Translate this page into:
Tilapia viscera protein hydrolysate maintain regulatory T cells and protect acute lung injury in mice challenged with lipopolysaccharide
⁎Corresponding author. putut.riyadi@live.undip.ac.id (Putut Har Riyadi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The utilization of fish by-products through hydrolysis methods may produce a high-grade product that increases its economic value and reduces pollution. Tilapia Viscera Protein Hydrolysate (TVPH) is reported to have a positive impact on health. However, there is a lack of evidence for the interplay of regulatory T cells (Tregs) and neutrophils in acute lung injury (ALI) influenced by TVPH. Our study investigated the implication of TVPH on Tregs and its protective effect on ALI in mice challenged with lipopolysaccharide (LPS).
Methods
Thirty-six male Balb/C mice were randomized into six groups: untreated, LPS, dexamethasone (DEX) + LPS, and TVPH at doses 150, 300, 450 mg/kg BW, respectively + LPS. Mice were challenged with LPS after seven days of treatment via intraperitoneal injection. After 6 h, mice were sacrificed. Spleen was harvested for flow cytometry analysis, and lung was collected for histological and immunofluorescence analysis. Tregs was labelled as CD4+CD25+CD62L+, CD4+CD25+IL-10, and CD4+CD25+TGF-β+. Neutrophil activation was labeled as a combination of CD66a and MPO antibodies.
Results
The CD4+CD25+CD62L+ subsets finding as well as CD4+CD25+IL-10+, CD4+CD25+TGF-β+ subsets, the expression of CD66a and MPO, and lung histopathological imaging confirmed that TVPH and DEX attenuate LPS-induced ALI significantly (p < 0.05).
Conclusion
Our finding proposed that TVPH protects LPS-induced ALI through maintaining neutrophils and Tregs. TVPH might be a promising food nutraceutical candidate by reducing the impact of inflammation.
Keywords
Hydrolysate
Lung Injury
Neutrophils
Regulatory T Cells
Tilapia Viscera
- AhR
-
Aryl hydrocarbon receptor
- ALI
-
Acute Lung Injury
- ARDS
-
Acute respiratory distress syndrome
- AU
-
Anson Units
- BW
-
Body weight
- CD
-
Cluster of differentiation
- DEX
-
Dexamethasone
- EAA
-
Essential amino acid
- FFO
-
Fermented fish oil
- HE
-
Hematoxylin-eosin
- IL
-
Interleukin
- LPS
-
Lipopolysaccharide
- MPO
-
Myeloperoxidase
- NSAIDs
-
Non-steroidal anti-inflammatory drugs
- SD
-
Standard of deviation
- TGF-β
-
Transforming growth factor β
- Tregs
-
Regulatory T cells
- TVPH
-
Tilapia Viscera Protein Hydrolysate
Abbreviations
1 Introduction
Inflammation is an early immune system reaction that protects the host against bacterial inflammation or cell/tissue damage (Atho’illah et al., 2021). LPS are unique properties of gram-negative bacteria, commonly from Escherichia coli, which are considered a potent inducer of proinflammatory cytokines to initiate inflammation (Abu-Taweel, 2020; Riyadi et al., 2019d). The unsolved inflammation during host-pathogen interaction would over-activate the innate immune system, leading to severe immune dysregulation (Wuryandari et al., 2021). Along with excessive inflammation, rapid cytokines production following with immune cells recruitment into the infected tissue may develop acute lung injury (ALI) (Zhao and Du, 2020).
ALI and the later form, acute respiratory distress syndrome (ARDS), are considered critical care medicine and are substantially associated with morbidity and mortality. ARDS is characterized by lung inflammation, hypoxemia, and edema due to the alteration of lung permeability, hyaline membrane, and alveolar hemorrhage (Matthay et al., 2019). ALI and/or ARDS are characterized by the infiltration of immune cells, particularly neutrophils. Myeloperoxidase (MPO) is a heme-containing enzyme considered the primary marker of neutrophil activation. MPO expression reflects the neutrophil infiltration and is closely related to pulmonary inflammation-induced lung damage (An et al., 2021). In contrast, regulatory T cells (Tregs) have been demonstrated to play a crucial role in suppressing inflammation and preventing autoimmunity in several diseases. Some previous studies reported that Tregs are involved in bacterial clearance, repairing the lung epithelium, and increasing the survival rates of animals induced with LPS (Tan et al., 2019). These findings provide a critical insight that balancing neutrophils and Tregs might be beneficial for protecting the lung from ALI driven by inflammation.
Fish are a significant animal source of food for millions of people, supplying roughly 20% of the average animal-protein consumption per capita throughout the world. The global fish production reached 179 million tons by 2018, with 87% consumed by humans. Indeed, fish consumption is expected to grow after 2018, and it is anticipated to increase around 18 percent by 2030 (FAO, 2020). However, the growing demand for fish consumption is frequently accompanied by increased by-products, including head, skin, fins, tail, bones, viscera, and scales (Darmanto et al., 2017). Interestingly, fish by-products still contain a high level of micronutrients (FAO, 2016). Some previous studies reported that fish by-products possessed anti-allergic, antioxidant, and anti-inflammatory properties (Aryani and Riyadi, 2021; Kim et al., 2018; Pan et al., 2016).
Regarding aquaculture products in Indonesia, Tilapia is one of the vital fish commodities predicted to reach 2.0 million tons in 2030 (Tran et al., 2017). The previous study was reported to generate a rich peptide successfully from Tilapia by-products by hydrolysis methods (Riyadi et al., 2019a; Riyadi et al. 2019c). Tilapia Viscera Protein Hydrolysate (TVPH) has shown promising results as an antihypertensive and immunomodulator (Riyadi et al., 2020a; Riyadi et al., 2020c). TVPH showed anti-inflammatory activity based on predictions using PASS online and SwissADME (Riyadi et al., 2020b; Riyadi et al., 2021). However, although TVPH has demonstrated beneficial impacts on health, it is still unclear whether TVPH is also involved in regulating Tregs and preventing tissue damage. Herein, we evaluated the TVPH effect in mice challenged with LPS to understand better its role in maintaining neutrophils and Tregs by protecting the infected mice from ALI. In addition, we investigated the effect of different doses of TVPH and compared it with DEX. Our study may provide a new direction for TVPH as an alternative nutraceutical food candidate for reducing the caused by inflammation.
2 Materials and methods
2.1 Defatting and hydrolysis
The viscera of Tilapia were collected from PT Aquafarm Nusantara, Semarang, Indonesia. Briefly, viscera were rinsed with water and removed their fat. The extraction methods to obtain viscera-rich peptides was following the previous study (Riyadi et al., 2019a). First, the viscera were added with distilled water at 1:1 (w/v) and stood for 20 min at 85 °C. The viscera mixtures were then defatted by 5,800 rpm centrifugation for 20 min at 10 °C. The pellet obtained was extracted with distilled water 1:1 (w/v) three times. Next, the 50 mL of protein extract was added with 1.5% alcalase enzyme (cat# 126741, Sigma-Aldrich, ST Louis, MO, USA) with ≥ 0.75 AU/mL activity and then incubated for 1.5 h at 55.8 °C with pH 7.9. The viscera protein hydrolysate obtained stood for 20 min at 85 °C to inactivate the alcalase and then cold centrifuged at 5,800 rpm. The residue obtained was then freeze-dried to obtain TVPH and evaluated their chemicals and amino acid composition.
2.2 Animal
Male Balb/C mice 6–7 weeks old with a bodyweight of 22–25 g were obtained from the Department of Pathology, Faculty of Medicine, Brawijaya University. Mice were placed 6 per cage with free access to food and fresh water in 12 h light/dark cycle at a constant temperature and humidity. Mice were acclimatized for seven days before treatment was given. The Animal Care and Use Committee of Brawijaya University approved all animal housing and experiments with approval number: 030-KEP-UB-2021 in accordance with the Guide to the Care and Use of Laboratory Animals (National Institutes of Health, United States).
2.3 Experimental design
Thirty-six mice were equally and randomly divided into six group: (i) untreated, (ii) LPS (E. coli serotype 0111:B4, cat# trlr-eblps, InVivoGen, San Diego, CA, USA) 5 mg/kg BW, (iii) DEX 1 mg/kg BW + LPS, (iv) TVPH 150 mg/kg BW + LPS, (v) TVPH 300 mg/kg BW + LPS, and (vi) TVPH 450 mg/kg BW + LPS. DEX and TVPH were orally administered for seven consecutive days before LPS injection. Mice in untreated and LPS groups received normal saline intragastrically for the same period. Thirty minutes after the final administration, all groups injected LPS at dose 5 mg/kg BW intraperitoneally. Meanwhile, the untreated group was received a normal saline injection intraperitoneally. Mice were monitored for their survival rates every one h up to 6 h. six hours after LPS injection, and mice were anesthetized and sacrificed. Spleen and lung were harvested and prepared for further experiments.
2.4 Cell staining and flowcytometry
The fresh spleen was collected and isolated into a single-cell suspension (Safitri et al., 2018). The cells were stained with FITC anti-mouse CD4 (clone GK1.5, BioLegend, San Diego, USA), PE anti-mouse CD25 (clone 3C7, BioLegend, San Diego, USA), and PE/Cy5 anti-mouse CD62L (clone MEL-14, BioLegend, San Diego, USA) as a Tregs marker for 30 min at 4 °C in low light condition (Atho’illah et al., 2017). On the other hand, the cells stained with FITC anti-mouse CD4 and PE anti-mouse CD25 (without PE/Cy5 anti-mouse CD62L) were added with cytofix/cytoperm buffer, then washed using perm/wash buffer. The intracellular staining was either with PE/Cy7 anti-mouse IL-10 (clone JES5-16E3, BioLegend, San Diego, USA) or PerCP/Cy5.5 anti-mouse TGF-β1 (clone TW7-16B4, BioLegend, San Diego, USA) addition for 30 min at 4 °C in low light condition. Cells were acquired for each sample using BD FACS CaliburTM. According to the stained used, the cells population were then analyzed using FlowJo v10 for Windows.
2.5 Lung injury assessment
The lung lobe was fixed in 10% formalin, dehydrated, embedded in paraffin, cut into four μm sections, and stained with hematoxylin-eosin (HE). The stained slides of the lung were then observed and captured for its histopathological imaging using Olympus BX51 equipped with Olympus XC10 digital camera system. The lung injury was assessed blindly by two experts at least from twenty different random fields for each section due to the inconsistent presence of ALI (Matute-Bello et al., 2011). At least five featured were evaluated to assess lung injury, and the graded result for each parameter was then calculated as the final injury score with an overall score ranging from 0 to 1, as followed by the previous study taken (Yaxin et al., 2014).
2.6 Immunofluorescence staining
The prepared lung tissue in section 2.5 was deparaffinized, rehydrated, washed three times, and then immersed in citrate buffer pH 6.0 at 100 °C for 20 min. The slides were then washed in TBS-T three times and blocked with 3% BSA for 2 h in low-light conditions. BSA was removed carefully, and then the slides were treated with combination antibodies of FITC anti-mouse CD66a (clone Mab-CC1, BioLegend, San Diego, USA) and PE-Cy5 anti-rabbit Myeloperoxidase (bs-1061R-Cy5, Bioss, Massachusetts, USA). Next, the slides were incubated overnight at 4 °C in low light conditions. After that, the slides were washed three times using TBS-T, mounted, and then examined using Olympus IX51 light microscope (Olympus, Tokyo, Japan) connected with a workstation installed with Fluoview software. The image acquired was then analyzed using ImageJ.
2.7 Statistical analysis
All data were examined by one-way ANOVA followed by Tukey HSD as a post-hoc test. Data were shown as mean ± standard deviation (SD). P-value<0.05 was defined as the significant value. GraphPad Prism 8.0 program assisted the statistical analysis.
3 Results
3.1 Protein, amino Acids, and chemical constituents identification
The protein percentage of dried TVPH showed a higher result than the wet TVPH (Table 1). The amino acids identification resulting in lysine has the highest content than other essential amino acids (g/100 g). Meanwhile, glutamine/glutamate has the highest content of other non-essential amino acids found in TVPH (Table 1). The chemical characteristics of TVPH demonstrated that TVPH contains alkaloids, tannins, triterpenoids, polyphenols, and saponins. In contrast, flavonoids and steroids were absent in TVPH (Table 1).
Component(s)
Results
Reference
Protein (%)
(Riyadi, et al., 2019a)
Wet
55.55 ± 0.18
Dry
62.81 ± 0.18
Essential amino acids (g/100 g)
(Riyadi, et al., 2019a)
Arginine
1.93
Histidine
2.04
Isoleucine
1.56
Leucine
2.19
Lysine
2.82
Methionine
0.88
Phenylalanine
1.07
Threonine
1.26
Tryptophan
0.42
Tyrosine
1.42
Valine
2.78
Total
18.37
Non-essential amino acids (g/100 g)
Alanine
1.56
Asparagine + aspartate
3.15
Cysteine
0.32
Glutamine + glutamate
3.85
Glycine
1.27
Proline/ hydroxy proline
0.99
Serine
1.19
Total
12.33
Chemicals
(Riyadi, et al., 2019b)
Flavonoids
Negative
Alkaloids
Positive
Tannins
Positive
Steroids
Negative
Triterpenoids
Positive
Polyphenols
Positive
Saponins
Positive
3.2 TVPH restored Naïve regulatory T-cells in LPS-challenged mice
Our result demonstrated that naïve Tregs have reduced in mice challenged with LPS 5 mg/kg BW. TVPH showed its protecting effect in the present study by maintaining the naïve Tregs subsets from fall after LPS stimulation (Fig. 1A). Furthermore, TVPH administration elevated the naïve Tregs subsets significantly (p < 0.05) in a dose-dependent manner (Fig. 1B) compared to the LPS group only. Interestingly, TVPH in all doses did not significantly differ with DEX 1 mg/kg BW (Fig. 1B).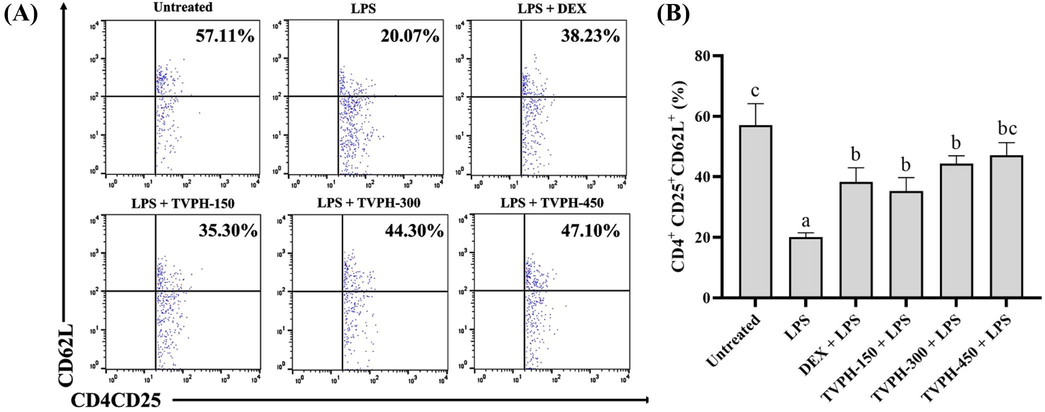
Effect of TVPH on the naïve Tregs subsets in LPS-challenged mice. (A) The detection of naïve Tregs (CD4+CD25+CD62L+) expression by flow cytometry analysis. (B) The comparison of naïve Tregs after stimulation with LPS showed a protecting effect by TVPH. All data displayed as mean ± SD (n = 5). Mean with different notation (a-c) in the chart are significantly different, and vice versa at p < 0.05 based on Tukey HSD’s test.
3.3 TVPH improves IL-10 and TGF-β secreted by regulatory T-cells in LPS-challenged mice
As displayed in Fig. 2A, LPS stimulation declined the IL-10 expression by CD4+CD25+ subsets compared to the untreated group. TVPH restored IL-10 expression significantly (p < 0.05) compared to the LPS group (Fig. 2B). In accordance with naïve Tregs results, TVPH enhances IL-10 expression (Fig. 2A-B). Interestingly, TVPH at dose 450 mg/kg BW showed a better effect than DEX. As following with the CD4+CD25+IL-10+ subsets result, LPS stimulation also declines TGF-β expression by CD4+CD25+ subsets (Fig. 2C). TVPH administration improved TGF-β expression significantly (p < 0.05) compared to the LPS group (Fig. 2D). TVPH at dose 300 and 450 mg/kg BW had a similar effect with DEX 1 mg/kg BW to elevate the TGF-β expression by CD4+CD25+ (Fig. 2C-D).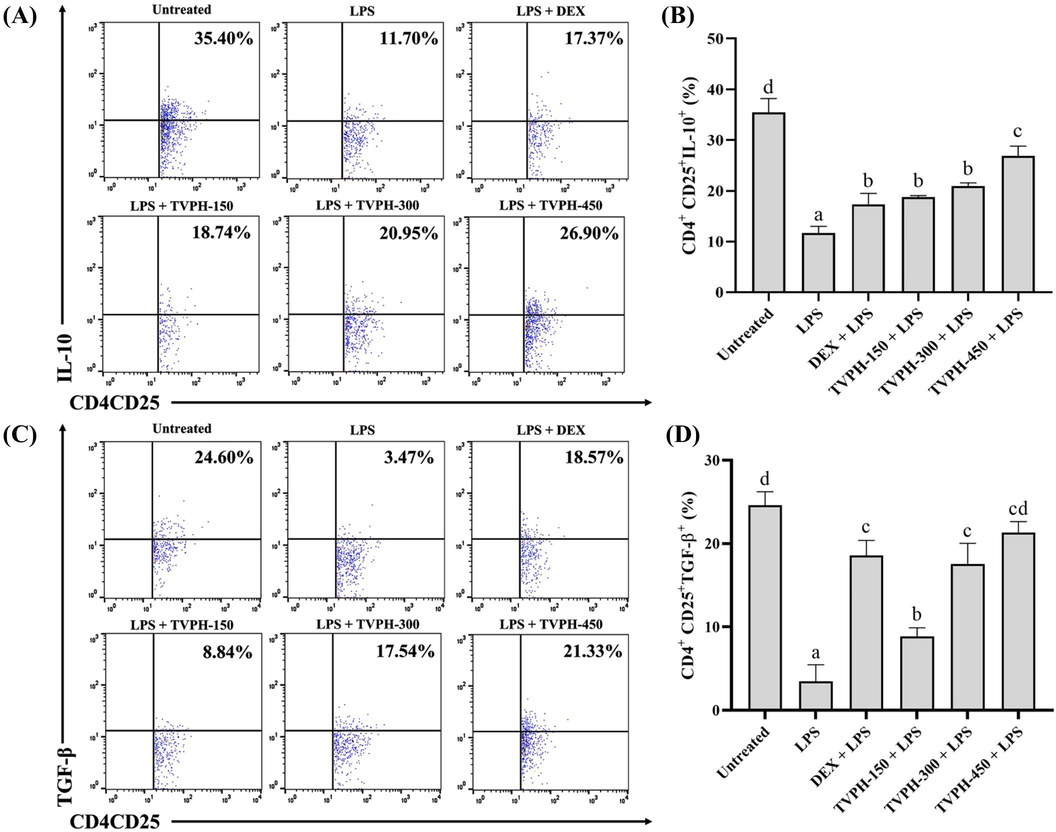
Effect of TVPH on the IL-10 and TGF-β secreted by CD4+CD25+ in LPS-challenged mice. (A) The detection of IL-10+ expressed by CD4+CD25+ subsets by flow cytometry analysis. (B) The comparison of IL-10+ expressed by CD4+CD25+ after stimulation with LPS. (C) The detection of TGF-β+ expressed by CD4+CD25+ subsets by flow cytometry analysis. (D) The comparison of TGF-β+ expressed by CD4+CD25+ after stimulation with LPS. All data displayed as mean ± SD (n = 5). Mean with different notation (a-d) in the chart are significantly different, and vice versa at p < 0.05 based on Tukey HSD’s test.
3.4 TVPH protected lung from LPS-induced lung injury
Our result demonstrated an inflammatory change in the lung after LPS injection, including neutrophil in alveolar and interstitial space, hyaline membrane formation, proteinaceous debris found in the lung airspaces, and alveolar alteration thickness. No lung histological alteration was observed in the untreated group. On the contrary, the alveolar thickness was observed explicitly after LPS injection. (Fig. 3A). The expression of CD66a and MPO was stronger in the LPS groups than in untreated groups. TVPH administration reduces the expression of CD66a and MPO in parallel with the dosage of TVPH given (Fig. 3A). Intriguingly, the merged image illustrated the gradual decrease in MPO expression intensity caused by TVHP, from yellow to green-dominant color. (Fig. 3A). The lung histology result was following the lung injury scores, which described that TVPH significantly reduces (p < 0.05) the features of lung injury in a dose-dependent manner (Fig. 3B). In parallel with the LIS result, the expression of CD66a and MPO was declined significantly (p < 0.05) in TVPH groups. TVPH administration protects against lung injury by reducing the inflammatory sign (Fig. 3C).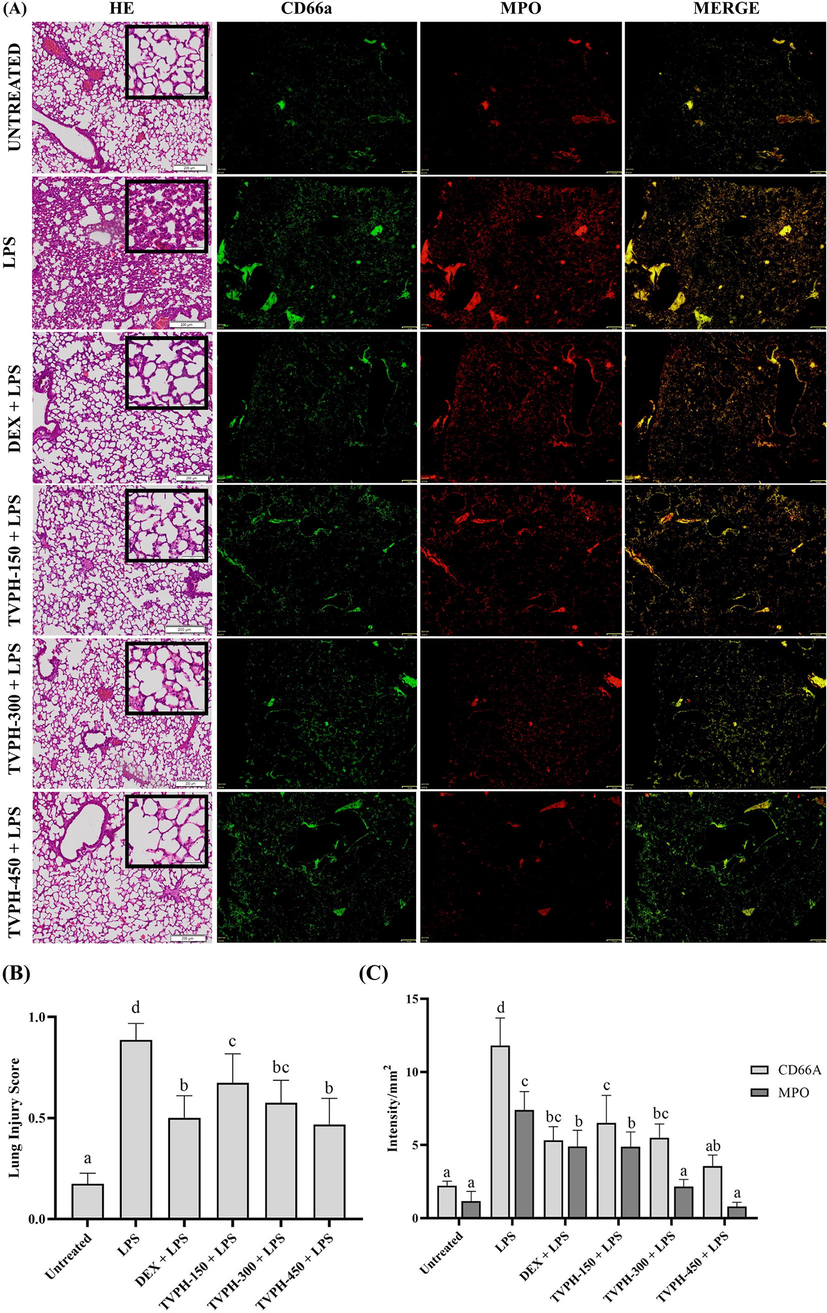
Lung histological changes after 6 h LPS injection in untreated/treated mice. (A) HE and immunofluorescence of lung tissue for detecting neutrophil and MPO after 6 h LPS injection. The black square indicated higher magnification in 50 μm scale (B) TVPH showed a protective effect significantly against LIS after 6 h LPS injection. Lung damages were evaluated in 400x magnification with scale bar 100 μm. (C) The expression intensity of neutrophil (CD66a) and MPO in lung tissue was diminished significantly by TVPH administration. Mean with different notation (a-d) in the chart are significantly different, and vice versa at p < 0.05 based on Tukey HSD’s test.
4 Discussion
Currently, both non-steroidal anti-inflammatory drugs (NSAIDs) and steroidal anti-inflammatory drugs are commonly used for treating pain and inflammation. However, both frequently induced undesirable effects in long-term use (Wongrakpanich et al., 2018). In addition, the term “back to nature” is growing and gaining much interest in research due to the excellent health effect offered. Interestingly, there is a trend to utilize food or its derivative and food by-products to treat some diseases (Kim et al., 2018; Sila and Bougatef, 2016). Fish by-products are frequently considered waste or discarded directly after processing a fish. However, fish by-products utilization produce bioactive peptides and other high-quality nutrients, which exhibit antioxidant activity (Riyadi et al., 2019a, 2019b; Riyadi et al., 2020a).
In the present study, we focused on using a fish by-product from the viscera of Tilapia through hydrolysis methods to evaluate its anti-inflammatory properties. Our result demonstrated that TVPH maintains the naïve Tregs subsets after six h injection with LPS. Tregs are well described to have an essential role in suppressing inflammation and orchestrating the immune response. A previous study reported that aryl hydrocarbon receptor (AhR) is involved in Tregs induction. Ahr is broadly expressed in various tissues and immune cells and recruited to the Foxp3 promoter, activating Tregs (Wang et al., 2012). Tryptophan (Trp), an essential amino acid (EAA), is reported to be involved in Tregs differentiation/action, while TVPH contained high Trp (Gargaro et al., 2021; Riyadi et al., 2019a). Further, Trp catabolites, L-Kyurenine, interact with Ahr to support the formation of Tregs. Interestingly depleted Trp catabolism and tyrosine would suppress Tregs establishment (Campesato et al., 2020). In long-term stimulation, Trp catabolites induce the phenotype CD4+CD25+CD62L+ and might restore its suppressive function (Fallarino et al., 2006). Our study suggested that biopeptide contained in TVPH might maintain the naïve Tregs subsets after LPS injection.
IL-10 and TGF-β are the primary inflammatory cytokines secreted by Tregs. Our finding suggested that LPS down-regulated IL-10 and TGF-β, expressed by CD4+CD25+, were reversed by TVPH (Fig. 2A-D). Tregs secretes IL-10 and TGF-β to reduce inflammatory response by inhibiting inflammatory immune cells (Shariati et al., 2019). Another previous study reported that fish by-products from tuna cooking drip up-regulated IL-10 concentration in a dose-dependent manner after splenocytes stimulated by LPS. Thus, the high dose of tuna cooking drip might assume to trigger the immune regulatory response (Kim et al., 2018). Similar to our results, TVPH administration showed the elevated of IL-10 in a dose-dependent manner (Fig. 2A-B). Besides, fermented fish oil (FFO) products were reported to increase TGF-β and IL-10 concentration. Moreover, the FFO also up-regulated Foxp3 expression, further improving the inflamed sites (Han et al., 2012). Interestingly, other studies reported that EAA levels in circulation could be sensed by T-cells and implicated for Tregs differentiation. Further, EAA and TGF-β have synergized effect in Foxp3 expression (Cobbold et al., 2009). These molecular mechanisms might restrict T-cells proliferation and delay inflammation.
In line with Tregs results, our finding indicated that TVPH protects from LPS-induced ALI by diminished MPO generated by neutrophils and improving lung histological features. We proposed two mechanisms how TVPH could protect mice from ALI after LPS induction. First, TVPH improves lung architectures via interplay between neutrophils and Tregs. As we stated before, TVPH administration elevated the naïve Tregs and its anti-inflammatory cytokines, IL-10 and TGF-β. Neutrophils infiltrate the alveolar spaces immediately after LPS induction and release a massive MPO during acute lung inflammation. The elevated level of MPO reflected the neutrophil migration on the alveolar cavities and lung parenchyma and might be represented lung damage (Mao and Huang, 2018). Based on our findings, TVPH significantly reduced CD66a and MPO in lung tissue. These findings suggested that TVPH could restrict the neutrophils activation in the lung tissue. Tregs could limit the neutrophils accumulation and modulate neutrophils function by generating IL-10 (Okeke and Uzonna, 2019). Besides, Tregs could induce lung repair by orchestrating T helper (Th)1 and Th17 cells (Tan et al., 2019).
Second, we assumed that TVPH could protect from ALI due to its antioxidant presence. Some previous studies reported that peptides, which have smaller molecules than protein, are more potent to regulate free radicals and terminate the lipid peroxidation cycles (Sila and Bougatef, 2016). The fraction with molecular weight 3–10 kDa and 10–100 kDa has better antioxidant activity than the fraction of < 3 kDa in red Tilapia scale protein hydrolysate. Different amino acids such as proline, methionine, lysine, phenylalanine, aspartic acid, and glutamine are responsible for their antioxidant activity (Sierra et al., 2021). In the present work, glutamine/glutamate displayed the highest abundance in TVPH (Table 1). Gln modulates IL-8 response via IκB/NFκB signaling pathway after stimulation by LPS which diminishes immune response, proinflammatory cytokines, and chemokines (Liboni et al., 2005). Moreover, Gln in its single, di-, and tripeptide form was represented about 40% of the total bioactivity observed in commercial fish protein hydrolysate (Fitzgerald et al., 2005). TVPH acts as an exogenous antioxidant, promoting enzymatic antioxidants, including superoxide dismutase, to lower lipid peroxidation and protect from renal injury (Riyadi et al., 2020a). Our finding suggests that TVPH may serve as a novel immunomodulator for protecting the lung from damage.
5 Conclusion
The present study suggested that TVPH at dose 450 mg/kg BW showed a promising effect on Tregs and lung improvement. Tilapia viscera protein hydrolysate attenuated inflammation through maintaining naïve Tregs and elevated the expression of IL-10 and TGF-β by CD4+CD25+. TVPH reduces the expression of CD66a and MPO on the lung and protects mice from lung injury after LPS injection. TVPH might be a promising candidate as a food nutraceutical or pharmaceutical in the future to treat inflammation caused by LPS. Therefore, further research is needed to discover the detailed mechanism of TVPH that interferes with the inflammation signaling pathway.
CRediT authorship contribution statement
Putut Har Riyadi: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. Romadhon Romadhon: Data curation, Investigation, Writing – original draft. Apri Dwi Anggo: Data curation, Investigation, Writing – original draft. Mochammad Fitri Atho'illah: Data curation, Formal analysis, Visualization, Writing – original draft. Muhaimin Rifa'i: Resources, Supervision, Writing – review & editing.
Acknowledgements
We want to thank the Department of Fish Product Technology at the University of Diponegoro, and the Department of Biology, Brawijaya University, for the research facilities.
Ethical Approval
The Animal Care and Use Committee of Brawijaya University approved all animal housing and experiments with approval number: 030-KEP-UB-2021 in accordance with the Guide to the Care and Use of Laboratory Animals (National Institutes of Health, United States).
Source of Funding
The Research Institute and Community Service (LPPM) of Diponegoro University funded this research in 2020-2022. Research fund through RPP (233-84/UN7.6.1/PP/2021).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Celery ameliorating against neurobehavioral and neurochemical disorders of perinatal lipopolysaccharides exposure in mice offspring. J. King Saud Univ. - Sci.. 2020;32:1764-1771.
- [CrossRef] [Google Scholar]
- Bergamottin alleviates LPS-induced acute lung injury by inducing SIRT1 and suppressing NF-κB. Innate Immun.. 2021;27:543-552.
- [CrossRef] [Google Scholar]
- Characterization of Charcoal from Kerandang (Channa pleurophthalma Bleeker) Fish Fins Waste as A Source of Hydroxiapatite. IOP Conf. Ser. Earth Environ. Sci.. 2021;750:012033
- [CrossRef] [Google Scholar]
- Elicited soybean extract attenuates proinflammatory cytokines expression by modulating TLR3/TLR4 activation in high-fat, high-fructose diet mice. J. Ayurveda Integr. Med.. 2021;12(1):43-51.
- [Google Scholar]
- Atho’illah, M.F., Widyarti, S., Rifa’i, M., Elicited soybean (Glycine max L.) extract improves regulatory T cell activity in high fat-fructose diet mice 2017 AIP Publishing Malang, Indonesia pp. 020004–1-020004–6 10.1063/1.4983415.
- Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat. Commun.. 2020;11:4011.
- [CrossRef] [Google Scholar]
- Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc. Natl. Acad. Sci.. 2009;106:12055-12060.
- [CrossRef] [Google Scholar]
- Characteristic of Taro (Colocasia esculenta) and Seaweed (Eucheuma cottoni) Based Analogue Rice Fortified with Fishes Bone Collage (A Promising Anti-Diabetic Functional Food) J. Eng. Appl. Sci.. 2017;12:3055-3060.
- [CrossRef] [Google Scholar]
- The Combined Effects of Tryptophan Starvation and Tryptophan Catabolites Down-Regulate T Cell Receptor ζ-Chain and Induce a Regulatory Phenotype in Naive T Cells. J. Immunol.. 2006;176:6752-6761.
- [CrossRef] [Google Scholar]
- FAO, 2020. The State of World Fisheries and Aquaculture 2020. FAO, Rome, Italy. https://doi.org/10.4060/ca9229en.
- Fao Fish and their by-products No. 8717 2016 FAO, Singapore.
- Reparative properties of a commercial fish protein hydrolysate preparation. Gut. 2005;54:775-781.
- [CrossRef] [Google Scholar]
- Tryptophan Metabolites at the Crossroad of Immune-Cell Interaction via the Aryl Hydrocarbon Receptor: Implications for Tumor Immunotherapy. Int. J. Mol. Sci.. 2021;22:4644.
- [CrossRef] [Google Scholar]
- Fermented fish oil suppresses T helper 1/2 cell response in a mouse model of atopic dermatitis via generation of CD4+CD25+Foxp3+ T cells. BMC Immunol.. 2012;13:44.
- [CrossRef] [Google Scholar]
- Immune-enhancement effects of tuna cooking drip and its enzymatic hydrolysate in Balb/c mice. Food Sci. Biotechnol.. 2018;27:131-137.
- [CrossRef] [Google Scholar]
- Glutamine Modulates LPS-Induced IL-8 Production through IκB/NF-κB in Human Fetal and Adult Intestinal Epithelium. J. Nutr.. 2005;135:245-251.
- [CrossRef] [Google Scholar]
- Myricetin attenuates lung inflammation and provides protection against lipopolysaccharide-induced acute lung injury by inhibition of NF-κB pathway in rats. Trop. J. Pharm. Res.. 2018;16:2585.
- [CrossRef] [Google Scholar]
- An Official American Thoracic Society Workshop Report: Features and Measurements of Experimental Acute Lung Injury in Animals. Am. J. Respir. Cell Mol. Biol.. 2011;44:725-738.
- [CrossRef] [Google Scholar]
- The Pivotal Role of Regulatory T Cells in the Regulation of Innate Immune Cells. Front. Immunol.. 2019;10:680.
- [CrossRef] [Google Scholar]
- Preparation and identification of antioxidant peptides from protein hydrolysate of skate (Raja porosa) cartilage. J. Funct. Foods. 2016;25:220-230.
- [CrossRef] [Google Scholar]
- Tilapia viscera hydrolysate extract alleviates oxidative stress and renal damage in deoxycorticosterone acetate-salt-induced hypertension rats. Vet. World. 2020;13(11):2477-2483.
- [Google Scholar]
- Riyadi, P.H., Romadhon, Anggo, A., Suharto, S., Tanod, W., Aryani, A., 2021. Anti-Inflammatory Potential from Tilapia (Oreochromis niloticus) Viscera Hydrolysate with Bioinformatics Analysis (Prediction of Activity Spectra for Substances – PASS). IOP Conf. Ser. Earth Environ. Sci. 750, 012044. https://doi.org/10.1088/1755-1315/750/1/012044.
- PASS and ADMET analyses for eight compounds from Nile tilapia (Oreochromis niloticus) viscera waste hydrolysate as anti-inflammatory nutraceutical. AACL Bioflux. 2020;13:2630-2638.
- [Google Scholar]
- Chemical Characteristics and Amino Acids Profile of Protein Hydrolysates of Nile Tilapia (Oreochromis niloticus) Viscera. World’s Vet. J.. 2019;9:324-328.
- [CrossRef] [Google Scholar]
- Screening of Chemical Components in the Protein Hydrolyzate Extract from Viscera of Tilapia (Oreochromis Niloticus) with Color Assay. Russ. J. Agric. Socio-Econ. Sci.. 2019;90:339-345.
- [CrossRef] [Google Scholar]
- Optimization of protein hydrolysate from visceral waste of Nile tilapia (Oreochromis niloticus) by response surface methodology. Bioflux. 2019;12:2347-2358.
- [Google Scholar]
- Effects of Nile Tilapia (Oreochromis niloticus) Viscera Hydrolyzate on Blood Pressure, TNF-α and IL-6 Expression in Rats (Rattus norvegicus) Induced by DOCA-Salt. Indian. J Anim. Res. 2020:1-6.
- [CrossRef] [Google Scholar]
- Effects of dichloromethane Sarcophyton spp. extract on the lipopolysaccharide-induced expression of nuclear factor-kappa B and inducible nitric oxide synthase in mice. Vet. World. 2019;12:1897-1902.
- [CrossRef] [Google Scholar]
- The Effects of Elicited Soybean (Glycine max) Extract on Hematopoietic Cells of High Fat-Fructose Diet Balb/C Mice Model. Jordan J. Biol. Sci.. 2018;11:241-246.
- [Google Scholar]
- Silymarin Restores Regulatory T Cells (Tregs) Function in Multiple Sclerosis (MS) Patients In Vitro. Inflammation. 2019;42:1203-1214.
- [CrossRef] [Google Scholar]
- Antioxidant peptides derived from hydrolysates of red tilapia (Oreochromis sp.) scale. LWT. 2021;146:111631
- [CrossRef] [Google Scholar]
- Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods. 2016;21:10-26.
- [CrossRef] [Google Scholar]
- Regulatory T-cells promote pulmonary repair by modulating T helper cell immune responses in lipopolysaccharide-induced acute respiratory distress syndrome. Immunology. 2019;157:151-162.
- [CrossRef] [Google Scholar]
- Indonesian aquaculture futures: An analysis of fish supply and demand in Indonesia to 2030 and role of aquaculture using the AsiaFish model. Mar. Policy. 2017;79:25-32.
- [CrossRef] [Google Scholar]
- Dietary Flavonoid Naringenin Induces Regulatory T Cells via an Aryl Hydrocarbon Receptor Mediated Pathway. J. Agric. Food Chem.. 2012;60:2171-2178.
- [CrossRef] [Google Scholar]
- A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis.. 2018;9:143.
- [CrossRef] [Google Scholar]
- Wuryandari, M.R.E., Atho’illah, M.F., Laili, R.D., Fatmawati, S., Widodo, N., Widjajanto, E., Rifa’i, M., 2021. Lactobacillus plantarum FNCC 0137 fermented red Moringa oleifera exhibits protective effects in mice challenged with Salmonella typhi via TLR3/TLR4 inhibition and down-regulation of proinflammatory cytokines. J. Ayurveda Integr. Med. 100531. https://doi.org/10.1016/j.jaim.2021.10.003.
- Resolvin D1 attenuates lipopolysaccharide induced acute lung injury through CXCL-12/CXCR4 pathway. J. Surg. Res.. 2014;188:213-221.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and protective effects of D-carvone on lipopolysaccharide (LPS)-induced acute lung injury in mice. J. King Saud Univ. - Sci.. 2020;32:1592-1596.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102020.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







