Thermodynamic equilibrium modeling of biomass gasification: Effects of operating conditions on gasifier performance
⁎Corresponding author. deki@vinca.rs (Dejan Cvetinović),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Biomass has remarkable potential to reduce harmful emissions and ensure stable and sustainable energy production. In this paper, various parameters such as operating temperature, type of gasifying agent, air–fuel ratio and steam-fuel ratio are investigated on the qualitative characteristics of the syngas obtained from biomass gasification. The qualitative indicators considered were the percentage of combustible components under the energy aspect and the percentage of undesirable components under the environmental aspect. The composition of the syngas was determined for a temperature range of 500–1000 °C as an equilibrium composition using the Gibbs free energy minimisation method. The results showed that increasing the gasification temperature above 900 °C had a positive effect on the energy and environmental properties of the syngas. Air and water vapour were selected as possible gasifying agents. The results showed that water vapour was significantly more favourable than air as a gasifying agent in terms of syngas quality. In the best case, the H2 yield for gasification with air is 35 %vol, while this value reaches 65 %vol for gasification with steam. In addition to the type, the ratio of the gasifying agent to the amount of fuel was also analysed. The analysis showed that it was more favourable to carry out the gasification process at lower air-to-fuel and steam-to-fuel ratios, which is consistent with the work of other authors.
Keywords
Biomass
Gasification
Gasification agent
Air-to-fuel ratio
Steam-to-fuel ratio
Gibbs free energy minimization
1 Introduction
The industrial age, powered by fossil fuels, has shaped the world's future and improved people's standard of living at the cost of severe ecological damage to the environment. However, limited fossil fuel resources pose a major problem for future energy production around the world. Replacing fossil fuels with renewable energy sources should significantly reduce emissions of harmful gases and still allow people to maintain their accustomed standard of living. Renewable energy sources such as wind power and photovoltaics have grown the fastest in the last two decades, while sales of electric vehicles have reached a new record, as reported by the International Energy Agency (IEA-International Energy Agency, World Energy Outlook, 2022). The crucial question today is how future renewable energy systems can be organised to meet the world's ever-increasing energy needs.
Despite the popularity of solar panels and wind turbines, they are still highly dependent on meteorological and geographical conditions, so some electricity must be produced if they cannot meet demand. Lee et al. (2022) provide an overview of the hybrid technologies developed for hydrogen production by water splitting using sustainable and renewable energy. Qamar et al. (2023) provide a detailed overview of the latest technologies in the field of bio-oil production from biomass to assess the sustainability of using certain technologies. Biomass is one of the prominent renewable energy sources that can play a crucial role in achieving the goal of reducing greenhouse gas emissions while providing energy stability and sustainability (Narnaware and Panwar, 2022). Several advantages of biomass over fossil fuels have contributed to its status as a source with high potential for meeting the energy needs of modern societies (Cao, 2021; Patuzzi, 2021). The aforementioned advantages primarily relate to biomass being the CO2 carbon neutral fuel that can improve employment in rural areas, as well as the variety of energetically valuable products obtained from biomass through various conversion processes. The best-known thermochemical conversion processes of biomass are pyrolysis (Niu, 2022), torrefaction (Gao, 2017), combustion (Nunes, 2016) and gasification (Sikarwar, 2016). There are many useful products from biomass gasification, including syngas, heat, electricity, biofuels, fertiliser and biochar. Synthesis gas can be further processed into methanol, dimethyl ether and other chemical feedstocks using the Fischer-Tropsch process. Cao et al. (Cao, 2021) believe that gasification owes its popularity to the variety of high calorific value products derived from biomass, such as syngas, methanol, dimethyl ether (DME), ethanol and hydrogen. At the same time, Korberg et al. (Korberg, 2021) added that this process accepts a variety of inputs such as agricultural waste, biogas digestate and even used tyres.
Gasification involves the conversion of organic or carbonaceous materials, such as biomass, in the presence of a gasifying agent at temperatures above 700 °C into a mixture of valuable gases, usually called syngas (consisting mainly of CO, H2, CO2 and CH4), biochar, ash and tars (Situmorang, 2020; Tezer, 2022). Further conversion of the gases into liquid fuels can be done via Fischer-Tropsch synthesis, or they can be used to produce heat and energy for power generation plants (Mishra and Upadhyay, 2021). During gasification, biomass goes through four main stages: drying, pyrolysis, oxidation and reduction or gasification. Since the moisture content of fresh biomass can be in the range of 30–60 % and has a significant impact on the quality and composition of the synthesis gas produced, drying is a necessary step to reduce the moisture content below 15 % (Situmorang, 2020). In the pyrolysis stage, the biomass is broken down into volatile compounds and solid residues called biochar (char), where the volatiles consist of hydrocarbons, hydrogen, carbon monoxide, carbon dioxide, water vapour and liquids such as tar (Nunes, 2022). In the third stage, oxygen from the gasifying agent reacts with the previously obtained products to form CO, CO2 and H2O. The reduction stage aims to reduce the tars in the gas produced by bringing them to a high temperature, as their excessive content reduces the overall efficiency of the conversion and can also cause severe damage to the system components (Machin, 2015). After the reduction stage, the main products are syngas, CO, H2 and CH4 components, and the remaining solid residue is ash. The gasification process consists of many endothermic and exothermic reactions that can occur simultaneously, and some of them are listed in (Nunes, 2022) and (Erić et al., 2022).
One of the biggest challenges in biomass gasification is the amount and number of various impurities in the syngas that need to be removed before the syngas can be used. The problem occurs mainly in gas cooling, where ash and tar particles or their mixture can cause clogging of the system components (Larsson, 2021). Due to the many types of biomasses, such as agricultural, forestry, waste, waste sludge, etc., the operating parameters for gasification may vary. Therefore, it is important to determine optimal operating conditions that reduce the amount of tar produced while increasing the yield of energetically valuable combustible components such as CO and H2. Jahromi et al. (Jahromi, 2021) developed a two-dimensional computational fluid dynamics (CFD) model for the gasification of sugarcane bagasse in a downdraft fixed-bed gasifier and validated it on a 25-kW pilot-scale experimental set-up. The authors investigated the effects of steam-to-air ratio (S/A), inlet velocity and preheating temperature of air/steam, and moisture content of the biomass. They concluded that the optimal operating parameters are an inlet velocity of 20 m/s, a preheating temperature of 1500 K, an S/A ratio of 0.67 and a moisture content of 1.14 %. At the aforementioned optimal conditions, the composition of the syngas in mole percent CO, H2, CH4 and CO2 was 24.2 %, 14.4 %, 5.4 % and 8.3 %, respectively, while the conversion efficiency achieved was 69.14 %. Ramos A. and Rouboa A. (Ramos and Rouboa, 2020) concluded that the type of biomass has a significant influence on the quality of the syngas. With the increase of temperature, the CO and H2 content as well as the lower heating value (LHV) and cold gas efficiency (CGE) of Miscanthus were increased. At the same time, the LHV and CGE of peach kernel decreased at higher temperatures. Koukouzas et al. (Koukouzas, et al., 2008) experimentally analysed the influence of different ratios of oxygen and nitrogen in the gasifying agent. The authors concluded that increasing the oxygen concentration positively influences the yield of valuable combustible components in the syngas. Kuo et al. (Kuo, 2014) were interested in the influence of the air–fuel ratio in the gasification process. They reported that the increase of the air content is related to the decrease of the combustible component content.
This work presents results of simulations using a one-dimensional model of biomass gasification based on the minimum Gibbs free energy. The aim is to estimate the effects of different parameters on the quality of the syngas, in particular on the yield of the combustible components CO and H2. The main advantage of the present model is the simplicity of the input data. The model can be applied to different types of biomass and other materials used in thermal decomposition processes, as evidenced by the comparison of the results with the work of other authors.
2 Methodology
2.1 Equilibrium composition model
To determine the thermodynamic equilibrium composition of the system being decomposed at high temperatures, it is necessary at the beginning to define the number of components of the system that will be taken into consideration, here and after, denoted by j.
The Gibbs free energy is essentially the maximum non-expansion work that can be obtained from the interaction of the system and the environment if the system is at constant pressure and temperature (Belov, 1999), and it can be calculated as:
By differentiating Eq. (1) and combining the Gibbs-Duem equation, we get the expression for the change of the Gibbs free energy at constant pressure and temperature:
According to Dalton's law, the pressure in a system consisting of j components is equal to the sum of the partial pressures of all components. This means that the chemical potential of the component in the mixture must be expressed as a function of its partial pressure. Then, for chemical equilibrium, eq. (4) becomes:
By grouping the elements relating to the chemical potential of products and reactants under standard conditions and by introducing activities, equation (5) becomes:
Bearing in mind that the chemical equilibrium constant is equal to:
The modelled system is considered as isothermal and the equilibrium composition is calculated iteratively using the method of minimizing the Gibbs function at several points in the desired temperature range.
2.2 Mass balance
The biomass consists of moisture, volatiles, solid carbon and ash. The combustible parts of biomass are volatiles and solid carbon, while moisture and ash are the non-combustible parts. Since water vapour undergoes various transformations during the gasification process and is involved in many reactions, e.g. water–gas reaction, steam-methane reforming, water–gas shift reaction and steam reforming of tar, moisture is considered an important component of biomass. However, this model does not consider ash.
The general equation describing the gasification process is presented as follows:
where
The air-to-fuel ratio (AFR) is defined as the ratio between the air entering the gasifier and the fuel provided in the gasifier, assuming that the fuel is biomass:
3 Results and discussion
3.1 Model validation
The accuracy of the developed model was measured by comparing the composition of the obtained syngas from biomass gasification with the experimental work of Jayah et al. (Jayah, 2003). The gasification process was simulated under the same conditions described in their work in terms of biomass composition, air–fuel ratio AFR = 2.03 and temperature T = 1000 °C as shown in Table 1. The results obtained are shown in Fig. 1.
|
The proximate analysis of rubberwood (% as received) |
The ultimate analysis of rubberwood (% as received) |
||
|---|---|---|---|
| Volatile matter | 80.1 | C | 50.6 |
| Fixed carbon | 19.2 | H | 6.5 |
| Ash content | 0.7 | N | 0.2 |
| O | 42.0 | ||
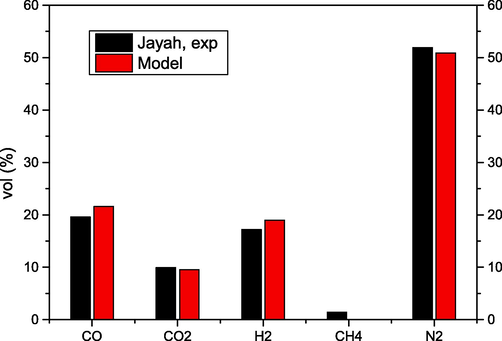
- Comparison of the syngas composition determined with the authors' model with the experimental data of Jayah et al. (Jayah, 2003).
The developed model showed good agreement with the comparative work with relative errors for CO, CO2, H2 and N2 of 9.17 %, 3.55 %, 9.33 % and 1.89 %, respectively. The results differed slightly in the case of CH4, where the model gave a value close to zero, while the experimental work found 1.17 %. Kuo et al. (Kuo, 2014) came to a similar conclusion, also based on the minimum Gibbs free energy, where the value for CH4 was close to zero. Considering the deviations of the real from the theoretical experimental conditions, which are taken into account when determining the input parameters and basic assumptions of the non-stoichiometric thermodynamic balance model, some deviations in the yields of the individual components of the system are to be expected. The overestimation of the yields of CH4 and other components cannot be defined in general terms and depends on how close the reactor conditions are to the ideal state. The closer the conditions in the reactor are to the ideal state, the smaller the deviation.
3.2 Parametric study
In this section, the effects of operating temperature, type of gasifying agent, and air–fuel ratio (AFR) and steam-fuel ratio (SFR) on syngas composition in biomass gasification were discussed. During the parametric studies, one parameter was varied and the other parameters were kept constant. The operating temperature was varied in the range of 500–1000 °C. The AFR reached values of 1–5, while the SFR was 0.5–2.5. Two gasifying agents were considered: air and steam. The biomass used for the analysis had the composition shown in Table 2.
| The proximate analysis of biomass (% as received) | The ultimate analysis (% dry ash free basis) | ||
|---|---|---|---|
| Volatile matter | 69.76 | C | 45.50 |
| Fixed carbon | 17.50 | H | 7.00 |
| Ash content | 4.36 | N | 0.10 |
| Moisture content | 8.38 | S | 0.10 |
| O | 47.30 | ||
3.2.1 Air gasification
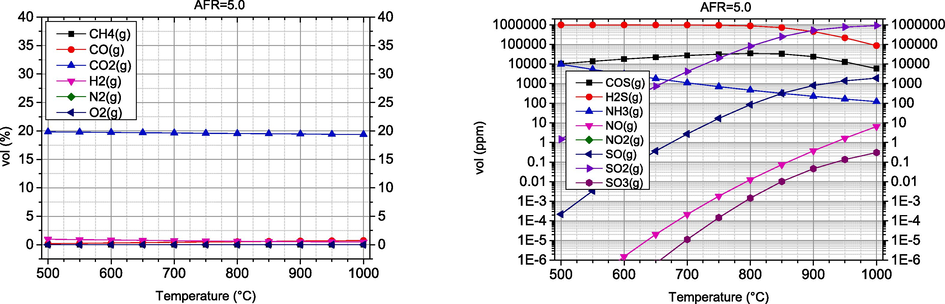
The most common gasifying agent is air because it is cheap and readily available. Although this technique is widely used in biomass gasification, air gasification produces a syngas with a low heating value (LHV) in the range of 4–7 MJ/Nm3 (Jayah, 2003) and a low hydrogen content (Cao, 2021). In addition, the nitrogen content of the air has a negative effect on the calorific value of the synthesis gas and causes the formation of nitrogen oxides. Oxygen gasification processes using pure oxygen as a gasifying agent produce high quality syngas, but the cost of obtaining pure oxygen is very high. In this section, parametric analysis of air gasification was presented in the temperature range of 500–1000 °C, while AFR values were in the range of 1–5. The results are shown in Fig. 2, where the graphs on the left side refer to the volume fractions of the main products of the gasification, while the right side represents the undesired products of the process.
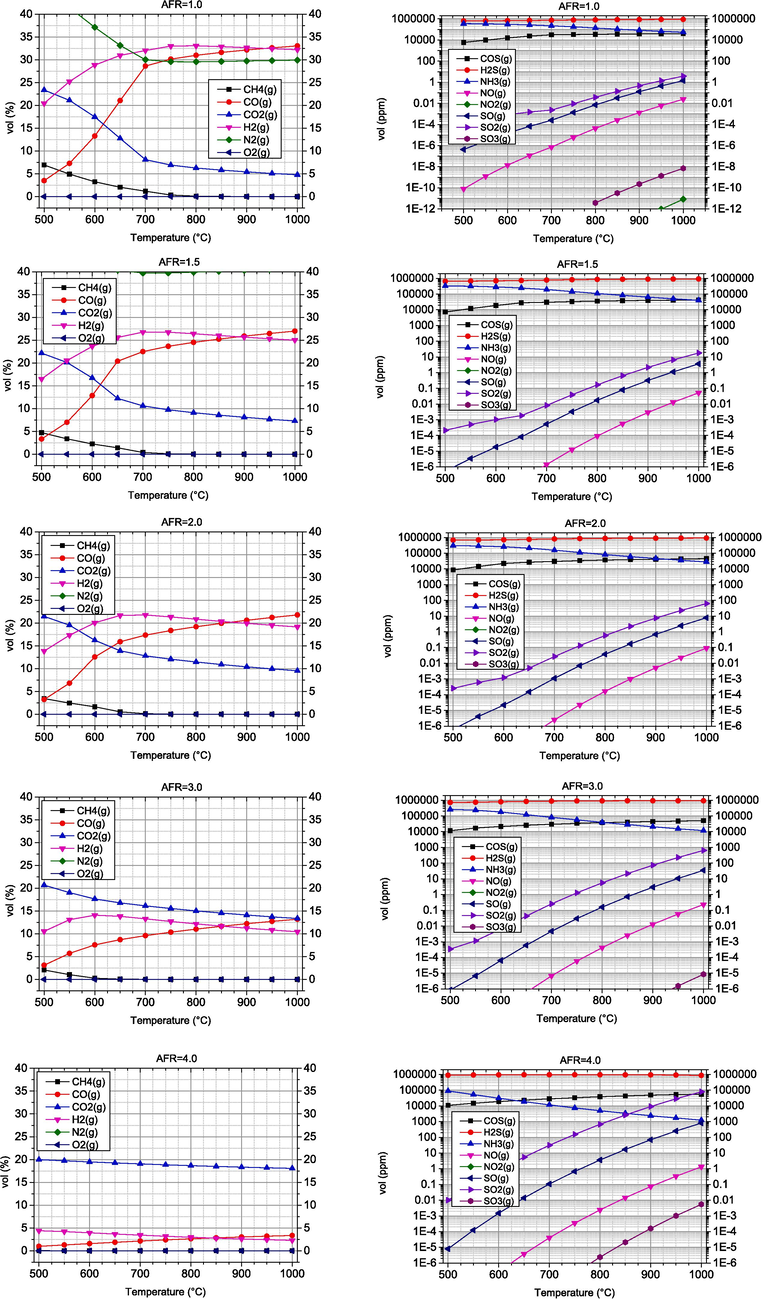
- Volume fractions of combustible and undesired components obtained by air gasification of biomass.
- Volume fractions of combustible and undesired components obtained by air gasification of biomass.
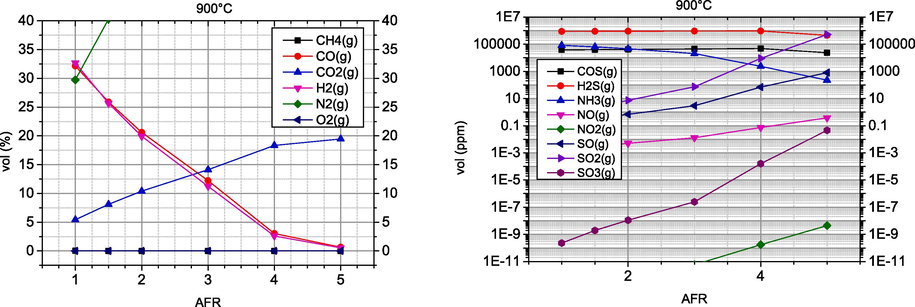
With the increase of temperature in the range of 500–700 °C, the H2 concentration increases significantly, while the further increase of temperature from 700 °C to 1000 °C leads to a decrease of the content, with the highest value being reached at 700 °C. This behaviour was also reported by Kartal et al. (Kartal and Özveren, 2021). A strong influence of temperature is observed for the concentrations of CO and CO2. As the temperature increases, the CO2 concentration decreases, which is consistent with the studies previously reported by Cao et al. (Cao, 2021). The highest CO2 concentration is observed at 500 °C. Similar findings were reported by Hoang et al. (Hoang, 2022). The highest concentrations of the combustible components CO and H2 are observed at AFR = 1, 33 %vol and 32 %vol, respectively, Fig. 3. An increase in temperature at AFR values of 1 to 3 leads to a decrease in the concentration of CO2 and CH4 and an increase in the main syngas components CO and H2. This trend is caused by two important parameters, temperature and the amount of oxidant, in this case oxygen. Lower AFR coefficients imply an insufficient amount of oxygen for complete combustion, so that partial oxidation reactions (1) and (8) are favoured (see Table 1). In these reactions, CO and H2 are formed instead of the products of complete combustion of CO2 and H2O. Higher temperatures favour endothermic reactions (8) and (10), which also favour higher concentrations of CO and H2 in the absence of oxygen. Most CO2 comes from exothermic reactions such as oxidation (combustion) and water–gas shift reaction. Since higher temperatures in exothermic reactions favour the reactants and not the products, its concentration decreases with increasing temperature, which correlates with Zhou et al. (Zhou, 2009). At higher values of the AFR coefficient of 4–5, the influence of the mentioned reactions is lower, as the gasification process is oxygen-rich and closer to the combustion process.
- Effect of air-to-fuel ratio (AFR) on syngas composition.
The main undesirable components produced during biomass gasification are H2S, COS, NH3 and NOx. All except NH3 showed a similar trend, increasing with increasing temperature. These harmful compounds should be considered when analysing the performance of the gasifier.
With the increase of AFR in the investigated range, the concentrations of the combustible gases CO and H2 decrease drastically from 32 %vol to zero, while the harmful gases increase, especially NH3, nitrogen and sulphur oxides. A significant N2 concentration is found in the syngas, which increases rapidly with the increase in AFR, which is the expected result.
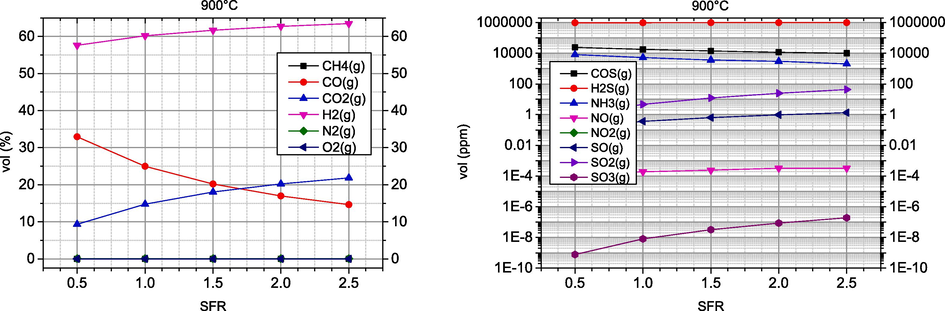
As expected, the proportion of fuel components decreases significantly with the increase of the amount of air involved in the gasification process, which decreases the quality of the synthesis gas obtained. Increasing the amount of air also has an unfavourable effect on the environmental parameters, as the proportion of undesirable components increases. As for the process temperature, the optimal values of the energy and environmental parameters reach their maximum in the temperature range of 800–1000 °C.
According to the previously discussed results, air gasification should be carried out at temperatures above 900 °C and lower values of AFR. In addition, higher temperatures have a positive effect on tar reduction, although this is outside the scope of this work.
3.2.1.1 Steam gasification
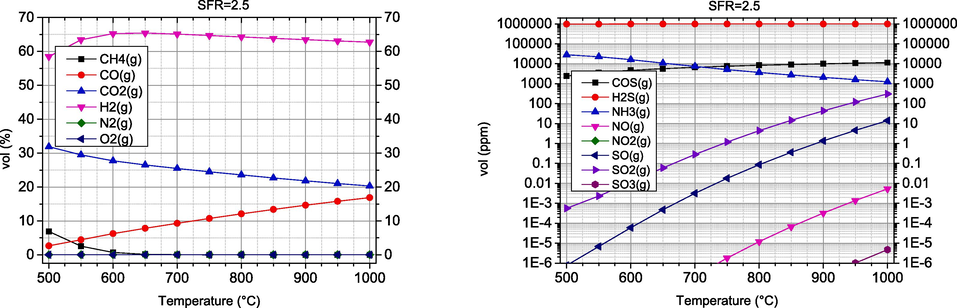
Water vapour is another interesting gasifying agent that produces syngas with a higher LHV than air gasification (Basu and Kaushal, 2009). In this section, the SFR is varied in the range of 0.5–2.5 and the temperature in the range of 500–1000 °C. The results obtained are shown in Figs. 4 and 5.
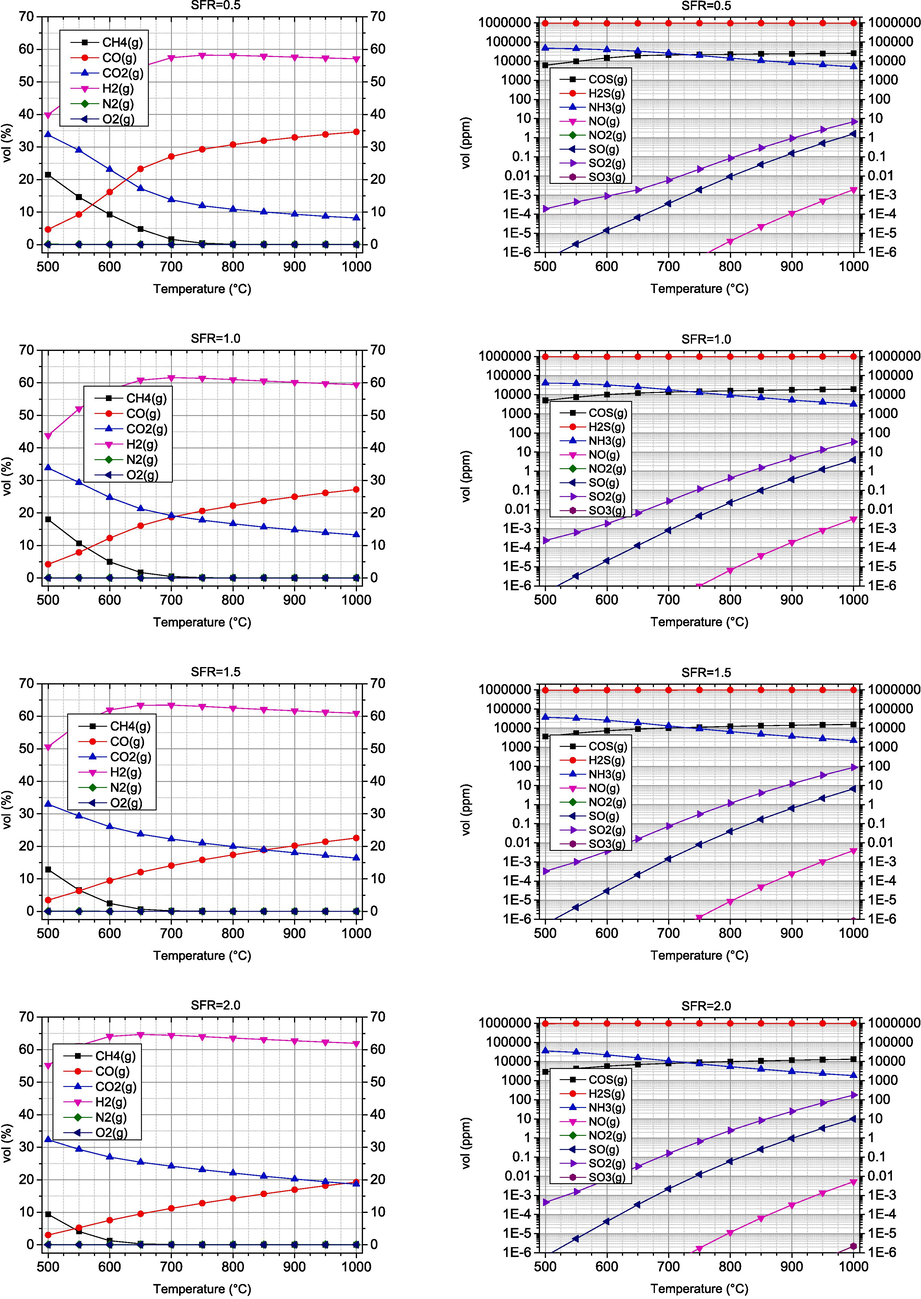
- Volume fractions of combustible and undesired components obtained by steam gasification of biomass.
- Volume fractions of combustible and undesired components obtained by steam gasification of biomass.
- Effect of steam-to-fuel ratio (SFR) on syngas composition.
The results obtained regarding the dependence of syngas composition on temperature show a similar pattern to air gasification, where the CO concentration increases and the CO2 concentration decreases, while the H2 concentration initially increases up to about 600–700 °C and then slightly decreases. A significant difference can be observed in the H2 concentration, as it is many times higher compared to air gasification. The same has been reported by other authors (Mishra and Upadhyay, 2021). The influence of SFR is to be expected. With the increase of SFR, the concentrations of H2 and CO2 increase, while the CO concentration decreases. At maximum gasification temperatures above 900 °C, H2 values range between 57–63 %vol, while CO concentrations range between 13–35 %vol. However, there is a negative effect that leads to a decrease in CO concentration and an increase in CO2. The reduction of CO has a negative effect on the energetic properties of the synthesis gas obtained, despite the slight increase of H2.
The dependence of concentrations of undesirable components on temperature is similar to air gasification, where there is an increase in nitrogen and sulphur oxides at higher temperatures. COS and H2S are quite resistant to temperature changes. In contrast, NH3 concentration decreases with increasing temperature. As the SFR coefficient increases, an increase in the concentration of nitrogen and sulphur oxides is also observed, which is due to the increased presence of oxygen originating from the gasifying agent.
In this case of steam gasification, the influence of the SFR coefficient on the syngas composition is much smaller than the influence of the AFR coefficient in the case of air gasification. When the SFR coefficient increases, the proportion of H2 increases, but the proportion of CO also decreases. Furthermore, an increase in the SFR coefficient leads to an increase in the proportion of undesirable components, especially sulphur oxides. The optimal temperature range of steam gasification is similar to that of air gasification and is 900 °C. In the case of CO, an increase in the SFR coefficient leads to a decrease in CO.
4 Conclusion
The developed model, based on the minimisation of Gibbs free energy, is able to predict the equilibrium composition of the components of biomass gasification. The results obtained have shown satisfactory agreement with experimental results obtained by other authors. In air gasification, increasing the temperature in the range of 500–700 °C leads to a significant increase in the main syngas components CO and H2. In steam gasification, the increase in temperature leads to a continuous increase in CO concentration, which is, among other things, a consequence of the reaction of fixed carbon and CO2. The AFT and SFR coefficients have the opposite effect. An increase in the AFR coefficient results in a lower yield of CO and H2 in the syngas, while an increase in the SFR coefficient results in a higher yield of these fuel components.
The analysis of the synthesis gas composition with air and steam as gasification agents and at different temperatures showed that steam gasification has a higher concentration of H2, the main combustible component of the synthesis gas, between 57–63 %vol. The yield of this component in air gasification was about 32 %vol. Furthermore, the model showed that the proportions of the main components of the synthesis gas in biomass gasification fit better to the lower ratios of gasification agents and fuels AFR and SFR. As for the SFR coefficient, when it is increased, there is a slight increase in the H2 concentration in the syngas obtained, but also a significant decrease in CO. A higher gasification temperature of over 900 °C also has a favourable effect on the yield of combustible components of the synthesis gas, especially CO. This temperature is also desirable to solve the problem of tar, which is an undesirable component of the synthesis gas. However, higher temperatures have a negative effect on the increase in the concentrations of the undesirable components NOx and SOx, but this is an unavoidable phenomenon that can be easily solved by secondary removal methods.
The optimal gasification temperature, either with air or with steam, is around 900 °C. For gasification with air, the optimal value of the AFR coefficient should be as low as possible, while for gasification with steam, an increase in the SFR coefficient causes an increase in the H2 concentration and a decrease in the CO concentration. The optimal value of the SFR coefficient is therefore about 2.
The results obtained can be of great use in further analysis of the gasification process. This could include energy and exergy analyses as well as the kinetics of the mentioned process.
Acknowledgement
The Ministry of Science, Technological Development and Innovation of the Republic of Serbia: Grant no. 451-03-66/2024-03/200017 («VINCA» Institute of Nuclear Sciences, National Institute of the Republic of Serbia, University of Belgrade) and additionally from Science Fund of the Republic of Serbia - Green Program of Cooperation between Science and Industry - project STABILISE.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Modeling of pyrolysis and gasification of biomass in fluidized beds: a review. Chem. Prod. Process Model.. 2009;4:1.
- [CrossRef] [Google Scholar]
- IVTANTHERMO for Windows - Database on Thermodynamic Properties and Related Software, Calphad: Computer Coupling of Phase Diagrams and Thermo-Chemistry. 1999;23(2):173-180.
- [CrossRef]
- Air-steam gasification of biomass based on a multi-composition multi-step kinetic model: A clean strategy for hydrogen-enriched syngas production. Sci. Total Environ.. 2021;753:141690
- [CrossRef] [Google Scholar]
- Combined parametric modelling of biomass devolatilization process. Renew. Energy. 2022;193:13-22.
- [CrossRef] [Google Scholar]
- Formation of PCDDs and PCDFs in the torrefaction of biomass with different chemical composition. J. Anal. Appl. Pyrol.. 2017;123:126-133.
- [CrossRef] [Google Scholar]
- L.V. Gurvich, I. Veyts, eds. Thermodynamic Properties of Individual Substances: Elements and Compounds. Vol. 1-5, ISBN 0891167609, CRC press, United States, 1990.
- Characteristics of hydrogen production from steam gasification of plant-originated lignocellulosic biomass and its prospects in Vietnam. Int. J. Hydrogen Energy. 2022;47(7):4394-4425.
- [CrossRef] [Google Scholar]
- Biomass gasification in a downdraft fixed-bed gasifier: Optimization of operating conditions. Chem. Eng. Sci.. 2021;231:116249
- [CrossRef] [Google Scholar]
- Computer simulation of a downdraft wood gasifier for tea drying. Biomass Bioenergy. 2003;25(4):459-469.
- [CrossRef] [Google Scholar]
- A comparative study for biomass gasification in bubbling bed gasifier using Aspen HYSYS. Bioresource Technology Reports. 2021;13:100615
- [CrossRef] [Google Scholar]
- The role of biomass gasification in low-carbon energy and transport systems. Smart Energy. 2021;1:100006
- [CrossRef] [Google Scholar]
- Koukouzas, N., et al., Fixed Bed Gasification of Biomass Fuels: Experimental Results, ENERGY FOR SUISTANABLE FUTURE, Edited by Petar Varbanov, Jirzí Klemeš, Igor Bulatov, University of Pannonia, Veszprém, Hungary, 5-6 May 2008, ISBN 978-963-9696-38-9, Available from: https://www.researchgate.net/publication/238683808_Fixed_Bed_Gasification_of_Biomass_Fuels_Experimental_Results [accessed Oct 25 2023]. (2008), pp. 5–6.
- Gasification performances of raw and torrefied biomass in a downdraft fixed bed gasifier using thermodynamic analysis. Fuel. 2014;117:1231-1241.
- [CrossRef] [Google Scholar]
- Steam gasification of biomass-Typical gas quality and operational strategies derived from industrial-scale plants. Fuel Process. Technol.. 2021;212:106609
- [CrossRef] [Google Scholar]
- A review on integrated thermochemical hydrogen production from water. Int. J. Hydrogen Energy. 2022;47(7):4346-4356.
- [CrossRef] [Google Scholar]
- Tar reduction in downdraft biomass gasifier using a primary method. Renew. Energy. 2015;78:478-483.
- [CrossRef] [Google Scholar]
- Review on biomass gasification: Gasifiers, gasifying mediums, and operational parameters. Materials Science for Energy Technologies. 2021;4:329-340.
- [CrossRef] [Google Scholar]
- Biomass gasification for climate change mitigation and policy framework in India: A review. Bioresource Technology Reports. 2022;17:100892
- [CrossRef] [Google Scholar]
- Synergistic effect on thermal behavior and product characteristics during co-pyrolysis of biomass and waste tire: Influence of biomass species and waste blending ratios. Energy. 2022;240:122808
- [CrossRef] [Google Scholar]
- Biomass combustion systems: A review on the physical and chemical properties of the ashes. Renew. Sustain. Energy Rev.. 2016;53:235-242.
- [CrossRef] [Google Scholar]
- Biomass gasification as an industrial process with effective proof-of-concept: A comprehensive review on technologies, processes and future developments. Results in Engineering. 2022;14:100408
- [CrossRef] [Google Scholar]
- State-of-the-art of small-scale biomass gasification systems: An extensive and unique monitoring review. Energy. 2021;223:120039
- [CrossRef] [Google Scholar]
- Feasibility-to-applications of value-added products from biomass: Current trends, challenges, and prospects. Chem. Eng. J.. 2023;Volume 454, Part 2(15):140240
- [CrossRef] [Google Scholar]
- Syngas production strategies from biomass gasification: Numerical studies for operational conditions and quality indexes. Renew. Energy. 2020;155:1211-1221.
- [CrossRef] [Google Scholar]
- An overview of advances in biomass gasification. Energ. Environ. Sci.. 2016;9(10):2939-2977.
- [CrossRef] [Google Scholar]
- Small-scale biomass gasification systems for power generation (<200 kW class): A review. Renew. Sustain. Energy Rev.. 2020;117:109486
- [CrossRef] [Google Scholar]
- Biomass gasification for sustainable energy production: A review. Int. J. Hydrogen Energy 2022
- [CrossRef] [Google Scholar]
- Biomass-oxygen gasification in a high-temperature entrained-flow gasifier. Biotechnol. Adv.. 2009;27(5):606-611.
- [CrossRef] [Google Scholar]







