Translate this page into:
The type species of Amphorellopsis and Tintinnopsis (Protozoa:Ciliophora): A new ciliary pattern and some comments in Tintinnina
⁎Corresponding authors. miaomiao@ucas.ac.cn (Miao Miao), xiaozhonghu@ouc.edu.cn (Xiaozhong Hu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
As dominant components of planktonic microeukaryotes, the tintinnine ciliates act as a trophic link between microbial food web and traditional planktonic food chain in aquatic ecosystems. However, traditionally the taxonomy and systematics of tintinnines have been based on the lorica. It is now accepted that lorica features alone are insufficient and that cytological and molecular information are also needed. The systematics of the genera Tintinnopsis and Amphorellopsis is ambiguous, mainly due to the lack of ciliary and molecular information on their type species, T. beroidea and A. acuta.
Result
In the present study, specimens of both species were collected from coastal waters of Qingdao, China. The morphology of each was investigated based on observations of live and protargol-stained specimens, and their SSU rDNA- and LSU rDNA-based phylogenies were analyzed. Tintinnopsis beroidea presents the genus-typical ciliature. The somatic kineties in Amphorellopsis acuta were found to be evenly distributed without differentiation into distinct ciliary fields. The phylogenetic analyses revealed that Tintinnopsis is non-monophyletic, and that T. beroidea has a close relationship with T. nana and T. baltica. The monophyly of Amphorellopsis is also not supported by SSU rDNA analysis.
Conclusion
This study first reveals the ciliary pattern and two nuclear rRNA gene sequences of two tintinnine type species, and thus expands knowledge and database of tintinnines. Amphorellopsis represents an intermediate lineage between tintinnines (loricate form) and aloricate choreotrichids.
Keywords
Ciliary pattern
Lorica
Phylogeny
rRNA gene sequence
Tintinnine
1 Introduction
Tintinnine ciliates are planktonic microeukaryotes characterized by the possession of a lorica, and act as a trophic link between microbial food web and traditional planktonic food chain in aquatic ecosystems (e.g., Dolan, 2010; Xu et al., 2017). They comprise approximately 1000 extant species, most of which are recognized only by features of the lorica (Hu et al., 2019; Kofoid and Campbell, 1929; Zhang et al., 2012). Recent studies revealed that the lorica can be polymorphic and may vary in size and shape in response to environmental factors or in different stages of the life cycle, which infers lorica features alone are insufficient for species separation (Agatha and Strüder-Kypke, 2013; Laval-Peuto, 1981; Xu et al., 2012). Furthermore, DNA sequencing of closely-related species has indicated examples of polymorphic and cryptic species (Santoferrara et al., 2013).
The members of genus Amphorellopsis Kofoid and Campbell, 1929 are characterized by their vase-shaped lorica with blade-like fins (Kofoid and Campbell, 1929). But their ciliary patterns are unknown for any member of this genus including the type species, A. acuta (Schmidt, 1902) Kofoid and Campbell, 1929.
Tintinnopsis Stein, 1867 is one of the most confused genera within the suborder Tintinnina (Bai et al., 2020b, 2020a; Wang et al., 2020) and is distributed among more than ten polyphyletic clades in phylogenetic trees based on small subunit ribosomal DNA (SSU rDNA) sequence data (Santoferrara et al., 2013). However, neither ciliary nor molecular data are available for the type species, T. beroidea Stein, 1867.
In the present study, the morphology and molecular phylogeny of the type species of Amphorellopsis and Tintinnopsis, collected from coastal waters of China, were investigated. This includes observations of live and silver-stained specimens, and phylogenetic analyses of nuclear rDNA. This study aims to provide archives for determining the taxonomy and systematics of these two genera by using an integrative approach that combines lorica, cell and molecular data.
2 Material and methods
Amphorellopsis acuta and Tintinnopsis beroidea were collected from surface coastal waters off Qingdao, China (36°03′35′'N 120°18′53′'E) on September 9, 2017 (water temperature = 25 °C; salinity = 30) and March 19, 2019 (water temperature = 16 °C; salinity = 30), respectively (Fig. S1). Live cells were microscopically observed at magnifications of 100–1000×. Lorica measurements were calculated from photomicrographs taken at magnifications of 100–1000×. The protargol staining method according to Wilbert (1975) was used to reveal the ciliary patterns and the nuclear apparatus with manually synthesized protargol (Pan et al., 2013). Identifications were based on original descriptions, the earliest authoritative redescription and some above-mentioned references (Entz, 1884; Schmidt, 1902; Stein, 1867). Terminology and classification follow Agatha and Riedel-Lorjé (2006) and Adl et al. (2019), respectively.
DNA extraction, PCR amplification, sequencing (including other 104 sequences of ciliates in SSU rDNA tree and 45 sequences of other ciliates in LSU rDNA tree with the sequences of Halteria grandinella and the hypotrichs were used as outgroup taxa) and data analyses refer to Bai et al. (2020a).
3 Results
Amphorellopsis acuta (Schmidt, 1902) Kofoid and Campbell, 1929 (Fig. 1A–J, 2A–O; Table 1, S1) Lorica data are based on living observations, others are based on protargol-stained specimens. Measurements are in μm. Abbreviations: CV = coefficient of variation in %; M = median; Max = maximum; Mean = arithmetic mean; Min = minimum; N = number of specimens examined; SD = standard deviation.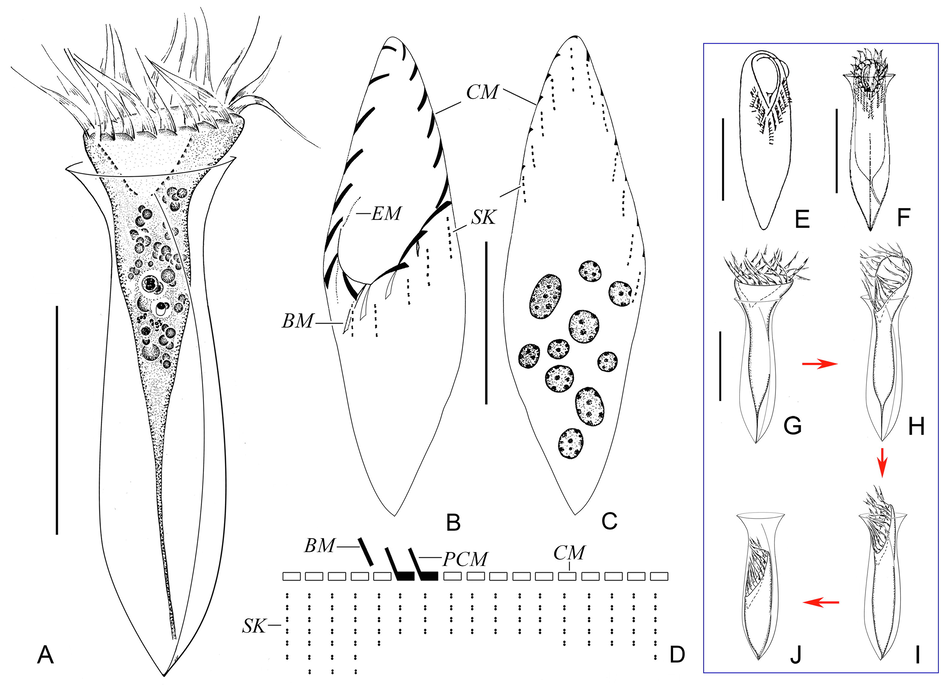
Drawings of Amphorellopsis acuta in vivo (A, E–J) and after protargol staining (B–D). A, Lateral view of a representative individual. B, C, Ciliary pattern of ventral (B) and dorsal (C) sides. D, Kinetal map of a morphostatic specimen. E, F, Sketch of living cells from Small et al. (1985) (E) and Laval-Peuto and Brownlee (1986) (F). G–J, Contraction process of lateral view of a contracted individual. Abbreviations: BM, buccal membranelle; CM, collar membranelle; EM, endoral membrane; PCM, prolonged collar membranelles; SK, somatic kineties. Scale bars: 50 μm (A, F, G), 30 μm (B, C, E).
Charactera
Min
Max
Mean
M
SD
CV
N
Lorica, total length
77
126
106.7
111
15.0
14.1
15
53
75
65.9
66
5.4
13.4
12
Lorica, bowl width
19
32
25.4
26
3.8
14.9
15
30
45
40.4
40
5.4
13.4
12
Lorica, anterior opening diameter
31
44
37.5
37
3.9
10.4
15
29
44
37
37
4.0
10.9
12
Lorica, collar length
9
22
15.4
17
3.9
24.0
15
–
–
–
–
–
–
–
Lorica, total length: anterior opening diameter, ratio
2.1
3.4
2.9
3.0
0.4
20.6
15
1.6
1.9
1.8
1.8
0.1
4.3
12
Lorica, diameter of narrowed portion
12
29
20.1
21
4.1
15.5
15
–
–
–
–
–
–
–
Cell proper, length
57
102
81.2
84
14.8
18.2
15
40
61
48.6
49
6.4
13.1
12
Cell proper, width
14
27
18.9
19
3.7
19.7
15
24
36
28.8
29
3.7
13.0
12
Macronuclear nodules, number
8
12
9.4
9
3.1
33.0
15
2
2
2
2
0.0
0.0
12
Macronuclear nodules, length
3
9
5.1
5
1.6
32.1
15
8
14
10.2
9.5
1.8
17.7
12
Macronuclear nodules, width
3
6
4.3
4
1.0
22.5
15
5
11
8.1
8
1.6
19.4
12
Ventral kinety, length
–
–
–
–
–
–
–
27
39
32.8
33
3.9
11.9
12
Ventral kinety, number of kinetids
–
–
–
–
–
–
–
19
32
24.4
24
3.4
14.1
12
Dorsal kinety, length
–
–
–
–
–
–
–
31
47
37.0
37
4.7
12.6
12
Dorsal kinety, number of kinetids
–
–
–
–
–
–
–
15
26
21.3
22
3.2
15.1
12
Posterior kinety, length
–
–
–
–
–
–
–
12
32
18.1
18
2.2
12.1
12
Posterior kinety, number of kinetids
–
–
–
–
–
–
–
7
9
8.1
8
0.8
9.8
12
Longest somatic kinety, length
6
11
7.8
7
1.3
16.9
15
–
–
–
–
–
–
–
Longest somatic kinety, number of kinetids
6
7
6.4
6
0.5
7.9
15
–
–
–
–
–
–
–
Shortest somatic kinety, length
3
5
4.1
4
0.8
20.2
15
–
–
–
–
–
–
–
Shortest somatic kinety, number of kinetids
4
5
4.1
4
0.4
8.5
15
–
–
–
–
–
–
–
Right ciliary field, number of kineties
–
–
–
–
–
–
–
6
8
6.8
7
8.3
12.2
12
Left ciliary field, number of kineties
–
–
–
–
–
–
–
5
7
5.4
5
0.7
12.3
12
Lateral ciliary field, number of kineties
–
–
–
–
–
–
–
10
14
11.8
12
1.3
11.3
12
Adoral zone of membranelles, width
10
25
17.7
18
3.6
20.5
15
18
32
24.1
24
4.3
18.0
12
Collar membranelles, number
17
18
17.8
18
0.4
2.3
15
17
18
17.2
17
0.4
2.3
12
Prolonged collarmembranelles,number
2
2
2.0
2
0.0
0.0
15
3
4
3.4
3
0.5
15.1
12
Buccal membranelles, number
1
1
1.0
1
0.0
0.0
15
1
1
1.0
1
0.0
0.0
12
Improved diagnosis (based on present population and original population): Lorica about 77–126 μm long, composed of fusiform bowl about 26 µm wide and flared collar about 15 μm high; opening 31–44 μm across, constricted portion about 20 μm across; posterior portion of lorica with three curved fins and pointed end. Extended cell proper obconical, size about 50–105 × 30–45 μm in vivo and on average 81 × 19 μm after protargol staining. Oral portion asymmetric, dorsal side longer than ventral side. Eight to 12 macronuclear nodules. Adoral zone with 17 or 18 collar membranelles, two of which extend into buccal cavity, and one buccal membranelle. Seventeen or 18 evenly distributed dikinetidal somatic kineties.
Deposition of voucher materials: Two protargol slides with voucher specimens were deposited in Laboratory of Protozoology, Ocean University of China (registration number: BY201709090101 and BY201709090102).
3.1 Redescription
Lorica hyaline, about 77–126 μm long, composed of fusiform bowl 19–32 μm in width, and flared collar, about 9–22 μm high; opening 31–44 μm across with smooth rim (Fig. 1A, 2A–D, H); constricted portion about 12–29 μm across; posterior end of lorica pointed (Fig. 1A, 2A–D). Three blade-like fins commence at constricted lorica portion (4–16 μm posterior to opening) and extend to posterior end of lorica (Fig. 1A, 2E, F, H).
Extended cell proper obconical, size about 50–105 × 30–45 μm in vivo and 57–102 × 14–27 μm after protargol staining; anterior portion asymmetric, i.e., dorsal side longer than ventral side in extended live cells and adoral zone obliquely oriented in contracted specimens (Fig. 1A, G–J, 2A–D). Eight to 12 ovoidal macronuclear nodules, each about 3–9 × 3–6 μm in size after protargol staining (Fig. 1C, 2I, M). Micronuclei, striae, tentaculoids, accessory comb, contractile vacuole, cytopyge, and capsules not observed. Locomotion by swimming forward while rotating about main cell axis.
Kinetids of each ciliary row ostensibly connected by argyrophilic fibers (Fig. 2K, L). Seventeen or 18 somatic kineties, each about 3–11 μm long, each comprising 4–7 dikinetids, kineties in dorsal side commence about 4 μm below anterior cell end (Fig. 1B–D, 2I–L). Both basal bodies of anteriormost dikinetid of each kinety are ciliated whereas for remaining dikinetids only posterior basal body bears a cilium; somatic cilia about 3 µm long after protargol staining, invisible in vivo (Fig. 2K, L).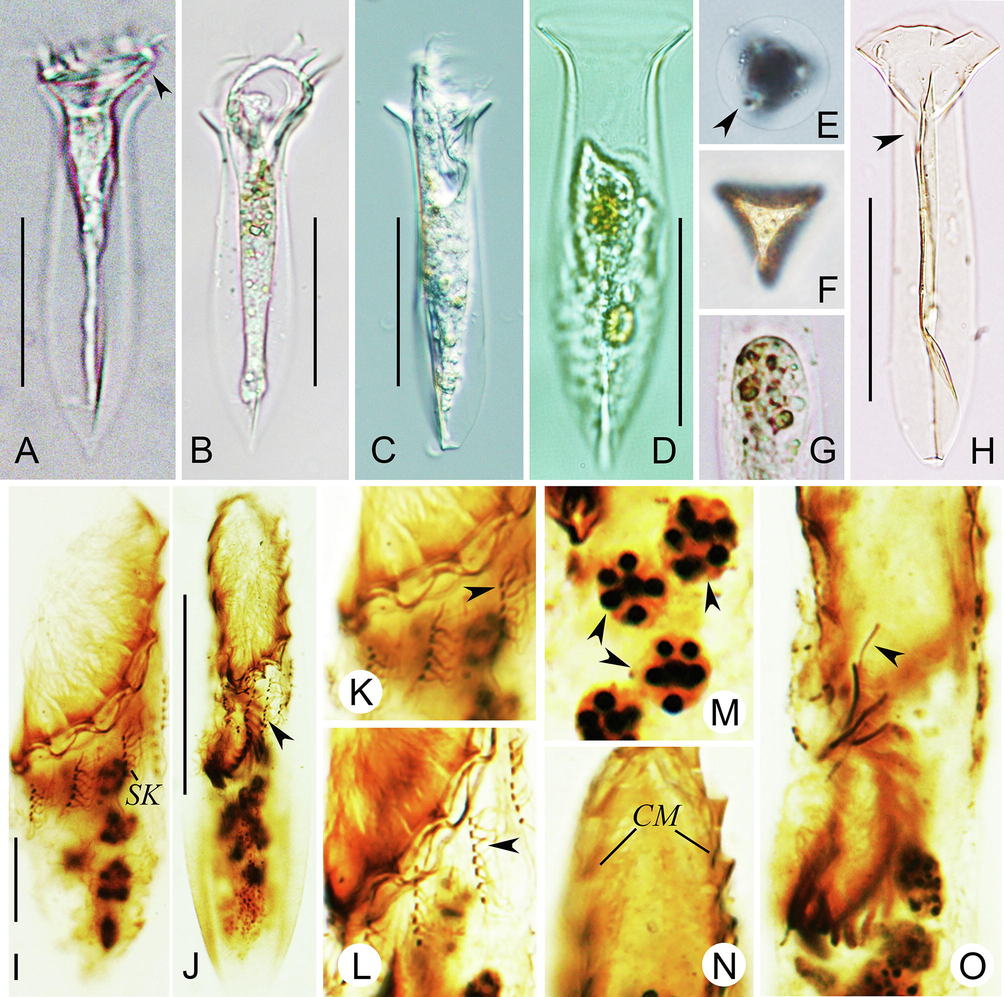
Photomicrographs of Amphorellopsis acuta in vivo (A–H) and after protargol staining (I–O). A, Lateral view of an individual with cell proper extending out of lorica opening, arrowhead indicates dorsal side of oral portion. B, Lateral view of the sequenced individual. C, Lateral view of a partly contracted individual with an artificial compressional deformation in the posterior end. D, Lateral view of a contracted individual. E, Apical view of lorica, arrowhead marks one blade fin. F, Antapical view of lorica. G, Showing food vacuoles. H, Lateral view of a flattened lorica with an artificial compressional deformation in the posterior end, arrowhead shows a bladed fin from posterior end to constrict portion. I, Somatic kineties and macronuclear nodules. J, Ventral view of an early divider, arrowhead shows elongated somatic kinety. K, L, Details of somatic kineties, arrowheads show ciliated dikinetids (K) and cilia and fibers of dikinetid at mid-portion (L). M, Macronuclear nodules (arrowheads). N, Collar membranelles. O, Anterior portion of the same divider as shown in (J), arrowhead shows endoral membrane. Abbreviations: CM, collar membranelle; SK, somatic kinety. Scale bar, 50 μm (A–D, H), 10 μm (I), 30 μm (J).
Adoral zone composed of 17 or 18 collar membranelles, of which two extend into buccal cavity, and one buccal membranelle (Fig. 1B–D, 2I, N, O); polykinetids of each membranelle about 10–15 μm long, kinetal structure of which is unavailable; cilia of membranelles 15–25 μm long in vivo. Three early dividers were observed; endoral membrane only observed in one early divider; oral primordium inverted C-shape, forms in right half of ventral side below buccal membranelle and somatic kineties (Fig. 2J, O).
Tintinnopsis beroidea Stein, 1867 (Fig. 3A–Q; Table 1, S1)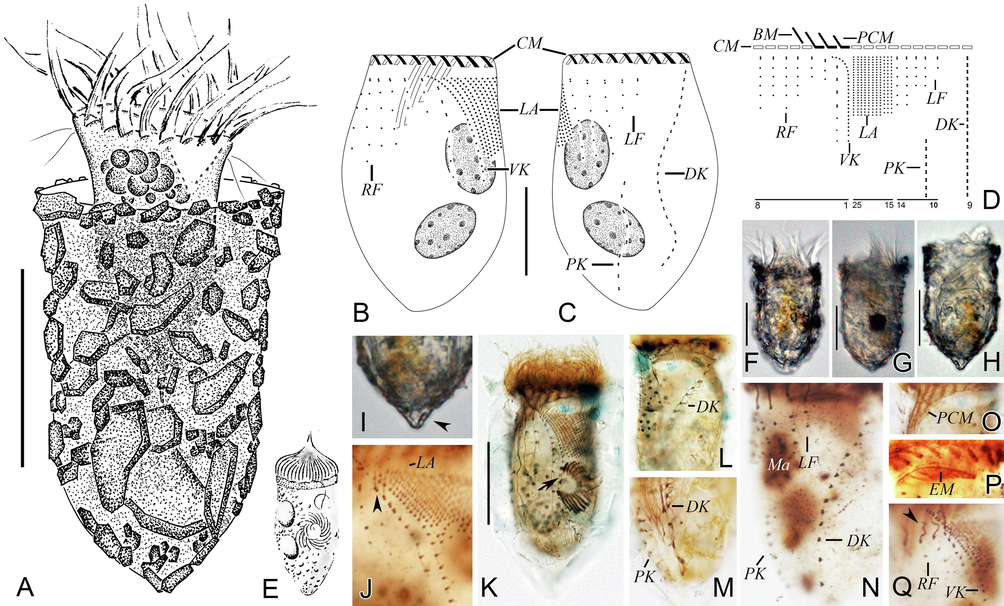
Morphology and infraciliature of Tintinnopsis beroidea in vivo (A, E–I) and after protargol staining (B–D, J–Q). A, Lateral view of a representative specimen with cell proper extending partly out of lorica opening. B, C, Ciliary pattern and macronuclear nodules of ventral (B) and dorsal (C) sides of the same specimen. D, Kinetal map of a morphostatic specimen. E, An early divider (from Entz, 1884). F–H, Lateral views of different individuals with cell proper contracted into lorica, (G) shows the sequenced individual. I, Pointed posterior end of lorica (arrowhead). J, Ventral kinety, lateral ciliary field, and the first kinety of right ciliary field, arrowhead indicates the anterior four dikinetids. K, An early divider within lorica, arrow shows oral primordium differentiating into new membranelles. L, Anterior portion of dorsal kinety. M, Posterior portion of dorsal kinety and posterior kinety. N, Dorsal side, showing the left ciliary field, dorsal kinety, and posterior kinety. O, Collar membranelles. P, Collar membranelles and endoral membrane. Q, Ventral side, showing the ventral kinety, right ciliary field, and lateral ciliary field, arrowhead shows the anterior elongated cilia. Abbreviations: BM, buccal membranelle; CM, collar membranelles; EM, endoral membrane; DK, dorsal kinety; LA, lateral ciliary field; LF, left ciliary field; Ma, macronuclear nodules; PCM, prolonged collar membranelles; PK, posterior kinety; RF, right ciliary field; VK, ventral kinety. Scale bars, 30 μm (A), 20 μm (B, C), 25 μm (F–H, K).
Improved diagnosis (based on present population and Entz, 1884): Lorica campanulate, boundary of collar and bowl inconspicuous, 53–80 μm in length, opening 29–60 μm in diameter. Two ellipsoidal macronuclear nodules. On average 17 collar membranelles, three or four of which extend into buccal cavity; one buccal membranelle. Ventral kinety with an average of 24 densely arranged monokinetids. Right, left and lateral ciliary fields consisting of seven, five and 12 kineties on average, respectively. Dorsal kinety composed of about 50 dikinetids. Posterior kinety composed of about eight dikinetids, positioned below left ciliary field.
Deposition of voucher materials: Two protargol slides with voucher specimens were deposited in the Laboratory of Protozoology, Ocean University of China (registration number: BY201903190101 and BY201903190102).
3.2 Redescription
Lorica campanulate, about 53–75 μm long, collar slightly flared with irregular rim with an opening 29–44 μm across (Fig. 3A, F–H). Bowl conical, diameter similar to opening, posterior end blunt, sometimes pointed (Fig. 3A, F–I).
Cell proper about 40–60 × 20–35 μm in vivo when fully extended and 40–61 × 24–36 μm after protargol staining with a peduncle about 30 μm long attached to bottom of lorica (Fig. 3A, F, G). Two ellipsoidal macronuclear nodules, 8–14 × 5–11 μm in size after protargol staining (Fig. 3B, C, N). Micronuclei, striae, tentaculoids, accessory comb, cytopyge, and capsules not observed. Locomotion by swimming forward while rotating about main cell axis.
Somatic ciliary pattern comprised of single ventral, dorsal, and posterior kineties as well as a right, left, and lateral ciliary field (Fig. 3B–D, J–N, Q). Ventral kinety 27–39 μm long with 19–32 densely spaced monokinetids, commences about 4 μm below anterior cell end, and curves leftwards before extending posteriad parallel to kineties of lateral ciliary field (Fig. 3B, D, J, K, Q). Right ciliary field comprised of six to eight kineties, all kineties commence about 4–8 μm below anterior cell end, except for first kinety which commences about 2 μm below level of remaining kineties; second and third kineties comprise two or three kinetids only, others with six or seven widely spaced kinetids; all kineties composed of monokinetids and one anterior dikinetid, except for first kinety which has three or four anterior dikinetids (Fig. 3B, D, J, K, Q). Left ciliary field consisting of five to seven kineties, each kinety commences about 4–8 μm below anterior cell end and consists of one anterior dikinetid and one to seven widely spaced monokinetids (Fig. 3C, D, N). In left and right ciliary fields, only anterior basal body of each dikinetid bears a cilium about 8 μm long in vivo and 5 μm long after protargol staining; cilia of monokinetids are about 1–2 μm long after protargol staining (Fig. 3A, N, Q). Lateral ciliary field with 10–14 monokinetidal kineties of similar length, each of which commences about 4–8 μm below anterior cell end; cilia about 1–3 μm long after protargol staining (Fig. 3B–D, J, K, Q). Dorsal kinety commences about 3–4 μm below anterior cell end and between left and right ciliary fields, about 3 μm from right and 10 μm from left ciliary field, respectively; extends in a curve towards left-posterior of cell proper; 31–47 μm long and consists of 15–26 dikinetids, only posterior basal body of each dikinetid bears a cilium that is about 5 µm long after protargol staining (Fig. 3C, D, L–N). Posterior kinety 12–32 μm long, commences below middle kinety of left ciliary field (14–31 μm below anterior cell end) and curves leftwards; consists of seven to nine dikinetids, only posterior basal body of each dikinetid bears a cilium that is about 5 µm long after protargol staining (Fig. 3C, D, M, N).
Adoral zone of membranelles composed of 17–18 collar membranelles, three or four of which extend significantly into buccal cavity, and single buccal membranelle (Fig. 3B, D, O, P). Bases of collar membranelles about 8–20 μm, kinetal structure of which could not be recognized; cilia of membranelles 15–20 μm long after protargol staining (Fig. 3A–D, F, G, K, O, P). Endoral membrane composed of a single row of basal bodies, extends across peristomial field and right wall of buccal cavity (Fig. 3P). One early divider was observed in protargol preparations; oral primordium located left of ventral kinety and below lateral ciliary field (Fig. 3K).
3.3 Sequence comparison and phylogenetic analyses
The length, G + C content and GenBank accession numbers of the SSU rDNA and LSU rDNA sequences of two newly investigated species are documented in Table S2. For each gene marker, the topologies of the ML and BI trees were similar, therefore only the ML trees are shown (Figs. 4 and 5). The phylogenetic analyses indicate that neither Tintinnopsis nor Amphorellopsis is monophyletic and their main phylogenetic features are described below (Figs. 4 and 5).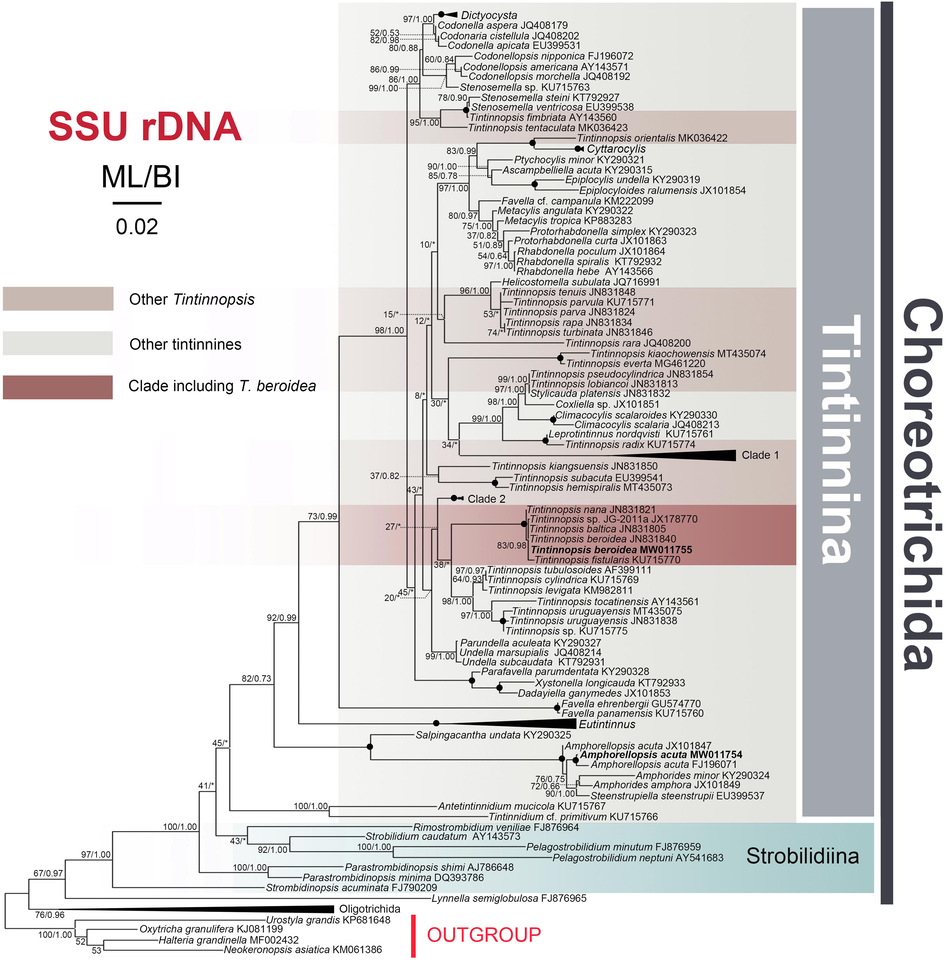
Maximum likelihood (ML) tree inferred from SSU rDNA sequences showing nodal support for ML and BI analyses. Newly sequenced species are shown in bold. Clade 1 comprises sequences of Tintinnopsis major (JN831818), T. dadayi (AY143562), T. buetschlii (JN831809), and T. tubulosa (AB640683). Clade 2 includes sequences of T. ventricosoides (KU715776), T. brasiliensis (KU715768), T. urnula (JN831852). The Eutintinnus clade comprises E. perminutus (KT792926), E. stramentus (JX101859), E. medius (KY290320), E. pectinis (AY143570), and E. lususundae (MK03642). The Oligotrichida clade comprises sequences of Cyrtostrombidium longisomum (KJ609953), Novistrombidium apsheronicum (FJ876958), Spirotontonia turbinata (FJ422994), and Strombidium sulcatum (FJ377546). Asterisks (*) reflect disagreements in topology between the BI and ML trees; black circles reflect fully-supported nodes (100%ML/1.00BI). The scale bar corresponds to 0.02 expected substitutions per site.
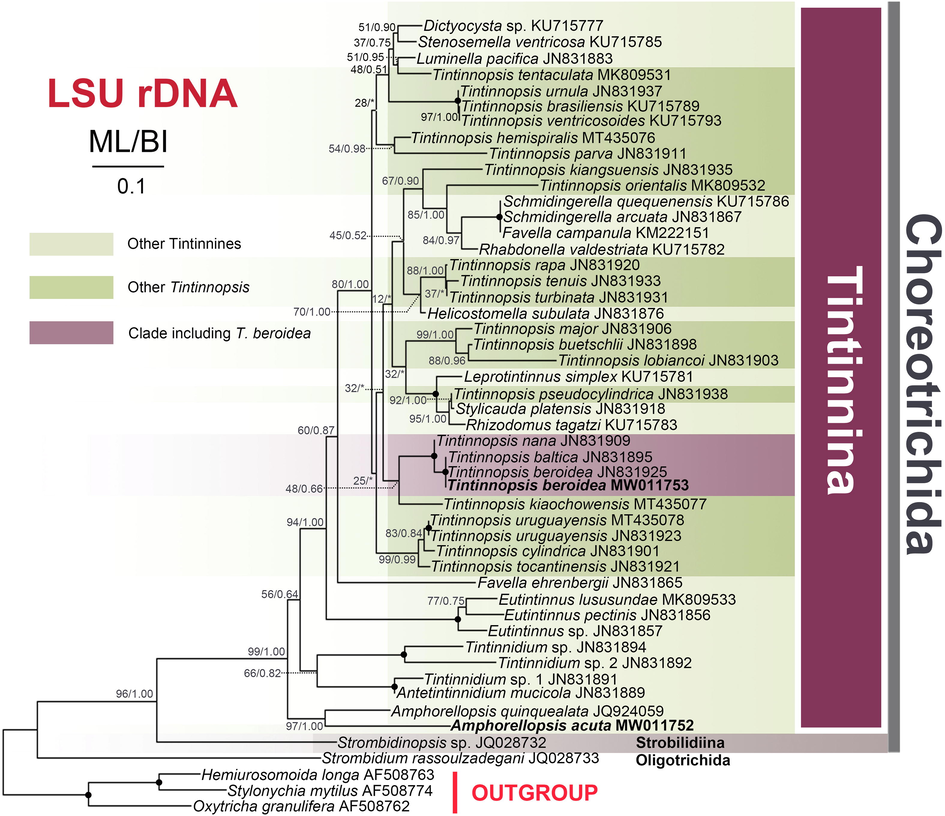
Maximum likelihood (ML) tree inferred from LSU rDNA sequences showing nodal support for ML and BI analyses. Newly sequenced species are shown in bold. Asterisks (*) reflect disagreements in topology between the BI and ML trees; black circles reflect fully-supported nodes (100%ML/1.00BI). The scale bar corresponds to 0.1 expected substitutions per site.
The SSU rDNA sequences of two Qingdao populations of Amphorellopsis acuta (including the newly sequenced population and FJ196071) have a 99.5% similarity and form a strongly supported group (99%ML/1.00BI) that is sister to the clade formed by Amphorides amphora (JX101849), A. minor (KY290324) and Steenstrupiella steenstrupii (EU399537). This assemblage groups with the Korean population of A. acuta (JX101847) and then clusters with Salpingacantha undata (KY290325) with maximal support (100%ML/1.00BI) (Table S3). Tintinnopsis beroidea forms a fully-supported clade with the American population of T. beroidea, T. baltica, T. fistularis, and T. sp. JG-2011a, all of which then clusters with T. nana (JN831821).
In our LSU rDNA tree, the newly sequenced Amphorellopsis acuta clusters with A. quinquealata (JQ924059) in a highly supported (97%ML/1.00BI) clade. Additionally, Antetintinnidium + Tintinnidium branch more basally than the Amphorellopsis + Amphorides + Steenstrupiella + Salpingacantha clade in the SSU rDNA tree but less basally in the LSU rDNA tree. Our T. beroidea sequences are 100% similar to six sequences of American T. beroidea population (JN831839 to JN831845 for SSU and JN831924 to JN831930 for LSU rDNA, although only one sequence was used in our SSU and LSU rDNA analysis, respectively) and T. baltica in both SSU and LSU rDNA.
4 Disscusion
4.1 Amphorellopsis acuta
4.1.1 Comparison with other populations
Amphorellopsis acuta is the type species of the genus and has been described many times (e.g., Kofoid and Campbell, 1929; Nie and Cheng, 1947; Schmidt, 1902). We identified the present population as A. acuta because it matches well with the original population in terms of its lorica length (77–126 μm vs. 91–98 μm), diameter of the lorica opening (31–44 μm vs. 31–32 μm) and invariably having three bladed fins (Schmidt, 1902). It is noteworthy that the lengths of the bladed fins are rather variable among different populations. In the drawings of specimens from the original and most of other population (e.g., Kofoid and Campbell, 1929; Nie and Cheng, 1947; Schmidt, 1902), the bladed fins only extend over the posterior half of the lorica, whereas in both the present population and that described in Xu et al. (2001), the fins extend up to the constricted lorica portion. Laval-Peuto and Brownlee (1986) and Small et al. (1985) provided simple diagrams of the ciliature of A. acuta but without giving any descriptions (Fig. 1E, F). Both show the oblique adoral zone of membranelles and undifferentiated somatic kineties in this species, which corresponds well with our specimens.
4.2 Congeneric comparison
Amphorellopsis comprises nine species. Amphorellopsis acuta can be distinguished from its congeners by having only three (vs. 4 or 5) bladed fins (Kofoid and Campbell, 1929).
The ciliary pattern of A. acuta is characterized by an oblique adoral zone of membranelles and uniform somatic kineties, which is more similar to aloricate choreotrichids and is considered to represent a precursor of the tintinnid pattern (Agatha and Strüder-Kypke, 2013). However, Antetintinnidium mucicola and freshwater Tintinnidium were discovered by possessing simpler ciliary pattern than other tintinnines but more specialized than Amphorellopsis acuta, i.e., kineties 1–3 are shortened and kinetids are densely arranged in the last three kineties in A. mucicola and possessing ventral organelles in freshwater Tintinnidium vs. all somatic kineties homogeneously arranged and equidistantly spaced in A. acuta (Foissner et al., 1999; Ganser and Agatha, 2019). Nevertheless, in the SSU rDNA tree, Antetintinnidium and freshwater Tintinnidium species branch more basally than Amphorellopsis (Fig. 4) whereas the reverse is true in the LSU rDNA tree (Fig. 5), albeit with low support values (45%ML/*BI and 56%ML/0.64BI, respectively). The topology of the LSU rDNA tree is more congruent with the morphological data, although given the level of undersampling, lack of infraciliature data and the low support, it is difficult to draw any firm conclusion. Nevertheless, we speculate that Amphorellopsis and Antetintinnidium/Tintinnidium may represent an intermediate group between the aloricate choreotrichids and representative tintinnines.
5 Tintinnopsis beroidea
5.1 Comparisons with other populations
Tintinnopsis beroidea, the type species of Tintinnopsis, was first reported by Stein (1867) without any measurements and illustrations. Entz (1884) described the lorica as having a cylindrical collar and an obconical bowl with a pointed posterior end, gave the size as 60–80 μm × 50–60 μm and provided several illustrations. In addition, Entz (1884) assigned T. beroidea to the genus Codonella, but this classification was not accepted by subsequent researchers (Jörgensen, 1912; Kofoid and Campbell, 1929) (Table S4). von Daday (1887) described a variety of T. beroidea, namely T. beroidea var. acuminata, based on a population with two macronuclear nodules, and concluded that there is only one macronucleus drawn in the illustrations of T. beroidea supplied by Entz (1884), and then his variety was raised to species level as T. acuminata by Kofoid and Campbell (1929) as its bowl is slender than T. beroidea. However, one of Entz’s illustrations shows an early divider with two macronuclear nodules but, obviously, its nuclear division has not been performed yet (Fig. 3E). Furthermore, it is now known that the diameter of the lorica opening is more systematically informative than the ratio of length:opening because the lorica length is variable according to the life history of certain populations of Tintinnopsis species (Agatha and Strüder-Kypke, 2013; Laval-Peuto, 1981). Hence, we consider that Daday (1887) misinterpreted the data and made a misidentification so that T. acuminata is invalid (Table S4).
In the last 70 years, numerous populations of Tintinnopsis beroidea have been discovered worldwide (e.g., Balech, 1959; Hada, 1932; Nie and Cheng, 1947). Our population is consistent with these in terms of lorica shape (i.e., cylindrical collar and obconical bowl) and size (total length 53–75 μm vs. 42–113 μm; opening diameter 29–44 μm vs. 26–56 μm) (Balech, 1959; Hada, 1932; Nie and Cheng, 1947). The sequenced specimens named Tintinnopsis sp.4 in Santoferrara et al. (2013) (i.e., T. acuminata in NCBI; JN831839 to JN831845 for SSU rDNA and JN831924 to JN831930 for LSU rDNA) with opening diameter of 31.8–47.5 μm should be classified as T. beroidea.
5.2 Congeneric comparison
In terms of its small lorica size, cylindrical collar, and obconical bowl, two congeners, namely T. baltica Brandt, 1896, and T. parvula Jörgensen, 1912, should be compared with the Qingdao population of T. beroidea. Tintinnopsis parvula can be distinguished from T. beroidea by having an inflated bowl (vs. widest portion of lorica is the opening) and a conspicuous (vs. inconspicuous) constriction between the collar and the bowl (Jörgensen, 1912). SSU rDNA similarities among T. beroidea (Qingdao population), T. bacillaria and T. parvula (Zhang et al., 2017) are less than 98%. Tintinnopsis baltica differs from T. beroidea by the presence (vs. absence) of a conspicuous narrowed portion between the collar and the bowl (Brandt, 1896), however both the SSU rDNA and LSU rDNA sequences of T. beroidea and T. baltica are identical (JN831840). This incongruence between the phenotype and genotype needs further investigation.
6 Conclusion
In the present paper, we redescribed two poorly known type species of tintinnines, namely Amphorellopsis acuta and Tintinnopsis beroidea. Their ciliary patterns and the rDNA sequences are reported for the first time thereby expanding knowledge and increasing understanding of the taxonomy and phylogeny of tintinnines. Moreover, the findings of A. acuta suggest that Amphorellopsis may represent an intermediate lineage between tintinnines and aloricate choreotrichids. Our data of T. beroidea contribute to the general understanding of the phylogeny and diversity and provide a benchmark for reclassification of the genus as more information of other species become available in the future.
7 Authors’ contributions
XH conceived and guided the study. YB and RW conducted sampling and performed laboratory work. XH and YB identified the species. YB performed the phylogenetic analyses and drafted the manuscript. YB, MM, KA and XH made further revisions. All authors read and approved this manuscript.
Acknowledgements
We thank Prof. Weibo Song, Ocean University of China for his long-term support. We are grateful to Dr. Alan Warren, Natural History Museum, UK, for his suggestions on this manuscript. Thanks are also due to Mrs. Yajuan Li, Ocean University of China for her advice on the molecular experiments.
Fundings
This work was supported by the National Natural Science Foundation of China (project number: 41776133 and 31672279) and Researchers Supporting Project (RSP-2020/10), King Saud University, Saudi Arabia for financial support, and the Fundamental Research Funds for the Central Universities.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Revisions to the classification, nomenclature, and diversity of eukaryotes. J. Eukaryot. Microbiol.. 2019;66:4-119.
- [Google Scholar]
- Redescription of Tintinnopsis cylindrica Daday, 1887 (Ciliophora: Spirotricha) and unification of tintinnid terminology. Acta Protozool.. 2006;45:137-151.
- [Google Scholar]
- Systematics and evolution of tintinnid ciliates. In: Dolan J.R., Montagnes D.J.S., Agatha S., Coats W.D., Stoecker D.K., eds. The Biology and Ecology of Tintinnid Ciliates: Models for Marine Plankton. Oxford: Wiley-Blackwell; 2013. p. :42-84.
- [Google Scholar]
- Redescriptions of three tintinnine ciliates (Ciliophora: Tintinnina) from coastal waters in China based on lorica features, cell morphology, and rDNA sequence data. Eur. J. Protistol.. 2020;72:125659.
- [Google Scholar]
- Redescription of five tintinnine ciliates (Alveolata: Ciliophora: Oligotrichea) from coastal waters of Qingdao, China. Mar. Life Sci. Technol.. 2020;2:209-221.
- [Google Scholar]
- Monographie der Familie der Tintinnodeen. Mitt. Zool. Stn. Neapel.. 1887;7:473-591.
- [Google Scholar]
- Morphology and ecology in tintinnid ciliates of the marine plankton: correlates of lorica dimensions. Acta Protozool.. 2010;49:235-244.
- [Google Scholar]
- Über Infusorien des Golfes von Neapel. Mitt. D. Zool. Station. Neap.. 1884;5:289-444.
- [Google Scholar]
- Identification and Ecology of Limnetic Plankton Ciliates. Bavarian State Office for Water Management; 1999.
- Redescription of Antetintinnidium mucicola (Claparède and Lachmann, 1858) nov. gen., nov. comb. (Alveolata, Ciliophora, Tintinnina) J. Eukaryot. Microbiol.. 2019;66:802-820.
- [Google Scholar]
- The Tintinnoinea from the Sea of Okhotsk and its neighborhood. J. Fac. Sci. Hokkaido. Imp. Univ. Zool.. 1932;2:37-59.
- [Google Scholar]
- Ciliate Atlas: Species Found in the South China Sea. Beijing: Science Press; 2019.
- Bericht über die von der schwedischen Hydrographisch-Biologischen Kommission in denschwedischen Gewässern in den Jahren 1909–1910 eingesammelten Planktonproben. Sven. Hydrogr. Biol. Komm. Skr.. 1912;4:1-20.
- [Google Scholar]
- A conspectus of the marine and fresh-water Ciliata belonging to the suborder Tintinnoinea, with descriptions of new species principally from the Agassiz Expedition to the Eastern Tropical Pacific 1904–1905. Univ. Calif. Publ. Zool.. 1929;34:1-403.
- [Google Scholar]
- Construction of the lorica in Ciliata Tintinnina. In vivo study of Favella ehrenbergii: variability of the phenotypes during the cycle, biology, statistics, biometry. Protistologica. 1981;17:249-272.
- [Google Scholar]
- Identification and systematics of the Tintinnina (Ciliophora): evaluation and suggestions for improvement. Ann. Inst. Oceanogr.. 1986;62:69-84. (with French summary)
- [Google Scholar]
- Tintinnoinea of the Hainan region. Contr. Biol. Lab. Sci. Soc. China.. 1947;16:41-86.
- [Google Scholar]
- Protargol synthesis: an in-house protocol. J. Eukaryot. Microbiol.. 2013;60:609-614.
- [Google Scholar]
- Utility of genetic markers and morphology for species discrimination within the order Tintinnida (Ciliophora, Spirotrichea) Protist.. 2013;164:24-36.
- [Google Scholar]
- Schmidt, J., 1902. Some tintinnodea from the Gulf of Siam. Videnskabellige Meddeleljer fra den Natur Historiske Forening i Kjobenhaun.
- Small, E.B., Lynn, D.H., Lee, J.J., Hutner, S.H., Bovee, E.C., 1985. Phylum Ciliophora Doflein, 1901. In: Lee, J.J., Hutner, S.H., Bovee, E.C., (Eds.), An illustrated guide to the Protozoa. Allen Press, Lawrence Kansas, pp. 375–393.
- Stein, F., 1867. Der Organismus der Infusionsthiere nach eigenen Forschungen in systematischer Reihenfolge bearbeitet (Vol. 2). Engelmann.
- Morphological redescriptions and neotypification of two poorly known tintinnine ciliates (Alveolata, Ciliophora, Tintinnina), with a phylogenetic investigation based on SSU rRNA gene sequences. Int. J. Sys. Evol. Microbiol.. 2020;70:2515-2530.
- [Google Scholar]
- Eine verbesserte Technik der Protargolimprägnation für Ciliaten. Mikrokosmos.. 1975;64:171-179. (in German)
- [Google Scholar]
- Species boundaries in tintinnid ciliates: a case study – morphometric variability, molecular characterization, and temporal distribution of Helicostomella species (Ciliophora, Tintinnina) J. Eukaryot. Microbiol.. 2012;59:351-358.
- [Google Scholar]
- Distribution and diversity of microbial eukaryotes in bathypelagic waters of the South China Sea. J. Eukaryot. Microbiol.. 2017;64:370-382.
- [Google Scholar]
- Studies on tintinnine ciliates in the Taiwan Strait (Ciliophora: Tintinnina) Acta. Zootaxonom. Sin.. 2001;26:454-466.
- [Google Scholar]
- Three rDNA loci-based phylogenies of tintinnid ciliates (Ciliophora, Spirotrichea, Choreotrichida) J. Eukaryot. Microbiol.. 2017;64:226-241.
- [Google Scholar]
- An Illustrated Guide to Contemporary Tintinnids in the World. Beijing: Science Press; 2012.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.10.006.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







