Translate this page into:
Thymoquinone nanoparticles protect against cisplatin-induced nephrotoxicity in Ehrlich carcinoma model without compromising cisplatin anti-cancer efficacy
⁎Corresponding authors at: Special Infectious Agents Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia (S. Harkaeh). Pharmaceutical Research Institute, Albany College of Pharmacy and Health Sciences, 1 Discovery Drive, Rensselaer, NY 12144, USA (S. Mousa). sharakeh@gmail.com (Steve Harakeh), shaker.mousa@acphs.edu (Shaker Mousa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Cisplatin (CISP) is an effective chemotherapy used in the treatment of various types of cancer, but it causes nephrotoxicity and other adverse effects. Thymoquinone (THY) is an effective anti-inflammatory and antioxidant compound, which might protects against many chemotherapies associated toxicities. However, THY applications are hindered by its poor solubility and low bioavailability. This study evaluated the efficacy of a novel nanoparticle (NP) encapsulting THY to overcome its poor solubility, enhance its bioavilability, efficacy for the protection against CISP-induced nephrotoxicity in an Ehrlich solid carcinoma (ESC) mice model.
Methods
Four treatment groups were included: 1) control, 2) tumour, 3) CISP, and 4) CISP + NP THY.
Results
The results showed that NP THY was effective in preventing CISP-induced kidney toxicity in ESC mice and improved its function and pathology. NP THY effectively ameliorated CISP-induced oxidative stress conditions in the kidney tissue via increasing the levels of antioxidants both non-enzymatic (GSH) and enzymatic (SOD and CAT). NP THY, also, significantly reduced CISP-induced kidney inflammation by reducing TNF-α, IL-1β, and NF-kB levels. NP THY didn’t hinder the antitumor activity of CISP as shown by tumour weight and histological examination data.
Conclusions
In conclusion, NP THY could be an adjuvant therapy to CISP cancer treatment to prevent associated nephrotoxicity and other adverse effects without compromising CISP antitumor efficacy.
Keywords
Cisplatin
Thymoquinone
Nephrotoxicity
Ehrlich
Antioxidant
Anti-inflammatory
Nanoformulation
1 Introduction
A variety of different types of cancers are treated with cisplatin (CISP). CISP’s ability to destroy cancer cells is based on its ability to bind to DNA and induce cytotoxic DNA destruction (Siddik, 2003) .However, it impairs the efficacy of the cell repair and induces apoptosis (Desoize and Madoulet, 2002). The side effects that are associated with the use of CISP are not desirable at all and include myelosuppression, vomiting and nausea (Tsang et al., 2009). Also, nephrotoxicity is considered as a serious complication associated with the use of CISP (Miller et al., 2010). CISP mainly affects the proximal tubule in laboratory animals (Leibbrandt et al., 1995). Massive necrosis is the main histopathological characteristics that is associated with CISP-induced nephrotoxicity in laboratory animals followed by subsequent regeneration of renal proximal tubular cells (Dobyan et al., 1980). There is a lot of controversy regarding the mechanism of action by which of CISP-induced nephrotoxicity is produced. Many mechanisms have been proposed including apoptosis, free radicals, inflammation, and hypoxia (Uehara et al., 2011).
Complementary therapy when applied with conventional treatment may lead to better results with less desirable side effects and lower drug resistance. Thymoquinone (THY) (2-methyl-5-isopropyl-1, 4-benzoquinone), a monoterpene, is the main active ingredient of (30–48%) of Nigella sativa known as either black cumin or black seed (Hajhashemi et al., 2004). It has anti-inflammatory and antioxidant characteristics and used against a wide range of cancers (Wanner et al., 1993). THY has inhibited proliferation and induced apoptosis in leukemia (Shoieb et al., 2003, Harakeh et al., 2008; Harakeh et al., 2017). Some studies have shown that a high dose of THY has potentiated the nephrotoxic side effects exerted by CISP in cancer free female rats (Dirican et al., 2016). On the other hand, several studies have reported that low doses of THY protected against CISP induced nephrotoxicity in rats (Badary et al., 1997; Nessa et al., 2011; Ulu et al., 2012; Dirican et al., 2016; Farooqui et al., 2017). Another recently published study indicated that a high dose THY combined with curcumin protects against CISP-induced nephrotoxicity (Al Fayi et al., 2020). THY-chemotherapeutics combination enhanced the antitumor activity of several chemotherapeutic agents and prevented their cytotoxic consequences on the normal cells and tissues by its antioxidant activities (El-Far et al., 2020). The drawbacks of considering THY as a primary therapeutic drug is related to its poor solubility which in turn affects its bioavailability (Ganea et al., 2010; Elmowafy et al., 2016).
The employment of nanotechnology in the area of herbal medicine is one of the quickest-growing fields. The utilization of nanotechnology has several benefits that have contributed to an increase in the use of herbal medicine in the management of many cancer and chronic diseases (El-Shitany et al., 2019). The formulation of natural remedies nano-particles (NP) enhances its bio-availability and effectiveness (Watkins et al., 2015).
This study aimed to: (1) prepare and characterize NP THY, (2) evaluate the beneficial role NP THY against CISP-induced nephrotoxicity in Ehrlich solid carcinoma (ESC) mice model, (3) investigate the possible protective mechanism (4) examine the impact of NP THY on the anticancer effect of CISP using ESC mice model.
2 Materials and methods
2.1 Preparation and characterization of nanoparticles thymoquinone (NP THY)
NP THY was synthesized by double emulsion methods of thymoquinone encapsulted into PLGA and PVA natural polymers and Pluronic 127 non anionic surfactant (Feczkó et al., 2008; Alam et al., 2012). In brief, 60 mg PLGA, 20 mg Pluronic and 30 mg THY were dissolved in 0.1 ml DMSO under stirring for 30 min. The entire solution was then emulsified with 0.7 ml 2% PVA under prob sonication for an additional 90 s. forming the first nano emulsion. Then, the emulsion was sonicated for 60 s with 0.2 ml 1% chitosan forming the second emulsion. NP THY was washed twice with deionized water using centrifugation (14,800×g, 4 °C, 60 min). The precipitate was dispersed in 1 ml of chitosan.
2.2 Characterization of NP THY
The size distribution and zeta potential of NP THY in aqueous dispersions was determined using Dynamic Light Scattering (DLS) and Electrophoretic Light Scattering (ELS) techniques using a Malvern zeta sizer (Malvern Instrumentation Co, Westborough, MA, USA). Ten µl of the prepared NP were resuspended in 1 ml of water. THY concentration was determined spectroscopically by establishing standards calibration curve.
2.3 Encapsulation efficiency (EE) and loading ratio (LR)
The EE of NP THY as determined by analyzing the THY encapsulated in the NP compared to the amount of THY fed initially. After lyophilization, weighed nano-encapsulated powder was dispersed in 3 ml of DMSO for 30 min. The amount of THY in the DMSO was determined spectroscopically by calibration curve. The EE was calculated according to equation (1):
The LR was determined by measuring the amount of encapsulated THY and the weight of whole NP according to equation (2):
(2)
2.4 Animals and drugs
Eight weeks adult female Swiss albino mice (n = 20) weighing between 20 and 25 g were reared at the animal house unit, King Fahd Medical Research Centre, King Abdulaziz University (KAU), Jeddah, Saudi Arabia. The animals were treated in a very humane way and all handling of animals were done according to the set rules put forward by the ethical committee at KAU university. The experimental design was approved by the Unit of Biomedical Ethics Research Committee, Faculty of Medicine, King Abdulaziz University (KAU), Jeddah, Saudi Arabia (Refernce No. 331.19). Before the experiment, animals will be acclimatized for 1 week with the new surrounding fed commercial diet and allows access to water ad libitum. CISP of Mylan Institutional LLC, Rockford, IL, USA, was used in this study.
2.5 Induction of Ehrlich solid carcinoma
ESC was induced in the mice using Ehrlich ascites carcinoma (EAC) cells purchased from the National Cancer Institute, Cairo, Egypt. The viability of ESC cells was measured using the trypan blue exclusion assay, and it was found to be 98 percent. On day zero 2.5 × 106 viable EAC cells (0.2 ml PBS/mouse) were injected intramuscularly in the left thigh of each mouse to induce a solid tumour (Aldubayan et al., 2019; Mohamed et al., 2020). The tumor grew in 100% of the mice, with a palpable solid tumor mass within 10 days of implantation. The mice holding the tumour were divided into 3 equal groups (n = 5) besides a group of non-tumours bearing mice served as the control group. The tumour groups were designed as follows: tumour group, tumour + CISP (3.5 mg/kg), and tumour + CISP + NP THY (3 mg/kg). The dose of CISP were according to a study published by (Elkhawaga et al., 2019). CISP was intraperitoneally injected as a single dose while NP THY was orally administered daily for 2 weeks. Both the control and the tumour groups were orally injected daily with PBS.
2.6 Samples collection and storage
Two weeks after CISP, CISP + NP THY, and PBS injection, the mice were ether anesthetized and blood was withdrawn via cardiac puncture, centrifuged at 3,000 rpm to separate the serum. Serum samples were collected and stored at −80 °C. The mice were then sacrificed, and the tumour samples and kidney samples were excised and weighed and were formalin-fixed for histopathology. Parts of the kidney samples were kept frozen at −80 °C for assessment of the markers of oxidative stress, antioxidants, and inflammation.
2.7 Determination of serum kidney function markers
Serum concentrations of urea, uric acid, creatinine, and cystatin C were determined using ELISA kits of MyBioSource (USA) catalogue No. MBS2600001, MBS7606443, MBS749827, and MBS763996, respectively according to the manufacturer's instructions.
2.8 Determination of kidney tissue oxidative stress/antioxidants markers
Kidney tissue concentrations of oxidative stress marker, malondialdehyde (MDA), and the antioxidants markers, reduced glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) were determined using ELISA kits of MyBioSource (USA) catalogue No. MBS268427, MBS265966, MBS036924, and MBS726781, respectively according to the manufacturer's instructions.
2.9 Determination of kidney inflammatory markers
Kidney tissue concentrations of inflammatory markers, tumour necrosis factor alfa (TNF-α), interleukin-1beta (IL-1β), and nuclear factor kappa beta (NF-kB) were determined using ELISA kits of MyBioSource (USA) catalogue No. MBS2507393, MBS825017, and MBS268833, respectively according to the manufacturer's instructions.
2.10 Histopathological investigation
The preparation of haematoxylin and eosin (H & E) stained sections of the collected tumour and kidney samples was carried out as previously described by (Suvarna et al., 2018). The sections were investigated and photographed by a blind pathologist for the pathological findings.
2.11 Statistical presentation of data
Data was presented as mean ± SD (n = 5). Comparison among groups were carried out using ANOVA followed by Tukey HSD post-hoc test, using GraphPad Prism version 5. The level of significance was settled as p ≤ 0.05.
3 Results
3.1 Preparation and physicochemical characterization
Results of Z-average particle size and zeta potential were 210.9 nm and +32.8 mV, respectively (Fig. 1).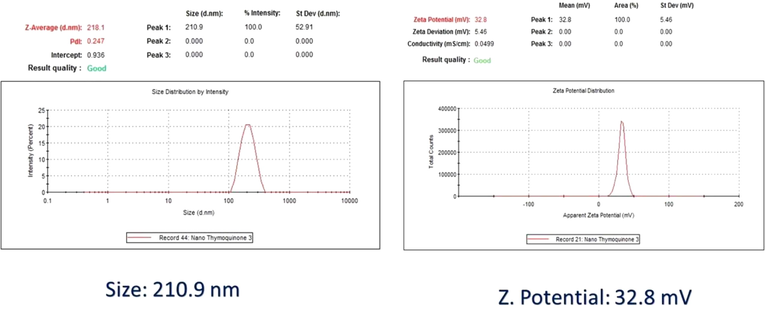
Nanoencapsulation of thymoquinone (THY) into PLGA-PEG nanoparticles.
3.2 Entrapment efficiency (EE) and drug loading capacity
The results showed that EE was 96% which reflects the success of the preparation method to prevent loss of the active drug. The drug loading ratio was 10.2% measured by HPLC-UV.
3.3 Effect of NP THY on-serum kidney function markers measured during treatment with CISP in Ehrlich solid carcinoma mice model
Treatment of mice bearing Ehrlich solid tumour with CISP significantly increased kidney function markers (urea, uric acid, creatinine, and cystatin C) compared to both the control and Ehrlich tumour groups. The mice treated with NP THY together with CISP showed improvement in kidney function as significantly decreased urea, uric acid, creatinine, and cystatin C was observed compared with the CISP group (Fig. 2). These results showed that adding NP THY to CISP protects the kidney against CISP-induced toxicity.![Effect of nanoparticle thymoquinone (NP THY) on serum kidney function markers [A] urea, [B] uric acid, [C] creatinine, and [D] cystatin C measured during treatment with cisplatin (CISP) in Ehrlich solid carcinoma mice model. Results are expressed as mean ± SD (n = 5). asignificant difference against the control group. bsignificant difference against Ehrlich tumor group. csignificant difference against CISP group. ***p ≤ 0.001 and **p ≤ 0.01.](/content/185/2022/34/1/img/10.1016_j.jksus.2021.101675-fig2.png)
Effect of nanoparticle thymoquinone (NP THY) on serum kidney function markers [A] urea, [B] uric acid, [C] creatinine, and [D] cystatin C measured during treatment with cisplatin (CISP) in Ehrlich solid carcinoma mice model. Results are expressed as mean ± SD (n = 5). asignificant difference against the control group. bsignificant difference against Ehrlich tumor group. csignificant difference against CISP group. ***p ≤ 0.001 and **p ≤ 0.01.
3.4 Effect of NP THY on-kidney tissue oxidative stress/antioxidant markers measured during treatment with CISP in Ehrlich solid carcinoma mice model
Treatment of mice bearing Ehrlich solid tumour with CISP significantly increased kidney tissue oxidative stress marker (MDA) compared to both the control and Ehrlich tumour groups. The mice treated with NP THY together with CISP showed a significantly decreased MDA concentration compared with the CISP group (Fig. 3A). Treatment of mice bearing Ehrlich solid tumour with CISP significantly reduced kidney tissue antioxidants markers (GSH, SOD, and CAT) compared to both the control and Ehrlich tumour groups. The mice treated with NP THY together with CISP showed significantly increased GSH, SOD, and CAT concentrations compared with the CISP group (Fig. 3B, C, and D). These results indicated that adding NP THY to CISP enhances the antioxidant protection against CISP-induced oxidative stress in the kidney tissue.![Effect of nanoparticle thymoquinone (NP THY) on kidney tissue oxidative stress marker [A] MDA and antioxidant markers [B] GSH, [C] SOD, and [D] CAT measured during treatment with cisplatin (CISP) in Ehrlich solid carcinoma mice model. Results are expressed as mean ± SD (n = 5). asignificant difference against the control group. bsignificant difference against Ehrlich tumour group. csignificant difference against CISP group. ***p ≤ 0.001.](/content/185/2022/34/1/img/10.1016_j.jksus.2021.101675-fig3.png)
Effect of nanoparticle thymoquinone (NP THY) on kidney tissue oxidative stress marker [A] MDA and antioxidant markers [B] GSH, [C] SOD, and [D] CAT measured during treatment with cisplatin (CISP) in Ehrlich solid carcinoma mice model. Results are expressed as mean ± SD (n = 5). asignificant difference against the control group. bsignificant difference against Ehrlich tumour group. csignificant difference against CISP group. ***p ≤ 0.001.
3.5 Effect of NP THY on-kidney tissue inflammatory markers measured during treatment with CISP in Ehrlich solid carcinoma mice model
Treatment of mice bearing Ehrlich solid tumour with CISP significantly increased kidney tissue inflammatory markers (TNF-α, IL-1β, and NF-kB) compared to both the control and Ehrlich tumour groups. The mice treated with NP THY together with CISP showed significantly decreased TNF-α, IL-1β, and NF-kB concentrations compared with the CISP group (Fig. 4). These results indicated that adding NP THY to CISP prevents CISP-induced inflammatory reaction in the kidney tissue.![Effect of nanoparticle thymoquinone (NP THY) on kidney tissue inflammatory marker [A] TNF-α, [B] IL-1β, and [C] NFkB measured during treatment with cisplatin (CISP) in Ehrlich solid carcinoma mice model. Results are expressed as mean ± SD (n = 5). asignificant difference against the control group. bsignificant difference against Ehrlich tumor group. csignificant difference against CISP group. ***p ≤ 0.001.](/content/185/2022/34/1/img/10.1016_j.jksus.2021.101675-fig4.png)
Effect of nanoparticle thymoquinone (NP THY) on kidney tissue inflammatory marker [A] TNF-α, [B] IL-1β, and [C] NFkB measured during treatment with cisplatin (CISP) in Ehrlich solid carcinoma mice model. Results are expressed as mean ± SD (n = 5). asignificant difference against the control group. bsignificant difference against Ehrlich tumor group. csignificant difference against CISP group. ***p ≤ 0.001.
3.6 Effect of CISP alone and combined with NP THY on-kidney tissues pathology in Ehrlich solid carcinoma mice model
Treatment of mice bearing Ehrlich solid tumour with CISP caused marked disorganization of renal parenchyma with deformity and atrophy of renal corpuscle and glomeruli. CISP also induced marked degenerative changes (swollen cells, unstained cytoplasm, and deformed small nuclei) in the kidney tubule lining epithelium. In the sections of CISP treated mice, the infiltrated neoplastic cells looked unviable and degenerated. The mice treated with NP THY together with CISP showed marked preservation of kidney parenchyma structure with slight deformity of some renal corpuscles. The kidney tubules showed healthy lining epithelium like that of the control sections with slight luminal dilation in the cortical region. The infiltrated neoplastic cells also showed features of degenerative involution. These results showed that adding NP THY to CISP markedly ameliorates CISP-induced kidney histopathological changes in Ehrlich solid carcinoma model in mice (Fig. 5).![Effect of cisplatin (CISP) alone and combined with nanoparticle thymoquinone (NP THY) on kidney tissues pathology in Ehrlich solid carcinoma mice model. Sections in mice kidney cortex and medulla show renal corpuscles (white arrows), glomerular capillaries (black star) and kidney tubules (black arrows) stained by H&E. [A and E]: Control sections show normal renal corpuscle and glomerular capillaries (white arrow). Kidney cortical and medullary tubules show also normal narrow lumina and lining epithelium (black arrows). [B and F]: Ehrlich sections show aggregation of infiltrating viable neoplastic cells (white star), mild decrease in renal corpuscle (white arrow) and glomerular size (white arrow) and dilation of tubular lumina (black arrows). [C and G]: CISP sections show marked disorganization of renal parenchyma with deformity and atrophy of renal corpuscle (white arrow) and glomeruli (black star). Kidney tubule lining epithelium (Black arrows) showed marked degenerative hydropic changes (swollen cells, unstained cytoplasm, and deformed small nuclei). Neoplastic cells (white star) near blood vessels (BV) looked unviable and degenerated (dark pyknotic nuclei). [D and H]: CISP + NP THY sections show marked preservation of kidney parenchyma structure against CISP toxicity, slight deformity of some renal corpuscles. Kidney tubules show healthy lining epithelium similar to that of the control sections with slight luminal dilation in the cortical region (black arrows). Neoplastic cells near blood vessels (white star) show also features of degenerative involution.](/content/185/2022/34/1/img/10.1016_j.jksus.2021.101675-fig5.png)
Effect of cisplatin (CISP) alone and combined with nanoparticle thymoquinone (NP THY) on kidney tissues pathology in Ehrlich solid carcinoma mice model. Sections in mice kidney cortex and medulla show renal corpuscles (white arrows), glomerular capillaries (black star) and kidney tubules (black arrows) stained by H&E. [A and E]: Control sections show normal renal corpuscle and glomerular capillaries (white arrow). Kidney cortical and medullary tubules show also normal narrow lumina and lining epithelium (black arrows). [B and F]: Ehrlich sections show aggregation of infiltrating viable neoplastic cells (white star), mild decrease in renal corpuscle (white arrow) and glomerular size (white arrow) and dilation of tubular lumina (black arrows). [C and G]: CISP sections show marked disorganization of renal parenchyma with deformity and atrophy of renal corpuscle (white arrow) and glomeruli (black star). Kidney tubule lining epithelium (Black arrows) showed marked degenerative hydropic changes (swollen cells, unstained cytoplasm, and deformed small nuclei). Neoplastic cells (white star) near blood vessels (BV) looked unviable and degenerated (dark pyknotic nuclei). [D and H]: CISP + NP THY sections show marked preservation of kidney parenchyma structure against CISP toxicity, slight deformity of some renal corpuscles. Kidney tubules show healthy lining epithelium similar to that of the control sections with slight luminal dilation in the cortical region (black arrows). Neoplastic cells near blood vessels (white star) show also features of degenerative involution.
3.7 Effect of adding NP THY to CISP on the antitumor activity of CISP in Ehrlich solid carcinoma mice model
Treatment of mice bearing Ehrlich solid tumour with CISP significantly decreased tumour weight compared to Ehrlich tumour group. The mice treated with NP THY together with CISP showed also a significantly decreased tumour weight compared to Ehrlich tumour group. There was no significant difference observed between the tumour weight observed in the mice treated with CISP alone and those treated with CISP combined with NP THY (Fig. 6).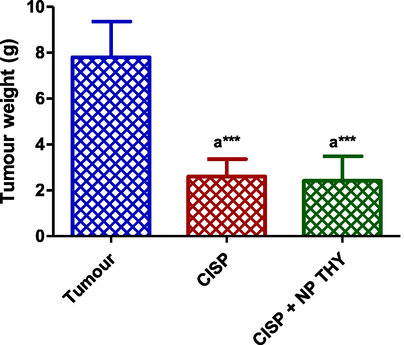
Effect of cisplatin (CISP) alone and combined with nanoparticle thymoquinone (NP THY) on Ehrlich solid carcinoma tumour weight. Results are expressed as mean ± SD (n = 5). asignificant difference against Ehrlich tumour group. ***p ≤ 0.001.
These results were confirmed by the histopathological findings observed when examining the H & E-stained tumour sections of the various study groups. The tumor sections show a highly vascular bed and massive proliferating neoplastic cell infiltration with pleomorphic vesicular active nuclei and numerous mitotic figures. CISP treatment-induced decreased both tumor vascularity and neoplastic cell density. In sections of CISP treatment wide regions of tumor necrosis and small degenerated pyknotic nuclei were observed. with NP THY together with CISP produced more decrease in proliferating cell density, wider regions of tumor necrosis, degeneration of neoplastic cells, and fragmented darkly stained nuclei (Fig. 7).![Effect of cisplatin (CISP) alone and combined with nanoparticle thymoquinone (NP THY) on Ehrlich solid carcinoma tumour pathology. Sections are H & E-stained low power (A, C, and E x40) and high power (B, D, and F x600). [A and B]: Tumour sections show highly vascular bed. Notice the massive proliferating neoplastic cells infiltrating the muscles which have pleomorphic vesicular active nuclei with numerous mitotic figures (arrows). [C and D]: CISP sections show decreased tumor vascularity (star). Neoplastic cells markedly decreased in density with wide regions of tumor necrosis (pink stained area) and small degenerated pyknotic nuclei (arrows). [E and F]: CISP + NP THY sections show more decrease in proliferating cell density. More wider regions of tumor necrosis, degeneration of neoplastic cells become more evident with fragmented darkly stained nuclei (arrows).](/content/185/2022/34/1/img/10.1016_j.jksus.2021.101675-fig7.png)
Effect of cisplatin (CISP) alone and combined with nanoparticle thymoquinone (NP THY) on Ehrlich solid carcinoma tumour pathology. Sections are H & E-stained low power (A, C, and E x40) and high power (B, D, and F x600). [A and B]: Tumour sections show highly vascular bed. Notice the massive proliferating neoplastic cells infiltrating the muscles which have pleomorphic vesicular active nuclei with numerous mitotic figures (arrows). [C and D]: CISP sections show decreased tumor vascularity (star). Neoplastic cells markedly decreased in density with wide regions of tumor necrosis (pink stained area) and small degenerated pyknotic nuclei (arrows). [E and F]: CISP + NP THY sections show more decrease in proliferating cell density. More wider regions of tumor necrosis, degeneration of neoplastic cells become more evident with fragmented darkly stained nuclei (arrows).
3.8 Effect of NP THY on body weight trend and percent survival observed during treatment with CISP in Ehrlich solid carcinoma mice model
Treatment of mice bearing Ehrlich solid tumour with CISP alone and combined with NP THY was not observed with any improvement in body weight throughout the experimental period. However, CISP alone and when added with NP THY increased survival percent tumor along the experimental period (Fig. 8).![Effect of nanoparticle thymoquinone (NP THY) on [A] body weight trend and [B] percent survival observed during treatment with cisplatin (CISP) in Ehrlich solid carcinoma mice model.](/content/185/2022/34/1/img/10.1016_j.jksus.2021.101675-fig8.png)
Effect of nanoparticle thymoquinone (NP THY) on [A] body weight trend and [B] percent survival observed during treatment with cisplatin (CISP) in Ehrlich solid carcinoma mice model.
4 Discussion
THY, which is derived from black cumin (Nigella sativa), is an antioxidant and anti-inflammatory natural compound, but apparently has low aqueous solubility and bioavailability (Ganea et al., 2010; Dehghani et al., 2015; Elmowafy et al., 2016). Bioavailability of a drug to the cells, whether in-vitro or in-vivo, is critical for its optimal efficacy (Dehghani et al., 2015). In the present work a NP THY was formulated and characterized to improve the solubility of THY. Consequently, a low dose of NP THY was used in this experiment (3 mg/kg/day orally). Cisplatin is an effective chemotherapy, but it causes serious renal, neuronal, and other organs via its po-inflammatory (NF-κB) and the generation of reactive oxygen species (Adalı et al., 2016; Farooqui et al., 2017; Kandeil et al., 2019). The protective effect of NP TQ, on chemotherapy-induced nephrotoxicity was demonstrated in our study and others (Farooqui et al., 2017). These positive effects are mediated a reduction in lipid peroxidation and increasing activity of antioxidant enzymes in the renal tissue of chemotherapy-treated animals. These data suggest that TQ and its nanoformulation might be useful adjunct nanonutraceuticals in cisplatin chemotherapy to ameliorate the accompanying nephropathy in long term cancer chemotherapy treatment. These protective effects were achieved without compromising the anti-cancer effects of cisplatin but rather enhanced it.
In this study, we wanted to simulate what happens in cancer patients. The researchers usually examine the effect of cancer chemotherapy toxicity in healthy animals (i.e., without cancer). While what happens is the emergence of associated side effects of chemotherapy on cancer patients being treated with these drugs. Therefore, this study investigated the possible protective effect of NP THY on the nephrotoxicity of CISP in mice bearing Ehrlich solid tumour. The results of this study showed that NP THY was effective in preventing CISP-induced kidney toxicity in ESC mice. This was confirmed by the decreased kidney function markers and improved kidney pathology observed in this study. NP THY effectively ameliorated CISP-induced oxidative stress conditions in the kidney tissue via increasing the levels of antioxidants both non-enzymatic (GSH) and enzymatic (SOD and CAT). NP THY also significantly reduced CISP-induced kidney inflammation by reducing TNF-α, IL-1β, and NF-kB levels. This is the first study to show the protective effect of this novel formulation of NP THY against CISP in an in vivo cancer model. Furthermore, this study showed that NP THY didn’t hinder the antitumor action of CISP in this in vivo ESC model as shown by the tumour weight and the histological examination results. Good evidence supports the key role of increased oxidative stress in the etiology of nephrotoxicity triggered by CISP (Kuhlmann et al., 1997; Santos et al., 2007). Besides, the severity of CISP-induced kidney toxicity was associated with increased kidney concentration of TNF-α and IL-6 (Zhang et al., 2007; Saifi et al., 2019). The researchers reported that TNF-α is the most important cytokine induced during CISP nephrotoxicity and they proved that inhibition of TNF-α could protects the kidney against the deleterious action of CISP (Ramesh and Reeves, 2002).
A recent study also reported the protective effect of THY against acute renal failure of gentamicin by reducing oxidative stress and stimulating antioxidants (Alsharidah et al., 2021).
The published data concerning the impact of THY on CISP-induced kidney toxicity showed contradictory results. Although many previous studies showed that THY might protect against CISP-induced nephrotoxicity in normal animals, other studies reported that THY does not protection. An early study carried by (Badary et al., 1997) reported a nephroprotective effect of THY (50 mg/L) administered in drinking water for 10 days against CISP-induced nephrotoxicity in non-tumorigenic rats. The researchers also reported that THY potentiated the antitumor effect of CISP in EAC model in mice. A study showed more detailed mechanism of THY (10 mg/kg in drinking water for 5 days) protection against CISP nephrotoxicity in rats (Ulu et al., 2012). The authors found that THY significantly reduced kidney oxidative stress markers (MDA and 8-isoprostane). They also reported a significant elevation in the expression of OATs and OCTs and downregulation of MRPs (MRP2 and MRP4) in the kidney. The study of (Farooqui et al., 2017) showed that administration of THY (2 ml/kg orally) significantly attenuated the CISP induced renal function impairment (Farooqui et al., 2017). THY precluded CISP induced changes in the function of carbohydrate metabolism enzymes and in the enzymatic and non-enzymatic antioxidant parameters. A recent study presented that treatment of rats induced with CISP nephrotoxicity with a combination of THY (50 mg/kg) and curcumin (100 mg/kg) for 5 days significantly prevent CISP-induced kidney damage (Al Fayi et al., 2020). The researchers suggested that THY mediated up-regulation of survival signals (Akt, Nrf2/HO-1) and attenuation of KIM-1 and NFkB.
A synergistic effect of THY and CISP has been reported based on both in vitro and in vivo cancer models including many types of cancers (Jafri et al., 2010; Nessa et al., 2011; Al-Malki and Sayed, 2014; Hafiza and Latifah, 2014; Wilson et al., 2015). The data from the latter studies indicated the THY downregulated the growth promoting oncogenic proteins (cMyc), pro-angiogenic factors (VEGF), and anti-apoptotic proteins (Bcl-2) mediated via the inhibition of the NF-kB activation pathway. The use of the combination treatment with THY and CISP resulted in a decrease in tumorigenesis associated with a reduction in the expression of certain important proliferation markers, a concomitant enhancement of double strand DNA break and an induction in apoptotic cell death were also noted (Jafri et al., 2010; Wilson et al., 2015). Furthermore, in agreement of our results, proliferation of MCF7 cells was significantly inhibited by THY and NP THY. THY-loaded nanoparticles proved more effective compared to regular THY (Dehghani et al., 2015). Another recently published article documented that adding a combination of THY and pentoxifylline to CISP excreted a synergistic chemotherapeutic action and induced attenuation in tumour growth mainly through Notch suppression (Mosalam et al., 2020).
THY treatment resulted in a significant attenuation of tumorigenic signalling, especially those mediated by TGF-β, EGF, FGF, VEGF and many other pro- angiogenic, metastatic factors, and mitogenic with a dose-dependent inhibition of invasion migration, and proliferation (Yi et al., 2008; Ahmad et al., 2013; Khan et al., 2015; Rajput et al., 2015). Moreover, THY mediated its efficacy via the activation of various transcriptional factors like NF-kB, as well as the signal transducer and activator of transcription-3 (STAT-3) and the nuclear factor erythroid-related factor-2 (Nrf-2), that cause the transcriptional activation of the genes that are involved in encoding certain proteins that are associated with angiogenesis, cell proliferation, and metastasis (Kundu et al., 2014; Zhang et al., 2016).
5 Conclusions
In conclusion, the results of this study provided evidence that nano formulation of THY could be an adjuvant therapy to CISP in treatment of cancer. The novel combination of NP THY and CISP prevents CISP-induced nephrotoxicity. The underlying mechanism may be the antioxidant plus the anti-inflammatory potential of THY.
Study limitations and future directions
The anticancer effect of NP THY still need more investigations to determine its efficacy in comparison to conventional THY and chemotherapeutic agents. Also, A prospective study should be designed to investigate the protective effect of NP THY in healthy rats and compare it with its effect in a mice tumor model. Also we did not conduct dose-ranging studies to determine PK and PD for TQ nanoformulation versus TQ, which will be the plan for another study. Additionally, we will determine if the enhanced anti-cancer efficacy with cisplatin is additive or synergistic based on different doses of cisplatin versus optimal dose of NP-TQ.
Animal protocol approval
The protocol of the current study was in accordance to the guidelines set by the animal care facility at KAU following all regulations issued by “The National Committee of Bio and Medical Ethics-King Abdulaziz City for Science and Technology”.
Conflict of interest
The authors declared that they don't have any conflict of interest.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. G: 750-140-1441. The authors, therefore, acknowledge with thanks The DSR for their technical and financial support.
References
- Effects of thymoquinone against cisplatin-induced cardiac injury in rats1. Acta cirurgica brasileira. 2016;31:271-277.
- [Google Scholar]
- Thymoquinone suppresses metastasis of melanoma cells by inhibition of NLRP3 inflammasome. Toxicol. Appl. Pharmacol.. 2013;270(1):70-76.
- [Google Scholar]
- Thymoquinone and curcumin combination protects cisplatin-induced kidney injury, nephrotoxicity by attenuating NFκB, KIM-1 and ameliorating Nrf2/HO-1 signalling. J. Drug Target.. 2020;28(9):913-922.
- [Google Scholar]
- Thymoquinone attenuates cisplatin-induced hepatotoxicity via nuclear factor kappa-β. BMC complementary Alternative Med.. 2014;14(1):1-8.
- [Google Scholar]
- Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: a pharmacoscintigraphic study. Int. J. Nanomed.. 2012;7:5705.
- [Google Scholar]
- Aldubayan, M. A., Elgharabawy, R. M., Ahmed A.S., Tousson E. (2019). Antineoplastic activity and curative role of avenanthramides against the growth of ehrlich solid tumors in mice. Oxidative medicine and cellular longevity 2019.
- Thymoquinone, but not metformin, protects against gentamicin-induced nephrotoxicity and renal dysfunction in rats. Appl. Sci.. 2021;11(9):3981.
- [Google Scholar]
- Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can. J. Physiol. Pharmacol.. 1997;75(12):1356-1361.
- [Google Scholar]
- The comparison of anticancer activity of thymoquinone and nanothymoquinone on human breast adenocarcinoma. Iranian J. Pharm. Res: IJPR. 2015;14(2):539.
- [Google Scholar]
- Particular aspects of platinum compounds used at present in cancer treatment. Crit. Rev. Oncol/Hematol.. 2002;42(3):317-325.
- [Google Scholar]
- Thymoquinone enhances cisplatin-induced neprotoxicity in high dose. J. Oncol. Sci.. 2016;1:17-24.
- [Google Scholar]
- Mechanism of cis-platinum nephrotoxicity: II. Morphologic observations. J. Pharmacol. Exp. Ther.. 1980;213(3):551-556.
- [Google Scholar]
- Thymoquinone-chemotherapeutic combinations: New regimen to combat cancer and cancer stem cells. Naunyn-Schmiedeberg's Arch. Pharmacol.. 2020;393:1581-1598.
- [Google Scholar]
- Nanoparticles ellagic acid protects against cisplatin-induced hepatotoxicity in rats without inhibiting its cytotoxic activity. Int. J. Pharmacol.. 2019;15(4):465-477.
- [Google Scholar]
- Evaluation of anti-tumor activity of metformin against Ehrlich ascites carcinoma in Swiss albino mice. Egypt. J. Basic Appl. Sci.. 2019;6(1):116-123.
- [Google Scholar]
- Enhancement of bioavailability and pharmacodynamic effects of thymoquinone via nanostructured lipid carrier (NLC) formulation. Aaps Pharmscitech. 2016;17(3):663-672.
- [Google Scholar]
- Oral administration of Nigella sativa oil and thymoquinone attenuates long term cisplatin treatment induced toxicity and oxidative damage in rat kidney. Biomed. Pharmacother.. 2017;96:912-923.
- [Google Scholar]
- Comparison of the preparation of PLGA–BSA nano-and microparticles by PVA, poloxamer and PVP. Colloids Surf., A. 2008;319(1–3):188-195.
- [Google Scholar]
- Delivery of phytochemical thymoquinone using molecular micelle modified poly (D, L lactide-co-glycolide)(PLGA) nanoparticles. Nanotechnology. 2010;21(28):285104
- [Google Scholar]
- Potential implications of GRP58 expression and susceptibility of cervical cancer to cisplatin and thymoquinone-based therapy. OncoTargets Ther.. 2014;7:1375.
- [Google Scholar]
- Black cumin seed essential oil, as a potent analgesic and antiinflammatory drug. Phytother. Res.. 2004;18(3):195-199.
- [Google Scholar]
- Epigallocatechin-3-gallate induces apoptosis and cell cycle arrest in HTLV-1-positive and-negative leukemia cells. Med. Oncol.. 2008;25(1):30-39.
- [Google Scholar]
- Harakeh, S., J. Semaan, M. El Sabban, S. K. Al Jaouni, M. Diab-Assaf and R. Azar (2017). Thymoquinone Inhibits Proliferation and induces apoptosis in leukemic cells, AACR.
- Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J. Exp. Clin. Cancer Res.. 2010;29(1):87.
- [Google Scholar]
- Thymoquinone and geraniol alleviate cisplatin-induced neurotoxicity in rats through downregulating the p38 MAPK/STAT-1 pathway and oxidative stress. Life Sci.. 2019;228:145-151.
- [Google Scholar]
- Thymoquinone inhibits cancer metastasis by downregulating TWIST1 expression to reduce epithelial to mesenchymal transition. Oncotarget. 2015;6(23):19580.
- [Google Scholar]
- Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol. Dialysis Transplant.. 1997;12(12):2478-2480.
- [Google Scholar]
- Thymoquinone induces heme oxygenase-1 expression in HaCaT cells via Nrf2/ARE activation: Akt and AMPKα as upstream targets. Food Chem. Toxicol.. 2014;65:18-26.
- [Google Scholar]
- Critical subcellular targets of cisplatin and related platinum analogs in rat renal proximal tubule cells. Kidney Int.. 1995;48(3):761-770.
- [Google Scholar]
- Growth retardation and apoptotic death of tumor cells by Artemisia herba-alba oral administration in Ehrlich solid carcinoma bearing mice. Revista Brasileira de Farmacognosia. 2020;29:763-772.
- [Google Scholar]
- Thymoquinone and pentoxifylline enhance the chemotherapeutic effect of cisplatin by targeting Notch signaling pathway in mice. Life Sci.. 2020;244:117299
- [Google Scholar]
- Synergism from combinations of cisplatin and oxaliplatin with quercetin and thymoquinone in human ovarian tumour models. Anticancer Res.. 2011;31(11):3789-3797.
- [Google Scholar]
- Thymoquinone restores radiation-induced TGF-β expression and abrogates EMT in chemoradiotherapy of breast cancer cells. J. Cell. Physiol.. 2015;230(3):620-629.
- [Google Scholar]
- TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Investig.. 2002;110(6):835-842.
- [Google Scholar]
- Protective effect of nanoceria on cisplatin-induced nephrotoxicity by amelioration of oxidative stress and pro-inflammatory mechanisms. Biol. Trace Elem. Res.. 2019;189(1):145-156.
- [Google Scholar]
- Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch. Toxicol.. 2007;81(7):495-504.
- [Google Scholar]
- In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone. Int. J. Oncol.. 2003;22(1):107-113.
- [Google Scholar]
- Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22(47):7265-7279.
- [Google Scholar]
- Suvarna, K.S., Layton, C., Bancroft, J.D. (2018). Bancroft's theory and practice of histological techniques E-Book, Elsevier Health Sciences.
- Comparative nephrotoxicity of cisplatin and nedaplatin: mechanisms and histopathological characteristics. J. Toxicol. Pathol.. 2011;24(2):87-94.
- [Google Scholar]
- Regulation of renal organic anion and cation transporters by thymoquinone in cisplatin induced kidney injury. Food Chem. Toxicol.. 2012;50(5):1675-1679.
- [Google Scholar]
- Wanner, M., Koomen, G., Dung, N.X. (1993). Thymoquinone from Eupatorium ayapana. Planta Med. 59(01): 99-99.
- Natural product-based nanomedicine: recent advances and issues. Int. J. Nanomed.. 2015;10:6055.
- [Google Scholar]
- Thymoquinone enhances cisplatin-response through direct tumor effects in a syngeneic mouse model of ovarian cancer. J. Ovarian Res.. 2015;8(1):1-10.
- [Google Scholar]
- Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol. Cancer Ther.. 2008;7(7):1789-1796.
- [Google Scholar]
- Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-α produced by renal parenchymal cells. Kidney Int.. 2007;72(1):37-44.
- [Google Scholar]
- Thymoquinone chemosensitizes colon cancer cells through inhibition of NF-κB. Oncol. Lett.. 2016;12(4):2840-2845.
- [Google Scholar]







