Translate this page into:
The role of the hot foot gene in the fertility of The in vitro fertilization and embryonic development of young adult and old mice as a model for assisted reproductive technology
⁎Corresponding author. ahimaidi@ksu.edu.sa (Ahmad Alhimaidi),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The hotfoot mutation first occurred in strain of mice C57BL/Ks in 1964. The homozygous (ho/ho) hotfoot mutation show a quick pattern motion that produces a progressive neuromuscular disability of the hind legs. The (ho/ho) is an autosomal recessive mutation affects fertility and neuromuscular system in mice.
Objective
This study was implemented to determine the nature and causes of infertility, to ascertain potential value as an animal model due to the presence of similar mutation in human and other mammals.
Methods
The experimental design to evaluate the fertility and sterility of young and old adult female and male hotfoot mice by utilizing the assisted reproduction technology (ART) via the in vitro fertilization, the in vitro embryo development, and the normal matting, the growth of the new born hotfoot mice.
Results
Demonstrated that the young adult and the old hotfoot females produce about similar rate of ova number following superovulation. In addition, they yielded similar rate of embryo development in vitro form 1 cell to the 16 cell stage, morula and blastula stage compared to normal females. The old hotfoot females show better rate in the in vitro fertilization IVF and embryo development 53.6%, and the rate of the degenerating ova (46.4%) compared to the young adult females 38.5%, 61.35, respectively. The hotfoot males show sterility (22%) of the homozygous mutant males compared to phenotypically normal males. Regarding the normal matting, the hotfoot female mating with males had less litter size compared to normal mice mating.
Conclusion
The hotfoot gene had a noticeable effect on the in vitro fertilization IVF of young adult compared to old adult hotfoot female, also the body weight growth and litter size of hotfoot less than normal mice.
Keywords
Hot foot mutation
Fertility
In vitro fertilization
- hCG
-
Human chorionic gonadotropin
- hoho
-
homozygous hotfoot
- +ho
-
Heterozygous hotfoot
- IVF
-
In vitro fertilization
- PMSG
-
pregnant mare's gonadotropin
Abbreviations
1 Introduction
The hotfoot (ho/ho) mutation was first occurred in C57BL/Ks mice in 1964, later similar mutations at the same locus found at the Jackson Laboratory: “ho 2j” in the AKR/J strain, and “ho 3j” in the C3H/HEJ strain (Green, 1981). The hotfoot (ho) gene in mice is located on chromosome 6, (Southard, 1981). Hotfoot mice demonstrate progressive neuromuscular disability of the hind legs, and show a quick pattern motion of their feet, hence the name, hotfoot (Dickie, 1966). The hotfoot mutation is classified as an ataxic and convulsive disorder (Oda and Kameyama, 1986). So far, at least 20 alleles, arising either spontaneously or through the random insertion of transgenes, documented (Motohashi et al., 2007). The hotfoot mutation is one of at least five mutations in the mouse, which was reported to affect the neuromuscular system and fertility, (Green, 1981). The hotfoot mutation characterized by cerebellar ataxia, and it mapped with at least eight alleles (Lalouette et al., 1998). Different mutations in the glutamate receptor inotropic delta 2 (GRID2) gene cause cerebellar ataxia in humans (Taghdiri et al., 2019). The infertility associated with the hotfoot mutation may occur at five major stages of reproductive cycle: a) The structural and functional development of sexual organs, b) pre-fertilization, c) pre-implantation, d) post-implantation, and e) perinatal and neonatal. The mice used as human model in the in vitro fertilization (IVF) for multiple purposes such as developing embryo culture media, and procedural training for embryology staff. In addition, the manufacturing companies use the mouse embryo assay as a means of quality control for the development of embryo culture media and medical devices. Also to meet the standards of testing for FDA approval of new products (Esfandiari and Gubista, 2020).
The aim of the current study is to examine the effect of the hotfoot mutation on fertility in male and female mice via regular mating and assisted reproduction technique (ART). Also to determine any impact of the hotfoot mutation on the body weight, the litter size and normal growth of newborn in mice.
2 Materials and methods
2.1 Part I: to study the fertility of hotfoot mice: via the following
2.1.1 Regular mating
The hotfoot mutation mice bought from the Jackson Memorial Laboratory C57BL/6J-Grid2ho-, and the gene maintained on a heterogeneous genetic background in C57BL/6J strain in the lab. Animals maintained on a 14-hrs light, 10 hrs. Dark cycle, and provided with food and water ad libitum. Thirty-three breeding cages were used in this study; each male housed with one female in 10 cages, or in 23 cages with more than one female. The number of mating, litter size, and offspring, with their sex genotype and postnatal survival, maintained and weighted until the end of the 4th week.
2.1.2 Study of the effect of the hotfoot gene on body weight and litter size
A total of 91 animals used: 36 males, and 55 females. Records about number of mating, litters, and offspring, with their sex genotype and postnatal survival.
The offspring from 24 different gene type littermates weighed every other day from day 1 or 2 until day 30, then every 5 days from day 30 up to day 45, and again at day 60.
2.2 Part II: Assisted reproductive technique (ART)
2.2.1 Sperm collection, count and motility
A total of 30 mice males (10 male from ho/ho, ho/+and +/+) of proven fertility. The sperm collection dish were prepared at least 30 min before start sperm collection, and place the dish in an incubator at 37 °C and 5% CO2 in air. For sperm collection,1 or2 male each time were enthused with ether, and the cauda epididymis immediately removed and the epididymal contents squeezed into to a 200 μl drop of modified Tyrode's medium under mineral oil (Sigma: Cat. #M8410). Capacitation allowed proceeding for 1–2 hrs at 37 °C incubator. Sperm concentrations were determined with a haemocytometer (Summers et al., 2000).
2.2.2 Superovulation and ova collection for the in vitro fertilization (IVF)
Female mice (20 female from each: ho/ho, ho/+ and +/+) used for superovulation, three females, one from each genotype were taken at the same time, and one male was utilized for the IVF. The superovulation of each female was done by intra peritoneal i.p. injection of 5 IU of pregnant mare's serum gonadotropin (PMSG) (Sigma) followed by an i.p. injection of 5 IU of human chorionic gonadotropin (hCG) (Sigma) 48 hrs. later. The oocytes collection dishes were prepared at least 30 min before oocytes collection, and placed in an incubator at 37 °C and 5% CO2 in air. Females were enthused with ether the ova were collected between 14 and 16 hrs post-hCG administration by removing entire oviducts and placing them into 1 ml drops of M2 medium (Sigma), at 37 °C. Cumuli with ova collected from an oviduct. Then the fertilization in vitro carried out in 100 ul drops of potassium simple optimize medium (KSOM) under mineral oil, by adding the capacitated sperm suspension to the freshly ovulated ova, then incubated for 4–6 hrs. The ova were then washed through several changes of medium and finally incubated in 50 μl drops of medium under mineral oil and kept in the incubator at 37.5 °C, with 5% CO2 and 95% air. After 24 hrs, the fertilization assessed by recording the number of 2-cell embryos (Alhimaidi and Umar, 1998).
2.2.3 Embryo development evaluation
The medium and culture dishes were prepared at least 30 min before use. Then fertilized oocytes and the dish placed in a CO2 incubator for equilibration. For embryo development evaluation over 3–4 day, embryos observed at × 100 on a warmed microscope stage (35 °C) of an Olympus dissecting microscope. Over this time, the ova classified as follows (Ahimaidi and Al Amro, 2002).
Developed ova or embryo: In vitro fertilized ova were confirmed by the presence of the 2nd polar body and two pronuclei (6–8 hrs post sperm addition); and in the next day reach the two cell stage of development. The majority of the ova developed to 2, 4, 8, 16 cell stages, and then reached the morula and blastula stage within 3.5–5 days of insemination (Fig. 1).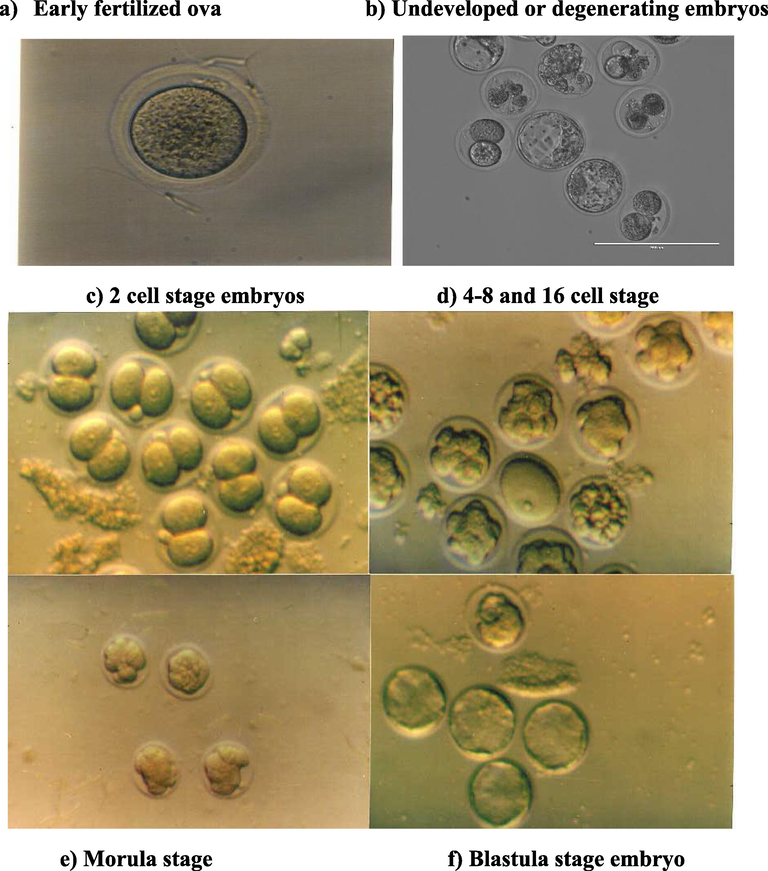
The in vitro embryo development stages of the hotfoot mutation and normal in mice.
Undeveloped ova: Included the degenerating ova, wherein the ova appeared as the zona pellucida filled with debris. Unfertilized ova, that showed no signs of a 2nd polar body or the formation of pronuclei 6–8 hrs post sperm addition. Fragmented or parthenogenetic ova: including oocytes with numerous cytoplasmic fragments of varying size, or ova that divided with unequal cell sizes.
2.3 Statistical analysis
Data analyzed using SPSS according to the following: Simple t-test or chi square for all comparisons between two means for body weight, testes weight, sperm concentration, and ova flushed from different mice genotypes. Two-way analysis of variance: 32 factorial design, with a two-way model with interaction used to test for differences among male and females of the three genotypes with respect to offspring survival or embryo development. For the t-test, F-test significance was set at P < 0.05 (SAS Institute, Cary, NC 1995).
3 Results
3.1 Part I: Effects of the hotfoot mutation on fertility
3.1.1 Breeding results
From the total of 91 animals were used: 36 males (14 ho/ho, 17 +/ho, and 5 +/+ males), and 55 females (27 ho/ho, 22 +/ho, and 6 +/+ females). The overall mating yielded 184 litters, containing 1.186 progeny. Regarding female fertility, the overall mean litter size was 6.45 progeny/female. The mating of homozygous mutant hotfoot (ho/ho × ho/ho) litter size was 6.05 progeny/female, the heterozygous (+/ho ×+/ho) litter size was 6.50 progeny/female, and the normal or wild type (+/+ × +/+) litter size was 8.50 progeny/female (Table 1a). Overall, the 27 (ho/ho) females produced 88 litters with 533 offspring, the +/ho females produced 80 litter with a total of 517 progeny, while the 6 normal +/+ females produced 16 litters with 136 offspring. If the phenotypically normal females (+/+ and +/ho) pooled,as phenotypically normal, they produce 653 progeny from 96 litters, with an overall mean litter size of 6.80, which was higher than that observed for ho/ho females (Table 1a). Regarding male fertility, of the 14 ho/ho males, 4 were sterile compared to only one male among the 22 normal +/+ and +/ho males. When the total number of offspring sired by the three different male genotypes compared, ho/ho males sired 499 mice from 77 litters, with a mean litter size of 6.46. While the +/ho males sired 505 mice from 80 litters, with a mean litter size of 6.31; and the +/+ males produced 182 progeny from 27 litters, with a mean litter size of 6.74. These results showed no significant differences between males, while the females show a significant difference between ho/ho females and normal females (P < 0.05). There were no significant differences in the mean litter size between the female and male genotypes (Table 1b). *Mean litter size different significantly at P < 0.05 for +/+ female vs ho/ho females based on LSD mean separation test. *Mean litter size different significantly at P < 0.05.
Mice genotype and no used
Total litters (ho/ho,+/ho, +/+)
Total progeny
(ho/ho, +/ho, +/+)Mean Litter size
Standerd Deviation
Female ho/ho (27)
88 (28, 47, 13)
533 (182, 287, 64)
6.05
2.72
Female +/ho (22)
80 (48, 29, 3)
517(308,186, 23)
6.47
2.53
Female +/+ (6)
16 (1, 4, 11)
136 (9,32,95)
8.5 *
2.22
Male ho/ho (14)
77(28, 48,1)
499 (182,308,9)
6.48
2.71
Male +/ho (17)
80 (47, 29, 4)
505(287,186,32)
6.31
2.44
Male +/+ (5)
27 (13, 3, 11)
182(64,23,95)
6.74
3.21
Entire population
184
1186 (male or female)
6.45
2.67
Source of Variation
Degree of freedom df
F value litter size
Sig of F litter size
F value offspring survival
Sig of F offspring survival
Male genotype
2
0.12
0.889
1.76
0.183
Female genotype
2
3.98
0.020 *
0.28
0.76
Male × Female genotype
4
0.78
0.537
047
0.759
3.1.2 Influence on postnatal survival of newborn mice
The percentage of offspring raised by ho/ho females was 71.8%, by the +/ho females 77.2%, and by the +/+ females 70.8%. The mortality rate for ho/ho females was 28.2%, the + ho females 22.8%, and +/+ females 29.2%. The highest incidence of postnatal mortality observed during the first week of life among all offspring. Table 2a shows the sex and genotype of the survived progeny that produced from the breeding cages. There was a significant difference in the male to female offspring ratio involving the hotfoot male with ho/ho female mating, but there was a higher number of hotfoot female offspring than their hotfoot male or normal offspring (P < 0.05) (Table 1b and 2b). *Significantly at P < 0.05.
Type of mating Male X female
Total number offspring
Born 28 day survivalThe present of Weekly survival %
1st week 2nd week 3rd week 4th week
ho/ho X ho/ho
182
125
78.1
74.8
73.2
68.8
ho/ho X +/ho
308
229
85.7
84.4
79.2
74.3
ho/ho X +/+
9
9
100
100
100
100
+ /ho X ho/ho
287
189
85.0
84.3
79.0
65.8
+ ho X +/ho
186
148
88.7
87.6
84.4
79.6
+/ho X +/+
32
24
100
100
78.1
75.0
+/+ X ho/ho
64
45
78.5
87.5
78.5
70.3
+/+ X +/ho
23
17
100
95.6
95.6
73.9
+/+ X +/+
95
65
85.2
82.1
82.1
68.4
Type of mating
Male X femaleChi-square (X2) Value
F value
ho/ho X ho/ho
4.232
0.040 *
ho/ho X +/ho
6.3013
0.0978
ho/ho X +/+
0.111
0.739
+/ho X ho/ho
3.106
0.376
+/ho X +/ho
2.869
0.412
+/ho X +/+
2.2857
0.1306
+/+ X ho/ho
0.20
0655
+/+ X +/ho
0529
0.467
+/+ X +/+
0.0154
0.9013
3.1.3 Effect of the hotfoot mutation on postnatal growth
Total of 24 litters, containing of 142 offspring weighed every other day up to 60 days. These litters comprised 38 hotfoot males and 18 females, 19 +/ho males and 23 females, and 6 +/+ normal males and 8 females, with unknown phenotype (+/?) 18 males and 12 females. Of the 38 newborn ho/ho males, 6 died during day 30 period, while none of the +/+ or +/ho males died during the experimental period. Among the 18 ho/ho newborn females, 6 females died, while only one newborn +/ho female died during the observation period. The mean body weight of the new born hotfoot males was 1.40 g, 1.51 g in the +/ho males, and 1.58 g in the +/+, +/? males. The mean body weight at the age of day 60 was 23.93 g. for hotfoot males, the +/ho male 25.7 g. and the +/+ males 24.66 g. with the +/? 25.27 g males. For females, the mean body weight of new born hotfoot was 1.32 g, 1.51 g for the +/ho females, and 1.42 g for the +/+ or +/? females. The mean body weight of the females at (day 60) was 17.95 g for ho/ho females, 21.06 g for +/ho females, and 21.03 g for +/+or +/? females. During the first week, the body weight of normal males and females was higher than the hotfoot mice. In addition, the overall male mean body weight was higher than that of females. All new born gained weight from day 1 until 60 day, except the hotfoot males and females, which had a very low gained weight rate (0.32 g/day after the 3rd week) (Figs. 2 and 3). The regression line of body weight with age (from the 2nd to 4th wk. of age) indicated that a significant difference existed between normal males and females compared to the hotfoot males and females.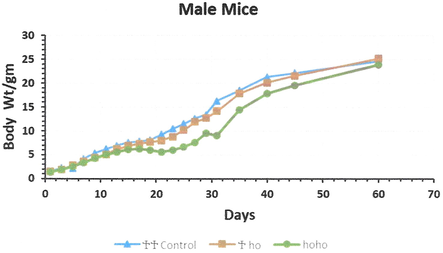
The mean body weight growth of the male new born of the 3 genotype groups hotfoot mutation and normal group (++, +ho, and hoho) in mice. *Young male were separated from their mothers at day 30 of age.
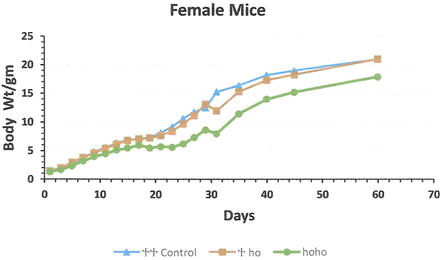
The mean body weight growth of the female new born from the 3 genotype groups hotfoot mutation and normal group (hoho, +ho, and ++) mice. *Young female were separated from their mothers at day 30 of age.
3.2 Part II: Results of assisted reproduction technology (ART)
3.2.1 Sperm collection, concentration, and motility
The mean number of sperm collected were (4.12, 5.84, and 6.20 × 106) form ho/ho, +/ho, and +/+ males, respectively. The highest number of sperm recorded for ho/ho, males was 13.5 × 106 sperm/ml. The number of sperm in the +/ho males varied from (1.3 to 19.2 × 106 sperm/ml). In normal males +/+, the highest recorded number of sperm was 17.25 × 106 sperm/ml. The mean sperm motility of the hotfoot males was 46.8%, which was lower than that in the +/+ and + ho males (59.5%, and 59.8% P < 0.001, respectively). Moreover, there was a significant difference in the mean sperm motility of right and the left epididymis between ho/ho and the +/+ or the +/ho males (P < 0.05 and P < 0.025, respectively) (Table 3). (1). Significantly difference at p value (P < 0.025) (t-test value 2.358) of the sperm count of +/+ compared to the (ho/ho) hotfoot male. (2). Significantly difference at p value (P < 0.01) (t-test value 2.53) of the sperm count of +/? male compared to the (ho/ho) hotfoot male. (a) Significantly difference at p value (P < 0.05) (t-test value 3.437,) of the left testes weight (ho/ho) compared to the right testes hotfoot. (b) Significantly difference at p value (P < 0.05) (t-test value 4.163) of the left testes weight (+/+) compared to the right testes (+/+) male. (c) Significantly difference at p value (P < 0.01) (t-test value 2.477) of the each +/ho or +/+ male sperm motility compared to the ho/ho. (d) Significantly difference at p value (P < 0.005) (t-test value 3.041) of the pooled +/? males sperm motility compared to the hoho. (*) Significantly difference at p value (P < 0.05) (t-test value 1.993) of the pooled left testes weight compared to the hotfoot male.
Male genotype and No.
Mean body wt. (gm)
Mean age (days)
Mean Tests wt.(mg)
Mean sperm count
×106 Sp/mlMean sperm motility %
Right
Left
Hotfoot ho/ho (10)
24.8
94
96.29a
93.68a
4.12 ×106(1)
46.8% c,d
+/ho male (10)
27.94
97
102.2
100.26
5.84 × 106
59.8% c
Normal +/+ male (10)
27.94
88
100.79b
98.59b
6.2 × 106 (1)
59.5% c
Pooled +/+& +/ho (20)
27.94
92
101.52
99.45*
6.1 × 106 (2)
59.6% d
3.2.2 Superovulation and in vitro fertilization of young adult females
The mean age and body weight of the young adult females (∼2–3 month) used for superovulation were as follows: ho/ho females, 86 days and 20.6 g; the +/ho females, 96 days and 24.4 g; and the +/+ females 77 days and 22.6 g. The hotfoot females had a lower body weight than either the +/+ or +/ho females (P < 0.01 and P < 0.05, respectively). In addition, the normal females had a lower body weight than the +/ho females (P < 0.025) (Table 4). *All ova were in vitro fertilized by sperm collected from young adult ++ males). (a)Significantly difference at p value (P < 0.01) (t-test value 2.804) of the body weight (+/+) females compared to the hotfoot females. (b)Significantly difference at p value (P < 0.025) (t-test value 2.138) of the body weight (+/ho) female compared to the (ho/ho) female. (c) Significantly difference at p value (P < 0.005) (t-test value 4.867) of the mean body weight of the (+/ho) female compared to the hotfoot (ho/ho) female. (d) Significantly difference at p value (P < 0.005) (t-test value 4.261) of the mean body weight of the (+/?) pooled (+/+ and+/ho) females compared to the (ho/ho) female. (*) Significantly, difference at p value (P < 0.05) of the total undeveloped ova compared to the developed ova in the all-young adult females mice. (**)Significantly difference at p value (P < 0.05) within all the developed embryos stages within the same group of female genotype. **Significantly difference at p value (P < 0.01) (t-test value 5.72) of the mean body weight of the (+/ho) female compared to the hotfoot (ho/ho) female. (aa) Significantly, difference at p value (P < 0.05) of the total embryo developed compared to the undeveloped ova in old females.
Female genotype & no.
Mean age (days)
Mean body wt. (gm.)
No and mean of ova flushed
Total of developed embryo and % *
Developed embryo cell stags
1, 2, 4, 8, 16,Mor, Blas
and it %Total undeveloped ova &%
Young Hotfoot ho/ho (20)
86
20.6
(a)436
(21.9)168*
38.53%97, 40, 12, 15, 2, 1 + 1 56.5,23.8,7.1., 8.9,1.2,%
0.59, 0.59 0%**268*
61.46%
Young +/ho (20)
96
24.4 (b)
426 (21.3)
116*
27.23%52, 38, 15, 2, 3, 3, 3
49.1,32.7, 12.9,2.3,3.4% 3.48, 3.48%**310*
72.77%
Young +/+ (20)
77
22.6 (c)
432
(21.6)149*
34.49%75, 48, 12, 11, 1, 1, 1
50.3, 32.2, 8.1, 7.3, % 0.67 0.67, 0.67 %**283*
65.51%
young +/ho &++ (40)
86
23.5 (d)
858
(21.45)265*
30.89%*127, 86, 27, 13, 4, 4, 4
47.9, 32.4, 10.2, 4.9,1.5 % 1.5, 1.5 %**593*
69.11%
Old +/ho (20)
254
28.1**
402
(20.1)233 57.97% (aa)
98, 60, 44, 6, 11, 7, 7
42.1, 25.7,18.9 2.6,4.7,% 3.0, 3.0 %169
42.03%
Old ho/ho (20)
244
24.5
504
(25.2)265
53.6%
(aa)115, 87, 32, 5, 11, 8, 7, 43.4, 32.8,12.1,1.9,4.1,% 3.1, 2.6 %
239
46.4%
A total of 1294 ova flushed from all (60) young adult females, There were no significant differences in the flushed ova with a mean number of around 21 ova/female. The ova flushed from the super-ovulated hotfoot females, 327 ova, with a mean of 21.8 ova/female collected. The total of 426 ova, with a mean ova number of 21.3 ova/female were collected from heterozygote +/ho females. The total number of ova flushed from +/+ females was 432, with a mean of 21.6 ova/female with no significant differences found between them (Table 4).
3.2.3 In vitro fertilization and embryo development
The percentage of fertilized and developed ova counted as follows: Total developed ova (embryo)/Total developed embryo + Total undeveloped ova × 100.
From the 1294 ova flushed from all (60) females, only 433 ova with a percent of (33.46%) were fertilized and developed into embryos. The remaining 861 (66.53%) undeveloped ova. The percentage of embryos developed in vitro of: 34.49% for +/+, 27%. for +/ho 23%, and for ho/ho females 38.53% developed embryos at different stages. There were significant differences in the percent of fertilized and developed ova found between the different genotypes. If the tow phenotypically normal females pooled (+/ho with +/+) the rate of fertilized and developed ova were 30.49%. The rate number of developed embryos for each female was 8.4 for hotfoot female, 5.8 for the +/ho female, and 7.45for the +/+ female. Regarding the fertilization rate, or one cell stage, the proportion was 50% for the +/+ females, 49.1% for the +/ho females, and 56.5% for ho/ho females. The percentage of the developed embryos decreased significantly with the embryo development day or age, and the lowest proportion among the developed embryo was the 16 cell, morula and blastula stages of embryo development for all females (Table 4) and (Fig. 1). The percentage of undeveloped ova in the young adult +/+ females was 65.51%, 72.27% for +ho females, and 61.46% for ho/ho females, and there were significant differences among the three type of the young adult females.
3.2.4 Superovulation and in vitro fertilization of aged or old females
The mean age of the old female the +/ho 245 days, and 244 days for ho/ho female, with a mean body weight of 28.3 g and 24.4 g, respectively. The total number of flushed ova from the +/ho old females was 402, with a mean of 20.1 ova/female; and ho/ho produced 504 ova, with a mean of 25.2 ova/female. The total number of ova fertilized and developed from the +/ho was 233 embryos with a rate of 11.65 developed ova/female and 57.97% of fertilized ova and developed embryo. These consisting of 98 fertilized ova or zygotes, 60 embryo at 2 cell stage, 44 at 4 cell stage, 4 embryo at 8 cell stage, 11 embryo at 16 cell, with 7 morula and 7 blastula stage. For ho/ho females, a total of 250 ova and embryos were developed with rate of 12.5 fertilized ova and developed /female, and 53.97% fertilized ova and developed embryo. Which comprised of 115 ova at one cell stage, 87embryos at 2-cell stage, 32 at 4-cell stage, with five embryo at 8-cell stage, and 11embryos at 16-cell stage, 8 embryo at morula and 7 at blastula stage. The number of undeveloped ova from the +/ho old females was 169 with 42.03% ova. While the old ho/ho females showed the total of 239 fertilized and developed with 46.4% ova were undeveloped ova (Table 4).
3.2.5 The comparison between the young and old super ovulated females
If a comparison made between the old and young adult females utilized in the ART, the old females hotfoot mutation females showed significantly a better embryonic development rate (53.6%) with 13.25 fertilized and developed embryo/female compared to all young adult females (38.53% and 8.4 ova/female), although they showed about the same rate of flushed ova. In addition, the old hotfoot female showed less undeveloped ova rate 46.4% significantly compared to the all-young adult female more than 61% (Table 4).
4 Discussion
In 1966, Dickie, reported that hotfoot females are fertile, but that hotfoot males were infertile. The results of the current study indicate that both males and females with hotfoot are capable of breeding, and it is possible to maintain the gene by breeding homozygous mutant males with homozygous mutant females. A low incidence of sterility was observed among the hotfoot males compared to the normal (+/+ or +/ho) males. However, there were some indications of differences in the litter size of the hotfoot females compared to the normal females. Our results demonstrate that hotfoot females less than the phenotypically normal (+/+ and +/ho) females in the litter size. This could be due to the energy-consuming process of reproduction, (Peters, 1983), or because hotfoot mice cannot stand as well during eating, which might causes food restriction and a dramatic inhibition of both body weight and reproductive development in females (Hamilton and Bronson, 1985). The lack of major effect of the hotfoot gene on male reproduction also supported by the observation that only slight differences were noted between hotfoot and phenotypically normal males with regards to testis weight, sperm concentration, and sperm motility. Although the hotfoot males produce fewer sperm than normal males, the number were sufficient to facilitate normal fertilization. Thus, hotfoot male infertility may be related to either incapacity to copulate effectively because of physical impairment, such as that shown for the hemimelic extra toes (Hx) mutation, which causes male-specific infertility. Alternatively, as the mutant stubby (stb) mouse appears to be infertile because the mice are impotent, which may involve the central nervous system. The trembler (Tr) mutant resembles the hotfoot somewhat in its effects, in the paralysis of the hind legs. It considered possible that the hotfoot mutation might have differential effects on body weight, reproduction aging, sperm count, and ovulation. This was the rational for the study of induced ovulation and embryonic development in vitro in older females. Although a difference in body weight was still evident in the aged mice females, the difference in reproductive characteristics appeared not similar. The older females show better in body weight than young adult, hence that lead to better fertility and embryo development.
Cendelin 2014 described the use of ataxic mutant mice to represent models of cerebellar degenerative disorder. These models have roles in the investigation of cerebellar function, pathogenesis of degenerative processes, Hotfoot, Purkinje cell degeneration, and Niemann-Pick disease, with special regard to cerebellar pathogenesis, functional changes, and possible therapeutic influences (Cendelin, 2014). So our study indicate that the hotfoot mutation leads to indirect effects on reproduction, could be a good model for human. Single gene mutation of the waltzer syndrome as well as several other neurological mutations the hotfoot mutation have effect on growth, viability and fertility. Mice with such mutations typically exhibit a reduction in weight that occurs with the first appearance of neurological /behavioral abnormalities. The hotfoot mice are smaller and although the weight reduction is not as severe as in many neurological mutation, the general pattern of growth is similar and the mice continue to gain weight throughout the animals' life span. The hotfoot mother were somewhat less successful than +/+ and +/ho female in rearing young to weaning. Although a difference in body weight was still evident in the aged hotfoot females, reproductive characteristics appeared similar. The aged ho/ho females produced significantly more super-ovulated and developed embryos than old +/ho and young adult females. Again, lending support to the view that the slight reduction in litter size at term observed in ho/ho is a result of some event-taking place late in pregnancy or it could be due to less feeding behavior of hotfoot. Postnatal viability also reduced in hotfoot mice. The effect seems to be a direct effect of the gene as hotfoot progeny had similar survival regardless of the type of female reared them.
In conclusion, the hotfoot gene had a noticeable effect on the IVF of young adult compared to old adult hotfoot female, also the body weight growth and litter size of hotfoot less than normal mice.
Additional studies on hormonal levels, as well as studies on behavior, mating, and maternal behavior would be worth exploring further.
Acknowledgement
The authors would like to thank the Researchers Supporting Project for their funding this work at King Saud University, Riyadh, Saudi Arabia.
Funding
This research was funded by the Researchers Supporting Project number (RSP-2021/232), King Saud University, Riyadh, Saudi Arabia.
Competing interests
The authors declare no competing or financial interests.
Ethics approval
All the experiments conducted according to the Guidelines of the Committee of Zoology Dept. and Institutional Animal Care at King Saud University. CITI program, Animal Biosafety, Record ID 34305335.
Author contributions
Ahmad Alhimaidi: The idea of the research, animal and material purchasing, writing the manuscript, group coordinator, correspondent author.
Muath Al-Ghadi: The IVF and embryo culture follow up of the hot foot mice with data collecting.
Aiman Ammari: Animal matting, pregnancy with new borne mice weight and animal follow up.
Hissah Alhusani: Animal handling ova and Sperm collection from mice.
Ramzi Amran: Preparation of IVF and culture medium and chemical solution.
Khalid Alanazi: Statistical analysis of data, Article review.
Mohammad Alhimaidi: Animal feeding with animal follow up and take care.
References
- The in vitro fertilization and early embryonic development of two mouse strains and their hybrids, cultured in simple and complex culture media. J. Egypt Ger. Soc. Zool.. 2002;37:1-11.
- [Google Scholar]
- In vitro fertilization of mouse ova obtained by three ways and their cleavage in commercially and chemically defined media. Saudi J. Biol. Sci.. 1998;6:82-89.
- [Google Scholar]
- Genetic variants and strains of the laboratory mouse. Stuttgart: Gustav Fischer Verlag; 1981.
- Hamilton, G.D., Bronson, F.H., 1985. Food restriction and reproductive development in wild house mice. Biol. Reprod. 32, 773–778.
- Hotfoot mouse mutations affect the delta 2 glutamate receptor gene and are allelic to lurcher. Genomics. 1998;50:9-13.
- [Google Scholar]
- Ho15J: A new hotfoot allele in a hot spot in the gene encoding the delta2 glutamate receptor. Brain Res.. 2007;6:153-160.
- [Google Scholar]
- Mouse embryo assay for human in vitro fertilization quality control: a fresh look. J. Assist. Reprod. Genet.. 2020;37(5):1123-1127.
- [Google Scholar]
- Animal models of hereditary a taxia with special reference to rolling mouse nagya. In: Itsuro S., ed. TRH and spinocerbellar degenaration sobue (1st ed.). Amsterdam: Elsevier; 1986. p. :117-124.
- [Google Scholar]
- The ecology implication of body size. Cambridge: Cambridge University Press.UK; 1983.
- Southard, J.L., 1981. Hotfoot. Mouse News Lett. 64, 60.
- IVF of mouse ova in a simplex optimized medium supplemented with amino acids. Hum. Reprod.. 2000;15:1791-1801.
- [Google Scholar]
- Further delineation of the phenotype caused by a novel large homozygous deletion of GRID2 gene in an adult patient. Clin. Case Rep.. 2019;7(6):1149-1153.
- [CrossRef] [Google Scholar]







