhang et al. (2016) suggested more phenolic derivatives were extracted from M. citrifolia leaves. Among the medicinal plants, M. citrifolia is the most favourable plant used as a traditional medicine having anti-microbial, anti-cancer, anti-oxidants and anti-tuberculosis agent. More bioactive compounds has been derived from M. citrifolia notably, anthraquinones, flavonoids and coumarins. Recently, the anti-oxidants and anti-inflammatory properties form M. citrifolia has been proved in the previous reports of Zhang and Zhang (2022).
M. citrifolia leaves, root, steam, bark, fruits and flower each parts are involved in different combination for almost 40 known herbal remedies. It has more phenolic derivatives of asperuioside, acubin, quinones and some other chemical constituents were showed with anti-bacterial agents. Then the two new derivatives of anthraquinones demonstrated as an anti-viral agent. Other than the reported parts of the plants, fruit juices of M. citrifolia is one of the significant agent for various infectious diseases like high blood pressure, arthritis, heart diseases, cancer, AIDS, gastric ulcer, atherosclerosis, mental depression and blood vessel problems (Wang et al., 2002). The diabetic induced rat treated with fermented noni juice were reduced the blood glucose level, nothing but it also induces the stimulation of β-cells to survive and secrete more insulin. Anti-oxidative property is the vital advantage in M. citrifolia above all. In addition, M. citrifolia enhances the anti-oxidative ability by decreasing tissue acidosis, acid-basic balance and increasing tissue oxygenation on the whole. It shows anti-oxidative property higher than both α-tocopherol and BHT because of the potential to scavenging the free radicals (Zin et al., 2017). Taking to the advantages, fruit juices of M. citrifolia is the most significant anti-cancer agent, particularly affect the DNA of carcinogenic infections. It also intercepts cancer at early stage by antioxidant property.
EOs is complex, volatile compound having strong odour formed by plants. These are secondary metabolites plays a pivotal role in protecting plants as insecticides, antifungal, antibacterial, antifungal agent. Most of the EOs was obtained from different part of various aromatic plants. These are metabolites can inhibit or slowdown the growth of microbes and they will target cytoplasm, membrane and occasionally it may change the structure of the organism. Essential oil contains of any of these three compounds such as terpenes, aromatic compound and isoprenoids. M. citrifolia have EOs that the major component is octanoic acid and hexanoic acid is two major compound present in both ripe and un ripened fruit show high antibacterial activity properties. Then the antioxidative property also tested showing significant amount of hydrogen bond donation. So it has adequate amount of anti-oxidant property which prevent cancer early stage itself (Esath Natheer, 2012).
2 Materials method
2.1 Screening of important EOs
Based on the tap water washing, the uncontaminated leaves of M. citrifolia was collected from drought land, which is available in Pottanam Village, Namakkal District, Tamil Nadu, India. Initially, the surface contamination of the fungal infection, organic substances, floating materials and other epiphytes were successfully eliminated using water and 70 % ethanol solution. The samples were dried and ground fine powder using mortar and pestle. In this process, large quantities of the leaves were grinded and received the high yield for various application processes. Then the process was started with Clevenger model instrument of hydro distillation method for 12 h. In hydro distillation method, 10 g of plant material plus 1L of n-hexane were used to separate the EOs with the help of earliest report Ashrafi et al., 2019. The short procedure was given below, 1: 2 dilution concentration of plant material plus double distilled water was utilized to heat at 90 ℃ until the color clear changes appeared using heating mantle for heat. The process was run with uninterrupted time of 1 h for room atmosphere. Then, colloid behavior of oil layer containing cooled tube was collected separately from receiver tube and followed by separate the water plus EOs using separating funnel. Both the mixture was clearly filtered by each tubes and the quantity of liquids were measured by measuring cylinder. The final dried process of mixture EOs-water combination was started using sodium sulphate. Finally, the dried extract of EOs containing solution was separately stored in sterile tube for further use.
2.2 LC-MS detection of available EOs in M. citrifolia
Based on the ability of EOs production, the M. citrifolia extract has been subjected to LC-MS analysis for discover the complete chemical profile and EOs with evidenced procedure of Jamal et al. (2021). In LC-Ms, the capillary column of HP-5 MS was attached with the machine and the exact procedure of Chrome Pack of Slicon 5 and 30 m x 250 μm x 0.25 μm. The oven temperature of 40 °C was set initially and secondary at 120 °C with 10–15 min anf flow rate of 4 °C/min was maintained in whole process. The temperature of 50–260 °C was used constantly in injector and detector places. 40 s of carrier gas plus 1 mL/min flow rate were maintained. Lastly, the result was scanned by LC-MS. All those result was checked by previous reports for confirmation, and proved by NIST library, Sun Yat-Sen University, China.
2.3 Biological properties of EOs against MDRs pathogens
Antimicrobial activities of M. citrifolia EOs against P. mirabilis, E. coli, P. aeruginosa and gram positive S. aureus by agar well diffusion assay (De La Cruz Sanchez et al., 2019). Firstly, cultures were allowed to grown in nutrient broth until 48 h to receive matured cultures. The well matured log phase cultures were taken and spread on freshly prepared sterile muller hinton agar (MHA). Subsequently, wells cut on the agar surface with needed gap between the wells. Following, the different concentration of M. citrifolia EOs were treated into all wells at room temperature for one day. For control, ceftazidime was used as an antibiotic. Then, the zone formation around the EOs treated wells were monitored carefully and measured the zone of inhibition for confirmed the biological effect of the EOs.
2.4 Liquid broth dilution of bacterial inhibition using EOs
Weather the screened EOs having anti-bacterial activity at concentration dependent or not was effectively confirmed by liquid broth assay using 24-polystrene plate (Feng et al., 2020). In this liquid broth assay, the inhibition rate of the bacterial growth was quantified with 540 nm wavelength by UV-spectrophotometer using microtire reader. Briefly, 100–1000 µg/mL concentration of M. citrifolia EOs were treated into the 24-wells before filled with nutrient broth plus 25 µl of well grown bacterial cultures separately. The plates were gently rotate to spread the EOs evenly on the bacterial cultures and avoid without over flow the culture at 37 ℃. Then, more turbidity based bacterial growth inactivation was observed in all the wells and detected whether the M. citrifolia EOs were inhibited the MDRs bacteria at increasing concentration or not. Following, all the turbidity based results of the wells were utilized to analyzed their inhibition rate through microtitre plate reader at 600 nm and interpreted the results after compared with control O.D value result. Based on the inhibition at increased concentration was used to fix the MIC of M. citrifolia EOs. The bacterial growth arrest was checked based on standard chemical formula,
2.5 Time dependent bacterial killing assay
This experiment was used to detect the bacterial growth inactivation based on the time interval and this method (Rajivgandhi et al., 2020). First, the 24-well plate was filled by 1 mL of tryptic soy broth (TSB) and followed by add 25 mL of 24 h matured bacterial cultures of P. mirabilis, E. coli, P. aeruginosa and gram positive S. aureus and followed by M. citrofolia EOs were treated into the respective wells of the all the plates separately using increasing concentration of 100–1000 µg/mL. The plates were allowed to decrease the bacterial growth due to the influence of M. citrifolia EOs. Then, the plates containing cultures were shifted to centrifuged tubes and centrifuged at 7, 500 rpm for 20 m and followed by collected the pellet after discard the supernatant. Subsequently, the pellet of the each culture samples were diluted by rose well park memorial institute 1640 medium. This medium was used to provide the nutrient for live cells. Then, the plates were incubated at 37 ℃ for one day time interval. Here, every 6 h of the treatment process monitored and alalyzed the bacterial growth degradation using UV-spectroscopy at 600 nm O.D value. Finally, 6–24 h time interval of the M. citrifolia EOs treated cultures result was taken carefully by UV-spectroscopy at same 600 nm O.D value.
Parallel, the cell cycle and doubling time of the M. citrifolia EOs treated time interval was fixed to detect the efficiency of bacterial growth in nutrient agar (Maruthupandy et al., 2020). This experiment was followed by. The 6 h, 12 h and 24 h M. citrifolia EOs treated cultures were streaked on nutrient agar plate and put it in incubation at room atmosphere for one day. Then, the growth was emerged on the agar surface or not was clearly monitored for all the time interval and confirmed the M. citrofilia efficiency against selected bacteria.
2.6 Dual staining assay for detection of damaged cells
Weather the M. citrifolia EOs have destroy the internal cells or internal virulence factors into the selected bacteria P. mirabilis, E. coli, P. aeruginosa and S. aureus were confirmed by AO/EB based CLSM (Tebatso et al., 2022). M. citrifolia EOs treated bacteria was taken in separate centrifuge tube and done the centrifuge at 7, 500 rpm for 25 min two times. Then, pellet was filtered after removal of supernatant and mixed with PBS. Then, the PBS plus pellets were taken together into the centrifuge tube at 7, 500 rpm for 5 min and pellet was removed by AO/EB fluorescence dye in the concentration of each 10 µl. Then, the tubes were covered by black cloth and carefully fixed the tested samples on cover glass slips separately. Then, the treated and untreated fixed bacterial cultures of cover slips were visualized by CLSM (Shimadzhu, Japan). Finally, the damages or normal intracellular changes were interpreted for advantages of M. citrifolia EOs.
2.7 Cytotoxicity evaluation of M. citrifolia EOs
The dimethylthiazol-diphenyltetrazolium bromide (MTT) assay was performed for M. citrifolia EOs against A549 lung cancer cells. The defined 48 h culture was diluted in DMEM medium and taken into 96-well plate (Rajivgandhi et al., 2020). Then, M. citrifolia EOs was subsequently treated into 96-well containing culture at CO2 incubator at 5 % and 95 % humidity. For the treatment, 100–1000 µg/mL of M. citrifolia EOs was used and not treated wells as control. The incubation was maintained at 37 ℃ in CO2 incubator for one day. The detachment of the sample was taken and added MTT solution (30 µl) in all the wells. Then, PBS was used to dilute in the samples and packed by aluminium foil at dark room temperature for 6 h. After, plates were clearly cooled, then subsequently added the formazan solution into all the wells. The turbidity of the samples was monitored and checked the color intensity on the culture. Then, the spectrophotometer of the samples were analysed at 660 nm and detect the IC50 value.
Percentage of IC50 = M. citrifolia EOs treatment value - M. citrifolia EOs non-treatment value/ M. citrifolia EOs non-treatment value.
3 Result
3.1 Available EOs detection from M. citrifolia by LC-MS analysis
The LC-MS result of Fig. 1 confirmed that the EOs of α- pinene, borneol, β-pinene, α-terpineol, thujene, L-rubiadin, myrcene, α-pinene, terpinen-4-ol, α-copaene, P-aucubin, sabinene, β-farnesene, L-scopoletin, β-phellandrene, terpinene, sabinene, thymol, cubenol and octanone were screened. Also, the structures and chemical formula are highly matched to these EOs, and confirmed (Rajivgandhi et al., 2020). The availability of EOs was separated from other phytochemical derivatives and bioactive compounds using NIST, Wiley 2017 library information of Sun Yat-Sen University, India. Before separated the EOs, the 30 numbers of the peaks were received. Peaks are including the phytochemical derivatives, bioactive metabolites, organic compounds and EOs. Among these peaks, the highest peaks were checked based on the NIST Library result and confirmed that the peaks were EOs peak. In addition, the bioactive chemical compounds of ɑ-norenone, Thieno[3,2-e] benzofuran, 2-proppropenal, 3–91-aziridinyl)-3-(dimethylamino), L-scopoletin, 5-Pyrrolidino-2-pyrrolidine, 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane, 5-Pyrrolidino-2-pyrrolidine, nordamnacanthal, hexahydro-3, hexadecane, Phenol, 2,5-bis (1,1-dimethyl ethyl), morindadiol were present in the extract. All these chemical derivatives with various biological properties of larvicidal, anti-viral, anti-biofilm, anti-fungal and anti-cancer activities were reported previously by Suthagar Pillai et al. (2012); Su et al. (2005); Ahmed et al. (2020); Dey et al. (2022). In specific, most of the drug leading compounds were inhibited the various cancer cell. Previously, more researchers were reported that the M. citrifolia is the important plant and it has excellent anti-cancer properties against various cancer cell (Ramachandran et al., 2020). Compared with other plant and plant compounds, M. citrifolia has excellent anti-cancer compounds naturally including nordamnacanthal (Campos et al., 2017), morindadiol (Rajivagndhi et al., 2020), L-scopoletin (Marques et al., 2021), and ɑ-norenone (Suthagar Pillai et al., 2012). All these compounds were frequently reported from M. citrifolia extract with enhanced anti-cancer activities against various cancer cells. Also, this information was accepted by recently reported article of Viriya et al. (2011). In addition, the M. citrifolia leaves, fruits, stem, seeds, parks and various other parts were also possess proved anti-cancer properties (Su et al., 2005). LC-MS result, the 20 different EOs were highly occupied the 20 different places, and it covered 115460, 14562, 12340, 213463, 267543, 908324, 87610, 238765, 2378623, 1190267, 9011922, and 6740921. Also, the retention times of 64.2, 34,56, 40, 45, 60, 20, 34,70, 22, 34, 33, 09, 11,45, 20, 45, 13.45, 9,12, 18, 64 (Table 1). Sometimes, the environmental factors were also influenced the biological properties of M. citrifolia. Also, it has the ability to grow in dry land and paddy field of the clay, lake and water dumped places (Anvy Susan et al., 2017). All those factors were also contributed to influence the M. citrifolia growth and stimulate the hormones to produce excess level of anti-cancer properties of the compounds (Rajivagndhi et al., 2020). Further, the plant of M. citrifolia is the important plant in anti-microbial and anti-cancer study. Importantly, all the available compounds, phytochemical derivatives and EOs are very active against various cancer cells inactivation. So, in future, the complete phytochemical profile of M. citrifolia is used to help inhibit the various infectious diseases effectively. Also, LC-MS is the needed instrument to found the available chemical molecules of plant material.
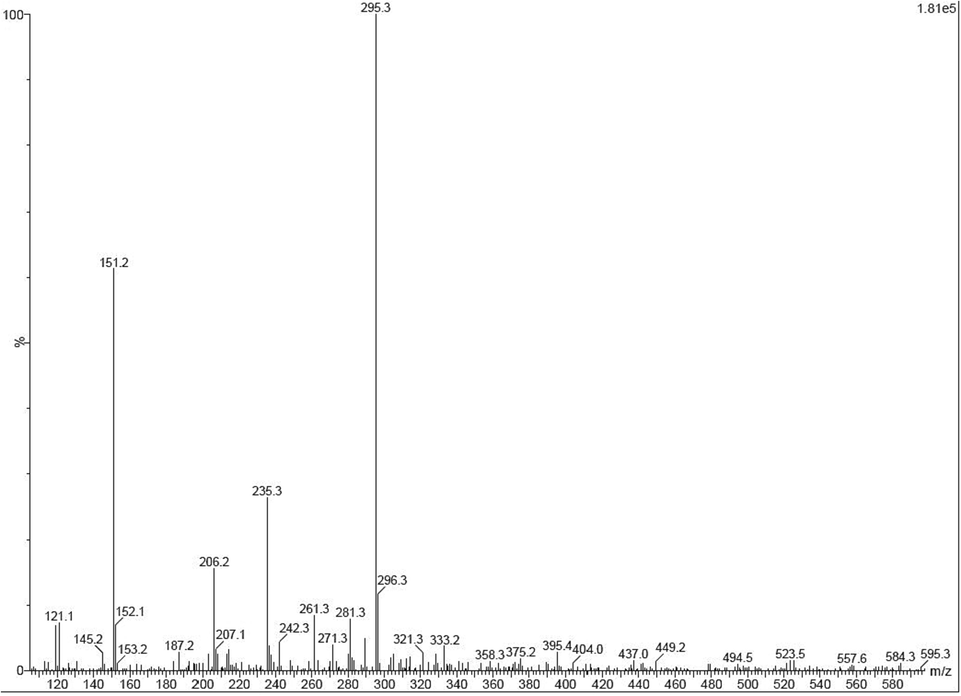
- Detection and confirmation of available EOs presence in M. citrifolia by LC-MS measurement.
| S. no | Compound name | Biological properties | Retention time | References |
|---|---|---|---|---|
| 1 | Thieno[3,2-e] benzofuran | Antimicrobial and anti-cancer activities | 30.8 | Hena and Shamsuzzaman, 2015 |
| 2 | 2-proppropenal | Anti-microbial activities | 22.2 | Izabela and Tomasz, 2019 |
| 3 | 3-(1-aziridinyl)-3-(dimethylamino) | Anti-microbial activities | 19.5 | Savin et al., 1979 |
| 4 | L-scopoletin | Anti-cancer activity | 28.4 | Dey et al., 2022 |
| 5 | 5-Pyrrolidino-2-pyrrolidine | Anti-cancer activity | 18.0 | Thangam et al., 2013 |
| 6 | 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane | Anti-biofilm activity | 16.0 | Rajivgandhi te al., 2020 |
| 7 | Nordamnacanthal | Anti-cancer activity | 15.2 | Saiful et al., 2021 |
| 8 | Hexadecane | Anti-microbial activity | 17.5 | Salima et al., 2021 |
| 9 | Phenol, 2,5-bis (1,1-dimethyl ethyl) | Anti-fungal activity | 14.5 | Thangarasu et al., 2021 |
| 10 | Morindadiol | Anti-cancer | 12.4 | Rajivgandhi et al., 2020 |
3.2 Inactivation of bacterial growth using M. citrifolia EOs
The result of anti-bacterial activity of this present study was suggested that the M. citrifolia is the excellent medicinal plant to perform against MDRs bacteria. In addition, the EOs of M. citrifolia was excellent choice to eradicate the bacterial viability and it the clear zones around the tested bacterial wells were shown at 1000 µg/mL concentration. The result of zone measurement was differed for bacteria to bacteria, but all the bacteria was shown highest inhibition level at 1000 µg/mL concentration. Among the MDRs pathogens, 24 mm, 22 mm, 26 mm and 18 mm zones around the wells of P. mirabilis, E. coli, K. pnumoniae and S. aureus were clearly measured at 1000 µg/mL concentration after 24 h time interval (Fig. 2a, b, c, d). The decreased zones M. citrifolia at 100 µg/mL concentration only started their toxicity against bacteria. Here, the inhibition of bacteria through various concentrations was available in Fig. 2f. However, the biological properties of the current result was evidently indicated, the MDRs pathogens growth was arrested due to the efficient of M. citrifolia EOs. M. citrifolia was deactivated at increasing concentration. Supportively, the antibiotic disc of ceftazidime was shown no any zones against all the tested pathogens and proved that the bacteria were MDR. Previously, De La Cruz Sanchez et al. (2019) suggested, M. citrifolia is a folk medicine and it has excellent toxicity nature to bacterial growth. Recently, the anti-bacterial and anti-cancer effect of the M. citrifolia was reported by Rajivgandhi et al. (2020). All the organic solvents of ethanol, ethyl acetate, alcohol, dichloromethane and petroleum ether are utilized to extract the bioactive compounds from M. citrifolia leaves, roots, barks and seeds (De La Cruz-Sánchez et al., 2019). Report of Zhang et al. (2016), the highest phenolic compounds were present in the extracted M. citrifolia leaves.
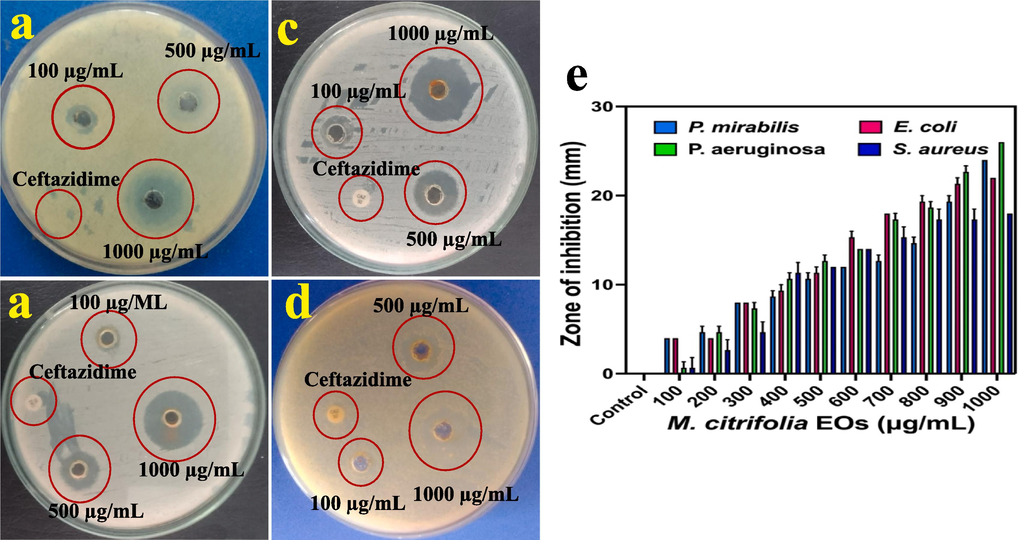
- Anti-bacterial ability of M. citrofolia EOs against multi drug resistant bacteria of P. mirabilis (a), E. coli (b), P. aeruginosa (c) and S. aureus (d) by agar well diffusion method. Various concentrations of M. citrofolia EOs against all the tested pathogens with their zone of inhibition (e).
3.3 Liquid broth dilution of bacterial inhibition using EOs
In liquid culture, the natural medicinal plant of M. citrifolia was effectively arrested bacterial growth in high number. Here, the consecutive biological efficiency of the M. citrifolia EOs excellent sources for MDRs strains of P. mirabilis, E. coli, P. aeruginosa and S. aureus. In treated wells of the 24-well plates were shown increased turbidity at increasing concentration (Fig. 3a). Result of O.D value was exhibited more bacterial death rate compared with control results and also the concentration of 1000 µg/mL. MDRs strains of P. mirabilis, E. coli, P. aeruginosa and S. aureus were compromised more in this concentration and could not produce the virulence factors to develop the bacterial multiplication. The half inhibition 50 % was detected at 600 µg/mL concentration for all the pathogens. It was also correlated with turbidity levels of the results. At 600 µg/mL concentration, 55 %, 51 %, 50 % and 52 % of inhibition were shown for P. mirabilis, E. coli, P. aeruginosa and S. aureus respectively. Instead, 100 µg/mL concentration of M. citrifolia was deactivated of 12 %, 16 % 14 % and 8 % was exhibited for P. mirabilis, E. coli, P. aeruginosa and S. aureus respectively. This result was indicated the information about M. citrifolia EOs, and it was started their inhibition level in liquid media at 100 µg/mL concentration and it increased in increased concentration. When we see the turbidity levels, the 1000 µg/mL concentration of the M. citrifolia EOs was shown more turbidity level in all the wells of the P. mirabilis, E. coli, P. aeruginosa and S. aureus respectively. Supportively, the inhibition rate of 97 %, 96 %, 94 % and 90 % were shown at in 1000 µg/mL concentration of M. citrifolia EOs treated wells of P. mirabilis, E. coli, P. aeruginosa and S. aureus respectively by UV-spectrometer after interpreted with control values (Fig. 3b). Altogether, both the turbidity and inhibition rate of the O.D values were clearly proved that the M. citrifolia EOs decreased the bacterial growth at minimum concentration and inhibited the complete growth at increasing concentration, Based on the present study, the lowest concentration with highest inhibition of 1000 µg/mL was fixed as a MIC and this MIC was could be used for further study to inhibit the bacteria. Previously, excellent pharmacological properties of the M. citrifolia were more effective against diabetics, tuberculosis, jaundice, liver infections and cancer diseases. All the parts of leaves, roots, seeds of M. citrifolia was very effective and it shown excellent anti-bacterial (Zhang et al., 2016), anti-oxidant (Xue et al., 2023), anti-cancer (Rajivgandhi et al., 2020) and some other infectious diseases (Karuppaiah et al., 2020).
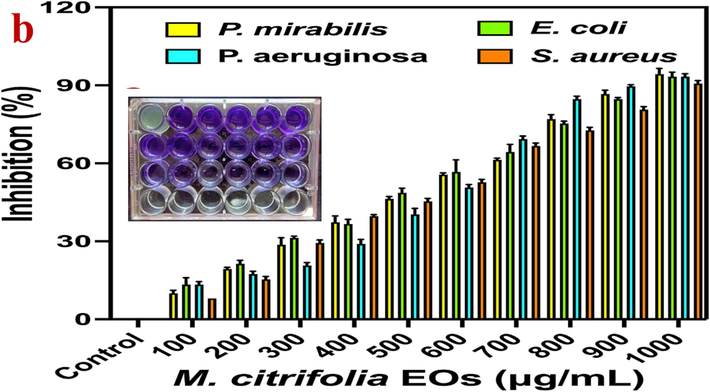
- Minimum inhibition concentration (a) and percentage of inhibition (b) of M. citrofolia EOs against multi drug resistant bacteria by microtitre plate assay.
3.4 Time dependent bacterial killing assay
To decrease the bacterial live condition, the M. citrifolia EOs was used in liquid medium of rose well park memorial institute 1640 medium. In this medium, the bacteria can be grown very fast and produced double time virulence particles in short period. In this experiment, we have tried the inhibition of bacteria and decreased complete bacterial growth at various time intervals and confirmed that the bacterial growth was completely arrested at 24 h time interval. As per MIC result, the concentration of 100–1000 µg/mL concentration was very effective against all the pathogens at 24 h compared with any other time interval. Among the various time interval, the 24 h time interval with 1000 µg/mL concentration of the M. citrifolia EOs were inhibited the bacteria growth of 98 %, 96 % 97 % and 94 % for P. mirabilis, E. coli, P. aeruginosa and S. aureus respectively. It was confirmed by reading of UV-spectroscopy after compared with untreated control results. In result of bacterial growth cell arrest, the inhibition rate of 25 %, 27 %, 32 % and 20 % for 6 h (Fig. 4a), 35 %, 42 %, 44 % and 30 % for 12 h (Fig. 4b), 58 %, 56 %, 52 % and 50 % for 18 h (Fig. 4c) and 98 %, 96 %, 97 % and 94 % for 24 h (Fig. 4d) were shown at 1000 µg/mL concentration. It was clearly indicated that the M. citrifolia EOs as effective anti-bacterial agent against MDRs bacteria. In addition, the inhibition was depends on the concentration and time dependent. In this time-dependent assay, the inhibition was started at 6 h and it reached half inhibition at 36 h after taken the O.D value of UV-spectrometer. Importantly, the complete bacterial growth was arrested for 24 h. Specifically, all the inhibition percentages were mentioned for 1000 µg/mL concentration. Hence, the result was clearly indicated that the M. citrifolia EOs as efficient biological agent against MDRs bacteria.
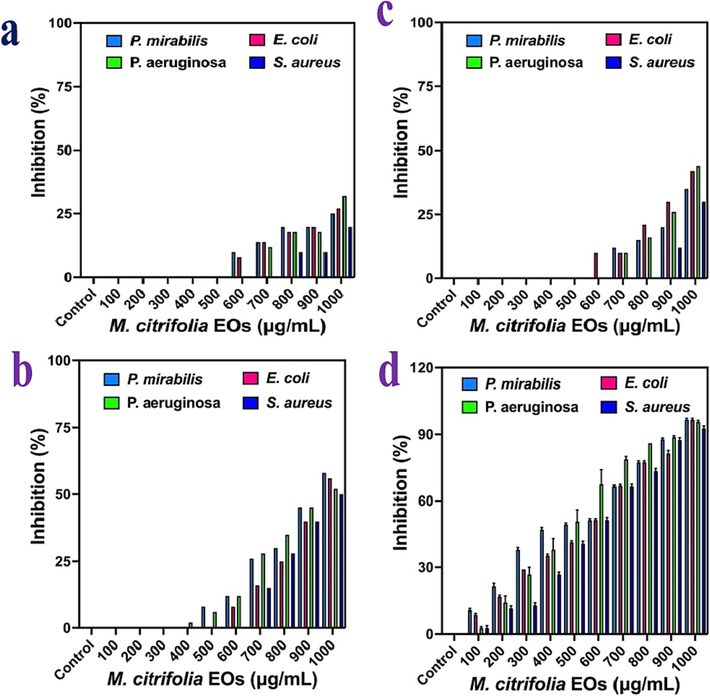
- Concentration and time-dependent inhibition of M. citrofolia EOs against all the MDRs bacteria at 6 h (a), 12 (b), 18 h (c) and 24 h (d) by UV-spectroscopy.
The anti-bacterial effect of M. citrifolia EOs against MDRs pathogens of P. mirabilis, E. coli, P. aeruginosa and S. aureus at 1000 µg/mL concentration for 24 h was further cross checked by MHA plates. The quadrant streak of untreated control plates were grown well matured clear growth in MHA plate for all the tested pathogens of P. mirabilis (Fig. 5a), E. coli (Fig. 5c), P. aeruginosa (Fig. 5e) and S. aureus (Fig. 5g), then the half viability for 12 h and decline phase of the bacterial growth for 18 h at 1000 µg/mL concentration in MHA plates were shown. Finally, there was no nay bacterial growth with clear MHA plates were shown at 1, 000 µg/mL concentration of M. citrifolia EOs for all the treated plates of P. mirabilis (Fig. 5b), E. coli (Fig. 5d), P. aeruginosa (Fig. 5f) and S. aureus (Fig. 5h) were observed at 24 h. Altogether, both the liquid interference assay of time-dependent inhibition and MHA plate assays result was proved that the MDRs pathogens of P. mirabilis, E. coli, P. aeruginosa and S. aureus was completely compromised to M. citrifolia EOs and the 1000 µg/mL concentration was more efficient for inhibition of tested bacteria at 24 h.
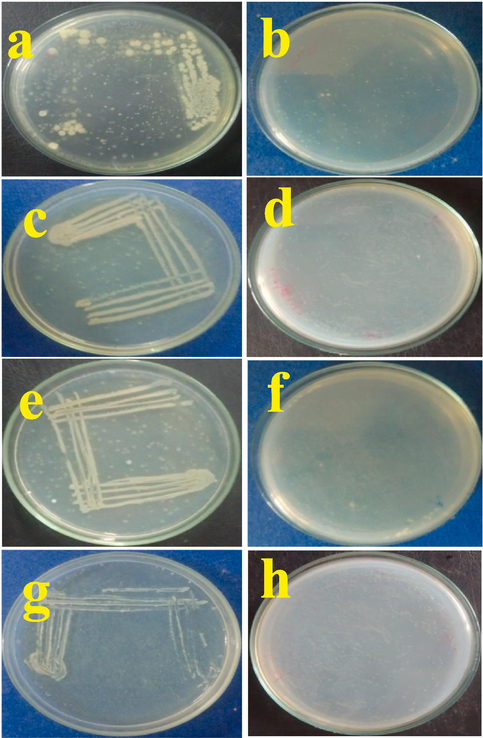
- Validation of time-dependent inhibition assay of M. citrofolia EOs untreated and treated P. mirabilis (a, b), E. coli (c, d), P. aeruginosa (e, f) and S. aureus (g, h) at 24 h by muller hinton agar plates.
3.5 Dual staining assay for detection of damaged cells
The fluorescence intensity based bacterial inhibition in intracellular level of the bacteria was proved by CLSM results. In our result, more intensity based damages of intracellular bacteria was visualized after seen in CLSM. In addition, the red cells intensity was very high in treated cells (Fig. 6) when compared with untreated control (Fig. 6). It was suggested that the intracellular cells of the bacteria was damaged due to the influence of M. citrifolia EOs. AO is the DNA based green florescence dye having high observation intensity in live cells, whereas EB is the red fluorescence dye having the ability to bind on leakage particles of DNA, nucleolus, chromatin based intracellular particles (Shenglan et al., 2022; Jing et al., 2020). As per the previous statement, the current result of untreated and treated bacterial cells of the all the pathogens P. mirabilis, E. coli, P. aeruginosa and S. aureus were clearly shown in CLSM. The CLSM images of untreated control cells were shown rod shape, clear and tightly interlocked structures with green color appearance, and M. citrifolia EOs treated bacterial cells were shown damaged rod, dispersed, unidentified structures with red colors for all the gram negative bacteria of P. mirabilis, E. coli and P. aeruginosa were observed. Whereas cocci shape with normal punch of growth like structure in control with green color appearance, and damaged cocci shape and dispersed arrangement of the cocci cells were shown with red color for S. aureus. Here, P. mirabilis (Fig. 6a, b) and P. aeruginosa (c, d) of M. citrifolia EOs treated and untreated cells were clearly shown in CLSM respectively. This result was more accordance with recently reported evidence of Shehabeldine et al. (2020). Hence, the result was clearly suggested, all the tested pathogens were compromised to M. citrifolia EOs. Among the medicinal plants, M. citrifolia is the most favourable plant used as a traditional medicine having anti-microbial, anti-cancer, anti-oxidants and anti-tuberculosis agent. More bioactive compounds has been derived from M. citrifolia notably, anthraquinones, flavonoids and coumarins. Recently, the anti-oxidants and anti-inflammatory effect of the effect of M. citrifolia was reported by Zhang and Zhang (2022).
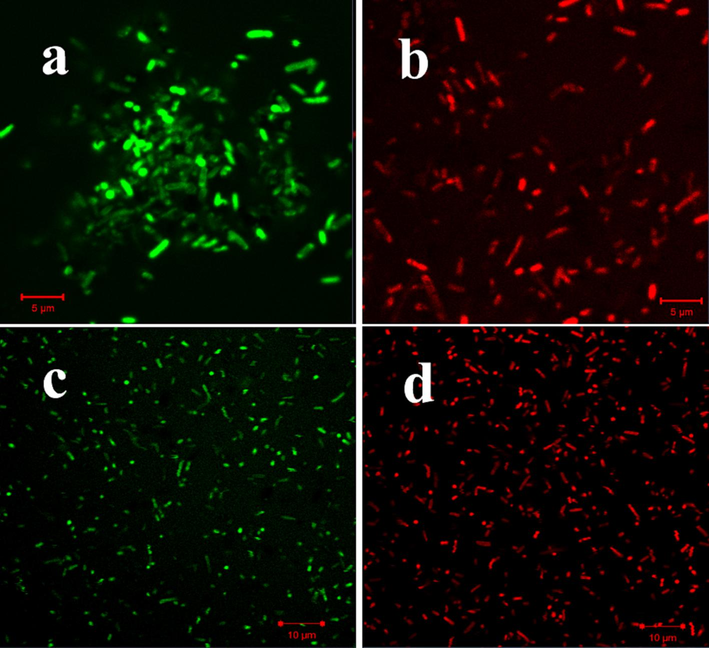
- Detection of intracellular damages of M. citrofolia EOs untreated and treated P. mirabilis (a, b) and P. aeruginosa (c, d) by CLSM.
3.6 Inhibition of cancer cells using plant essential oils
Cytotoxicity effect of this study result was more favours to EO rich M. citrifolia and it affected the A549 lung cancer cells. In result of MTT assay indicated increasing concentration of 1000 µg/mL was more effective and fixed as IC50. This concentration was clearly eradicated the lung cancer cells at increased dose of the EOs. The inhibition percentage was shown for 52 % (Fig. 7). Result of cytocompatibility and decreased growth of lung cancer cells in the treated cells were clearly indicated M. citrifolia is as effective natural anti-cancer agent (Suthagar Pillai, et al., 2012). M. citrifolia is the excellent medicinal plant having more anti-cancer compound through oxidative stress responses. In addition, Campos et al. (2017), M. citrifolia is an efficient natural medicine used for inhibit the apoptosis and necrotic cells. After addition of formazan, the intensity of the color was changed and indicated clearly. It led to more apoptosis mediated cell death through cellular enzyme degradation.
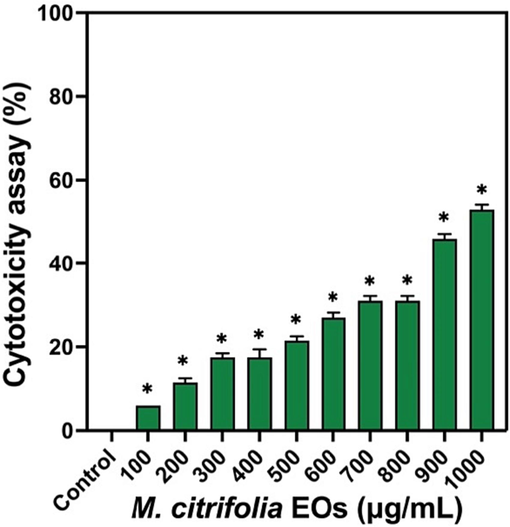
- Cytotoxicity effect of M. citrofolia EOs against A549 lung cancer cells by MTT assay.
4 Conclusion
The available EOs from M. citrifolia of the present study are very important to inhibition of MDRs pathogens. In current study, the M. citrifolia EOs have excellent biological properties against different MDRs pathogens. In addition, the cytotoxicity effect was very higher against A549 lung cancer cells. The invitro inhibition study results were suggested, M. citrifolia EOs have more biological molecules. The M. citrifolia EOs exhibited concentration dependent inhibition against bacteria and cancer cells. All the EOs of the LC-MS study report was interpreted, the M. citrifolia synthesized EOs were very effective than existing plant EOs. In addition, this kind of study was used to discover the new approach to eradicate bacterial infections and cancer cells. Hence, present study result was suggested, medicinal plant of M. citrifolia is the excellent medicinal plant and it can be used in pharmacological application for inhibit the MDRs bacterial infections and cancer cells.
Acknowledgment
The authors express their sincere appreciation to the Researchers Supporting Project Number (RSPD2023R679), King Saud University, Riyadh, Saudi Arabia. All the authors gratefully acknowledge the National Natural Science Foundation of China (Project Approval Numbers: 41950410573) and Postdoctoral Science Foundation of China (Project Approval Number: 2019M663213) for financial support for this work. G. Rajivgandhi and F. Quero acknowledge the financial support from ANID-FONDECYT (Chile) under the Postdoctoral Fellowship No. 3220019.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antimicrobial activity of certain natural-based plant oils against the antibiotic-resistant acne bacteria. Saudi J. Biolog. Sci.. 2020;27:448-455.
- [Google Scholar]
- Evaluation of cytotoxic activity of protein extracts from the leaves of Morinda pubescens on human cancer cell lines. Revis. Brasil. Farmacog.. 2017;27:99-104.
- [Google Scholar]
- Mentha piperita EOs loaded in a chitosan nanogel with inhibitory effect on biofilm formation against S. mutans on the dental surface. Carbohyd. Polym.. 2019;212:142-149.
- [Google Scholar]
- Morinda citrifolia lipid transfer protein 1 exhibits anti-inflammatory activity by modulation of pro- and anti-inflammatory cytokines. Int. J. Biolog. Macromol.. 2017;103:1121-1129.
- [Google Scholar]
- The noni fruit (Morinda citrifolia L.): A review of agricultural research, nutritional and therapeutic properties. Journal of Food Composition and Analysis. 2006;19:645-654.
- [Google Scholar]
- Antibacterial activity of Morinda citrifolia Linneo seeds against Methicillin-Resistant Staphylococcus spp. Microb. Pathog.. 2019;128:347-353.
- [Google Scholar]
- Antibacterial activity of Morinda citrifolia Linneo seeds against Methicillin-Resistant Staphylococcus spp. Microb Pathog.. 2019;128:347-353.
- [Google Scholar]
- An overview of the pharmacological activities of scopoletin against different chronic diseases. Pharmacological Research. 2022;179:106202
- [Google Scholar]
- Anti-bacterial activity of chitosan loaded plant essential oil against multi drug resistant K. pneumonia. Saudi Journal of Biological Sciences. 2020;27:3449-3455.
- [Google Scholar]
- Hena, K., Shamsuzzaman., Bioactive Benzofuran derivatives: A review, European Journal of Medicinal Chemistry, 2015, 483-504.
- Anti-biofilm activity of LC-MS based Solanum nigrum EOs against multi drug resistant biofilm forming P. mirabilis. Saudi Journal of Biological Sciences. 2021;28:302-309.
- [Google Scholar]
- Dual responsive linalool capsules with high loading ratio for excellent antioxidant and antibacterial efficiency. Colloids and Surfaces b: Biointerfaces. 2020;190:110978
- [Google Scholar]
- Anticancer and antioxidant efficacy of silver nanoparticles synthesized from fruit of Morinda citrifolia Linn on Ehrlich ascites carcinoma mice. Journal of King Saud University - Science. 2020;32:3181-3186.
- [Google Scholar]
- Evaluation of the Brazilian functional fruit Morinda citrifolia as phospholipases A2 and proteases modulator: Morinda citrifolia: enzymatic modulation. Phytomedicine plus. 2021;1:100071
- [Google Scholar]
- Maruthupandy, M., Rajivgandhi, G., Kadaikunnan, S., Veeramani, T., Naiyf, S.A., Muneeswaran, T., Jamal, M.K., Wen Jun, L., Khalid, F.A., 2020. Anti-biofilm investigation of graphene/chitosan nanocomposites against biofilm producing P. aeruginosa and K. pneumoniae, Carbohydrate Polymers, 230, 115646.
- Rajivgandhi, G., Ramachandran, G., Maruthupandy, M., Manoharan, N., Alharbi, N.S., 2020. Anti-oxidant, anti-bacterial and anti-biofilm activity of biosynthesized silver nanoparticles using Gracilaria corticata against biofilm producing K. pneumoniae, Colloids and Surfaces A, 600, 124830.
- Enhanced anti-cancer activity of chitosan loaded Morinda citrifolia essential oil against A549 human lung cancer cells. International Journal of Biological Macromolecules. 2020;164:4010-4021.
- [Google Scholar]
- Anti-biofilm compound of 1, 4-diaza-2, 5-dioxo-3-isobutyl bicyclo[4.3.0]nonane from marine Nocardiopsis sp. DMS 2 (MH900226) against biofilm forming K. pneumoniae. Journal of King Saud University - Science. 2020;32:3495-3502.
- [Google Scholar]
- Anti-carbapenamase activity of Camellia japonica essential oil against isolated carbapenem resistant Klebsiella pneumoniae (MN396685) Saud. J. Biolog. Sci.. 2020;27:2269-2279.
- [Google Scholar]
- Anticancer Potential of Damnacanthal and Nordamnacanthal from Morinda elliptica Roots on T-lymphoblastic Leukemia Cells. Molecules.. 2021;26:1554.
- [Google Scholar]
- Chemical Composition Related to Antimicrobial Activity of Moroccan Nigella sativa L. Extracts and Isolated Fractions. Evid. Based Complement. Alternat. Med. 20218308050
- [Google Scholar]
- Synthesis of 2-(dimethylamino-3H)-6-[bis(1-aziridinyl)phosphinylamino]-7-methylpurine (fopurine-3H) Pharm Chem J. 1979;13:62-63.
- [Google Scholar]
- Callistemon citrinus bioactive metabolites as new inhibitors of methicillin-resistant Staphylococcus aureus biofilm formation. Journal of Ethnopharmacology. 2020;254:112669
- [Google Scholar]
- Shenglan, L., Gang, Y., Zhirong, W., Yuheng, O., Shan, H., Bin, L., Aijun, L., Jianquan, K., 2022. Ultrasonic preparation of Tween-essential oil (Zanthoxylum schinifolium Sieb. et Zucc) oil/water nanoemulsion: Improved stability and alleviation of Staphylococcus epidermidis biofilm, Industrial Crops and Products, 188, 115654.
- Chemical constituents of the fruits of Morinda citrifolia (Noni) and their antioxidant activity. J. Nat. Prod.. 2005;68:592-595.
- [Google Scholar]
- Chemical composition, antioxidant and cytotoxicity activities of the EOs of Myristica fragrans and Morinda citrifolia. J. Sci. Food. Agricult.. 2012;92:593-597.
- [Google Scholar]
- Quorum sensing modulation and inhibition in biofilm forming foot ulcer pathogens by selected medicinal plants. Heliyon. 2022;8:09303.
- [Google Scholar]
- Antioxidant and in vitro anticancer effect of 2-pyrrolidinone rich fraction of Brassica oleracea var. capitata through induction of apoptosis in human cancer cells. Phytother Res.. 2013;27:1664-1670.
- [Google Scholar]
- Antifungal activity and molecular docking of phenol, 2,4-bis(1,1-dimethylethyl) produced by plant growth-promoting actinobacterium Kutzneria sp. strain TSII from mangrove sediments. Arch Microbiol.. 2021;203:4051-4064.
- [Google Scholar]
- Kirk Parkin, Isolation and synergism of in vitro anti-inflammatory and quinone reductase (QR) inducing agents from the fruits of Morinda citrifolia (noni) Food Res. Int.. 2011;44:2271-2277.
- [Google Scholar]
- Morinda citrifolia (Noni): A literature review and recent advances in Noni research. Acta Pharmacologica Sinica. 2002;23:1127-1141.
- [Google Scholar]
- Fabrication, characterization and biological properties of pectin and/or chitosan-based films incorporated with noni (Morinda citrifolia) fruit extract. Food Hydrocolloids. 2023;134:108025
- [Google Scholar]
- , U.W., Yan, Z., Amir, R., , S., Jin, Y.W., Lee, E.K., Loake., G.J., 2012. Plant natural products: History, limitations and the potential of cambial meristematic cells, Biotechnology and Genetic Engineering Reviews, 28, 47–50.
- Antibacterial Constituents of Hainan Morinda citrifolia (Noni) Leaves. J Food Sci.. 2016;81:1192-1196.
- [Google Scholar]
- Neuroprotective effects of Morinda officinalis How.: Anti-inflammatory and antioxidant roles in Alzheimer's disease. Front Aging Neurosci.. 2022;14:963041
- [Google Scholar]
- Antioxidative activity of extracts from Mengkudu root, fruit and leaf’. Food Chemistry. 2002;78:227-231.
- [Google Scholar]







