Translate this page into:
Therapeutic efficacy of geranium-thymol combination against murine eimeriosis with reference to its apoptotic activity
⁎Corresponding author. azema1@yahoo.com (Abdel-Azeem S. Abdel-Baki)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Eimeria is among the most harmful parasites that affect domestic animals. The development resistance in Eimeria to the majority of regularly used anticoccidials necessitates development of an effective alternative strategy. This study was therefore aimed to investigate the therapeutic efficacy of essential oils combination, Pelargonium graveolens (geranium) with thymol (PGT) against Eimeria papillata murine model. Three doses of 50, 100, and 150 mg/kg were tested in Eimeria experimentally infected mice to determine the combination's highest effective dose. The results showed that the 150 mg/kg dose was the most effective, reducing the oocyst output by 56.92 % (P ≤ 0.001). This result has been associated with a 66.19 % (P ≤ 0.001) reduction in the jejunal developmental stages. The antioxidant effect of PGT decreased lipid peroxidation (P ≤ 0.05) and increasing reduced glutathione (GSH) (P ≤ 0.001). Moreover, PGT decreased the jejunum's apoptotic cell count (P ≤ 0.001). The in vitro results showed that PGT significantly inhibited oocysts sporulation and destructed 96.33 % of treated oocysts. In conclusion, PGT has therapeutic effect as an anticoccidial alternative to control murine eimeriosis.
Keywords
Coccidiosis
Geranium
Thymol
Oxidative stress
Apoptosis
Sporulation
PGT
1 Introduction
The members of the genus Eimeria are the most common cause of coccidiosis. This disease is one of the most prevalent parasitic diseases in poultry industry, causing large yearly losses of up to USD 13 billion per year (Blake et al., 2020). Eimeria is an obligatory parasite in the phylum Apicomplexa with exogenous and endogenous life cycle phases. These parasites can infect and proliferate inside the mucosal epithelia in various parts of the gut via the oral route. As a result, they induce gastrointestinal injury (i.e., inflammation, watery/bloody faeces, etc.), morbidity, and mortality in poultry (Chapman, 2014). This pathogen can disturb the intestinal microbiota and predispose the intestinal environment to the growth of pathogenic bacteria such as Clostridium perfringens, resulting in necrotic enteritis (Chapman, 2014).
The primary strategies of preventing and controlling this infection include anticoccidial drugs such as ionophores, synthetic chemicals, coccidiocides, and vaccination(Chapman et al., 2010). Resistance has been established for all of the medications now in use, thus the development of innovative agents with distinct mechanisms of action is critical if chemotherapy is to remain the primary way of controlling this disease (Muthamilselvan et al., 2016; Thagfan et al., 2023). Furthermore, the use of anticoccidial drugs as feed additives for prevention has been rigorously prohibited in European countries since 2006, with a complete prohibition expected to take effect in 2021 (European Council Council Decision 2011/50/EU).
Essential oils (EOs) or aromatic compounds could be appropriate alternative strategies to control coccidiosis (Namdeo et al., 2020). Thymol (T) is a naturally occurring phenol monoterpene. It is one of the most significant nutritional components of thyme species (Nagoor Meeran et al., 2017). It has a long history of usage in traditional medicine and has been demonstrated to have a broad spectrum of pharmacological effects, including the treatment of neurological and respiratory disorders (Gavliakova et al., 2013, Yu et al., 2016,), antiparasitic and antihelminthic (Tisserand and Young, 2014), antioxidant, anticancer (Hashemipour et al., 2013).
Pelargonium graveolens (PG) is a medicinal herb in the Geraniaceae family. This plant's essential oil has a wide range of biological benefits, including insecticidal, antibacterial, antifungal, and antiparasitic characteristics. It is also used in aromatherapy to treat gastrointestinal disorders (Huang et al., 2021).
According to Remmal et al. (2013) and Boyko et al. (2021), both PG and T have oocysticidal activity against chicken Eimeria. As a result, the current study is intended to test the potential efficacy of a geranium-thymol mixture in the management of murine eimeriosis caused by Eimeria species of mice through in-vitro and in-vivo trials.
2 Material and methods
2.1 Pelargonium graveolens and thymol essential oil (PGT)
Trust Scientific for Natural Products in Cairo, Egypt provided the essential oils Pelargonium graveolens (PG) and thymol (T). These oils were dissolved in 10 % DMSO to prepare stock solution. For in vivo treatment, the stock solution was suspended in physiological saline to prepare the following concentrations: 5, 2.5, and 1.25 % (volume/volume). The binary combination of both oils was added at the following rates: (2.5 % PG:2.5 % T, 1.25 % PG:1.25 % T and 0.63 % PG:0.63 % T).
2.2 GC–MS of Pelargonium graveolens oil
A TRACE GC Ultra Gas Chromatograph (THERMO Scientific Corp., USA) has been used for the GC–MS analysis. A TR-5 MS column (30 m0.32 mm i.d., 0.25 m film thickness) was attached to the GC–MS system. The Nawah Scientific Educational Research Centre in Egypt (https://nawahscientifc.com/) conducted this analysis.
2.3 Coccidian parasite
A lab strain of Eimeria papillata was used in this study. It was maintained by routine passage through coccidian-free mice on a regular basis in the Parasitology Laboratory Research (Zoology Department, Faculty of Science, Beni-Suef University, Egypt). For the propagation of oocysts, six male Mus musculus laboratory mice (aged 9–12 weeks) were used. According to Abdel-Tawab et al. (2020), 1 × 103 sporulated oocysts were given orally to each mouse. Five days following infection, oocysts were recovered from the feces of the infected mice (Long et al., 1976). The collected oocysts were allowed to sporulate in 2.5 % (w/v) potassium dichromate (K2Cr2O7) at 24 °C in order to use them in the experiment. Then, sporulated oocysts were recovered by centrifuging them at 250 g for 5 min in a saturated saline solution, followed by washing with distilled water (Schito et al., 1996).
2.4 Experimental design
The study's ethical guidelines were authorized by the Faculty of Science at Beni-Suef University in Egypt, with approval number (022–384). All experiments were conducted in conformity with the necessary legislation and guidelines.
A total of forty male Swiss albino mice weighing 22–25 g and aged 6–7 weeks were divided into five groups as following:
G1. Negative control: non-infected non-treated group.
G2. Positive control: (1 × 103 sporulated E. papillata oocysts) infected non-treated group.
G3. E. papillata infected mice receiving 50 mg/kg PGT (one hour after infection).
G4. E. papillata infected mice receiving 100 mg/kg PGT.
G5. E. papillata infected mice receiving 150 mg/kg PGT.
PGT therapy was subsequently repeated daily for four days. Mice were maintained in pathogen-free circumstances with 12 h of light and 12 h of darkness, unrestricted access to water, and a standard mouse chow diet in pathogen-free conditions at a constant temperature of 21 °C. The oocyst count per gram (OPG) was calculated using the modified McMaster technique (Schito et al., 1996). Briefly, 50 ml of water were used to homogenize 100 g of fresh feces. In 60 ml of saturated sodium chloride solution, 6 g of this homogenate were suspended. Oocysts were counted per gram of feces by diluting this suspension based on the density of the oocysts and the numer of oocysts was calculated as:.
On day 5p.i., mice were euthanized and jejuna were collected for further studies.
2.5 Preparations of intestinal tissue for biochemical
As recommended by Tsakiris et al. (2004), pieces of the freshly excised jejunum were homogenized. The final homogenate was spun at 500 g for 10 min in a cooling centrifuge. The supernatant was then collected and stored in –20 °C the subsequent biochemical studies.
2.6 Histological studies
For the histological studies, several pieces jejunal were rapidly fixed in phosphate buffered formalin 10 %. This preserved tissue was subsequently processed, sectioned, and stained with hematoxylin and eosin (H&E) in order to evaluate the stages of parasite development in at least 10 well-oriented villi (Drury and Wallington, 1980).
2.7 Oxidative stress assays
The reduced glutathione (GSH) activity was determined using the Ellman method (Ellman, 1959), with minor adjustments indicated by Sedlak and Lindsay (1968). The technique is based on the formation of a yellow product from the reduction of 5,5 dithiobis (2-nitrobenzoic acid) (DTNB) with glutathione (GSH), and its absorbance can be measured at 405 nm. The peroxidation activity (MDA) was assayed following the method of Ohkawa et al. (1979). MDA interacts with thiobarbituric acid (TBA) to create a pink chromogen (TBARS), which can be detected at 535 nm.
2.8 Apoptosis immunohistochemistry and apoptotic score
Apoptosis has been detected in situ using a terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) apoptosis kit (ab206386; Abcam Biotechnology, Cambridge, UK). The jejunal sections were counterstained with methyl green for morphological evaluation and characterization of normal and apoptotic cells. The apoptotic score (VCU) was calculated by taking the average number of apoptotic cells from 10 better orientated villous crypt units. The average number of apoptotic cells per ten VCU was reported as the result.
2.9 The impact of PGT on sporulation of e. Papillata oocysts in vitro (Sporulation inhibition test)
An in vitro experiment was carried out to assess the effect of different PGT concentrations on the sporulation of E. papillata oocysts. PGT was added to 2.5 % potassium dichromate containing 1 × 104 oocysts in this test, with a final concentration ranging from 5 to 1.25 %. The oocysts in the control group were not treated. All treatments were performed in triplicate. The sporulation percentage was calculated for each treatment by counting the number of sporulated and unsporulated oocysts using the McMaster technique after each petri dish had been incubated at 28 °C for three days. Morphological changes, deformities, and destruction percentage were also assessed for each treatment.
2.10 Statistical analysis
One-way ANOVA (with Duncan post-hoc test for subsequent multiple comparisons) was performed for each experimental group using the statistical programme SPSS 22 (SPSS, Chicago, IL, USA). The significance level was set at p ≤ 0.05. The Pearson's method was used to run a simple linear correlation analysis in order to assess relationships between variables.
3 Result
3.1 GC–Mas analysis of PG
Pelargonium graveolens underwent GC/MS analysis, revealing 40 compounds, the most prevalent ones being citronellol (14.44 %), geraniol (11.08 %), linalool (7.74 %), and citronellol formate (7.66 %). The supplemental displays the constituents' amount and retention time (RT and %Area, respectively). (Table 1.).
Compound name
RT
Area %
β-Pinene
0.75
3.3
cis-Linalool oxide
0.55
3.89
Linalool
7.74
4.32
Rose oxide
4.72
0.76
Trans-p-menthone
0.33
5.17
Isomethane
4.35
5.41
Citronellal
0.44
5.73
Isopulegol
0.41
5.85
Citronellol
14.44
6.63
β-Geranial
0.4
6.94
Geraniol
11.08
7.31
Citronellyl formate; formic acid
7.55
7.66
Geranyl formate
3.91
8.01
Geranyl acetal
0.63
8.69
Citronellyl acetate
1.23
8.95
Copaene
1.05
9.44
a-Bourbonene
3.28
9.63
ι-Gurjunene
0.26
10.1
Caryophyllene
2.55
10.32
β-Copaene-4α-ol
0.36
10.49
Aromadendrene
1.73
10.75
ç-Muurolene
0.96
10.88
Humulene
0.83
10.99
Epi-β-Caryophyllene
0.48
11.13
Geranyl propionate
2.01
11.38
Germacrene D
2.63
11.56
Epi-β-Selinene
0.3
11.65
Elemene
1.6
11.85
Epicubebol
0.53
11.91
a-Farnesene
0.3
12.04
Naphthalene
0.89
12.19
σ-Cadinene
3.27
12.39
α-Gurjunene
0.3
12.64
γ-Costol
0.82
12.82
Geranyl butyrate
2.36
13.08
Spathulenol
1.13
13.48
Phenylethyl tiglate
2.71
13.6
Globulol
0.29
13.76
Neryl 2-methylbutyrate
0.41
13.91
Caryophylene oxide
0.3
14.03
3.2 Effect of PGT on faecal oocyst shedding
On day 5p.i., faeces oocyst output peaked at around 6761.66 × 103 ± 372.34 oocysts/g faeces in the infected group. In the groups treated with 50, 100, and 150 mg/kg of PGT suspension, oocyst output was reduced by 4.4, 23.59, and 56.92 %, respectively (Fig. 1). The 150 mg/kg dose was clearly the most effective in reducing faeces oocyst discharge. As a result, we only used the 150 mg/kg dose in subsequent studies.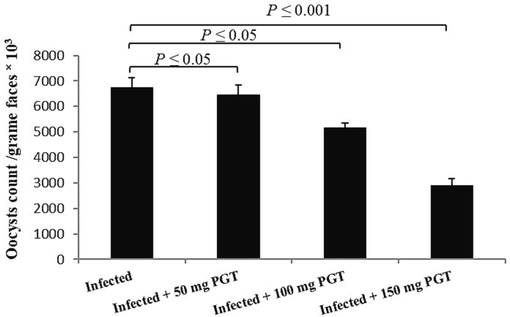
Effect of PGT at varying doses (50, 100, and 150 mg/kg) on the oocyst output patterns of mice infected with E. papillata on day 5p.i. (Data are given as Mean ± SEM). Significant difference as compared to control (P ≤ 0.05).
3.3 Histological observations
Treatment with 150 mg/kg PGT resulted in a 66.19 % (P ≤ 0.001) reduction in the number of parasite stages (meronts, microgametes and macrogametes) per ten villous-crypt units and 56.9 reduction in the number of shedded oocysts when compared to the infected group (Figs. 2-4), (Table 2). All values are means ± SEM. *** significance (P ≤ 0.001) between the infected and treated groups.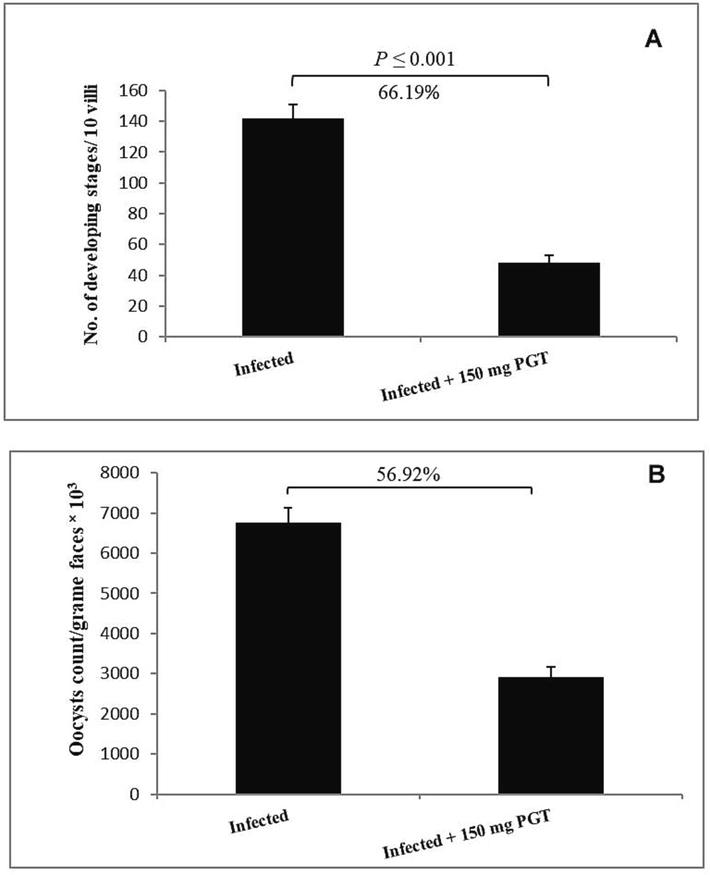
Effect of PGT at 150 mg/kg on the numbers of parasitic stages (A) and amount of shedded oocysts (B). (Data are given as Mean ± SEM). Significant difference as compared to control (P ≤ 0.05).
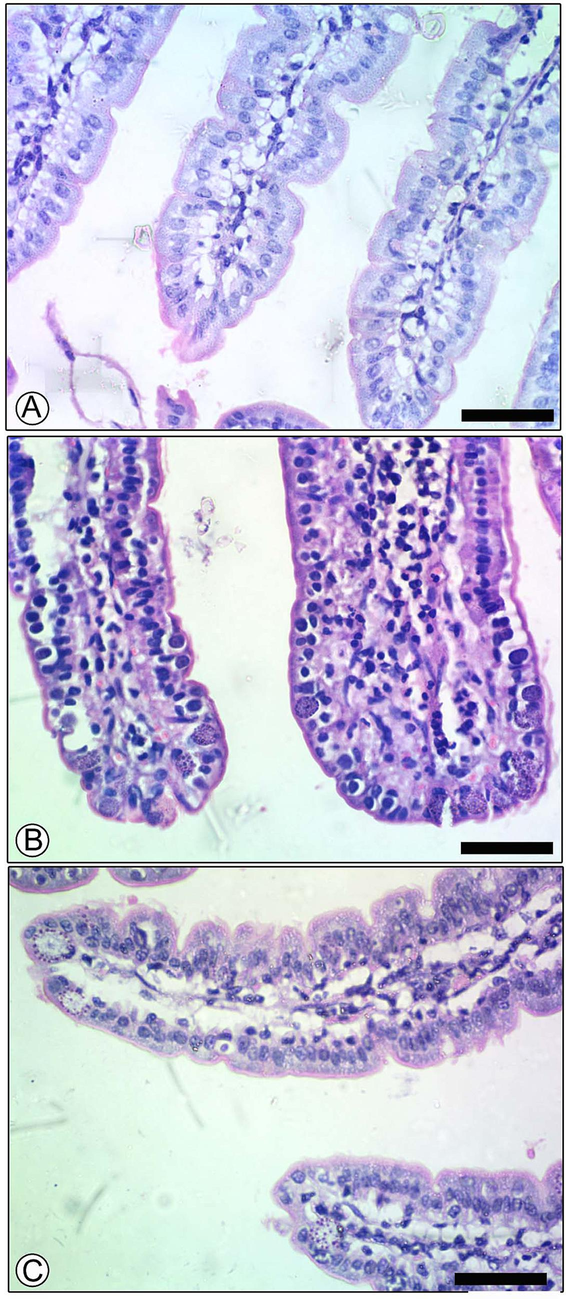
Histological sections of mouse jejunum stained with hematoxylin and eosin showed the effect of PGT at 150mg/kg on the parasitic stages of E. papillata. (A) Uninfected control group; (B) Infected group showed high numbers of developmental stages; (C) Infected treated group with reduced numbers of developmental stages. Scale-bar = 50 µm.
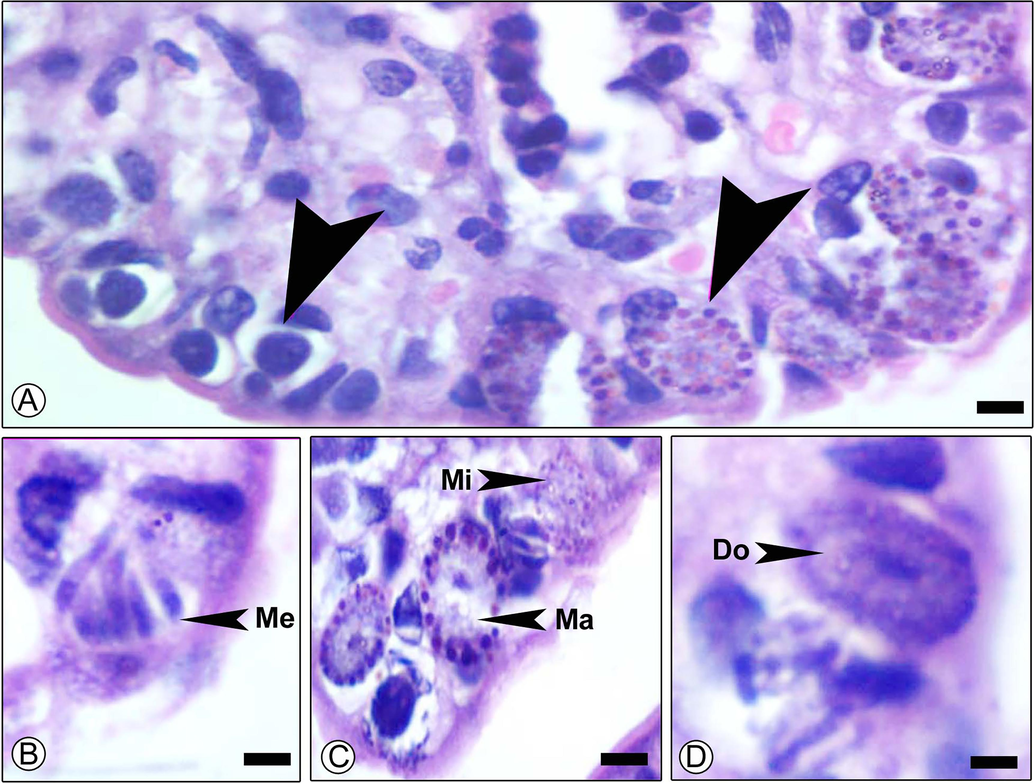
Section of mouse jejunum infected with E. papillata and, stained with hematoxylin and eosin on day 5p.i showing different developmental stages in inner epithelium. meronts (Me), macrogamont (Ma), microgamont (Mi) and developing oocyst (Do). Scale-bar = 100 µm.
Group
Meronts / 10 VCU
Male and female gamonts/ 10 VCU
Developing Oocysts / 10 VCU
Infected
78.00 ± 3.74
46.00 ± 4.00
18.00 ± 2.00
PGT
33.00 ± 4.36***
11.00 ± 1.55***
4.00 ± 1.18***
3.4 Apoptosis and apoptotic score
PGT treatment significantly reduced the number of apoptotic cells in the jejunum tissue (Fig. 5.), from 53.33 ± 8.81 to 20.00 ± 5.77 apoptotic cell/10 VCU (P ≤ 0.05) (Fig. 6).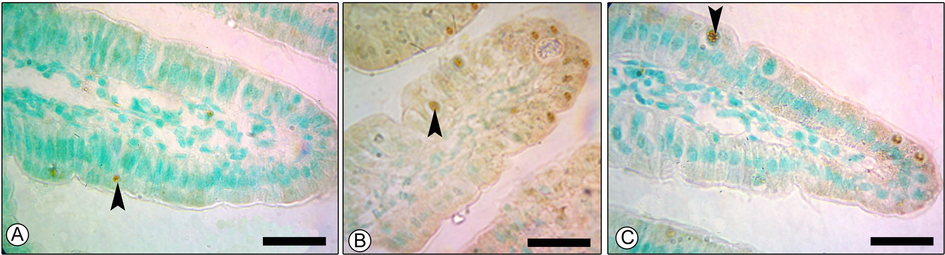
Effect of PGT on the apoptosis level in jejunum of mice. Apoptotic cells in control (A), E. papillata infected (B), and infected-treated (C). Black arrows indicating TUNEL positive cells. Scale-bar = 50 µm.
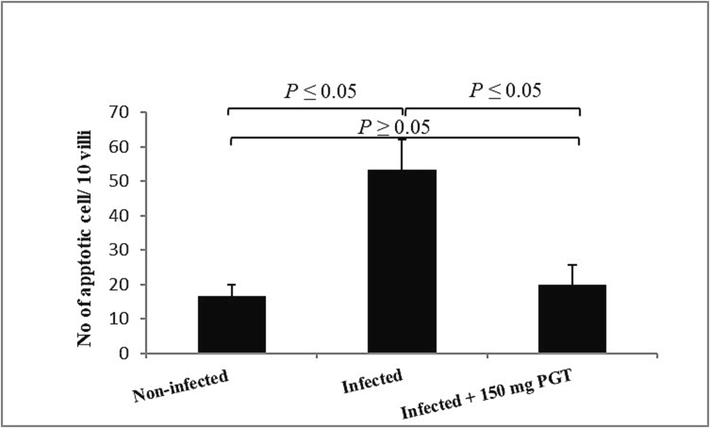
The number of apoptotic cells in different group. Significant change at P ≤ 0.05 with respect to the non-infected and the infected group.
3.5 Effect of PGT on oxidative stress in jejunal tissue
The infection of mice with E. papillata significant decrease in the glutathione (GSH) levels (P ≤ 0.001). When compared to infected groups, PGT treatment resulted in a significant increase in GSH (P ≤ 0.001) (Fig. 7A). Also, the infection caused a significant increase (P ≤ 0.01) in MDA levels while the treatment with PGT improved these MDA alterations (P ≤ 0.05) (Fig. 7B).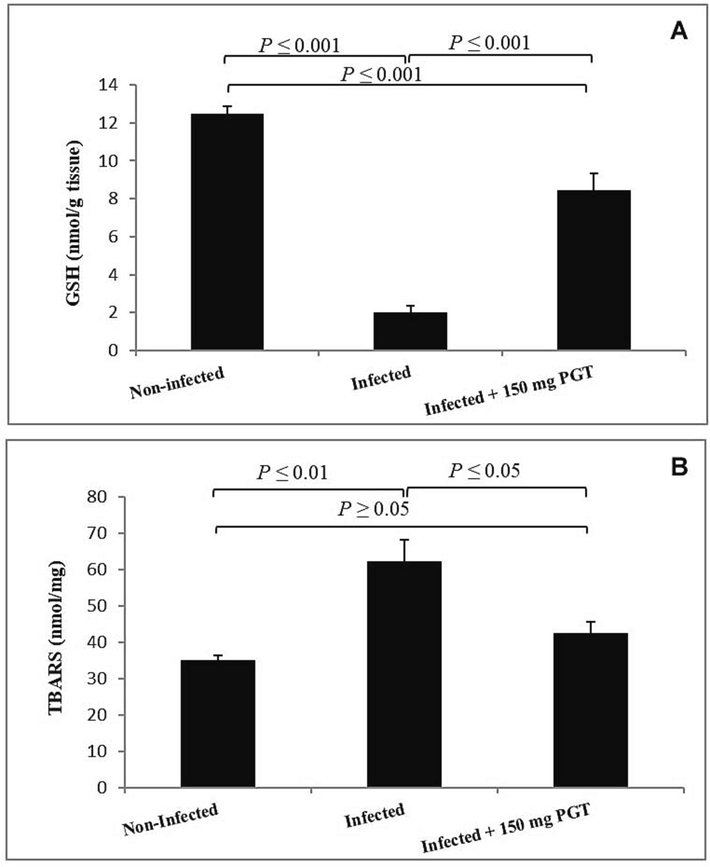
Effect of PGT on the level of reduced glutathione (A) and malondialdehyde (B) in mouse jejunum infected with E. papillata. (Data are given as Mean ± SEM). Significant difference as compared to control and infected group (P ≤ 0.05).
3.6 Sporulation inhibition test
The microscopic examination and counting of oocysts treated with PGT at various concentrations resulted in deformed oocysts with cracked walls and lysis (Fig. 8). The sporulation rate for the control after three days was 83 % ± 1.73. At the doses of 2.5 and 1.25 % (P ≤ 0.05), PGT significantly reduced sporulation rate (P ≤ 0.05) and it totally stopped at a dose of 5 %. The sporulation inhibition rates of PGT relative to control were 100 %, 81.13 %, and 61.85 % for 5, 2.5, and 1.25 mg doses, respectively (Table 3). Also, the oocysticidal effect was confirmed by the higher ratio of degenerated oocysts 96.33, 46.00 and 16.66 for 5, 2.5 and 1.25 % of PGT doses, respectively compared to the control. The liner equation showed the highest coefficient of determination (R2) values (P ≤ 0.001) for sporulation and destruction (Fig. 9). Means with different superscripts within a column are significantly different at P ≤ 0.05.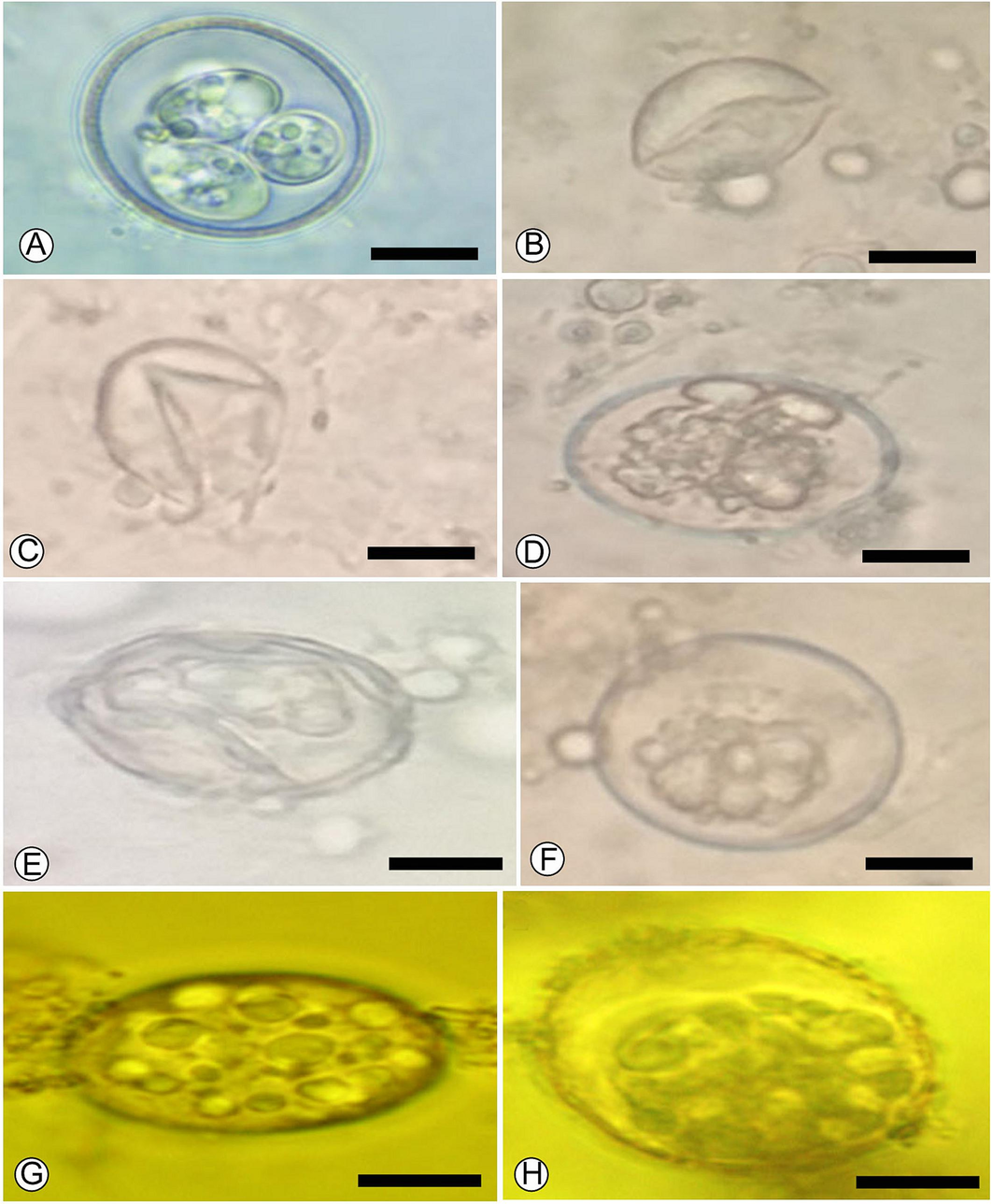
The morphology of E. papillata oocysts treated with PGT different concentrations. Typical sporulated oocyst from control (A). Destructed oocysts after incubation with PGT 5 % (B, C). Abnormal oocysts after incubation with PGT 2.5 % (D, E, F). Degenerated oocysts after incubation with PGT 1.25 % (G, H). Scale-bar = 10 µm.
Concentration/ Group
% Sporulated oocysts
%Unsporulated Oocyst
% Destructed oocyst
control
83.00 ± 1.73a
15.66 ± 1.45c
1.33 ± 0.33d
PGT 5 %
0.00 ± 0.00d
3.66 ± 0.88c
96.33 ± 0.88a
2.5 %
15.66 ± 2.3c
41.66 ± 7.26b
46.00 ± 6.65b
1.25 %
31.66 ± 1.66b
55.00 ± 2.88a
16.66 ± 1.66c
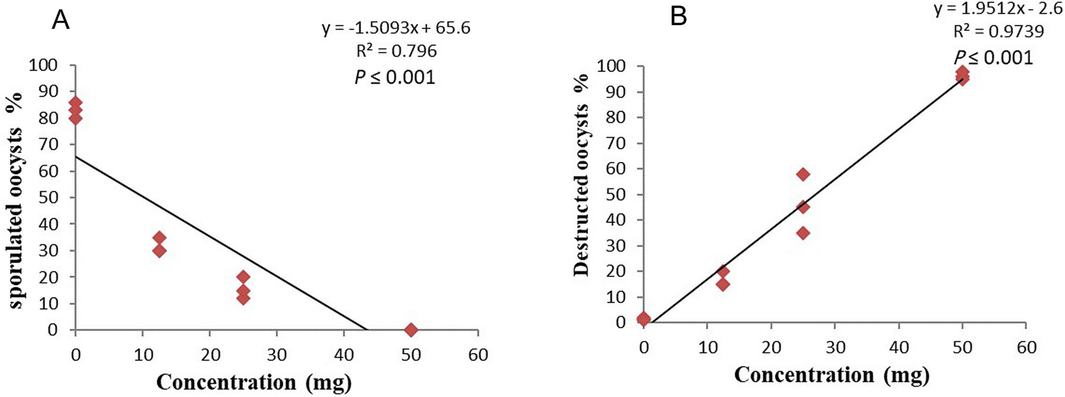
Liner correlation between sporulated and concentration of PGT oil (A), and destructed oocyst and concentration of PGT oil (B).
4 Discussion
The use of EOs and their metabolites may be an alternative tool to anticoccidial drugs for coccidiosis management (Githiori et al., 2006). Furthermore, the synergistic effects of the various compounds in EOs are another important aspect that demonstrated a greater mode of action than individual compounds. Because the dose of use is reduced, the synergism serves to lessen the environmental and toxicological impact (Haleem et al., 2019).
According to the GC–MS analysis of PG, its main components were citronellol (14.44 %), geraniol (11.08 %), and linalool (7.74 %). Citronellol and geraniol are the key components responsible for PG's antiparasitic action (de Mello et al., 2023).
In this study, the synergism between P. graveolens essential oils and thymol against E. papillata infection was investigated. Different doses of PGT (50,100,150 mg/kg) have been tested. In terms of anticoccidial activity, PGT might impede E. papillata development in the host and finally oocyst excretion in mouse faeces, with the highest dose (150 mg/kg) being the most potent.
According to the previously discussed issues, EOs have a high oocysticidal activity due to their hydrophobic characteristics and low molecular weight, as well as their bioactive compounds, which can destroy parasites including oocysts and sporozoites (Abbas et al., 2012, Quiroz-Castañeda and Dantán-González, 2015, Muthamilselvan et al., 2016).
Thymol showed oocysticidal action against Eimeria species of pigeons, with oocyst counts significantly decreased at 10 % thymol concentration. While P. graveolens did not prevent E. magna sporulation, 55–75 % of E. magna oocysts sporulated after being exposed to P. graveolens essential oils (Remmal et al., 2011, Remmal et al., 2013, Arafa et al., 2020). Also, Sárközi et al. (2007) found that thymol has antiparasitic activity against Eimeria spp., and that their mode of action is linked to the degradation of the sporozoite membrane and the subsequent loss of calcium ions from the parasite, which is essential for invasion. Furthermore, Küçükyilmaz et al. (2012) discovered that oregano essential oils, which are high in thymol and carvacrol, help to improve animal health during coccidia challenge and lower the quantity of oocysts shed in faeces. Similarly, thymol may inhibit enzymes involved in protozoal metabolism, such as dihydrofolate reductase. This enzyme catalyses the reduction of dihydrofolate to tetrahydrofolate, which is a precursor of cofactors needed for the biosynthesis of purines, deoxythymidine triphosphate (dTTP), and a number of amino acids in a NADPH-dependent manner. As a result, inhibiting dihydrofolate reductase causes cell growth to stop and death to occur (Hikal et al., 2021).
The histological analysis conducted in this study indicated that E. papillata infection damaged the intestinal tissue at the infection site. This damage was caused by the parasite's developmental stages, particularly merozoites, which broke out of the intestinal cells to invade other intestinal cells (Al-Quraishy et al., 2019).
Intestinal eimeriosis causes oxidative damage and antioxidant depletion, which leads to irreversible cell damage due to an increase in reactive oxygen species, which is associated with an increase in lipid peroxidation, protein oxidation, damaged DNA and cellular membranes, and ultimately cell death (Georgieva et al., 2006, El-Shahat et al., 2009). Essential oils can indirectly interfere with parasitic metabolism by increasing the host immune response and antioxidant defense systems, allowing for effective parasitic invasion control and eradication (Idris et al., 2017).
Our findings showed that PGT reduced oxidative stress in the infected jejunum, which was supported by its antioxidant activities. Similarly, Abdel Rahman et al., 2020 discovered that thymol and geranium supplementation enhanced antioxidant status and reduced MDA levels in broilers and common carp. Apoptosis is critical in the gastro intestinal system because it allows for the continual and rapid replacement of intestinal cells while maintaining tissue homeostasis. Nevertheless, several variables, including increased reactive oxygen and nitrogen intermediates and inflammatory cytokines within cells, contribute to apoptosis (Major et al., 2011). Our results indicated that the number of apoptotic cells in the jejunum of infected mice treated with PGT is significantly lower than in infected mice. Similarly, Green, 2000 demonstrated that E. papillata causes apoptosis, which might be attributed to the complicated host-parasitic relationship; parasite invasion and multiplication, which could cause significant stress to the host cell (Metwaly et al., 2014). PGT have substantial antioxidant and anti-inflammatory properties and thus reduce E. papillata-induced apoptotic effects in infected mice jejuna (El Aanachi et al., 2020, Gholami-Ahangaran et al., 2022).
The in-vitro study, our findings revealed the presence of destructed and deformed oocysts with cracked walls in the oocysts treated with PGT. Also, the rate of oocyst sporulation was significantly lower than in the control group, particularly at 5 % PGT dosage. These findings suggested that PGT could destroy a cell's permeable outer membrane (Boyom et al., 2003). The increased cytoplasmic membrane permeability promotes the release of lipopolysaccharides, porin proteins, cellular fatty acids, and the leaking of metabolites and enzymes such as aromatic amino acids and nucleotides (Remmal et al., 2011). As a result of their hydrophobic abilities, oocysts degenerate as a result of greater cell membrane penetration (Boyom et al., 2003). Therefore, essential oils have anticoccidial activities by diffusing across parasite cell membranes, causing permeability disruption of cell membranes, and interfering with cell metabolisms. The process causes ion, energy, and other cell components to seep out, resulting in cell death (Allen et al., 1997), and also induction of oxidative stress, which inhibits invasion and impairs Eimeria growth (Abbas et al., 2012). In addition, geraniol and citronellol the main component of geranium oil can act on cell wall and cell membrane via interfering with ergosterol biosynthesis (Pereira et al., 2015).
In conclusion, mixing geranium oil and thymol oil may be an effective way to boost the anticoccidial effectiveness of natural extracts. Our findings suggest that PGT decreases developmental stages, oocyst output, and can impair E. papillata sporulation. This protective impact could be attributed to PGT's antioxidant and anti-apoptotic characteristics.
CRediT authorship contribution statement
Heba AbdelTawab: Writing – original draft. Shawky M. Aboelhadid: Supervision, Writing – original draft. Almahy M. ElMallah: . Abdel-Azeem S. Abdel-Baki: Supervision, Writing – original draft. Saleh Al-Quraishy: . Ahmed O. Hassan: . Ebtesam A. Yousef: .
Acknowledgments
This work was supported by Researcher supporting Project (RSP-2023/3), King Saud University.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Botanicals: an alternative approach for the control of avian coccidiosis. World Poult. Sci. J.. 2012;68(2):203-215.
- [CrossRef] [Google Scholar]
- Anticoccidial and antioxidant activities of moringa oleifera leaf extract on murine intestinal eimeriosis. Acta Parasitol.. 2020;65(4):823-830.
- [CrossRef] [Google Scholar]
- Effects of components of Artemisia annua on coccidia infections in chickens. Poult. Sci.. 1997;76(8):1156-1163.
- [CrossRef] [Google Scholar]
- Salvadora persica protects mouse intestine from eimeriosis. Rev. Bras. Parasitol. Vet.. 2019;28:605-612.
- [CrossRef] [Google Scholar]
- Thymol efficacy against coccidiosis in pigeon (Columba livia domestica) Prev. Vet. Med.. 2020;176:104914.
- [CrossRef] [Google Scholar]
- Re-calculating the cost of coccidiosis in chickens. Vet. Res.. 2020;51(1):115.
- [CrossRef] [Google Scholar]
- Influence of essential oils on sporulation of Eimeria magna oocysts. Ann. Parasitol.. 2021;67(1):11-17.
- [CrossRef] [Google Scholar]
- Composition and anti-plasmodial activities of essential oils from some cameroonian medicinal plants. Phytochemistry.. 2003;64(7):1269-1275.
- [CrossRef] [Google Scholar]
- Milestones in avian coccidiosis research: a review. Poult. Sci.. 2014;93(3):501-511.
- [CrossRef] [Google Scholar]
- Forty years of monensin for the control of coccidiosis in poultry. Poult. Sci.. 2010;89(9):1788-1801.
- [CrossRef] [Google Scholar]
- Microscopic alterations in fasciola hepatica treated with the essential oils of Pelargonium graveolens and Citrus aurantium. Vet. Parasitol.. 2023;314:109863.
- [CrossRef] [Google Scholar]
- Carleton’s Histological Technique (5th Edition). New York: Oxford University Press; 1980.
- Phenolic contents and in vitro investigation of the antioxidant, enzyme inhibitory, photoprotective, and antimicrobial effects of the organic extracts of Pelargonium graveolens growing in Morocco. Biocatal. Agric. Biotechnol.. 2020;29:101819.
- [CrossRef] [Google Scholar]
- Altered testicular morphology and oxidative stress induced by cadmium in experimental rats and protective effect of simultaneous green tea extract. Int. J. Morphol.. 2009;27:757-764.
- [CrossRef] [Google Scholar]
- Antitussive effects of nasal thymol challenges in healthy volunteers. Respir. Physiol. Neurobiol.. 2013;187(1):104-107.
- [CrossRef] [Google Scholar]
- Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Vet. J.. 2006;172(3):488-492.
- [CrossRef] [Google Scholar]
- Thymol and carvacrol supplementation in poultry health and performance. Vet. Med. Sci.. 2022;8(1):267-288.
- [CrossRef] [Google Scholar]
- Use of plants in novel approaches for control of gastrointestinal helminths in livestock with emphasis on small ruminants. Vet. Parasitol.. 2006;139(4):308-320.
- [CrossRef] [Google Scholar]
- Apoptotic pathways: Paper wraps stone blunts scissors. Cell. 2000;102(1):1-4.
- [CrossRef] [Google Scholar]
- Effect of synbiotics and essential oils in ameliorating the negative drawbacks of necrotic enteritis and coccidiosis in broiler chicks. Benha Vet. Med. J.. 2019;36 187–198
- [CrossRef] [Google Scholar]
- Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci.. 2013;92(8):2059-2069.
- [CrossRef] [Google Scholar]
- Chemical composition and biological significance of thymol as antiparasitic. Open J. Ecol.. 2021;11(03):240.
- [Google Scholar]
- In vitro anti-parasitic activity of Pelargonium X. asperum essential oil against Toxoplasma gondii. Front. Cell Dev. Biol.. 2021;9:616340.
- [CrossRef] [Google Scholar]
- The potential of antioxidant rich essential oils against avian coccidiosis. World Poult. Sci. J.. 2017;73(1):89-104.
- [CrossRef] [Google Scholar]
- Effects of vaccination against coccidiosis, with and without a specific herbal essential oil blend, on performance, oocyst excretion and serum IBD titers. Ital. J. Anim. Sci.. 2012;1:e1.
- [CrossRef] [Google Scholar]
- A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet. Lat.. 1976;6(3):201-217.
- [Google Scholar]
- Intestinal mucin dynamic and leukocytic responses of chickens infected with Eimeria acervulina and fed oregano supplemented diet. Acta Vet. Brno.. 2011;80:147-156.
- [CrossRef] [Google Scholar]
- Anti-coccidial and anti-apoptotic activities of palm pollen grains on Eimeria papillata-induced infection in mice. Biologia. 2014;69(2):254-259.
- [CrossRef] [Google Scholar]
- Herbal remedies for coccidiosis control: A review of plants, compounds, and anticoccidial actions. Evid. Based Complement. Alternat. Med.. 2016;2016:2657981.
- [CrossRef] [Google Scholar]
- Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol.. 2017;8:380.
- [CrossRef] [Google Scholar]
- Essential oils: an potential substitute to antibiotics growth promoter in broiler diet. J. Entomol. Zool. Stud.. 2020;8:1643-1649.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2):351-358.
- [CrossRef] [Google Scholar]
- Antifungal activity of geraniol and citronellol, two monoterpenes alcohols, against Trichophyton rubrum involves inhibition of ergosterol biosynthesis. Pharm. Biol.. 2015;53(2):228-234.
- [CrossRef] [Google Scholar]
- Control of avian coccidiosis: future and present natural alternatives. Biomed. Res. Int.. 2015;2015:430610.
- [CrossRef] [Google Scholar]
- In vitro destruction of Eimeria oocysts by essential oils. Vet. Parasitol.. 2011;182(2–4):121-126.
- [CrossRef] [Google Scholar]
- Oocysticidal effect of essential oil components against chicken eimeria oocysts. Int. J. Vet. Med. Res. Rep.. 2013;2013:1-8.
- [CrossRef] [Google Scholar]
- Effect of natural phenol derivatives on skeletal type sarcoplasmic reticulum Ca2+-ATPase and ryanodine receptor. J. Muscle Res. Cell Motil.. 2007;28(2):167-174.
- [CrossRef] [Google Scholar]
- Comparison of four murine Eimeria species in immunocompetent and immunodeficient mice. J. Parasitol.. 1996;82(2):255-262.
- [Google Scholar]
- Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem.. 1968;25(1):192-205.
- [CrossRef] [Google Scholar]
- Biosynthesized nanosilver from ginger extract exhibits antioxidant and hepatic responses during Eimeria papillata infection. ACS Omega. 2023;8(26):23806-23811.
- [CrossRef] [Google Scholar]
- 2 - Essential oil composition. In: Tisserand R., Young R., eds. Essential Oil Safety (second Edition). St. Louis, Churchill: Livingstone; 2014. p. :5-22.
- [Google Scholar]
- Protective effect of l-cysteine and glutathione on the modulated suckling rat brain Na+, K+-ATPase and Mg2+-ATPase activities induced by the in vitro galactosaemia. Pharmacol. Res.. 2004;49(5):475-479.
- [CrossRef] [Google Scholar]
- Thymol reduces oxidative stress, aortic intimal thickening, and inflammation-related gene expression in hyperlipidemic rabbits. J. Food Drug Anal.. 2016;24(3):556-563.
- [CrossRef] [Google Scholar]







