Translate this page into:
The synergistic effect of capsicum aqueous extract (Capsicum annuum) and chitosan against multidrug-resistant bacteria
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
This study investigated the effects of chitosan, capsicum and chitosan and capsicum combined (Capsicum annuum) on Gram-positive pathogenic bacteria, such as Staphylococcus aureus, and Gram-negative bacteria, such as Salmonella typhimurium and Pseudomonas aeruginosa.
Methods
The obtained extracts were categorised as chitosan, capsicum and chitosan and capsicum combined. The antimicrobial action of the extracts was determined by the inhibition zone compared to the control. Antimicrobial bioassays using S. typhimurium, P. aeruginosa and S. aureus were performed on the extracts.
Results
The antimicrobial profiles showed that the chitosan and capsicum combined possessed antimicrobial effects. This is mostly attributed to the high concentration of components that possess antibacterial action. The growth of bacterial pathogens was inhibited by all samples of capsicum with chitosan, but the largest inhibition zone was against S. typhimurium (18 mm) with a capsicum and chitosan concentration of 10 mg/ml.
Conclusion
The combination of chitosan and capsicum can be effective against pathogenic bacteria. This is an innovative approach to enhancing these extracts’ antibacterial activity. Further investigation is required to explore further aspects of these extracts’ antimicrobial activity against pathogenic bacteria. The observed results showed that chitosan and capsicum were more active against S. aureus.
Keywords
Salmonella
Pseudomonas
S. aureus
E. coli
Capsicum extract (Capsicum annuum)
Chitosan
- ALP
-
Alkaline phosphatase
- NADH2
-
Nicotinamide adenine dinucleotide
- MIC
-
Minimum inhibitory concentration
- LD
-
Lactate dehydrogenase
- ROS
-
Reactive oxygen species
Abbreviations
1 Introduction
Chitosan as a linear polysaccharide consisting of 1–4 linked d-glucosamine (deacetylated and acetylated units) of N-acetyl-d-glucosamine. It used widely in industrial and medicinal applications. Chitosan is produced from the shell of shrimp, some fungi, seashells and other marine crustaceans and is widely used for multiple purposes (Wansapura et al., 2017; Sami et al., 2021b; Kumar and Neeraj, 2019). Chitosan is commonly used as an agent against a wide scope of microorganisms cellular components. Polysaccharide components have excellent properties, including nontoxic, antioxidant, antimicrobial, antifungal and good nutrient profiles (Kumar and Neeraj, 2019). This results in microbial death caused by the hydrolysis of peptidoglycans, including intracellular electrolytes (Rokayya et al., 2021; Sami, et al., 2021a, 2021b, 2021c, 2021d).
The charge given by chitosan chains results in the protonation of amino acid compounds by methylation, which brings up the thickened layer of charged polymeric particles. This then leads to proficient antimicrobial movement. Thus, in the current investigation, chitosan polymers were evaluated against Gram-positive and Gram-negative bacteria, specifically Staphylococcus aureus and Escherichia coli as classic microbes. Outcomes gained from turbidity estimations varied according to materials and S. aureus development, while the E. coli strain was less sensitive to the Capsicum annuum and chitosan. Moreover, antimicrobial adequacy was firmly subjected to complex, spatial polymer adaptation (Goy et al., 2016; Sami, 2021a, 2021b, 2021c, 2021d; Sami et al., 2021a).
The Capsicum spp. of peppers are a vital, economical crop part of the Solanaceae family. The crop farming of chili peppers has increased gradually worldwide over the last two decades, with 3.8 million/m2 of land devoted to the farming of this crop. In 2017, 40.7 million tonnes of peppers were produced (Kang et al., 2020). A previous work produced capsicum extracts using an agar diffusion method that had a deleterious effect on microbes, including Salmonella typhimurium, S. aureus, Listeria monocytogenes and Bacillus cereus (Dorantes et al., 2000a). Pseudomonas aeruginosa also showed sensitivity to the extracts (Dorantes et al., 2000b). Furthermore, all chitosan films enriched with pomegranate peel exhibited significant antibacterial activity against E. coli (Pratibha et al., 2021a, 2021b).
The aim of this work was to elucidate the antibacterial activity of chitosan microparticles combined with capsicum extracts on S. aureus, P. aeruginosa, E. coli and S. typhimurium. The extracts were prepared by combining chitosan microparticles and capsicum (10 μg ml−1 and 5 μg ml−1) to determine their impact on multidrug-resistant bacteria.
2 Materials and methodology
2.1 Microbial strains and culture conditions
S. typhimurium, S. aureus and P. aeruginosa were collected from the Microbiology Department of the College of Medicine at King Faisal University, Saudi Arabia, and were preserved in Mueller–Hinton agar. Strains were cultured in nutrient broth for up to 24 h at a temperature of 35 °C. Conditions were standardised to 0.5 using a McFarland nephelometer, with 108 CFU/ml used to prepare the inoculum (Warren, 1985). To create dilutions, a Meat Peptone 0.1 % solution and the plate count method were used while checking bacterial viability and the concentration of bacterial inoculum.
2.2 Preparing the capsicum aqueous extract
To prepare the capsicum aqueous extracts, bell peppers (Capsicum annuum) were purchased from a local market in Saudi Arabia (Al-Ahsa, Saudi Arabia). Crops and fruits were at the half-ripe maturity stage. Peppers were rinsed with water and blotted by towel. Then, an equal amount w/w of peppers with seeds and ethanol were homogenised using a blender (Panasonic). The mixture was then shaken for about 15 min before filtering with No. 4 paper. Then, 15 g of active charcoal was added to 1lL of the solution to separate interfering substances. The filtrate was collected after 10 min of evaporation in a vacuum at a temperature of 75 °C.
2.3 Preparing the chitosan
Chitosan was obtained from Biotech. Co. ltd. (Korea), and the molecular weight was approximately 50,000–190,000 Daltons based on viscosity with 75–85 % deacetylation. Capsicum extract and glycerol (1 %) as plasticizers were added to the prepared chitosan solutions and stirred for 60 min at room temperature (23 ± 2◦C). The chitosan solution amount was determined by dissolving it in 1 % acetic acid at a concentration of 5 or 10 ug ml−1.
2.4 Antibacterial bioassay
The extract with antibacterial potency created using capsicum and chitosan was assessed against the microbial activity of E. coli, S. aureus, P. aeruginosa and S. typhimuriu. Extract sensitivity was examined using the modified Kirby–Bauer disc diffusion susceptibility protocol. For the antibacterial analyses, sterilised paper discs 6 mm in diameter were inundated with 30 μl of extract. The dried soaked discs were placed on a nutrient agar medium with inoculum made of bacterial suspensions (0.85 g NaCl in 100 ml- 1 of distilled water). To ensure the diffusion of bioactive extract, plates were kept in the medium for almost 2 h at 4 °C and then incubated at 37 °C. As a negative control, the discs were sterilised with 30 μl of distilled water, while the positive control discs were treated with chloramphenicol (5 %) for comparison. The plates were then incubated for 24 h, during which the diameters of the inhibition zones (mm) were assessed. Data were taken in triplicate, after which the average and standard deviation (SD) were documented (Al dayel et al., 2020). The antimicrobial bioassay using an aqueous solution of capsicum and chitosan at 5 and 5 ug/ml against E. coli, S. aureus, P. aeruginosa and S. typhimurium, which are multidrug-resistant strains, was performed.
2.5 Colony-forming unit (CFU)
The culture was mixed with saline to a density of 5 × 105 CFU/ml (Wayne et al., 2008). The antibacterial activity was determined using the agar dilution technique involving Mueller–Hinton agar media. As the positive control, vancomycin 15 mcg/ml in the form of discs was used (VA, 30 μg, Oxoid, Basingstoke-Hampshire, UK). Petri dishes were used to incubate the samples for 24 h at 37 °C. The MIC did not show any bacterial growth, while MICs with 250 μg/ml of extract were considered.
2.6 Effects of chitosan and capsicum aqueous extract tested according to alkaline phosphatase in S. aureus, P. aeruginosa and S. typhimurium
2.6.1 Screening of ALP by bacteria
S. aureus, P. aeruginosa and S. typhimurium were tested for ALP activity by culturing on Heart Infusion Agar (Difco) with phenolphthalein bisphosphate tetrasodium salt 0.01 % (Sigma) and 10 % NaCl incubated at 37 °C for about 48 h. Pink colonies were selected to confirm isolates producing ALP. Strains were inoculated in 5 ml broth from JCM (no. 377) and incubated on a rotary shaker at 37 °C (150 rpm) for 24 h to culture the bacteria. The seed culture broth with 0.5 ml of inoculum was transferred to a 50 ml JCM no. 377 broth medium and incubated under the mentioned conditions. The culture was then centrifuged at 10,000 rpm (13,300 g) at 4 °C for about 10 min, and the obtained volume of supernatant was used to detect ALP enzyme activity (Barber & Kuper, 1951).
2.6.2 ALP activity assay
ALP activity was assessed using the Helianti method. The reaction mixture contained 1.0 ml of 10 mM p-nitrophenylphosphate (pNPP) by Sigma and 0.2 M Tris-HCl buffer with a pH of 10. The mixture had 5 mM MgCl2, and 0.1 ml of crude enzyme from the inoculum was taken for incubation at 37 °C for about 15 min. The procedure was stopped when 1 ml of 1 M NaOH was produced. Then, absorbance at a wavelength of 405 nm was measured (Helianti et al., 2007). The protein content was assessed using the optimised Lowry method involving bovine serum albumin (Lowry et al., 1951).
2.7 Effect of aqueous chitosan and capsicum extract on the membrane potential of S. aureus, P. aeruginosa and S. typhimuriu
2.7.1 Measurement of membrane potential
The protocol used by Sánchez et al. (2010) was followed, although with some modifications. The S. aureus and S. typhimurium cultures were incubated overnight and diluted in broth to achieve a cell density up to 1 × 107 CFU/ml. Suspensions treated with extracts were incubated at 25 °C for 10 min at 0.5 µg/ml. Fluorescence intensity was measured using a fluorescence spectrophotometer (Cary Eclipse G9800A, Agilent Technologies Trading Co., ltd., Shanghai, China) at wavelengths of 492 nm and 515 nm, respectively, and the membrane potential-sensitive fluorescent probe, specifically the DiBAC4 (bis-(1,3-dibutylbarbituric acid) trimethine oxonol) probe (Life Technologies, Eugene, OR, USA), was used in the dark for 5 min.
2.8 Effects of aqueous chitosan and capsicum extract on the LDH activity of S. aureus, P. aeruginosa and S. typhimuriu
2.8.1 Organism harvesting and growth
In the aerobic condition, the cells were cultured by incubation at 37 °C in a 2.5 L flask containing 0.5 to 1.0 L culture medium. The flask was placed on a reciprocal shaker at 80 cycles/min. The growth harvested during the late exponential phase was taken for centrifugation at a speed of 2,500 X g for 10 min at 4 °C and washed using 0.05 M potassium phosphate buffer having a pH of 6.5 before being stored at −20 °C.
2.8.2 Preparation of cell-free extracts
Cell culture suspensions with 20 ml of potassium phosphate buffer (0.05 M and pH 6.5) were maintained in a pre-cooled French pressure cell. To remove cell debris, centrifugation at 27,000 X g for 15 min at 4 °C left behind 0.3 mg/ml protein content.
2.8.3 LDH assay
The LDH assay was conducted for the extract with 1 ml potassium phosphate buffer at pH 6.5 (50 μmol; sodium pyruvate: 2.5 µmoles; NADH2: 0.136 μmol) and from 1 to 10 μg protein. Pyruvate was added to initiate the reaction to measure the endogenous NADH2 oxidase activity. The optical density (OD) was taken at 340 mA using a Zeiss M4 QIII spectrophotometer (Carl Zeiss, Inc., New York). Assays were conducted at 24 °C.
3 Statistical analyses
The data from all measurements were acquired using the STATISTICA 6.0 program for the analysis of variance (ANOVA) and multivariate ANOVA (MANOVA) (StatSoft, 2001). The mean difference between the treatment groups was evaluated at a probability level of p = 0.05.
4 Results
4.1 Antibacterial susceptibility testing
For the final concentration, the culture was diluted using sterile saline to 5 × 105 CFU/ml. Potential antimicrobial activity was evaluated using the agar dilution technique. Vancomycin was used as the positive control. The petri dishes were incubated for 24 h at 37 °C. The MIC of compounds at ≤ 250 μg/ml was considered effective.
4.2 Antibacterial activity of aqueous chitosan and capsicum extract
We assessed the antibacterial potential of chitosan and capsicum at different concentrations, including chitosan 10 mg/ml, capsicum + chitosan 10 mg/ml, and capsicum 10 mg/ml and capsicum + chitosan 5 mg/ml. The capsicum + chitosan 10 mg/ml concentration showed antibacterial responses according to their bacteriostatic and bactericidal properties mentioned in Table 1. Variances were detected for different concentrations. Capsicum + chitosan 10 mg/ml was the most active, while capsicum + chitosan 5 mg/ml showed the lowest activity. Interestingly, because capsicum + chitosan 10 mg/ml was found to be the most active, this suggests that capsicum + chitosan 10 mg/ml is an effective combination. The basic structure of naturally occurring chitosan can be seen in Fig. 1 and Fig. 2.
Bacteria
Extract
Positive control
chitosan 10 mg/ml
capsicum + chitosan 10 mg/ml
capsicum 10 mg/ml
capsicum + chitosan 5 mg/ml
Pseudomonas aeruginosa
250/250
>1000
>1000
>1000
125/250
Staphylococcus aureus
62.5/125
>1000
125/250
>1000
62.5/125
Salmonella typhimurium
62.5/250
>1000
62.5/500
>1000
62.5/125
Chemical structure of isolated and purified chitosan compounds (Jorge López-García et al., 2014).
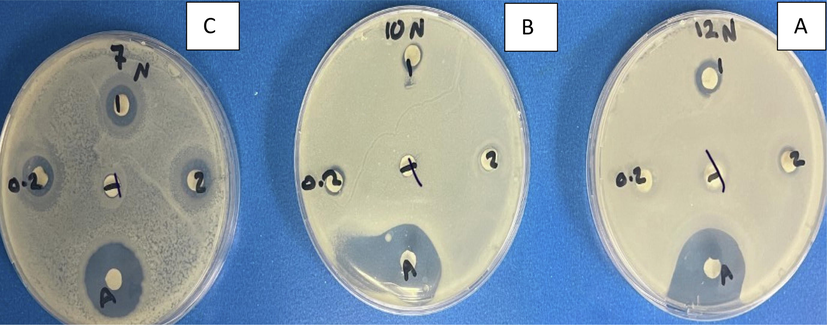
Antibacterial activity of chitosan and Capsicum annuum. Agar disc diffusion technique displaying the antibacterial action of chitosan and Capsicum annuum against three bacterial strains at MIC values. Data are expressed as the mean zone of inhibition in mm. A is S. aureus, B is Pseudomonas aeruginosa and C is Salmonella typhimurium. 1 is capsicum + chitosan 10 mg/ml,2 capsicum + chitosan 5 mg/ml, 0.2 is capsicum 10 mg/ml, T is chitosan 10 mg/ml and A is Imipenem 10 µg.
4.3 Antibacterial bioassay
Table 2 summarises the concentrations of the aqueous capsicum and chitosan extracts. Chitosan 10 mg/ml, capsicum 10 mg/ml and capsicum + chitosan 5 mg/ml were ineffective against S. aureus. However, capsicum + chitosan 10 mg/ml was effective. The yield from the negative control presented no inhibition zone (0 cm). *Means within a column followed by the same letter did not found significantly different at 0.05 level of probability according to L.S.D. test.
Bacterial pathogen
Treatment
Inhibition zone before exposure
S. aureus
Chitosan 10 mg/ml
5 ± 0.1 ab
S. aureus
Capsicum 10 mg/ml
6 ± 0.01 a
S. aureus
capsicum + chitosan 5 mg/ml
12 ± 0.2b
S. aureus
capsicum + chitosan 10 mg/ml
16 ± 0.2c
Pseudomonas aeruginosa
Chitosan 10 mg/ml
5 ± 0.06 ab
Pseudomonas aeruginosa
Capsicum
6 ± 0.1b
Pseudomonas aeruginosa
capsicum + chitosan 5 mg/ml
9 ± 0.1 ab
Pseudomonas aeruginosa
capsicum + chitosan 10 mg/ml
13 ± 0.2c
Salmonila typhimuriu
Chitosan 10 mg/ml
6 ± 0.05c
Salmonila typhimuriu
Capsicum 10 mg/ml
7 ± 0.1 a
Salmonila typhimuriu
capsicum + chitosan 5 mg/ml
13 ± 0.2ac
Salmonila typhimuriu
capsicum + chitosan 10 mg/ml
18 ± 0.3b
4.4 Effects of coumarin, LDH and ALP activity on membrane potential
The effects of chitosan and capsicum extracts on the membrane potential of bacteria were assessed. The results showed that chitosan 10 mg/ml, capsicum 10 mg/ml, capsicum + chitosan 5 mg/ml and capsicum + chitosan 10 mg/ml caused an increase in the electrostatic discharge on the membrane potential compared to other bacterial infections. The membrane potential of S. aureus was increased for capsicum + chitosan 10 mg/ml compared to the control and chitosan 10 mg/ml, capsicum 10 mg/ml alone and capsicum + chitosan 5 mg/ml, followed by P. aeruginosa and S. typhimurium which less increase than S. aureus. Only S. aureus was significantly altered by the chitosan and capsicum combination. Capsicum + chitosan 10 mg/ml yielded the highest broken membrane potential compared to the other concentrations in S. aureus, P. aeruginosa and S. typhimurium (Table 4).
The quantification of enzyme activity (LDH) suggested that the chitosan and capsicum extracts strongly affected the bacterial cell wall by increasing enzyme activity. The media containing the capsicum + chitosan 10 mg/ml was most effective compared to the other concentrations (Table 3). Among all concentrations, capsicum + chitosan 10 mg/ml showed the highest effect on ALP and LDH. These enzyme activity was higher and more significant in S. aureus, while only capsicum 10 mg/ml decreased LDH activity in S. aureus, P. aeruginosa and S. typhimurium as mentioned in Table 5. One unit of ALP defined as amount of enzyme yielding one micromole of p-nitrophenol within one minute per milligram protein under the optimized conditions. values (n = 3) ± SE expressed in mean followed as per ANOVA Duncan’s multiple range test. One unit of ALP defined as amount of enzyme yielding one micromole of p-nitrophenol within one minute per milligram protein under the optimized conditions. values (n = 3) ± SE expressed in mean followed as per ANOVA Duncan’s multiple range test. values (n = 3) ± SE are expressed in mean followed as per ANOVA Duncan’s multiple range test. values (n = 3) ± SE are expressed in mean followed as per ANOVA Duncan’s multiple range test.
Sample No.
S. aureus
S. typhimurium
P. aeruginosa
Control
a6.55b ± 0.07
a4.30a ± 0.05
a5.22c ± 0.05
chitosan 10 mg/ml
cd12.43b ± 0.13
c7.11a ± 0.07
cd11.30c ± 0.17
capsicum + chitosan 10 mg/ml
f16.44b ± 0.04
f11.22a ± 0.02
f14.11c ± 0.06
capsicum 10 mg/ml
b10.30b ± 0.20
b5.22a ± 0.05
b9.22c ± 0.30
capsicum + chitosan 5 mg/ml
c12.59b ± 0.3
cd7.86a ± 0.01
c10.53c ± 0.4
Sample No.
Relative fluorescent units
S. aureus
S. typhimurium
P. aeruginosa
Control
a-7.22c ± 0.05
a-5.56a ± 0.08
a-6.32b ± 0.07
chitosan 10 mg/ml
c-84.15c ± 0.18
c-62.34a ± 0.22
c-81.55b ± 0.12
capsicum + chitosan 10 mg/ml
e-116.15c ± 0.33
f-99.12a ± 0.03
e-112.65b ± 0.56
capsicum 10 mg/ml
b-58.66c ± 0.07
b-35.22a ± 0.2
b-52.26b ± 0.05
capsicum + chitosan 5 mg/ml
d-90.33c ± 0.16
d-64.44a ± 0.52
d-85.43b ± 0.12
Sample No.
Specific activity
S. aureus
S. typhimurium
P. aeruginosa
Control
a6.56c ± 0.01
a4.52b ± 0.05
a3.36a ± 0.04
chitosan 10 mg/ml
d19.27c ± 0.16
c7.11b ± 0.11
d16.17a ± 0.12
capsicum + chitosan 10 mg/ml
e24.46c ± 0.21
d13.17b ± 0.07
e21.56a ± 0.15
capsicum 10 mg/ml
b11.23c ± 0.05
a4.56b ± 0.17
b8.63a ± 0.07
capsicum + chitosan 5 mg/ml
c17.32c ± 0.11
b6.33b ± 0.23
c14.42a ± 0.14
5 Discussion
Chitosan is made commercially by deacetylating chitin. Chitosan is a bio-adhesive that easily adheres to negatively charged surfaces, including membranes. Nonviral gene transfer uses trimethyl chitosan as a derivative component. Chitosan also protects against fungal infections and has the capacity to clot blood quickly. Therefore, it is now used in bandages and other haemostatic agents (Bose & Wong, 2018).
Different studies have reported that bioactive isolates, including capsaicin and dihydrocapsaicin from capsicum, tend to exhibit antibacterial activity (Oyedemi et al., 2019; Füchtbauer et al., 2021). C. annuum extract has been used in wound healing processes, although no scientific literature has supported this finding (Ekom et al., 2021).
The current study’s findings suggest that the effects of the antibacterial activity of chitosan extracts vary according to different pathogenic phenotypes, especially multidrug-resistant bacteria (Koffi-Nevry et al., 2012; Oulaï et al., 2018). This could be due to the difference in the genetic and structural composition of bacterial strains or isolates. The potential antimicrobial feature was ensured by the presence of components, including alkaloids, polyphenols, flavonoids, anthocyanins, anthraquinones, tannins, triterpenes and saponins (Ekom et al., 2021). This upholds the findings of earlier studies (Koffi-Nevry et al., 2012; Samrot et al., 2018) that validated these groups as bioactive compounds in methanol extracts of C. annuum.
Our results corroborate those of Steve et al. (2021), who found that S. aureus and E. coli are sensitive to C. annuum extract (Fig. 2). One way to induce the antimicrobial activity of chitosan is based on the electrostatic interaction between negatively charged membranes and the molecules charged as cations (Chen et al., 2015; Felt et al., 2000; Rivera Aguayo et al., 2020).
The differential characteristics of chitosan, including its antibacterial activity, have been found to be associated with its structural properties, such as its physicochemical characteristics, environmental conditions and reactive hydroxyl groups at the C-3 and C-6 positions (Younes & Rinaudo, 2015; Xing et al., 2014; Dutta et al., 2011; Synowiecki & Al-Khateeb, 2003; Kong et al., 2010; Hosseinnejad & Jafari, 2016; Ma et al., 2017; Ing et al., 2012; Azuma et al., 2018). Chitosan performs its function by targeting extracellular functions, intracellular functions, or both to induce antimicrobial effects (Varlamov & Mysyakina, 2018; Kravanja et al., 2019; Kong et al., 2010; Hosseinnejad & Jafari, 2016; Raafat et al., 2008; Cheung et al., 2015).
The current work examined extracts of chitosan and capsicum for their antibacterial potential on bacterial species. All concentrations yielded adequate to tremendous effects on bacterial growth (Ekom et al., 2021), which is in agreement with the data presented in prior studies (Ke et al., 2021; Pratibha et al., 2021a; Pratibha et al., 2021b). Our results were found to align with those of previous studies (Ekom et al., 2021; Ke et al., 2021). They found that capsicum extract presented antibacterial properties related to the presence of phenolic compounds, flavonoids and tannins by inhibiting the formation of biofilms, thus affecting the ATPases/H+ proton pump and dehydrogenase activity by altering the bacterial cell wall, thus causing leakages of nucleic acids and reducing sugars and proteins outside the membrane (Ekom et al., 2021). The data were also compatible with concentrations ranging from 5 to 125 μg/ml, thus verifying the effects of capsicum extract on the biofilm action of S. aureus and P. aeruginosa.
Chitosan and capsicum extracts may affect the lipophilic properties of the cell membrane and influence its molecular structure, thereby increasing penetration into the cell. The suppressive action of the combination of chitosan and capsicum was influenced by the substitution patterns (Ke et al., 2021). The substitution of chitosan is exaggerated by the following factors: pH, MW and DDA. The physicochemical characteristics of C2-NH2, C3-OH (secondary hydroxyl), and C6-OH (primary hydroxyl) functional groups may likewise significantly influence antimicrobial characteristics against bacteria (Ke et al., 2021). The antimicrobial potential of chitosan and capsicum extracts due to C6-OH suggests that substitution is a risk factor, where polarity also plays an important role. The extracted chitosan and capsicum suspensions presented high antibacterial potential due to their passive diffusion, permitted by their molecular structure and lipophilic properties. Aromatic substitution and side chains may assist in diffusion (Ke et al., 2021). Hence, the mode of action is attributable to the bacterial cell membrane.
The diffusion of chitosan and capsicum extracts may possibly be prohibited by peptidoglycan barriers and other cellular components (Ke et al., 2021). These composites interact with cells and hinder the potential of the bacterial cell membrane (Arokiyaraj et al., 2014). Phytochemical compositions influenced by medicinal plants have been reported to alter the cell walls of Gram-positive and Gram-negative bacteria (Ke et al., 2021).
Pathogenic bacteria release ALP and LD upon exposure to stress (Arokiyaraj et al., 2014). The higher concentrations of enzymes showed that chitosan and capsicum generated an uneven environment for bacterial growth, resulting in the increased production of these enzymes. This is the first study to assess the effects of chitosan and capsicum on LDH and ALP activity. However, membrane damage activity was demonstrated to a lower scale. The released volume of LDH activity represented a high level, showing the mechanism of action for membrane damage to the bacterial cells. An increase in membrane permeability or the outflow of cell contents may be triggered by ROS.
6 Conclusions
The results showed the antibacterial effects of chitosan and capsicum combined against different bacteria. This combination caused significant damage to the membrane potential while increasing the production of LDH and ALP in S. aureus, P. aeruginosa and S. typhimurium. Therefore, the combination of chitosan derivative N,N,N-trimethyl chitosan (TMC) and capsicum at a concentration of 10 mg/ml, exhibited antimicrobial activity against Gram-positive and Gram-negative bacterial strains. The observed results showed that chitosan and capsicum extracts were more active against S. aureus.
Acknowledgements
The authors extend their appreciation to the Annual Funding track by the Deanship of Scientific Research and the Vice Presidency for Graduate Studies and Scientific Research of King Faisal University, Saudi Arabia [Project No. INST304].
Funding
This work was supported by the Annual Funding track governed by the Deanship of Scientific Research and Vice Presidency for Graduate Studies and Scientific Research of King Faisal University, Saudi Arabia [Project No. GRANT2015].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Investigating the applications of Chlorella vulgaris in agriculture and nanosilver production. J. Environ. Biol.. 2020;41(5):1099-1104.
- [CrossRef] [Google Scholar]
- Characterization of ambrette seed oil and its mode of action in bacteria. Molecules. 2014;20(1):384-395.
- [CrossRef] [Google Scholar]
- Chitin, Chitosan, and its derivatives for wound healing: old and new materials. J. Funct. Biomater.. 2018;9(2):104-142. 38
- [CrossRef] [Google Scholar]
- Identification of Staphylococcus pyogenes by the phosphatase reaction. J. Pathol. Bacteriol.. 1951;63(1):65-68.
- [CrossRef] [Google Scholar]
- Oral colon cancer targeting by chitosan nanocomposites. Appl. Nanocompos. Mater. Drug Deliv. 2018:409-429.
- [CrossRef] [Google Scholar]
- Optimization and evaluation of a chitosan/Hydroxypropyl methylcellulose hydrogel containing toluidine blue O for antimicrobial photodynamic inactivation. Int. J. Mol. Sci.. 2015;16(9):20859-20872.
- [CrossRef] [Google Scholar]
- Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs. 2015;13(8):5156-5186.
- [CrossRef] [Google Scholar]
- Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annum extracts. Int. J. Food Microbiol.. 2000;57(1–2):125-128.
- [CrossRef] [Google Scholar]
- Dorantes, L., Hernández-Sánchez, H., Acero-Ortega, C., López-Malo, A., 2000b. Effect of capsicum extracts on the growth of some microorganisms important in food products. In 2000 Institute of Food Technologists Annual Meeting Book of Abstracts (pp. 145–146). Institute of Food Technologists Chicago, Illinois, USA.
- Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides: a systematic study needs for food applications. Agron. Sustain. Dev.. 2011;18(1):3-34.
- [CrossRef] [Google Scholar]
- Antibacterial and therapeutic potentials of the capsicum annuum extract against infected wound in a rat model with its mechanisms of antibacterial action. Biomed Res. Int.. 2021;2021:1-17.
- [CrossRef] [Google Scholar]
- Chitosan as tear substitute: a wetting agent endowed with antimicrobial efficacy. J. Ocul. Pharmacol. Ther.. 2000;16(3):261-270.
- [CrossRef] [Google Scholar]
- Antibacterial properties of capsaicin and its derivatives and their potential to fight antibiotic resistance – A literature survey. Eur. J. Microbiol. Immunol.. 2021;11(1):10-17.
- [CrossRef] [Google Scholar]
- Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farmacogn.. 2016;26(1):122-127.
- [CrossRef] [Google Scholar]
- Characterization of thermostable native alkaline phosphatase from an aerobic hyperthermophilic archaeon, Aeropyrum pernix K1. Appl. Microbiol. Biotechnol.. 2007;74(1):107-112.
- [CrossRef] [Google Scholar]
- Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Maromol.. 2016;85:467-475.
- [CrossRef] [Google Scholar]
- Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater.. 2012;1–9
- [CrossRef] [Google Scholar]
- HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater.. 2014;5:43-57.
- [CrossRef] [Google Scholar]
- Transcriptome profiling of abiotic responses to heat, cold, salt, and osmotic stress of Capsicum annuum L. Sci. Data. 2020;7(1)
- [CrossRef] [Google Scholar]
- Antimicrobial actions and applications of chitosan. Polymers. 2021;13(6):904.
- [CrossRef] [Google Scholar]
- Antibacterial activity of two bell pepper extracts: Capsicum annuum L. and Capsicum frutescens. Int. J. Food Prop.. 2012;15(5):961-971.
- [CrossRef] [Google Scholar]
- Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol.. 2010;144(1):51-63.
- [CrossRef] [Google Scholar]
- Chitosan-based (Nano)Materials for novel biomedical applications. Molecules. 2019;24(10):1960.
- [CrossRef] [Google Scholar]
- Polysaccharide-based component and their relevance in edible film/coating: a review. Nutr. Food Sci.. 2019;49:793-823.
- [CrossRef] [Google Scholar]
- Protein measurement with the folin phenol reagent. J. Biol. Chem.. 1951;193(1):265-275.
- [CrossRef] [Google Scholar]
- Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: a review. Carbohyd. Polym.. 2017;176:257-265.
- [CrossRef] [Google Scholar]
- Chemical composition, antioxidant and antimicrobial activities of Capsicum annuum var. annuum concentrated extract obtained by reverse osmosis. GSC Biol. Pharm. Sci.. 2018;5(2):116-125.
- [CrossRef] [Google Scholar]
- Capsaicin and gingerol analogues inhibit the growth of efflux-multidrug resistant bacteria and R-plasmids conjugal transfer. J. Ethnopharmacol.. 2019;245:111871
- [CrossRef] [Google Scholar]
- Chitosan edible films enhanced with pomegranate peel extract: study on physical, biological, thermal, and barrier properties. Materials. 2021;14(3305):1-18.
- [CrossRef] [Google Scholar]
- Effect of active chitosan-pullulan composite edible coating enrich with pomegranate peel extract on the storage quality of green bell pepper. LWT. 2021;138(110435):1-12.
- [CrossRef] [Google Scholar]
- Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol.. 2008;74(12):3764-3773.
- [CrossRef] [Google Scholar]
- Antimicrobial and antibiofilm capacity of chitosan nanoparticles against wild type strain of pseudomonas SP. Isolated from milk of cows diagnosed with bovine mastitis. Antibiotics. 2020;9(9):551.
- [CrossRef] [Google Scholar]
- Application of nano-titanium dioxide coating on fresh Highbush blueberries shelf life stored under ambient temperature. LWT. 2021;137:110422
- [CrossRef] [Google Scholar]
- Effect of nano silicon dioxide coating films on the quality characteristics of fresh-cut cantaloupe. Membranes. 2021;11:140.
- [CrossRef] [Google Scholar]
- Enhancement in physico-chemical parameters and microbial populations of mushrooms as influenced by nano-coating treatments. Sci. Rep.. 2021;11:7915.
- [CrossRef] [Google Scholar]
- Performance study of nano/SIO2 films and the antimicrobial application on cantaloupe fruit shelf-life. Appl. Sci. 2021;11:3879.
- [CrossRef] [Google Scholar]
- Microscopic image segmentation and morphological characterization of novel chitosan/silica nanoparticle/nisin films using antimicrobial technique for blueberry preservation. Membranes. 2021;11:303.
- [CrossRef] [Google Scholar]
- Antibacterial and antioxidant activity of different staged ripened fruit of Capsicum annuum and its green synthesized silver nanoparticles. Bionanoscience. 2018;8(2):632-646.
- [CrossRef] [Google Scholar]
- Extracts of edible and medicinal plants damage membranes of Vibrio cholerae. Appl. Environ. Microbiol.. 2010;76(20):6888-6894.
- [CrossRef] [Google Scholar]
- StatSoft Inc., 2001. StatSoft STATISTICA for Windows, Version 6, 2300.
- Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003;43(2):145-171.
- [CrossRef] [Google Scholar]
- Chitosan in biology, microbiology, medicine, and agriculture. Microbiology. 2018;87(5):712-715.
- [CrossRef] [Google Scholar]
- Preparation of chitin-CdTe quantum dots films and antibacterial effect on Staphylococcus aureus and Pseudomonas aeruginosa. J. Appl. Polym. Sci.. 2017;134(22)
- [CrossRef] [Google Scholar]
- Warren, R., 1985. Manual of Clinical Microbiology, American Society for Microbiology, New York (1985), 1149. In: Lennette, E.H., Balows, A., Hausler, W.J., Shadomy, H.J. (Eds.). J. Infection 11 (3), 278–278. https://doi.org/10.1016/s0163-4453(85)93624-2.
- Wayne, P.A., 2008. Performance standards for antimicrobial susceptibility testing. 18th Informational Supplement M100-S18, Clinical and Laboratory Standards Institute, 120–126.
- Chitosan antimicrobial and eliciting properties for pest control in agriculture: a review. Agron. Sustain. Dev.. 2014;35(2):569-588.
- [CrossRef] [Google Scholar]
- Chitin and chitosan preparation from marine sources. Structure, Properties and Applications. Mar. Drugs. 2015;13(3):1133-1174.
- [CrossRef] [Google Scholar]







