Translate this page into:
Thiourea enriched cotton waste enhances biomass and nutrition contents in (White oyster) and (Phoenix oyster) mushrooms
⁎Corresponding authors at: Institute of Horticultural Sciences, University of Agriculture Faisalabad, Pakistan. fghulamhussain@gmail.com (Fozia), anam.zahidrana@gmail.com (Anam Zahid)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Oyster mushrooms (white oyster and Phoenix oyster) are edible, delicious and having medicinal properties. This study aimed to estimate the role of thiourea (TU) enriched with cotton waste for production of Pleurotus ostreatus (P1) and Pleurotus sajor-caju (P3). Undiluted cotton waste was used as control (T1 = 100% cotton waste) and accretion along different combination of TU (T2 = 2 mmol L−1, T3 = 4 mmol L−1, T4 = 6 mmol L−1, T5 = 8 mmol L−1 and T6 = 10 mmol L−1). This research was conducted in 2015–2016. The response of Oyster mushroom strains to TU was estimated initiation of spawn running, completion of mycelium growth (100%), biological efficiency (BE), days to completion of flushes (1st, 2nd and 3rd flush), yield, mineral contents (nitrogen (N), potassium (K), manganese (Mn), magnesium (Mg), calcium (Ca), iron (Fe), sodium (Na), zinc (Zn), phosphorus (P), Reducing sugars, total sugars and non-reducing, Vitamin C contents and total soluble solids (TSS)of mushrooms fruiting body and spectral characteristics of mushroom examined through Fourier transform infrared spectroscopy(FTIR) compared with control. Yield of mushroom and BE, were greater by increasing with an accretion in the various combinations of TU within the cotton waste as compared to control. Both strains of mushrooms indicated total soluble solids (TSS) (6.4–6.7 0Brix), total sugar (12.3–14%), non-reducing sugar (8.9–9.5%), reducing sugar (2.5–3.2%), Ascorbic acid (36–45.1 mg/100 g), carbohydrates (58–62.66%), crude protein (26–28%), crude fiber (26–41%), fat contents (35.3–37%) and ash content (4.35–16.01%) on different levels of TU supplemented with cotton waste. TU properly enriched the cotton waste suggested as a novel substrate for high-quality and nutrition rich mushrooms production.
Keywords
Thiourea
Biological efficiency
Total soluble solids
Fourier transform infrared spectroscopy
- TU
-
thiourea
- BE
-
Biological efficiency
- TSS
-
total soluble solids
- FTIR
-
Fourier transform infrared spectroscopy
Abbreviations
1 Introduction
White oyster mushroom (P. ostreatus) belongs to the family of Pleurotaceae, is a worldwide edible mushroom, utilized due to its good flavor and unique texture. Its fan shaped fruit cap having 5–25 cm length, dark-brown and white to grey and in color (Ayodele and Akpaja, 2007). The color of gills is white to creamy. White Oyster mushroom is rich source of lipids, carbohydrates, proteins, vitamin B and C, minerals and low in fat contents and calories (Caglarirmak, 2007). Different lingo-cellulosic materials, industrial deposits and agricultural waste materials such as bean straw, rice straw, wheat straw, cotton waste and sugarcane bagasse are utilized as important substrate (Oei, 2003; Peksen and Kucukomuzlu, 2004). Under the 10-21 °C temperature and 85–90% humidity is best and suitable condition for its fruiting body development (Chen, 2004). Although it has high food value, but still used in medicinal industry to cure different diseases such as cancer therapy and in diabetics (Sivrikaya et al., 2002). It has best remedial effects to cure hypertension and heart diseases (Ortega et al., 1992).
Thiourea (TU) is a simple and sulfur enriched compound. It prevents protein oxidation and substantially, helpful in the reduction of ascorbate copper-catalyzed oxidation. Application of TU separately or with combination of phosphorous (p) in Mung bean is not only improved the leaf area and nitrogen metabolism of the crop but it also amicably enhance the seed yield under the water deficient conditions (Burman et al., 2004). It upgrades sucrose H+ carrier complex structure; consequently, increases the processes involved in carbon dioxide fixation and photosynthates translocation in chickpea embryos. Thiourea improved the dry matter production, seed production, leaf area and growth of cluster bean (Garg et al., 2006). Application of thiourea increase the nutrient uptake like nitrogen, phosphorus and potassium in maize plant (Anjum et al., 2011; Kaya et al., 2015).
In accordance with the available information regarding bio-regulator effect of thiourea in crop plants the present study was conducted aiming to evaluate the effect of application of thiourea in different combinations along cotton waste for the growth and yield related traits in oyster mushrooms. The outcome of current study would ensure profitable production of mushrooms that would result in improvement of human diet and life quality.
2 Materials and method
2.1 Experimental material
We used potato dextrose agar (PDA) (PanReac AppliChem, Spain) media to culture the two different strains of oyster mushroom such as, P1 (V1) P. ostreatus (white oyster) and P3 (V2) P. sajor-caju (phoenix oyster). Medicinal and Mushroom lab, Institute of Horticultural Sciences at University of Agriculture, Faisalabad was used to conduct the whole experiment. The material preserved at 4 °C for further use.
2.2 Preparation of spawn
The spawn preparation is completed by using animal waste, wheat grains mix with Calcium sulfate (2% CaSO4) and Calcium carbonate (2% CaCO3) in 500 mL flask. Initially, we boiled the wheat grains till it reaches in to soften stage after that mixed with rest of material. Then it left for autoclave at 121 °C for the time duration of 20 min, cooled it overnight and potato dextrose agar (PDA) was used for the inoculated material of fresh mycelium. At the 17th of inoculation to get the a fairly homogenous collection of colonized grains the spawn was perturbed well and re-incubated to check the mycelium growth immersed on the grains for 8 days.
2.3 Substrate preparation and polythene bag fill-up
Substrate (cotton waste) was permeated in water and maintained pH by adding 2% percent lime in it. The water-soaked substrate was conglomerated and wrapped with polythene for the time duration of 4–5 days allowing substrate to undergo fermentation. Then substrate was left to remove the excessive amount of water by evaporation on the floor. Cotton waste substrate was treated with various levels of thiourea at 2 mM, 4 mM, 6 mM, 8 mM and 10 mM per liter. The experiment was conducted in three replication with CRD design. Thiourea in different concentration was added into substrate and mixed it thoroughly and left the substrate for one day under sun drying. At the end, the polypropylene bags of size (15 × 25) cm were filled with the substrate, mouths were loosely tied with rubber bands keeping bag weight of 800 g (Estrada and Royse, 2007).
2.4 Growth parameters
The inoculated bags were kept under light at 24 ± 2 °C and 80% relative humidity for mycelium colonization. After colonization, bags were moved in cropping room at the temperature18 ± 2 °C and relative humidity 80–90% to enhance fructification. Different growth-related parameters i.e., days to initiation of spawn running, days to completion of mycelium growth (100%), days to completion of flushes (1st, 2nd and 3rdflush) and days for pinhead formation, yield, biological efficiency (BE) were measured.
2.5 Estimation of sugar contents and TSS
The sugar contents (Total sugars, non-reducing sugar and reducing) and TSS calculated in mushroom extract following the method explained by (Hortiwitz, 1960).
2.6 Determination of macro nutrients and other minerals contents
Digestion of grinding mushroom samples was done by using methods described by (AOAC, 1990) for analysis of macro-nutrients and other minerals. Nitrogen (N) was estimated by using kjeldhal’s method while and Phosphorous (P) was determined by using vonadomolybdate spectrophotometric method described by (Afiukwa et al., 2013). Calcium (Ca) and Magnesium (Mg) were determined by EDTA complexion-metric titration method described by (Udoh and Ogunwale, 1986). Sodium (Na) and Potassium (K) were quantified by flame photometric method described in AOAC (1990), whereas manganese (Mn), lead (Pb) and zinc (Zn) were determined by Atomic Absorption Spectroscopy (AAS) (Afiukwa et al., 2013).
2.7 Ascorbic acid (mg/100 mL)
The concentration of ascorbic acid in mg/100 mL was calculated by analysis (Ruck, 1961). Whereas, it’s quantitative measurement was done by 2,6-dichloro-indo-phenol dye method (AOAC, 2002).
2.8 Estimation of proximate
The crude protein contents in mushroom were calculated by the macro-Kjeldahl method. Carbohydrates, proteins, fat and ash contents were measured by using AOAC procedures (AOAC, 1995).
2.9 FTIR-spectroscopy
The variations in the molecular study of two different oyster mushroom strains due to different application of thiourea were estimated by the grounded sample pellet with FTIR spectroscopy (Agilent 680, University of Engineering and Technology Lahore, Faisalabad campus, Pakistan). The samples of Oyster mushrooms were cleaned and kept under air for drying. Dried sample (1 mg) of fruiting body was milled with 100 mg KBr, pressed into capsule form and spectrum was noted in the range of 650–4000 cm−1.
2.10 Statistical analysis
The data were analyzed statistically adopting ANOVA (analysis of variance) and comparing mean differences at level of significance 5% along with least significance difference (LSD) estimation (Steel and Torrie, 1980).
3 Results
3.1 Initiation of spawn running, mycelium growth, pinhead formation (days)
In Table 1 analysis of variance elucidated the non-significant effects of thiourea treatments on both varieties regarding initiation of spawn running (days). The outcomes confirmed that varieties and thiourea treatments to great extent influence on completion of mycelium growth at 100%. Amongst various thiourea treatments, minimum (38 days) taken by treatment T6 and maximum time (50 days) was measured in T1 in variety V1 respectively. The same results were obtained for T6 (39 days) and (46 days) in T1 in variety (V2). These consequences described that substrate enriched along thiourea motivated growth of mycelium as compared with treatment (T1). Results demonstrated that varieties and thiourea treatments appreciably impact on days for initiation of pinhead formation. Between the different treatments of thiourea, minimum (65 days) expressed by T6 and maximum time (73 days) in T1 by variety V1. Although in V2, treatments T6 (61 days) and T1 (70 days) significantly involved the initiation of pinhead’s formation (days) contrast with other treatments.
3.2 Days for completion of flush and yield (g)
The significant effects of varieties and thiourea treatments on completion of flushes in days (Table 1) confirmed that different level of thiourea had considerably impact on accumulation of flushes (1st flush, 2nd flush and 3rd flush). Along with the various treatments of thiourea, minimum time was taken by treatment T6 and maximum time was measured by T1. The impact of thiourea on both varieties (V1, V2) demonstrates statistically different results. Mushroom yield attained from substrate (cotton waste) mixed with thiourea was considerably greater in treatments T6, T5 in both varieties as compared to control (Table 1). Mean value (n = 3) in the same column with the same following letter do not significantly differ (p < 0.05). (T1 = 100% cotton waste, T2 = 2 mM/lit Thiourea, T3 = 4 mM/lit Thiourea, T4 = 6 mM/lit Thiourea, T5 = 8 mM/lit Thiourea, T6 = 10 mM/lit); V1 (P1); V2 (P3).
Treatments
Initiation of spawn running (days)
Completion of mycelium growth (100%)
Days for pinhead formation
Completion of flushes (days)
Yield
Biological efficiency (BE)
V1
V2
V1
V2
V1
V2
V1
1st flushV2
1st flushV1
2nd flushV2
2nd flushV1
3rd flushV2 3rd flush
V1
V2
V1
V2
T1
1
1
50 ± 2a
46 ± 2a
73 ± 2.5a
70 ± 2.5a
80 ± 2a
77 ± 2a
90 ± 2a
87 ± 2a
103 ± 3a
100 ± 2a
182 ± 2f
246 ± 2f
45 ± 2e
61 ± 2e
T2
1
1
48 ± 2ab
44 ± 1.5ab
67 ± 2b
66 ± 2b
74 ± 2b
74 ± 2ab
84 ± 1.4b
83 ± 2.5ab
97 ± 2b
96 ± 2ab
249 ± 2e
338 ± 2e
64 ± 2d
84 ± 2d
T3
1
1
46 ± 2bc
45 ± 1ab
66 ± 2b
62 ± 2c
73 ± 2bc
72 ± 2bc
83 ± 2.5bc
83 ± 2b
96 ± 2.5bc
93 ± 2.5b
268 ± 2.5d
342 ± 3d
67 ± 2d
85 ± 2d
T4
1
1
44 ± 2c
42 ± 1.5b
67 ± 2b
64 ± 2bc
72 ± 2bc
71 ± 2bc
82 ± 2bc
83 ± 2b
95 ± 2bcd
95 ± 2b
298 ± 2c
404 ± 3c
74 ± 2c
101 ± 2c
T5
1
1
40 ± 3d
42 ± 2bc
66 ± 2b
66 ± 2b
72 ± 2bc
73 ± 2.5ab
81 ± 2bc
80 ± 2.5bc
92 ± 2d
96 ± 1.4ab
438 ± 2b
424 ± 3b
108 ± 3.1b
106 ± 2b
T6
1
1
38 ± 2 d
39 ± 2c
65 ± 2b
61 ± 2c
70 ± 2c
69 ± 3c
80 ± 2c
78 ± 2c
92 ± 2.5 cd
94 ± 3b
550 ± 2a
585 ± 2a
137 ± 2a
146 ± 2a
3.3 Biological efficiency (%)
Biological efficiency is the percentage conversion of dry substrate to fresh fruit bodies. Thiourea significantly enhanced the biological efficiency of white oyster and phoenix oyster mushroom. Amid the different treatments of thiourea, maximum biological efficiency was measured in treatments (T6 and T5) in both varieties (Table 1).
3.4 Total soluble solids (Brix0)
Two different strains of oyster mushroom showed appreciable amount of TSS (Brixo) cultivate on substrate of cotton waste mixed along different application of thiourea. Amid the treatments of thiourea, maximum TSS was recorded in treatment (T6) and lowest TSS was noticed in (T1). Greatest value of TSS was observed in V1 as compared V2. (Table 2). Mean value (n = 3) in the same column with the same following letter do not significantly differ (p < 0.05). (T1 = 100% cotton waste, T2 = 2 mM/lit Thiourea, T3 = 4 mM/lit Thiourea, T4 = 6 mM/lit Thiourea, T5 = 8 mM/lit Thiourea, T6 = 10 mM/lit); V1 (P1); V2 (P3).
Treatments
TSS (0brix)
Total sugars (%)
Reducing Sugar (%)
Non-reducing sugar (%)
Vitamin C (mg/100 g)
Nitrogen (mg/100 g)
Phosphorous (mg/100 g)
Potassium (mg/100 g)
V1
V2
V1
V2
V1
V2
V1
V2
V1
V2
V1
V2
V1
V2
V1
V2
T1
4.5 ± 0.2e
4.5 ± 0.1d
5.8 ± 0.2d
6.9 ± 0.2d
0.4 ± 0.2e
1.1 ± 0.2c
5.5 ± 0.2e
5.8 ± 0.2e
32.7 ± 0.3b
32.4 ± 1.5d
49.6 ± 2.5e
40 ± 2e
41.3 ± 3.2e
25.3 ± 2.5e
150 ± 3d
20.3 ± 3.5 cd
T2
4.7 ± 0.2e
5.1 ± 0.2c
9.1 ± 0.2c
8.6 ± 0.2 cd
1.2 ± 0.1d
1.4 ± 0.2c
7.8 ± 0.1d
7.1 ± 0.1d
33.7 ± 0.4b
33 ± 1 cd
60.3 ± 3.5d
45 ± 3de
64.6 ± 3.5d
32.0 ± 4d
154 ± 3 cd
18.3 ± 2.5d
T3
5.4 ± 0.2b
5.4 ± 0.2c
9.8 ± 0.1bc
10 ± 1bc
1.6 ± 0.2c
2.4 ± 0.3b
8.3 ± 0.1c
7.6 ± 0.2c
33.9 ± 0.2b
34.1 ± 0.8bc
65 ± 3 cd
50 ± 3 cd
80 ± 3c
36 ± 2d
156 ± 3bc
30.3 ± 3.5bc
T4
5.8 ± 0.2c
5.8 ± 0.2b
11 ± 2bc
11.3 ± 1.5ab
2.1 ± 0.2b
3 ± 0.2a
8.8 ± 0.2b
7.9 ± 0.2c
34 ± 2b
34 ± 0.3bcd
70.3 ± 2.5bc
54.6 ± 3.5c
95.3 ± 2.5b
42 ± 2c
158 ± 2bc
40 ± 4ab
T5
6.2 ± 0.2b
6.1 ± 0.2ab
11.5 ± 1.5b
12.8 ± 1.2a
2.4 ± 0.1ab
3.1 ± 0.2a
9.1 ± 0.2b
8.5 ± 0.1b
44.1 ± 2a
34.8 ± 0.2ab
75 ± 3b
62.3 ± 1.5b
118 ± 3a
48 ± 2b
160 ± 2.5ab
45.3 ± 2.5a
T6
6.7 ± 0.2a
6.4 ± 0.2a
14 ± 2a
12.3 ± 1.5a
2.5 ± 0.1a
3.2 ± 0.1a
9.5 ± 0.2a
8.9 ± 0.1a
45.1 ± 3a
36 ± 0.9a
84.3 ± 3.5a
78.6 ± 3.5a
122 ± 1.5a
55.3 ± 2.5a
164 ± 3a
48.2 ± 2.0a
3.5 N.P.K and ascorbic acid contents (mg/100 g)
Maximum nitrogen (84.3 mg/100 g), Phosphorus (122 mg/100 g) and potassium (164 mg/100 g) were measured in oyster mushroom cultivated on substrate augmented with thiourea at 10 mM per liter. Different concentrations of thiourea enhanced the quantity of Vitamin C in edible part of mushroom. Highest quantity of Vitamin C was noted among strains V1, V2 of oyster mushroom at the concentration of 10 mM, while lowest quantity of Vitamin C was obtained in control (Table 2).
3.6 Total sugars contents (%)
A positive significant trend in total sugars (reducing sugar and non-reducing sugar) in mushroom was observed at different treatments of thiourea enriched cotton waste. Treatment T6 (10 mM/L) among all other treatments showed high percentage of all sugar contents in both strains (Table 2).
3.7 Estimation of macro and micro minerals contents in mushroom
Both macro and micro mineral (Na, Fe, Zn, Mg, Mn and Ca) contents were observed in increased concentration under different treatments of thiourea in both varieties. The largest amount of minerals contents were recorded in T5 and T6, for Na (10.4 mg/100 g), Fe (2.5 mg/100 g), Zn (2.4 mg/100 g), Mg (28.6 mg/100 g), Mn (0.50 mg/100 g) and Ca (50.3 mg/100 g) on dry weight basis (Table 3). Mean value (n = 3) in the same column with the same following letter do not significantly differ (p < 0.05). (T1 = 100% cotton waste, T2 = 2 mM/lit Thiourea, T3 = 4 mM/lit Thiourea, T4 = 6 mM/lit Thiourea, T5 = 8 mM/lit Thiourea, T6 = 10 mM/lit); V1 (P1); V2 (P3).
Treatments
Na (mg/100 g)
Fe (mg/100 g)
Zn (mg/100 g)
Mg (mg/100 g)
Mn (mg/100 g)
Ca (mg/100 g)
V1
V2
V1
V2
V1
V2
V1
V2
V1
V2
V1
V2
T1
7.7 ± 0.1c
8.6 ± 0.2c
1.1 ± 0.02d
0.8 ± 0.1d
1.2 ± 0.1e
1.3 ± 0.2e
13.2 ± 2.0d
15.4 ± 1.5d
0.02 ± 0.01b
0.02 ± 0.02c
37.1 ± 2.0e
37.0 ± 2e
T2
7.7 ± 0.2c
8.8 ± 0.2c
1.5 ± 0.2 cd
0.9 ± 0.1d
1.5 ± 0.1d
1.5 ± 0.1de
17.0 ± 2 cd
19.0 ± 2 cd
0.04 ± 0.02b
0.05 ± 0.02bc
40.3 ± 1.5d
40.0 ± 2de
T3
8.1 ± 0.2bc
9.0 ± 0.2bc
1.7 ± 0.1bc
1.2 ± 0.1c
1.8 ± 0.1c
1.6 ± 0.1 cd
18.0 ± 2c
22.6 ± 3.5bc
0.06 ± 0.02b
0.07 ± 0.01bc
43.0 ± 2 cd
42.0 ± 2 cd
T4
8.3 ± 0.3ab
9.5 ± 0.4b
1.8 ± 0.1b
1.4 ± 0.1c
2.0 ± 0.1bc
1.9 ± 0.1bc
23.0 ± 2b
21.3 ± 1.5c
0.08 ± 0.02b
0.09 ± 0.01bc
45.0 ± 2bc
44.0 ± 2bc
T5
8.4 ± 0.2ab
9.5 ± 0.3b
2.0 ± 0.3b
1.7 ± 0.1b
2.2 ± 0.1ab
2.2 ± 0.2ab
26.0 ± 3ab
25.6 ± 2.5ab
0.19 ± 0.17b
0.23 ± 0.15b
47.0 ± 1b
46.0 ± 2ab
T6
8.7 ± 0.3a
10.4 ± 0.4a
2.5 ± 0.2a
2.0 ± 0.1a
2.4 ± 0.1a
2.4 ± 0.2a
28.6 ± 2.5a
28.0 ± 2a
0.40 ± 0.2a
0.50 ± 0.2a
50.3 ± 1.5a
48.0 ± 2a
3.8 Estimation of proximate contents in mushroom
Total fat, crude protein, carbohydrates, crude fiber and ash contents were also significantly enhanced (Figs. 1–5). Maximum amount of crude protein was calculated in T6 of variety V2 as compared to T1. Crude fiber ranges from 12% to 43% were estimated in fruit body of mushroom. Greatest value of carbohydrate was examined in treatment T6 of variety V2 as compared to T1 (control).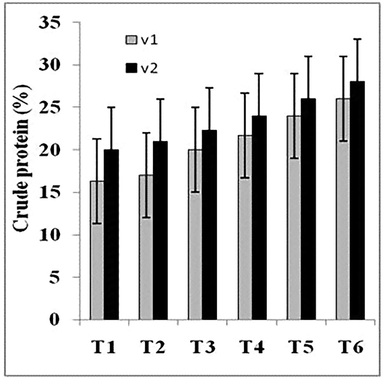
Crude protein (%) of two strains oyster mushroom cultivated on different concentration of TU enriched cotton waste. (T1 = 100% cotton waste, T2 = 2 mM/L TU, T3 = 4 mM/L TU, T4 = 6 mM/L TU, T5 = 8 mM/L TU, T6 = 10 mM/L TU); V1 (P1); V2 (P3).
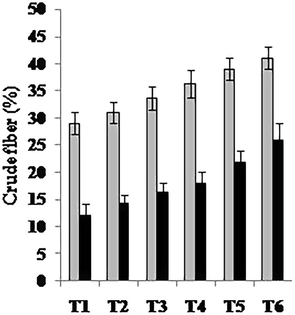
Crude fiber (%) of two strains oyster mushroom cultivated on different concentration of TU enriched cotton waste. (T1 = 100% cotton waste, T2 = 2 mM/L TU, T3 = 4 mM/L TU, T4 = 6 mM/L TU, T5 = 8 mM/L TU, T6 = 10 mM/L TU); V1 (P1); V2 (P3).
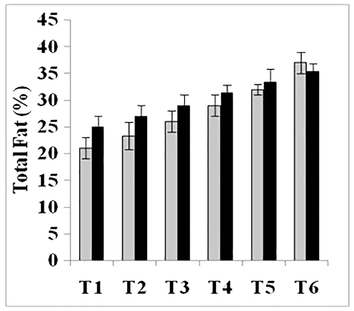
Total fat (%) of two strains oyster mushroom cultivated on different concentration of TU enriched cotton waste. (T1 = 100% cotton waste, T2 = 2 mM/L TU, T3 = 4 mM/L TU, T4 = 6 mM/L TU, T5 = 8 mM/L TU, T6 = 10 mM/L TU); V1 (P1); V2 (P3).

Carbohydrates (%) of two strains oyster mushroom cultivated on different concentration of TU enriched cotton waste. (T1 = 100% cotton waste, T2 = 2 mM/L TU, T3 = 4 mM/L TU, T4 = 6 mM/L TU, T5 = 8 mM/L TU, T6 = 10 mM/L TU); V1 (P1); V2 (P3).
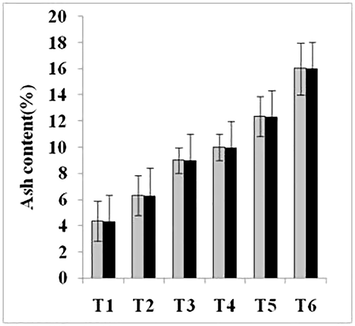
Ash contents of two strains oyster mushroom cultivated on different concentration of TU enriched cotton waste. (T1 = 100% cotton waste, T2 = 2 mM/L TU, T3 = 4 mM/L TU, T4 = 6 mM/L TU, T5 = 8 mM/L TU, T6 = 10 mM/L TU); V1 (P1); V2 (P3).
3.9 Features of FTIR spectra
FTIR spectrum characterized revealed complete biochemical contents of two different strains of Oyster mushroom (4000–1800 cm−1). Bands spectrum assessment in variety (V1) placed particular 3360, 2911, 2922, 2929 and 3255 cm-1, that might be allocated to C–H and O–H group. Distinguished bands located in spectrum of Pleurotus ostreatus (V1) as compared to T1, 3255 and 3360(C–H and O–H), 1610 (Carbonyl group), 1140 and 1026, 1023, 1019, 1015, 1013, 1375(β (1 → 3) glucan, C–O bond, cell wall, protein, polysaccharide) (Table 4). In variety (V1) the band spectrum showed the significant change as compared to Pleurotus sajor-caju (V2). Largest changes in spectrum bands were noted in both varieties treatment (T6) (Table 5). T1 = 100% cotton waste, T2 = 2 mM/L TU, T3 = 4 mM/L TU, T4 = 6 mM/L TU, T5 = 8 mM/L TU, T6 = 10 mM/L TU; V1 (P1). T1 = 100% cotton waste, T2 = 2 mM/L TU, T3 = 4 mM/L TU, T4 = 6 mM/L TU, T5 = 8 mM/L TU, T6 = 10 mM/L TU; V2 (P2).
T1V1
T2V1
T3V1
Peaks
Stretching
Peaks
Stretching
Peaks
Stretching
2911
C–H
2922
C–H
2922
C–H
1623
Carbonyl group
2152
CH2
2186
C≡C
1420
protein
2117
C≡C
1958
O–H bond
1013
C–O bond
2578
O–H
1943
O–H bond
1507
Lipids, protein
1584
Lipids, protein
1459
CH2
1518
Lipids, protein
1438
polysaccharide
1459
CH2
1401
protein
1405
polysaccharide
1313
O–H bending polysaccharides,
1375
β (1 → 3) glucan,
1244
C–O bond
1142
C–O bond, β (1 → 3) glucan, cell wall, polysaccharide
1189
C–O bond, β (1 → 3) glucan, cell wall, polysaccharide
1029
C–O bond, β (1 → 3) glucan.
697
lipids
669
carbohydrates
T4V1
T5V1
T6V1
Peaks
Stretching
Peaks
Stretching
Peaks
Stretching
2922
C–H
3255
O–H bond C–H
3360
O–H bond C–H
2857
CH3–CH2 lipid
2920
C–H
3304
O–H bond C–H
1584
Lipids, protein
2152
C≡C
3261
O–H bond C–H
1448
protein
1578
Lipids, protein
2920
C–H
1395
O–H bending polysaccharides,
1399
polysaccharides
2853
CH3–CH2 lipid
1375
O–H bending polysaccharides,
T1V2
T2V2
T3V1
Peaks
Stretching
Peaks
Stretching
Peaks
Stretching
3268
O–H bond, C–H
3280
O–H bond, C–H
3302
O–H bond, C–H
2920
C–H
3254
O–H bond C–H
2924
C–H
2853
CH3-CH2 lipids
2927
C–H
2851
CH3–CH2 lipids
1610
Amide I, chitin
2925
C–H
2119
C≡C
1399
Polysaccharides
2180
C≡C
2094
C≡C alkyne
1369
β (1 → 3) glucan,
1617
Amide I, chitin
1636
Amide I, chitin
1017
C–O
1449
polysaccharides
1541
Amide II, chitosan
1146
C–O bond, β (1 → 3) glucan, cell wall, polysaccharide
1395
β (1 → 3) glucan,
T4V2
T5V2
T6V1
Peaks
Stretching
Peaks
Stretching
Peaks
Stretching
3309
O–H bond, C–H
3326
O–H bond, C–H
3336
O–H bond, C–H
3257
O–H bond, C–H
3257
O–H bond, C–H
3218
O–H bond, C–H
2920
C–H
2922
C–H
2924
C–H
2079
O–H bond,
2855
C–H
2022
O–H bond,
2055
O–H bond,
2182
C≡C
1578
Amide II, chitosan
1619
Amide 1, chitin
1587
Amide II, chitosan
1522
protein
1407
protein
1146
C–O, C–C glycosides
1438
polysaccharides
1367
β (1 → 3) glucan,
1013
C–O
1397
β (1 → 3) glucan,
1148
C–O bond, β (1 → 3) glucan, cell wall, polysaccharide
1364
β (1 → 3) glucan,
1025
C–O bond, β (1 → 3) glucan, cell wall, polysaccharide
1146
C–O bond, β (1 → 3) glucan, cell wall, polysaccharide
4 Discussion
4.1 Initiation of spawn running, mycelium growth, pinhead formation (days)
The Thiourea has no effects on initiation of spawn strains of both mushrooms under feasible environment. In different species of mushroom the mycelium growth mostly relies on different factors like as accessibility of nutrients in substrate, type of substrate, rate of spawn. Mycelium growth also depends on the growth conditions such as temperature, light during incubation period, humidity, carbon dioxide and oxygen (Hassan et al., 2010). The early formation of pinhead started due to highly rich substrate and presence of macro and micro elements in it.
4.2 Days for completion of flushes, yield (g)
The number of mushroom flesh yield decreased subsequently by increasing the number of flushes presentence of nutrients in the substrate in very low quantity (Rizki and Tamai, 2011). These were in accordance to the findings of Amin et al. (2013) revealed that maize yield enhanced by the application of thiourea at different levels.
4.3 Biological efficiency (%)
Bhattacharjya et al. (2014) declared that if different agricultural waste material is amalgamated with various chemicals, the biological efficiency of oyster mushroom increases.
4.4 N,P,K and Vitamin C contents of mushroom (mg/100 g)
Nitrogen plays important role in the cultivation and growth of mushroom. Nitrogen considerably influences the growth of mycelium, spawn run, formation of pinheads, edible fruit body formation, protein concentration and cultivation of mushroom (Elhami et al., 2008). Nitrogen plays role in the composition of amino acids (production of proteins), polysaccharide, pyrimidines, vitamins and purines (chitin helps to make cell wall of mushrooms (Miles and Chang, 2004). In case of maize plant thiourea application considerably increases nitrogen (N) in the leaves; it is helpful to improve the biomass production of maize plant (Anjum et al., 2011).
Phosphorus is a major component which helps and promote plant growth. It is also helpful in different processes such as production of nucleic acid, energy generation, respiration, glycolysis, membrane stability and synthesis, activation and inactivation of enzyme, carbohydrate metabolism (Vance et al., 2003). Phosphorus is responsible for good trait and better yield of mushrooms (Beyer and Muthersbaugh, 1996). The exogenous application of thiourea plays a major contribution in cellular ion homeostasis and then increases the intake of phosphorus (P). Our result correlates with Kaya et al. (2015) thiourea profitably increases the phosphorus concentration in maize plant.
Potassium plays the very important role in the different mechanisms that are involves in the development (biosynthesis) and discrimination of cap and gills, enzyme activity, upholding carbohydrate metabolism, ionic balance (Griffin, 1996). The exogenous application of thiourea plays important role in cellular ion homeostasis and then increases the uptake of potassium (K). Our result correlates with Kaya et al. (2015) thiourea beneficially increases the potassium concentration in maize plant.
Ascorbic acid plays important role in plants, it is present generally in plants as the excessively existing micro-molecule substance. Ascorbic acid (Vitamin C) plays the important role in different physiological processes like as co-factor of some enzymes, cell division, cell elongation, plant defense against oxidization, plant development, plant growth and senescence (Horemans et al., 2000). Thiourea increases the concentration of ascorbic acid, our result similar to Babu and Yadav (2002) by the foliar spray of thiourea at full bloom stage increases the ascorbic acid contents in peaches.
4.5 Total soluble solids (Brix0)
Total soluble solids are the solid contents which are present in the substance. And, sugar is one of them and others such as, vitamins and minerals etc. The different solid contents contain substances called as total soluble solids. Total soluble solids of apple and some other fruits conifers the most important quality indicators which is related to the composition and texture (Peck et al., 2006). Thiourea helpful to increases the TSS in mushroom. Foliar application of thiourea significantly increases the TSS (total soluble solids) in maize plant (Amin et al., 2013).
4.6 Total sugars, reducing sugar and non-reducing sugar in mushroom (%)
Total soluble sugars contain the sucrose, sorbitol and glucose. These total sugars present in fruit tissues that are responsible for the fruit sweetness, quality and taste of fruits. In addition, sucrose balances the osmotic pressure, maintains the cell membrane structure, triggers the anti-oxidization system to eradicate free radicals and adjusts metabolic pathways (Nishizawa et al., 2008). Our study findings elucidated that total sugar (%) increased by increasing the concentration of thiourea. Earlier study by Jana and Das (2014) showed increasing the concentration of thiourea total sugar (%) also increased in Asian pear. Current study shows that by the application of thiourea reducing sugars increases. Foliar application and seed treatment with thiourea boost the reducing sugars in mung bean (Mathur et al., 2006). Thiourea increases the non-reducing sugar (%). Application of thiourea after fruit setting increases the non-reducing sugar (%) in peaches (Meitei et al., 2013).
4.7 Estimation of macro and micro minerals contents in mushroom
By foliar application of thiourea the uptake of mineral nutrients such as Ca, Mg, Mn, Zn, Na and Fe were higher because thiourea contribute in cellular ionic movements. Foliar application of thiourea increases the Ca contents in the maize leaves. Exogenous application of thiourea increases the Na concentration due to increase in the biomass production of root and shoot (Kaya et al., 2013). Singh and Paliwal (2017) noticed that application of thiourea through foliar application and seed soaking @ 500 ppm increased the zinc content in okra fruit.
4.8 Estimation of proximate contents in mushroom
Mahmoud and Ahmed (2018) discussed that by application of thiourea enhance the carbohydrates, ash, protein, fat and fiber contents in wheat grains.
4.9 Features of Fourier transform infrared spectroscopy
Different compounds with various functional groups was detected with Fourier transform infrared spectroscopy. Mushroom contains abundant amount of carbohydrates, protein, micro and macro-elements with low fats contents. Various bands and spectrum, formed through FTIR might well-ornate compositional analysis based on structural groups of compounds. The consequence of FTIR was its capacity for more precise classification of, proteins, sugars, nucleic acid, starch, lipids and macrostructure of functional groups (O’Gorman et al., 2010). Earlier studies demarcated chemical classification for Agaricus bisporus, many kinds of Amanita and truffles using bands and structural groups through FTIR spectroscopy. Major purpose of current study was to validate whether several treatments of TU influence the nutritional contents, molecular structure of two different strains of mushrooms by FTIR spectroscopy method.
5 Conclusion
The present studies concluded the potential influence of Thiourea enriched Cotton Waste on growth and Nutrition contents in Oyster mushroom. The significant increase in mushroom biomass and its nutritive value proves Thiourea as a potential substrate for good quality mushroom production.
Acknowledgements
This project was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R153), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. We are also thankful to the Beijing Forestry University, Beijing China and University of Agriculture Faisalabad, Faisalabad Pakistan for providing us an environment of learning. Without these platforms, the completion of this work was not an easy task. The authors also extend their appreciation to the deanship of Scientific Research at King Khalid University, Abha KSA for supporting this work under grant number (R.G.P.2/117/43).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Proximate and mineral element compositions of five edible wild grown mushroom species in Abakaliki, Southeast Nigeria. Res. J. Pharm. Biol. Chem. Sci.. 2013;4:1056-1064.
- [Google Scholar]
- Physiological effects of salicylic acid and thiourea on growth and productivity of maize plants in sandy soil. Commun. Soil Sci. Plant Anal.. 2013;44:1141-1155.
- [Google Scholar]
- Potential of foliar applied thiourea in improving salt and high temperature tolerance of bread wheat (Triticum aestivum) Int. J. Agric. Biol.. 2011;13:251-256.
- [Google Scholar]
- AOAC, 1995. Official Methods of Analysis. Association of Official Analytical Chemists. 16th Ed., Arlington, VA.
- AOAC. 2002. Official Methods of Analysis. Association of Official Analytical Chemists. 17th Ed., Washington, DC.
- AOAC, 1990. Official Methods of Analysis of AOAC International. 17th Edition. USA.
- Yield evaluation of Lentinus squarosulus, on selected sawdust of economic tree species supplemented with 20% oil palm fruit body fibers. Asian J. Plant Sci.. 2007;11:1098-1102.
- [Google Scholar]
- Nutrient supplements that influence later break yield of Agaricus bisporus. Can. J. Plant Sci.. 1996;76:835-840.
- [Google Scholar]
- Effect of Different saw dust substrates on the growth and yield of oyster mushroom (Pleurotus ostreatus) J. Agric. Vet. Sci.. 2014;7:38-46.
- [Google Scholar]
- Intractive effects of thiourea and phosphorus under stimulated water stress. Biol. Plant.. 2004;48:61-65.
- [Google Scholar]
- The nutrients of exotic mushrooms (Lentinula edodes and Pleurotus sp.) and an estimated approach to the volatile compounds. Food Chem.. 2007;105:1188-1194.
- [Google Scholar]
- Chen, A.W., 2004. Oyster mushroom cultivation (Handbook by mushroom growers). Chap. 3, ISSN 1739-1377.
- Effect of substrate type, different level of nitrogen and maganese on growth and development of oyster mushroom (Pleurotus florida) Dyn. Biochem. Process Biotechnol. Mol. Biol.. 2008;2(1):34-37.
- [Google Scholar]
- Yield, size and bacterial blotch resistance of Pleurotus eryngii grown on cottonseed hulls/oak sawdust supplemented with manganese, copper and whole ground soybean. Bioresour. Technol.. 2007;98:1898-1906.
- [Google Scholar]
- Influence of thiourea on photosynthesis, nitrogen metabolism and yield of clusterbean (Cyamopsis tetragonoloba (L.) Taub.) under rainfed conditions of Indian arid zone. Plant Growth Regul.. 2006;48:237-245.
- [Google Scholar]
- Fungal Physiology. John Wiley & Sons; 1996.
- Cultivation of the king oyster mushroom (Pleurotus eryngii) in Egypt. Aust. J. Basic. Appl. Sci.. 2010;4:99-105.
- [Google Scholar]
- Ascorbate function and associated transport systems in plants. Plant Physiol. Biochem.. 2000;38:531-540.
- [Google Scholar]
- Official and Tentative Method of Analysis. Washington D.C.: Association of the Official Agriculture Chemist; 1960. p. :320-341.
- Effect of dormancy breaking chemicals on flowering, fruit set and quality in Asian pear (Pyrus pyrifolia L.) Afr. J. Agric. Res.. 2014;9:56-60.
- [Google Scholar]
- Promotive effect of exogenously applied thiourea on key physiological parameters and oxidative defense mechanism in salt-stressed Zea mays L. plants. Turk. J. Bot.. 2015;39:786-795.
- [Google Scholar]
- Exogenous application of mannitol and thiourea regulates plant growth and oxidative stress responses in salt-stressed maize (Zea mays L.) J. Plant Interact.. 2013;8:234-241.
- [Google Scholar]
- Saline soil properties, quality and productivity of wheat grown with bagasse ash and thiourea in different climatic zones. Chemosphere. 2018;193:538-546.
- [Google Scholar]
- Improved productivity of mung bean by application of thiourea under arid conditions. World J. Agric. Sci.. 2006;2:185-187.
- [Google Scholar]
- Effect of chemical thinning on yield and quality of peach cv. Flordasun. Afr. J. Agric. Res.. 2013;8:3558-3565.
- [Google Scholar]
- Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact. CRC Press; 2004.
- Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol.. 2008;147:1251-1263.
- [Google Scholar]
- Appropriate Technology for Mushroom Growers. Mushroom Cultivation. Leiden. The Netherlands: Backhuys Publishers; 2003. p. :429.
- Use of Fourier transform infrared spectroscopy and chemometric data analysis to evaluate damage and age in mushrooms (Agaricus bisporus) grown in Ireland. J. Agric. Food Chem.. 2010;58:7770-7776.
- [Google Scholar]
- Bioconversion of sugarcane crop residues with white rot fungi Pleurotus species. World J. Microbiol. Biotechnol.. 1992;8:402-405.
- [Google Scholar]
- Apple orchard productivity and fruit quality under organic, conventional, and integrated management. Hort. Sci.. 2006;41:99-107.
- [Google Scholar]
- Yield potential and quality of some Pleurotus species grown in substrates containing hazelnut husk. Pak. J. Biol. Sci.. 2004;7(5):768-771.
- [Google Scholar]
- Effects of different nitrogen rich substrates and their combination to the yield performance of oyster mushroom (Pleurotus ostreatus) World J. Microbiol. Biotechnol.. 2011;27:1695-1702.
- [Google Scholar]
- Chemical Methods for Analysis of Fruit and Vegetables. Canada: Research Station Sumer Land, Research Branch Canada, Department of Agriculture; 1961. p. :1154.
- Studies on correlation between yield and yield contributory parameters and quality of okra [Abelmoschus esculentus (L.) Moench.] as influenced by zinc and bio-regulators. Int. J. Curr. Microbiol. Appl. Sci.. 2017;6:2458-2464.
- [Google Scholar]
- Trace elements in Pleurotus Sajor-caju cultivated on chemithermo mechanical pulp for bio-bleaching. Food Chem.. 2002;79:173-176.
- [Google Scholar]
- Principles and Procedures of Statistics, A Biometrical Approach. McGraw-Hill Kogakusha Ltd.; 1980.
- Laboratory Manual for the Analysis Soil, Plant and Water Samples (2nd ed.). London: McMillian; 1986. p. :67-137.
- Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist. 2003;157:423-447.
- [Google Scholar]







