Translate this page into:
The synthesis of polysaccharide crude nanoparticles extracts from Taif rose petals and its effect on eggplant seedlings under drought and salt stress
⁎Corresponding author. nmalabdallah@iau.edu.sa (Nadiyah M. Alabdallah),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Natural biopolymer nanoparticles are an emerging area of research in green electronic devices and biomedical and agricultural fields. However, because drought and salt are two keys environmental aspects that affect agricultural crop yields around the world, a new strategy has been suggested to valorize the waste of Taif rose petals (Rosa damascena Miller var. trigintipetala) in the production of polysaccharide material on the nanoscale. In this study, we explore the effects of drought and salinity stress on the physiology of eggplants.
Methods
The key objectives of this research were to investigate the effect of polysaccharide crude nanoparticles extract (PCE) in the alleviation of drought and salinity stress in eggplants.
Results
Reduced chlorophyll content, chlorophyll fluorescence (Fv/Fm), and chlorophyll stability index (CSI) were seen in the plants that had been subjected to drought and salt stress. Under drought and salt stress, proline, total soluble protein, and sugar levels were all shown to be higher. However, polysaccharide crude nanoparticles extract reverses the negative effects of drought and salt stress and increases the chlorophyll content, chlorophyll fluorescence (Fv/Fm), and chlorophyll stability index (CSI) in the presence of drought and salt stress. When PCE was supplemented, the accumulation of lipid peroxidation was reduced, and the activities of antioxidant enzymes (SOD, CAT, POX, GR) were raised.
Conclusion
In summary, the results suggest that exogenous PCE can mitigate the detrimental effects of drought and salt stress in eggplants by modulating antioxidant systems. Furthermore, the findings of this study give experimental proof that PCE can be used to increase drought tolerance as well as production in eggplant plants.
Keywords
Taif rose petals
Polysaccharides
Antioxidant enzymes
Environmental factors
Lipid peroxidation
Total soluble sugar
1 Introduction
Natural polysaccharides are biomaterials and have received considerable interest due to their significant structural features, physical and chemical properties, and biological activities (Xia et al., 2020). These biomaterials have the promising potential to be applied for green electronic devices, biomedical applications and agriculture (Bouissil et al., 2020; Torres and De-la-Torre, 2021). Polysaccharides can be found in plants, algae and animals. Among these natural sources of polysaccharides, floral waste consumed in social occasions and events were not fully examined as value-added product (Dutta and Kumar, 2021). Studies on waste Rosa damascena petals reported they can serve as a source of polysaccharides material (Slavov et al., 2017). However, the functionality of extracted polysaccharide can be affected by their dimensionality, shape and purity during the optimal synthetic procedure, leading to an increase in the range of potential applications (Torres et al., 2019). As a sustainable strategy could be suggested and targeted the application of polysaccharides in the protection of crop yields could be essential and gaining a great deal of attention.
Eggplant (Solanum melongena L.) is a member of the Solanaceae family and one of the most common agricultural products, ranking third in terms of production after potato and tomato (Rotino et al., 2014). Eggplant fruits are known for their low-calorie content and it also contains carbohydrates, vitamins, and high protein content (Saeedifar et al., 2014). In addition, it possesses antioxidant, ant-proliferative, and anti-inflammatory properties (Gürbüz et al., 2018). However, in their full lifecycle, eggplants are extremely vulnerable to several abiotic stresses and biological stimuli that can restrict their growth. They are quite susceptible to water shortages, as they require a large volume of water for continued growth (Fu et al., 2013). When water shortages rose from 20% to 40% of the field capacity, eggplant fruit yield declined by up to 60% (Karam et al., 2011). Eggplant is also relatively susceptible to the effects of salinity (Abbas et al., 2010). The optimal electrical conductivity (EC) for eggplant growing is between 1.1 and 1.5 dS m−1 (Ünlükara, 2010). Because it is such a valuable plant, understanding its sensitivity and tolerance to increased salinity and drought stress is crucial in order to build effective crop production techniques. Since eggplant is one of the most major crops in the Gulf, and changes in climate will increase the intensity and frequency of coupled pressures (drought and salt), addressing the problem of climate-resilient crops is essential.
Drought is the key environmental variables that that has a detrimental impact on crop yields around the world (Hasan et al., 2020; Alabdallah et al., 2021). Furthermore, rapid evaporation causes water loss, which exacerbates salt accumulation. Non-optimal irrigation practices can also cause soil salinity, resulting in agricultural production loss (Torres et al., 2019). Both stress and anxiety generate physiological and biochemical changes, such as the creation of reactive oxygen species (ROS) as a result of metabolic processes (Alabdallah and Hasan, 2021). The production of suitable solutes, antioxidant components, and the induction of secondary protection against oxidative stress by crops are all crucial to mitigate cellular damage caused by ROS accumulation in the environment. It has been demonstrated that drought and salinity stress cause a variety of interacting processes, such as the suppression of enzyme activity in metabolic pathways. Plant responses to environmental stressors such as drought and salinity, on the other hand, have received little attention. When exposed to abiotic stress, many plant species have been reported to accumulate suitable solutes such as proline (Hasan, 2018; Krasensky and Jonak, 2012). They have been shown to have an important function in crop osmoregulation (Hasan et al., 2021; Hasan et al., 2021).
It has been demonstrated in previous studies that exogenous application of many plant hormones can improve the abiotic stress tolerance of plants (Hasan et al., 2021; Jahan et al., 2021; Hasan, 2021). However, exogenous application of crude polysaccharides nanoparticles extracts from Taif rose petals (Rosa damascena Miller var. trigintipetala) (has never been reported) was not investigated. A polysaccharide is a carbohydrate macromolecule consisting of monosaccharide units that are covalently attached together by glycoside bonds, whether in a linear or branched setup. They are essential for the survival and activity of all organisms, and play an important role in their metabolic processes (Barbosa and de Carvalho Junior, 2021). The method used to extract polysaccharides from plants has a considerable impact on not only the extraction rate, but also the characteristics and bioactivities of the polysaccharides that have been extracted, all of which have an impact on the applications of the polysaccharides (Zhu et al., 2016). Despite the fact that numerous biochemical and physiological changes have been shown to be involved in the adaptation process of several crops to environmental challenges, however little understood about the sensitivities of eggplants to ROS production, which leads to oxidative stress. In the current research, we address the effects on plant development, antioxidant defense mechanisms, oxidative damage, and physiological and biochemical changes that occur as a result of drought and salinity stress in order to better understand the plant's adaptation to these stresses.
2 Materials and methods
2.1 Extraction of crude polysaccharides nanoparticles
Taif roseus were purchased from Al Qurashi Rose Factory, Al Hada, Taif, Saudi Arabia. Taif roseus polysaccharides nanoparticles were extracted. A 5 g of plant components were extracted in a 30% (w/v) potassium hydroxide solution at 90 °C for 120 min. The plant parts homogenate was combined with 100 ml of a 30% (w/v) potassium hydroxide (KOH) solution, which was boiled in water at 100 °C for 1 h before being chilled and centrifuged. The polysaccharide concentration of the upper solution was tested at neutral pH. The residue was sifted using Whatman filter paper. The filtrate was condensed using a rotary extractor at 60 °C and reduced pressure. The concentration was tripled by adding three times the amount of 98% ethanol. After that, the solution was left for 7 days to allow the polysaccharides to precipitate. The size of PCE crude nanoparticles was determined using TEM (2.33 to 4.36) and $Fourier-transform infrared (FTIR) $spectroscopy were used to confirm the isolation of polysaccharides from a particular plant. The FTIR samples were acquired in the band of 5000–400 cm−1 utilizing Fourier transmission infrared spectrometer (Perkin Elmer).
2.2 Separation and quantification
An Agilent liquid chromatographic system (USA) with the column SB-C18 and G 4226A software was used to separate and quantify gallic acid, quercetin, and tannic acid in plant materials (1.8 m, 4.6 150 mm). Finally, Taif rose polysaccharide extracts (0, 15, 35, and 65 mM) were obtained.
2.3 Chromatographic analysis of glucose and fructose
To identify glucose in the sample, 0.6% aqueous acetic acid solution (A) and methanol (B) (10: 90) (V/V) were used as the mobile phase. The samples were eluted using the gradient given below, with a flow rate of 0.700 ml/min and an injection volume of 1 l. The temperature in the column was kept constant at 25 °C. A chromatogram was acquired at a wavelength of 274 nm based on the absorption maxima of the tested materials (Fig. 2). The presence of glucose in the sample was determined by measuring its retention time and spiking it with glucose as a reference under the same conditions.
Acetonitrile solution (A) and methanol (B) were utilized as the mobile phase for identifying fructose sugar in the sample (70:30). The following gradient was used to elute the samples: The injection volume was 10 l and the flow rate was 1000 ml/min. The column temperature was kept constant at 25 °C. According to the absorption maxima of the examined samples, a chromatogram was acquired at a wavelength of 274 nm (Fig. 7). Retention time measurements and D-fructose spikes were used to assess the presence of fructose in the sample.
2.4 Plant growth, treatments, and experimental design
The two pot trials were carried out at Imam Abdulrahman Bin Faisal University and Umm Al-Qura University in Saudi Arabia, in their laboratories and green houses. Eggplant seeds were purchased in Altuajri, Saudi Arabia, and sterilized in 0.35% sodium hypochlorite for ten minutes before being properly cleaned with distilled water. To germinate the seeds, three seeds were placed in each of the 20 cm plastic pots, which contained a one-to-one volume ratio of potting soil and compost. To determine each pot's field capacity, FC (100 %) had to soak the soil with distilled water. Before being reused, all of the pots were covered with plastic sheets and allowed to drain for 48 h. After 48 h of draining and 24 h of drying at 105⁰C, the pot weights were recalculated. Field capacity was determined by comparing soil weight before draining and after drying to determine the soil moisture content. Drought stress was induced (50% FC, 35% FC) by allowing plants to dry out over a 15-day period by withholding water. Field capacity (FC) was maintained by weighing the pot. In order to impose salinity stress, NaCl at final concentrations of 0, 20, 50, 80, and 120 mM (at intervals of 3 days between each treatment) was supplied for a total of 15 days. Plants were maintained on a 25/19 °C day/night temperature cycle with a 16/8h light/dark cycle. At least three plants from each treatment were employed in each of the three biological replicates to assess growth and physiological parameters. The plants were harvested at 35 days.
2.5 Chlorophyll content determination
Chlorophyll content was extracted based on Aron method (Arnon, 1949). A minimum of 0.25 g of leaf samples were taken and grounded with 5 ml of acetone at room temperature, centrifugation was performed for 10 min at 3000 rpm at 400 °C. The supernatant absorbs at 663 and 645 nm was used to measure the amounts of chlorophyll a and b.
2.6 Chlorophyll fluorescence (Fv/Fm) and chlorophyll stability index measurements (CSI)
At midday, chlorophyll fluorescence (Fv/Fm) in eggplant seedlings was measured using a chlorophyll fluorometer (OS-30p, Opti-Science, Inc., Hudson, USA). The chlorophyll stability index (CSI) was calculated using the Murphy method (1962).
The CSI was calculated using the following formula:
2.7 Lipid peroxidation
Using the thiobarbituric acid (TBA) method devised by Heath and Packer (Heath and Packer, 1968), it was determined that the products of lipid peroxidation (which were indicative of MDA concentration) were present. The findings for these assays were obtained by combining 0.5% TBA with 1 ml of trichloroacetic acid (TCA) extract (prepared in 20% TCA). The mixture was incubated for 30 min at 95 °C before being chilled in ice for 10 min. To deliver the best results result, the specific absorbance of the objects was measured at 532 nm, and the nonspecific background absorbance at 600 nm was subtracted from the values. The MDA content was calculated using the molar extinction coefficient of 155 mM−1 cm−1.
2.8 Proline content
Bates et al. (1973) used a modified technique to determine the amount of proline in the sample. It was necessary to homogenize fresh leaf samples (0.1 g, for example) from each treatment in the mortar and pestle in order to obtain the homogenous solution. An equal amount of each homogenate was mixed with an equal amount of glacial acetic acid, which was then mixed with an equal amount of ninhydrin. Each homogenate was present in roughly 0.2 ml of the final mixture. For 30 min, the reaction mixture was heated to a boil in a 1000⁰C water bath before being quickly cooled in an ice bath. Toluene (400 µl) was added to the reaction mixture after the mixture had cooled. Chromophore-containing toluene was fully mixed before a spectrophotometer equipped with a UV–visible detector (Chemito Spectrascan, UV 2600) was used to measure the absorbance of red color created against a toluene blank.
2.9 Total soluble sugar (TSS)
Cock et al. (1976) discovered a method for measuring the amount of soluble sugars available in eggplant leaves. The total soluble sugar analysis was carried out using 0.5 ml of soluble sugar extract and 4.5 ml of 85% ethanol in a test tube. Slowly add 10 ml of anthrone reagent to the sample tubes in a cold-water bath until they are completely filled. The tubes were immersed in a boiling water bath for 7.5 min before being swiftly cooled in an ice water bath. The absorbance at 630 nm was measured one hour after cooling.
2.10 Total soluble protein (TSP)
500 mg homogenized mixture was centrifuged for 25 min at 16000 × g and 4 °C in 5 ml potassium phosphate buffer (pH 7.0, 10 mM, 4 percent polyvinylpyrrolidone, PVP). The absorbance at 595 nm was measured after adding 980 ml of Bradford solution to the supernatant (20 ml). Finally, to calculate the TSP content, a standard curve was constructed using various amounts of bovine serum albumin (Bradford, 1976).
2.11 Antioxidant enzyme activity
The extraction of antioxidant enzymes was proceeded as per the previous studies (Mukherjee and Choudhuri, 1983). The leaves were extracted with 10 ml of phosphate buffer and 0.5 g of freshly chosen material (pH 7). Afterwards, the homogenate was centrifuged for 10 min at 15,000g at 40 °C. Antioxidant enzyme activity was measured by heating the supernatant to 200 °C.
2.11.1 Superoxide dismutase (SOD) activity determination
SOD activity was measured using the nitro-blue-tetrazolium (NBT) reduction method (Hasanuzzaman et al., 2011). The reaction mixture contains roughly 3 ml of 50 ml enzyme extract, 150 ml riboflavin (13 mM), 2.5 ml methionine (13 mM), 250 ml NBT (63 mM), and 50 ml phosphate buffer (50 mM, pH 7.8). The absorbance at 560 nm was measured using a spectrophotometer.
2.11.2 Catalase (CAT) activity determination
The Aebi (Aebi, 1984) protocol was used to measure CAT activity. In a 40 ml container, the enzyme extract was combined with 0.016 ml H2O2 (30%) and a 10 mM phosphate buffer solution (pH 7). Finally, using a spectrophotometer, the absorbance at 240 nm was determined (LKB-Biochrom 4050).
2.11.3 Peroxidase (POX) activity determination
Hammerschmidt et al. (1982) established methods for measuring the activity of the peroxidase (POX) enzyme. In the solution, there was 0.25% (v/v) guaiacol and 100 mM H2O2 in a buffer solution of 100 mM sodium phosphate (pH 6.0). For three minutes, 100 L of crude enzyme extract were combined, and every 30 s, the absorbance at 470 nm was measured. g−1 FW was used to quantify POX activity.
2.11.4 Measuring glutathione reductase (GR) activity
$Glutathione reductase (GR) activity was assessed using the Foyer and Halliwell procedures in a mixture of sodium phosphate buffer (pH 7.8), NAD phosphate (0.01 mM), and 0.1 mM glutathione reductase in an assay mixture (Foyer and Halliwell, 1976).
2.12 Statistical evaluation
The data were analyzed using analysis of variance (ANOVA) in the MINITAB 17 statistical tool, with the treatment mean ± standard error (n = 3) provided (Hasan et al., 2021). At the p < 0.05 level, the LSD test indicates that bars with dissimilar letters are statistically significantly different.
3 Results
PCE nanoparticle was derived from the Taif rose. TEM analysis was employed to explore the structure and distribution of polysaccharide nanoparticles. Fig. 1 shows the TEM image and we clearly observe the spherical shape of polysaccharide nanoparticle with a size on the nanoscale. The FTIR spectra of a basic polysaccharide are shown in Fig. 2. Furthermore, we have investigated the chromatographic technique to analyze the glucose and fructose (Figs. 3-4).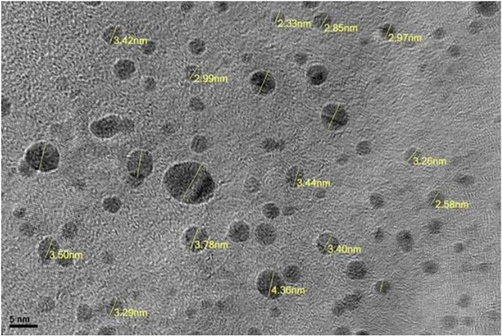
Images of polysaccharide crude extract derived from Taif rose.
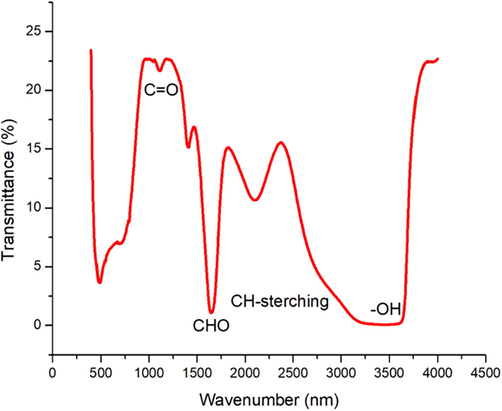
FTIR spectra identify a basic polysaccharide backbone with an intense peak: (3449 cm−1). The broad peak signifies the stretching vibrations of the OH group (Dutta and Kumar, 2021). (1651 cm−1) represent the carbonyl group of a carboxylic acid group. The region Blow 800 cm−l is known as the “skeletal region) and it is related to the carbohydrate skeletal vibrations.
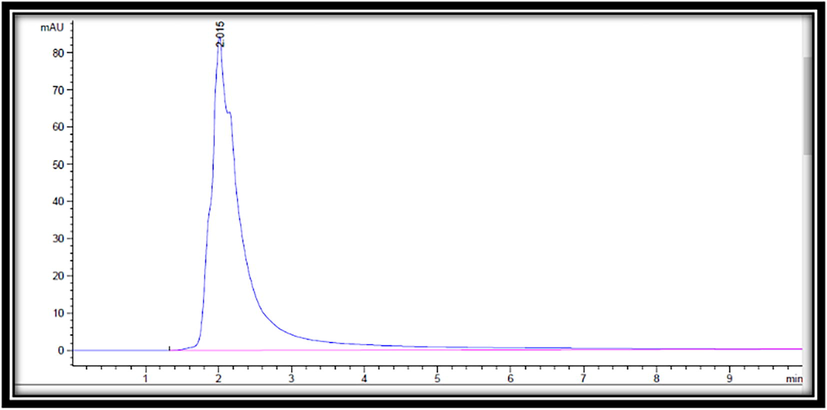
HPLC chromatograms of glucose.
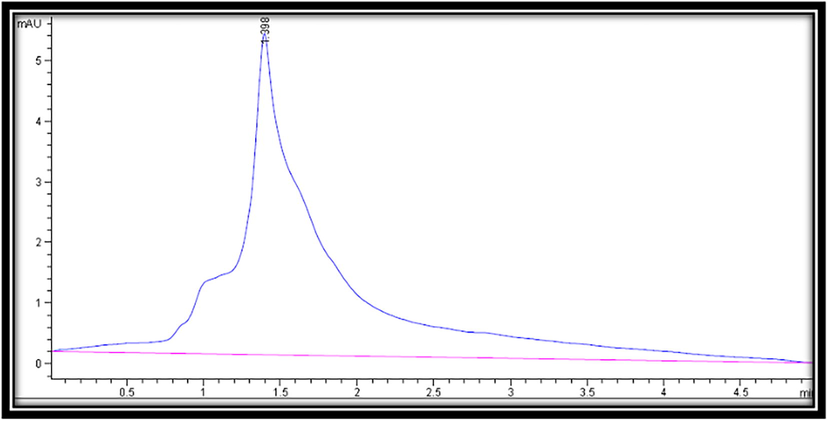
HPLC chromatograms of fructose.
We examined at a variety of physiological and biochemical parameters to see if PCE had any favorable impacts on eggplant seedlings. When eggplants were exposed to drought stress (50% and 35% FC) and varied salinity concentrations (20, 50, 80, and 120 mM), the levels of Chla, Chlb, carotenoids, total chl, and chlorophyll fluorescence (Fv/Fm) were drastically reduced (p < 0.001; Fig. 5). However, when eggplants were subjected to drought and salinity stress, PCE treatment increased these photosynthetic metrics dramatically.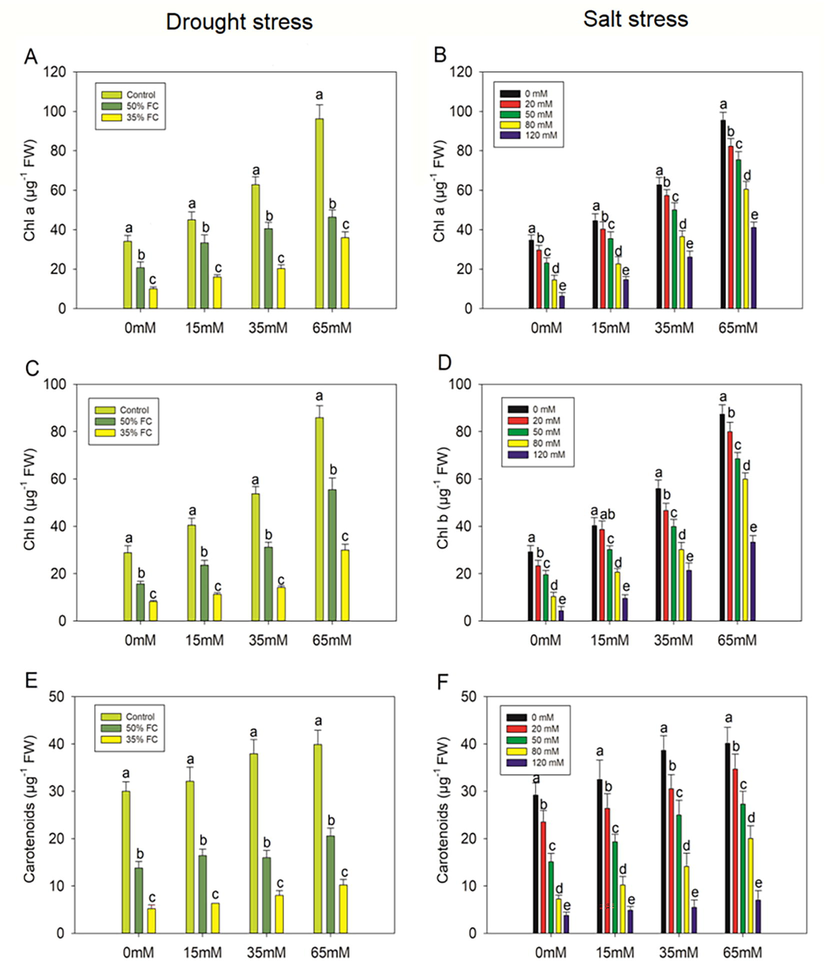
Effect of polysaccharide crude extract, PCE (0, 15, 35. and 65 Mm) on Chla, Chlb and carotenoids in the leaf of eggplant seedlings under drought and salt stress. The data displayed are the means (±SE) of three replicates, and bars of dissimilar letters differ significantly at the p = 0.05 level.
Drought treatments (50% and 35% FC) resulted in substantial (p < 0.001) decreases in CSI in eggplant seedlings of 42% and 71%, respectively, as compared to control seedlings. Externally administered PCE (15, 35, and 65 mM) significantly increased CSI by 36%, 49%, 64%, and 33%, 47%, and 72%, respectively, as compared to drought stressed (50% and 35% FC) drought plants alone (Fig. 3). In contrast, salt treatments of 20, 50, 80, and 120 mM reduced CSI in eggplants by 17%, 33%, 61%, and 84%, respectively, as compared to control seedlings that did not receive PCE (Fig. 3). Despite this, the exogenous infusion of PCE considerably (p < 0.001) boosted the CSI.
Drought stress (50% and 35% FC) significantly (p < 0.001) elevated MDA in eggplant seedlings by 13% and 46%, respectively, as compared to controls. Nonetheless, pretreatment with PCE (15%, 35%, and 65%) reduced MDA at 50% FC by 14%, 48%, and 70%, and at 35% FC by 13%, 41%, and 60%, respectively, compared to drought stressed drought plants alone (Fig. 6). The salt treatments (20, 50, 80, and 120 mM) raised MDA content in eggplant seedlings by 42%, 55 %, 58%, and 63%, respectively.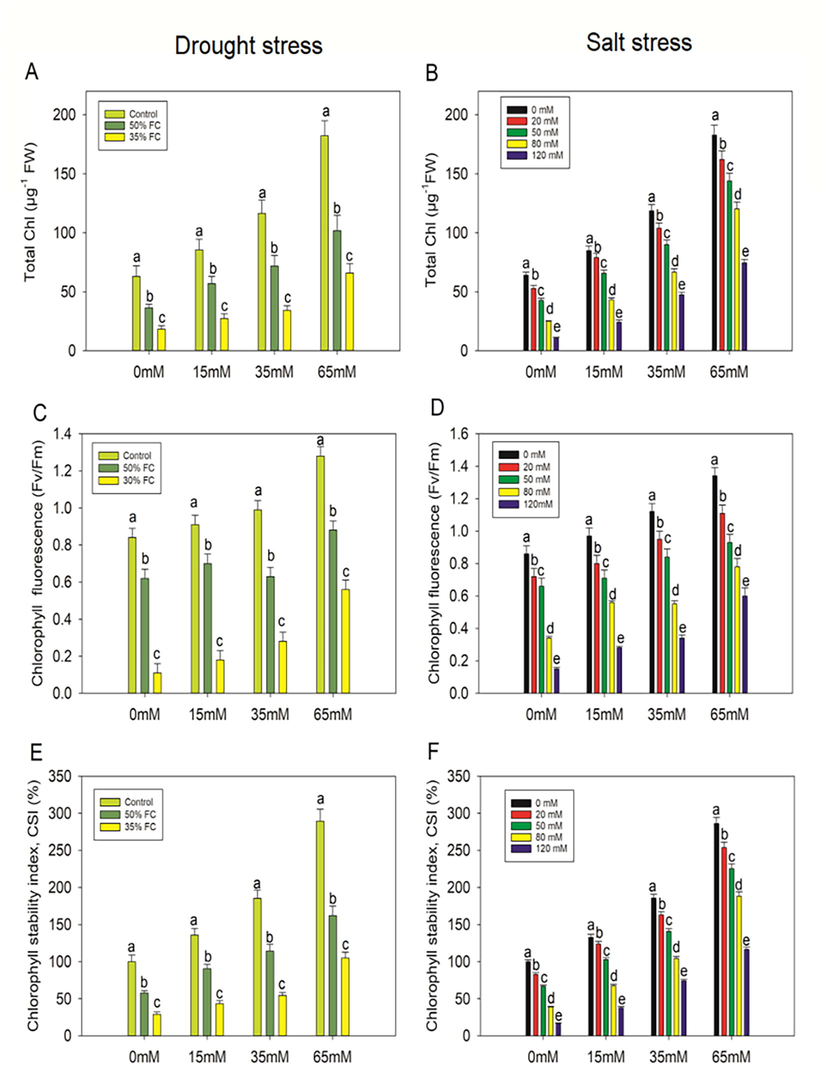
Effect of polysaccharide crude extract, PCE (0, 15, 35, and 65 Mm) on total chl, chlorophyll fluorescence (Fv/Fm), and chlorophyll stability index (CSI) in the leaf of eggplant seedlings under drought and salt stress. The data displayed are the means (±SE) of three replicates, and bars of dissimilar letters differ significantly at the p = 0.05 level.
However, the exogenous application of PCE decreased MDA content significantly (p ≤ 0.001), in comparison with those in the salt-stressed eggplant (Fig. 7).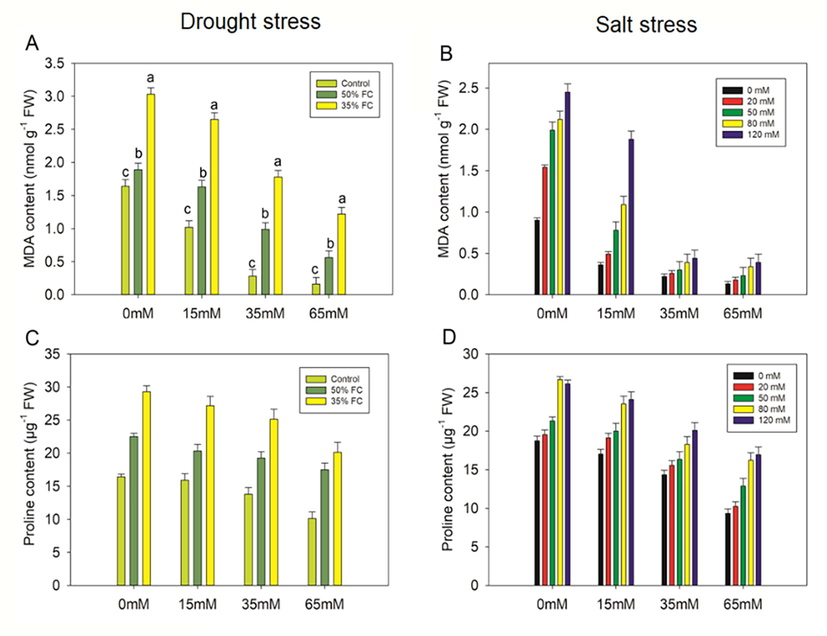
Effect of polysaccharide crude extract, PCE (0, 15, 35, and 65 Mm) on MDA and praline content in the leaf of eggplant seedlings under drought and salt stress. The data displayed are the means (±SE) of three replicates and bars of dissimilar letters differ significantly at thep = 0.05 level.
Drought treatments (50% and 35% FC) significantly (p < 0.001) raised proline content by 44% and 27%, respectively, and TSP by 37% and 26% which in turn strikingly increased the TSS by 23% and 23% relative to the control (Figs. 6-7). However, pretreatment of PCE (15, 35, 65 mM) decreased proline content by 10%, 15%, 22% and 7%,14%, 31%, TSP by 14%, 48%, 70% and 13%,41%, 60%, TSS by 16%, 27%, 43% and 4%,11%, 14%, when eggplant seedlings exposed to 50% and 35% FC. In response to salt stress (20, 50, 80, 120 mM), the content of proline, TSP, and TSS increased by 4%, 12%, 30%, 28%, 42%, 55%, 58%, 63%, and 4%, 39%, 49%, 51%, respectively. However, the external supply of PCE considerably (p < 0.001) reduced proline, TSP, and TSS content (Figs. 7-8).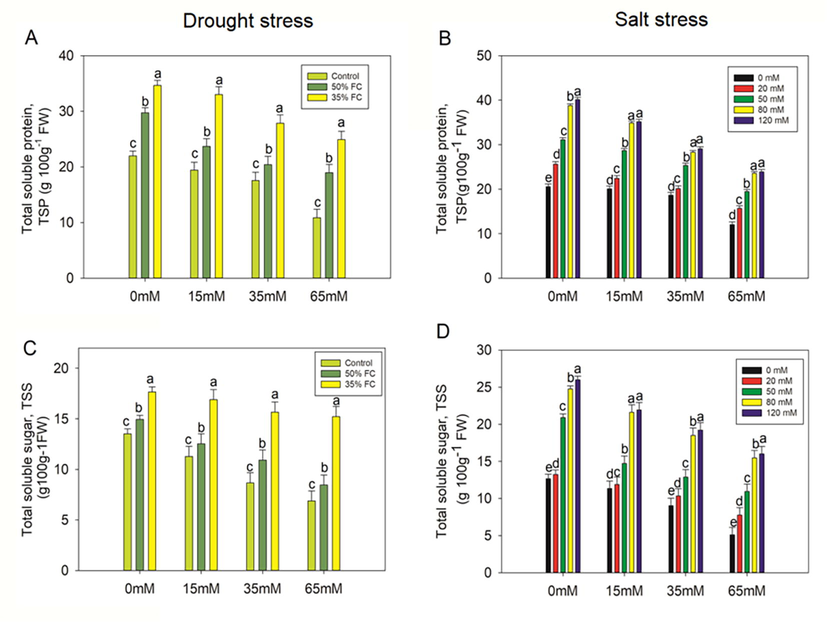
Effect of polysaccharide crude extract, PCE (0, 15, 35, and 65 Mm) on total soluble protein (TSP) and total soluble sugar (TSS) in the leaf of eggplant seedlings under drought and salt stress. The data displayed are the means (±SE) of three replicates, and bars of dissimilar letters differ significantly at the p = 0.05 level.
Drought treatments (50% and 35% FC) resulted in significant (p < 0.001) increases in SOD activity of 52% and 56%, CAT activity of 29% and 47%, and POX activity of 43% and 55%, respectively, which increased GR activity by 30% and 40% relative to the control (Figs. 9-10). However, PCE (15, 35, 65 mM) increased SOD activity by 7%, 11%, 15%, and 17%, 20%, 25%, CAT activity by 15%, 32%, 42% and 12%, 22%, 36%, POX activity by 11%, 29%, 44% and 8%, 25%, 44%, whereas GR activity increased by 4%, 25%, 28% and 4%, 22%,31%, respectively, in comparison to that observed in the drought-stressed (50% and 35% FC) plants. SOD; CAT; POX; and GR activities were increased in salt-stressed (20, 50, 80, 120 mM) eggplants by 15%, 42%, 47%, 48%; 4%, 27%, 32%, 39%; 8%, 19%, 31%, 38%; and 8%, 11%, 21%, 25%, respectively, compared to untreated plants. However, when eggplants were exposed to PCE treatments, their SOD, CAT, POX, and GR activities increased when compared to salt-stressed (20, 50, 80, and 120 mM) seedlings (Figs. 9-10).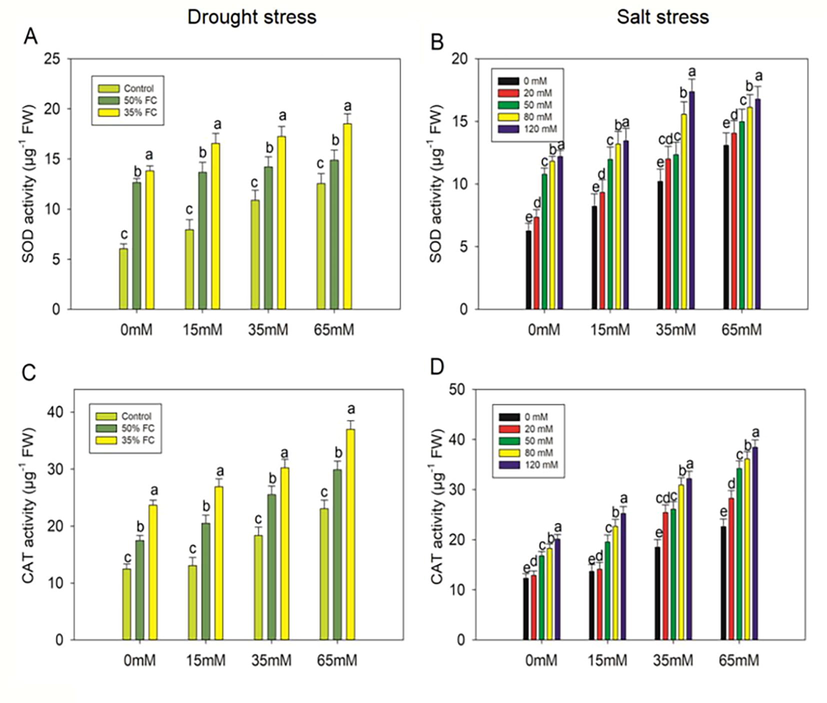
Effect of polysaccharide crude extract, PCE (0, 15, 35 and 65 Mm) on SOD and CAT activity in the leaf of eggplant seedlings under drought and salt stress. The data displayed are the means (±SE) of three replicates, and bars of dissimilar letters differ significantly at the p = 0.05 level.
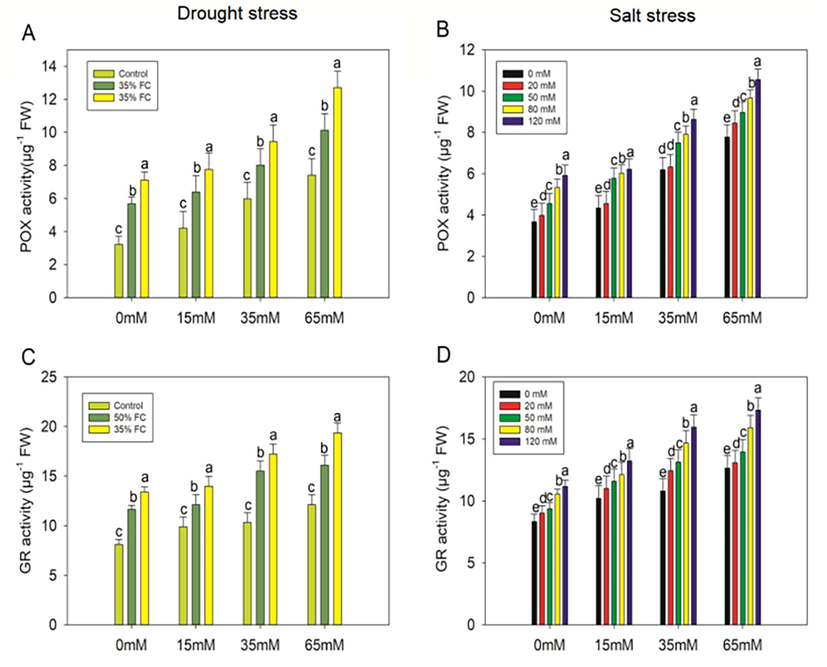
Effect ofpolysaccharide crude extract, PCE (0, 15, 35, and 65 Mm) on PDX and GR activity in the leaf of eggplant seedlings under drought and salt stress. The data displayed are the means (±SE) of three replicates, and bars of dissimilar letters differ significantly at the p = 0.05 level.
4 Discussion
This study examined the impact of drought and salinity stress on the physiology of eggplant. Chlorophyll is a vital component that allows plants to undergo photosynthetic activity. Photosynthesis-related features and chlorophyll pigments, which are the major factors of plant growth, often influence plant growth performance. In this study, we discovered that when eggplant leaves were subjected to dryness and salt, they lost chlorophyll. Drought and salt stress increase the generation of reactive oxygen species (ROS), which causes lipid peroxidation and, as a result, a decrease in plant chlorophyll content (Foyer et al., 1994). A decrease in chlorophyll content due to saltwater stress is common in drought and salt-sensitive plant species. This is due to dehydration and salt toxicity, which frequently results in burning of leaves or other fleshy parts, as well as the destruction of other pigments. PCE derived from the Taif rose, on the other hand, was found to be effective in the restoration of chlorophyll in eggplants. PCE may aid in the recovery of photosynthetic regulation by enhancing metabolites for eggplant growth, which in turn aids in the regeneration of chlorophyll. Furthermore, drought and salt stress reduced the chlorophyll stability index (CSI) as well as the maximal quantum yield of PSII (Fv/Fm) in the eggplant.
Chlorophyll fluorescence (Fv/Fm) is related to photosynthetic efficiency, and when plants are stressed, their photosynthetic efficiency decreases. As a result, chlorophyll fluorescence has become a key parameter for assessing plant chlorophyll content, classifying plants, and identifying abiotic stress in plants. Chlorophyll content, on the other hand, is directly related to chlorophyll fluorescence (Jahan et al., 2021; Urban et al., 2017).
Drought and salt stress cause a decrease in Fv/Fm value, indicating photoinhibition, indicating that the light-harvesting complex of PSII in stressed eggplant is disrupted. According to the outcomes of our study, PCE aids in the alleviation of photoinhibition in eggplants by increasing the Fv/Fm value. It is possible that the aforementioned traits are to blame for the significant increases in total soluble sugars and protein levels found in eggplants when treated to drought and salt stress. Proline buildup in stressed plants has been demonstrated to be an important defense response for maintaining osmotic pressure within the cell. Proline accumulation potential under stress conditions has been linked to stress tolerance in a range of plant species. More importantly, applying PCE to eggplants grown in drought and salt conditions has been shown to increase their tolerance to drought and salinity stress through biosynthetic modulation of osmolytes. According to the previous findings, the beneficial effect of osmolytes on stressed eggplants can be associated to the involvement of osmotic adjustment in membrane integrity and the prevention of physiological dehydration in plant cells. Under a wide range of stress conditions, a similar pattern in the accumulation of osmolytes was observed (Vighi et al., 2017). Despite the fact that proline concentration increased under both drought and salinity stress conditions, it was unable to offset the oxidative damage caused by the increase in H2O2 and the subsequent increase in lipid peroxidation. It has already been established that lipid peroxidation is connected with cellular injury induced by a variety of environmental stressors. In this research, we found that stress treatments significantly enhance lipid peroxidation, and that this increase is closely linked to the generation of ROS, which promotes membrane instability. Nevertheless, MDA content decreased by exogenous application of PCE, which may be accomplished via limiting ROS generation.
SOD, CAT, POX, and GR antioxidant enzymes were all more effective in eggplants that had been treated to drought and salt stress. When there is a lot of drought and salt stress, these enzymes become more active, which helps them resist the detrimental effects of stress by getting rid of reactive oxygen species (Hasan, 2021). Our findings are comparable with those of other researchers who have studied a wide range of plant species under a variety of environmental stresses (El-Banna and Abdelaal, 2018; Abdulmajeed et al., 2021). This study found that exogenous PCE modifies antioxidant enzyme activity and keeps reactive oxygen species levels tolerable, protecting eggplants from oxidative damage in a stress environment. The crucial involvement of PCE in increasing the activities of SOD, CAT, POX, and GR enzymes could be ascribed to PCE's role in attempting to control oxidative stress by reducing ion toxicity and the development of nucleoproteins that play a part in plant stress tolerance parameters. Taking into account all of the findings of this research, we infer that increasing drought and salinity stress treatments severely inhibited eggplant development by modifying multiple biochemical reactions in a differentiated manner. We found a clear relationship between stress-induced oxidative damage (caused by high MDA levels) and the accumulation of osmoprotectants, with stress resistance being regulated by the ability to effectively scavenge ROS from the environment.
5 Conclusion
The data gained from the study, we suggest that PCE obtained from Taif rose petals was advantageous and plays a crucial role in mitigating the harmful impacts of drought and salt stress on eggplant physiological and biochemical features. It triggered the stimulation of antioxidative metabolism mechanisms in eggplants, resulting in increased drought and salt tolerance. All this data brings a clear piece of knowledge on the physiological and biochemical pathways that underpin the response of eggplants to drought and salinity. Finally, we can conclude that using PCE to alleviate drought and salinity stress while also increasing water usage efficiency and crop yield in dry environments where drought-tolerant eggplant is grown.
Conflict of interest
None.
References
- Alleviation of saltinduced adverse effects in eggplant (Solanum melongena L.) by foliar-applied natural and synthetic glycinebetaine. Sci. Hort. 2010;125:188-195.
- [Google Scholar]
- Alleviation of copper phytotoxicity by acetylsalicylic acid and nitric oxide application in mung bean involves the up-regulation of antioxidants, osmolytes and glyoxalase system. J. Plant Interact.. 2021;16(1):201-212.
- [Google Scholar]
- Green synthesized metal oxide nanoparticles mediate growth regulation and physiology of crop plants under drought stress. Plants. 2021;10(8):1730.
- [Google Scholar]
- Plant-based green synthesis of silver nanoparticles and its effective role in abiotic stress tolerance in crop plants. Saudi J. Biol. Sci.. 2021;28(10):5631-5639.
- [Google Scholar]
- Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol.. 1949;24(1):1-15.
- [Google Scholar]
- Polysaccharides obtained from natural edible sources and their role in modulating the immune system: Biologically active potential that can be exploited against COVID-19. Trends Food Sci. Technol.. 2021;108:223-235.
- [Google Scholar]
- Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205-207.
- [Google Scholar]
- Use of alginate extracted from Moroccan brown algae to stimulate natural defense in date palm roots. Molecules. 2020;25(3):720.
- [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72(1–2):248-254.
- [Google Scholar]
- Cock, J., S. Yoshida, and D.A. Forno, Laboratory manual for physiological studies of rice. 1976: Int. Rice Res. Inst.
- Potential of value-added chemicals extracted from floral waste: A review. J. Cleaner Prod.. 2021;294:126280
- [Google Scholar]
- Response of strawberry plants grown in the hydroponic system to pretreatment with H2O2 before exposure to salinity stress. J. Plant Prod.. 2018;9(12):989-1001.
- [Google Scholar]
- The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133(1):21-25.
- [Google Scholar]
- Leaf morphological and ultrastructural performance of eggplant (Solanum melongena L.) in response to water stress. Photosynthetica. 2013;51(1):109-114.
- [Google Scholar]
- Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol.. 1982;20(1):73-82.
- [Google Scholar]
- Effects of magnetized water on phenolic compounds, lipid peroxidation and antioxidant activity of moringa species under drought stress. JAPS: J. Anim. Plant Sci.. 2018;28(3)
- [Google Scholar]
- ABA-induced stomatal movements in vascular plants during dehydration and rehydration. Environ. Exp. Bot.. 2021;186:104436
- [Google Scholar]
- Insights into 28-homobrassinolide (HBR)-mediated redox homeostasis, AsA–GSH cycle, and methylglyoxal detoxification in soybean under drought-induced oxidative stress. J. Plant Interact.. 2020;15(1):371-385.
- [Google Scholar]
- Spermine-mediated tolerance to selenium toxicity in wheat (Triticum aestivum L.) depends on endogenous nitric oxide synthesis. Antioxidants. 2021;10(11):1835.
- [Google Scholar]
- Spermine: its emerging role in regulating drought stress responses in plants. Cells. 2021;10(2):261.
- [Google Scholar]
- GABA: A key player in drought stress resistance in plants. Int. J. Mol. Sci.. 2021;22(18):10136.
- [Google Scholar]
- Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep.. 2011;5(4):353-365.
- [Google Scholar]
- Photooxidation in isolated chloroplasts. Arch. Biochem .Biophys.. 1968;125:189-198.
- [Google Scholar]
- Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem.. 2021;167:309-320.
- [Google Scholar]
- Yield and water use of eggplants (Solanum melongena L.) under full and deficit irrigation regimes. Agric. Water Manag.. 2011;98(8):1307-1316.
- [Google Scholar]
- Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot.. 2012;63(4):1593-1608.
- [Google Scholar]
- Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant.. 1983;58(2):166-170.
- [Google Scholar]
- Rotino, G., T. Sala, and L. Toppino, Alien gene transfer in crop plants. 2014, Springer Berlin, Germany.
- Nitrate and Heavy Metal Contents in Eggplant (Solanum melongena) cultivated in the farmlands in the south of Tehran-Iran. Int. J. Farming Allied Sci.. 2014;3(1):60-65.
- [Google Scholar]
- Combined recovery of polysaccharides and polyphenols from Rosa damascena wastes. Ind. Crops Prod.. 2017;100:85-94.
- [Google Scholar]
- Polysaccharide-based triboelectric nanogenerators: a review. Carbohydr. Polym.. 2021;251:117055
- [Google Scholar]
- Natural polysaccharide nanomaterials: an overview of their immunological properties. Int. J. Mol. Sci.. 2019;20(20):5092.
- [Google Scholar]
- Effects of salinity on eggplant (Solanum melongena L.) growth and evapotranspiration. Irrigation and Drainage: The Journal of the International Commission on. Irrig. Drain.. 2010;59(2):203-214.
- [Google Scholar]
- Assessing the effects of water deficit on photosynthesis using parameters derived from measurements of leaf gas exchange and of chlorophyll a fluorescence. Front. Plant Sci.. 2017;8:2068.
- [Google Scholar]
- Functional characterization of the antioxidant enzymes in rice plants exposed to salinity stress. Biol. Plant.. 2017;61(3):540-550.
- [Google Scholar]
- Application of polysaccharide biopolymer in petroleum recovery. Polymers. 2020;12(9):1860.
- [Google Scholar]
- Effects of extraction methods on the yield, chemical structure and anti-tumor activity of polysaccharides from Cordyceps gunnii mycelia. Carbohydr. Polym.. 2016;140:461-471.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102055.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







