Translate this page into:
The protective effect of Azadirachta excelsa leaves extract and quercetin treatment on the learning and memory impairments in relation with insulin and amylin levels in the brain of streptozotocin-induced diabetic rats
⁎Corresponding author. syaffinaz_zin@yahoo.com.my (Noor Syaffinaz Noor Mohamad Zin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Azadirachta excelsa increases the secretion of plasma insulin with subsequent reduction of fasting blood glucose. However, its protective effect towards brain amylin and insulin levels as well as the memory and learning functions remain elusive. Thus, this study aimed to ascertain the effect of A. excelsa and quercetin consumptions on the brain amylin and insulin concentrations as well as memory and learning abilities of the streptozotocin (STZ)-induced diabetic rats. Diabetic condition was induced in the male Sprague Dawley rats by the intraperitoneal injection of 60 mg/kg body weight (bwt) STZ. The experimental animals were divided into normal control (saline), diabetic control (saline), Metformin-treated diabetic (1000 mg/kg bwt) (positive control), A. excelsa-treated diabetic (250 mg/kg bwt) and Quercetin-treated diabetic (40 mg/kg bwt) rats. All treatments were administered orally by oral gavage once daily throughout the 8 weeks of the treatment period. Amylin and insulin concentrations were determined by using the commercial rat amylin and insulin immunoassay kits, while learning and memory consolidation parameters were conducted using Morris Water Maze (MWM) paradigm. Treatment with A. excelsa had significantly (p < 0.05) improved the concentration of both brain amylin and insulin. In MWM test, the administration of A. excelsa and quercetin significantly (p < 0.05) attenuated the learning and memory impairments in STZ-induced diabetic rats. These analytical results provide evidence that A. excelsa can improve learning and memory impairments due to diabetes by increasing the amylin and insulin levels. These also suggest that, quercetin might only improve the learning and memory impairment, but not be the one that contributed to the improvement in the brain amylin and insulin concentrations.
Keywords
Azadirachta excelsa
Streptozotocin-induced diabetes
Morris Water Maze
Cognitive impairment
Amylin
Insulin
- STZ
-
streptozotocin
- MWM
-
Morris Water Maze
- bwt
-
body weight
- NC
-
normal control
- DC
-
diabetic control
- DMET
-
metformin-treated diabetic rats
- DAE
-
A. excelsa-treated diabetic rats
- DQ
-
quercetin-treated diabetic rats
- FBG
-
fasting blood glucose
- EL
-
escape latency
- DT
-
distance travelled
- SS
-
swimming speed
- %TQ
-
percentage of time spent in target quadrant
- ANOVA
-
Analysis of Variance
Abbreviations
1 Introduction
A. excelsa is a species under the family of Meliaceae which is also known by several common names such as marrango tree, the Philippine neem tree, (Jack) Jacobs and “Sentang” (Orwa et al., 2009). The bitter old leaves of the plant have been consumed as a traditional medicine to treat dysentery and diarrhea (Hazandy, 2009). It has been found recently that A. excelsa ethanolic extract was able to significantly improve the fasting blood glucose by 60.7%, while the concentration of glycated hemoglobin was found to be 6.58% (Nurdiana et al., 2013). In addition, Nurdiana et al. (2013) also revealed the ability of A. excelsa to significantly increase the plasma insulin secretion in the diabetic rats. These might be contributed by the distribution of active compounds in the plant. Sithisarn et al. (2007) tested the presence of quercetin compound in all three species of Azadirachta leaves extract. Interestingly, average quercetin content in A. excelsa was higher as compared to A. indica A. Juss. var. siamensis Valeton and A. indica A. Juss.
Quercetin (3, 5, 7, 39, 49-pentahydroxyflavone), the most common flavonol found in plants contains various beneficial effects to human health. It is part of the polyphenols that is known to carry a strong antioxidant property to help prevent oxidant injury and cell death, which could lead to diverse health problems (Sirovina et al., 2013; Khanam et al., 2015). Recently, the ability of quercetin to combat against hyperglycemia and hyperlipidemia in diabetic rats were determined (Bhutada et al., 2010; Kim et al., 2011; Jadhav and Puchchakayala, 2012). Not only has that, antioxidant properties of quercetin also helped in improving spatial learning and memory in diabetic rats (Bhutada et al., 2010).
Learning and memory deficits are recognised to be one of the cognitive impairments due to diabetes. Cheng et al. (2012) revealed a higher risk factor of cognitive impairment among diabetic patients as compared to the general population. Various studies have suggested the contribution of amyloid-β deposition in the brain as the pathophysiological factor of cognitive impairment in both human and animal subjects (Despa et al., 2012; Srodulski et al., 2014). Interestingly, the accumulation of amyloid-β not only occurred in the transgenic rat models (Do Carmo and Cuello, 2013), but also in the STZ-induced diabetic rats (Liu et al., 2008, 2012b). Nevertheless, it has been found that the presence of monomeric amylin is important to reduce amyloid burden which then leads to the improvement in learning and memory consolidation (Zhu et al., 2015). In addition, the contribution of insulin deficiency and/or resistance in cognitive impairment has also been emphasised recently. According to De la Monte (2012), inhibition of insulin or insulin growth factor contributes to neurodegeneration via cellular and functional basis. Absence of insulin signaling disturbs several pathways and may lead to elevation of phosphorylated tau, accumulation of amyloid-β, oxidative and endoplasmic reticulum stress, generation of reactive oxygen and nitrogen species, mitochondrial dysfunction, activation of pro-inflammatory and pro-apoptosis cascades as well as the down regulation of neuronal plasticity, memory and cognition.
Various therapeutic agents have been developed to combat cognitive impairment due to diabetes. Pramlintide; the clinical analog of amylin with the substitution of prolines at positions 25, 28 and 29 amino acids have been used in animal models (Adler et al., 2014; Zhu et al., 2015). Meanwhile, exogenous insulin has been used in both human and animal models (Biessels et al., 1998; Reger et al., 2006; Reger et al., 2008; Viswaprakash et al., 2015; Claxton et al., 2015). Unfortunately, the consumption of those synthetic agents could bring various side effects which include weight loss (Adler et al., 2014) and nausea (Aronne et al., 2007) for pramlintide as well as the risk of hypoglycemic episodes for insulin (Ahren, 2013). These are, therefore, bring to the new insights on using the natural plant as a remedy for the cognitive impairment due to diabetes. In this study, the scope of the cognitive impairment was specified into the spatial learning and memory deficit in streptozotocin (STZ)-induced diabetic rats. This model was chosen as it might reveal either the deficiency of amylin and insulin in the brain could contribute to the learning and memory impairment. Thus, this study was conducted to assess the ability of A. excelsa and quercetin to improve the brain amylin and insulin as well as their contribution to the learning and memory impairment.
2 Material and methods
2.1 Collection and authentication of the plant specimen
A. excelsa leaves were collected from the Forestry Research Institute Malaysia (FRIM), Kepong, Selangor Darul Ehsan, Malaysia. The plant specimen was authenticated by Mr. Sani Miran from Herbarium Universiti Kebangsaan Malaysia (UKMB), Fakulti Sains dan Teknologi, Universiti Kebangsaan Malaysia, Bandar Baru Bangi, Selangor Darul Ehsan, Malaysia with voucher number: UKMB40314.
2.2 Preparation of A. Excelsa leaves extract
The leaves were washed, dried and ground before it were soaked in 70% ethanol (Nurul ‘Izzati et al., 2015) with 1:10, weight:volume ratio for two days at room temperature. The extract was obtained by filtering the suspension. The extract was then evaporated using the Buchi R-215 rotavapor (Buchi, Switzerland) at 40 °C until it was completely dried. A dark semi-solid material was stored at 4 °C until further used.
2.3 Identification of quercetin content in A. Excelsa
The dark semi-solid extract of A. excelsa was sent to third party (ChromaDex, Inc., USA) to conduct the High Performance Liquid Chromatography (HPLC) analysis on the quercetin content of the extract.
2.4 Experimental animals
Thirty male Sprague-Dawley rats weights between 200–250 g (Chenur Supplier, Selangor, Malaysia) were used in this study. Upon delivery, the rats were acclimatised for a week and exposed to 12-h dark/light cycle at 22 °C, housed in groups of six, and fed with a standard commercial rodent diet (Gold Coin Feedmills, Gold Coin Holdings, Malaysia) and plain water ad libitum. The care and maintenance of the experimental animals were closely observed and followed the standard guideline approved by the Committee of Animal Research Ethics, Universiti Tekonologi MARA (UiTM Care) (Ref No: 112/2015).
2.5 Experimental protocol
2.5.1 Induction of diabetes
After one week of acclimatisation, induction of diabetes was conducted through a single intraperitoneal injection of 0.5 ml STZ (Sigma Chemicals, USA) at 60 mg/kg bwt (Bhutada et al., 2010; Sabahi et al., 2016). STZ was freshly prepared in saline solution (0.9% sodium chloride, Sigma Chemicals, USA) at 4 °C. During the induction period, food and drink were provided ad libitum. To prevent from the drug-induced hypoglycemic shock, 5% D-glucose (Sigma Chemicals, USA) water was given orally by using oral gavage for two days immediately after the injection (Tuzcu and Baydas, 2006). On day seven, the blood sample was obtained by a “tail nick” using a 27G needle (Terumo, Canada) (Sang et al., 2015) and the blood glucose levels of the rats were estimated by using Glucometer ACCU-CHEK® Performa (Hoffmann-La Roche Ltd., Dubai). Post induction, the rats showing stable fasting blood glucose level of 11.0 mmol/L and above were considered diabetic and selected for further experimentation.
2.5.2 Experimental design
The experimental animals were grouped accordingly as shown in Table 1. Each group comprised of 6 rats. Treatments were given once a day for a duration of 8 weeks with 1 ml/100 g bwt volume for each treatment. The saline solution was made up of 0.9% sodium chloride (Sigma Chemicals, USA), while metformin was obtained from Hovid Berhad, Malaysia. The average fasting blood glucose (FBG) and body weight were determined at the beginning and the end of the experimental period. The treatment groups consist of NC (normal control), DC (diabetic control), DMET (diabetic treated with metformin, DAE (diabetic treated with A. excelsa extract) and DQ (diabetic treated with quercetin).
Groups
Treatment
Dosage
A
NC
Saline
–
B
DC
Saline
–
C
DMET
Metformin
1000 mg/kg bwt (Tahara et al., 2008)
D
DAE
A. excelsa extract
250 mg/kg bwt (Nurdiana et al., 2014; Nur Syimal’ain et al., 2017)
E
DQ
Quercetin
40 mg/kg bwt (Bhutada et al., 2010; Kandhare et al. 2012)
2.6 Morris Water Maze test
Animals were tested in a spatial version of MWM test (Morris et al., 1982; Bhutada et al., 2010). The apparatus consists of a circular water tank (180 × 60 cm of diameter and height) within which an invisible platform (12.5 × 38 cm of diameter and height) was placed. The apparatus was attached to the computer program (ANY-Maze Behaviour Tracking Software, Stoelting Co., USA) for automatically tracking and analyzing the swimming path of an animal in a large pool of water. The tank that was partially filled with water was maintained approximately at 25 ± 0.5 °C of temperature, and the platform was kept inside the tank and located in the middle of a selected quadrant about 22 cm of distance from the tank wall. There were several brightly colored cues visible around the water maze and be used by the experimental animals for spatial orientation. The position of the cues remains unchanged throughout the study. The escape platform was kept in the same position respective to the colored cues.
2.6.1 Learning test
A place navigation test was performed wherein the extent of learning was assessed. The rats were given five training trials daily in 5 consecutive days. Each trial has a ceiling time of 60 s and a trial interval of approximately 20 s. In each trial, the animals were gently placed in the random starting quadrant with the head facing the wall (Garthe and Kempermann, 2013; Gao et al., 2015). On Days 1 and 2, the task was conducted with a visible platform where it was placed slightly above the water surface to be visible by the rats. On Days 3 to 5, the task was conducted with hidden platform where it was submerged underneath the water surface. For each trial, the animal was left on the platform for 20 s after climbing on it before the commencement of the next trial. The escape platform was kept in the same position relative to the distal cues. The escape latency (EL), swimming speed (SS) and distance travelled (DT), were measured. If the rats failed to reach the escape platform within the maximally allowed time of 60 s, it was gently placed on the platform and remain there for 20 s.
2.6.2 Memory consolidation test
A spatial probe test was conducted wherein the extent of memory was assessed (Bhutada et al., 2010; Garthe and Kempermann, 2013). The time spent in the target quadrant indicates the degree of memory of rats after the completion of the training period. In the probe trial, the hidden platform was removed from the pool. The rat was placed in the pool at the quadrant opposite to the previously removed platform. The EL, SS, DT and percentage of time spent in the target quadrant (%TQ) were recorded in 60 s.
2.7 Preparation of tissue homogenate
Fresh brain slices from groups of treated rats were separately homogenized in ice-cold phosphate buffer (4 °C, pH 7.6; Sigma Chemicals, USA). It was then centrifuged at 3000 rpm for 15 min at 4 °C (Centrifuge 5417R; Eppendorf, Germany). The supernatants were then collected and stored at −80 °C until used.
2.7.1 Determination of the brain amylin and insulin concentrations
The amylin and insulin levels in the brain were determined by using Rat Amylin Enzyme Immunoassay Kit (Ray Biotech, USA) and Rat Enzyme Linked Immunosorbent Assay kit for insulin (Cloud-Clone Incorporation, USA), respectively. All procedures involved were provided by the manufacturer. Those hormonal activities were analysed using the microplate spectrophotometer (Epoch 2 microplate spectrophotometer; BioTek, USA).
2.8 Statistical analysis
All data were expressed as means ± standard error means (mean ± SEM) and dissected using the Statistical Package for the Social Sciences (SPSS) 20.0 software (IBM Corporation, USA). A paired t-test was conducted to analyse the differences in the mean of FBG and body weight at the onset and the end of the study. In the case of multiple mean comparisons of the brain amylin and insulin concentrations, the data were analysed by a one-way Analysis of Variance (ANOVA) followed by Duncan’s post hoc test. Two-way repeated measured ANOVA was conducted to analyse the behavioral data and was also followed by Duncan’s post hoc test. A p-value less than 0.05 was regarded as the criterion for significance.
3 Results
HPLC analysis that was conducted prior to the experiment had confirmed the presence of quercetin in A. excelsa extract. Figures 1(a) and (b) showed the chromatograms of A. excelsa ethanolic extract and the quercetin standard, respectively.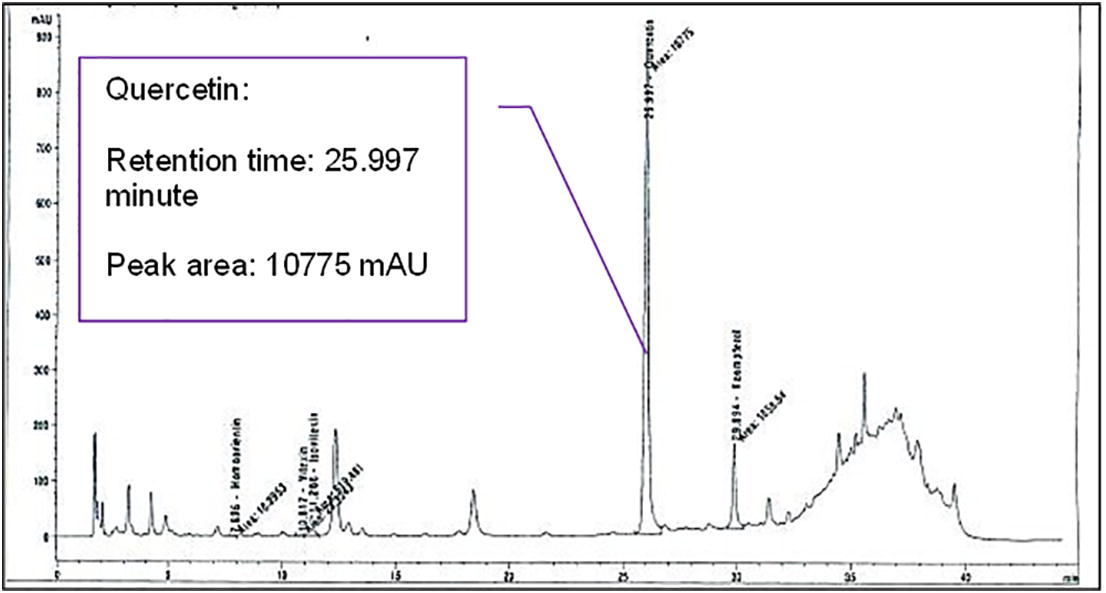
The Chromatogram of the Active Compound in A. excelsa Leaves Extract.
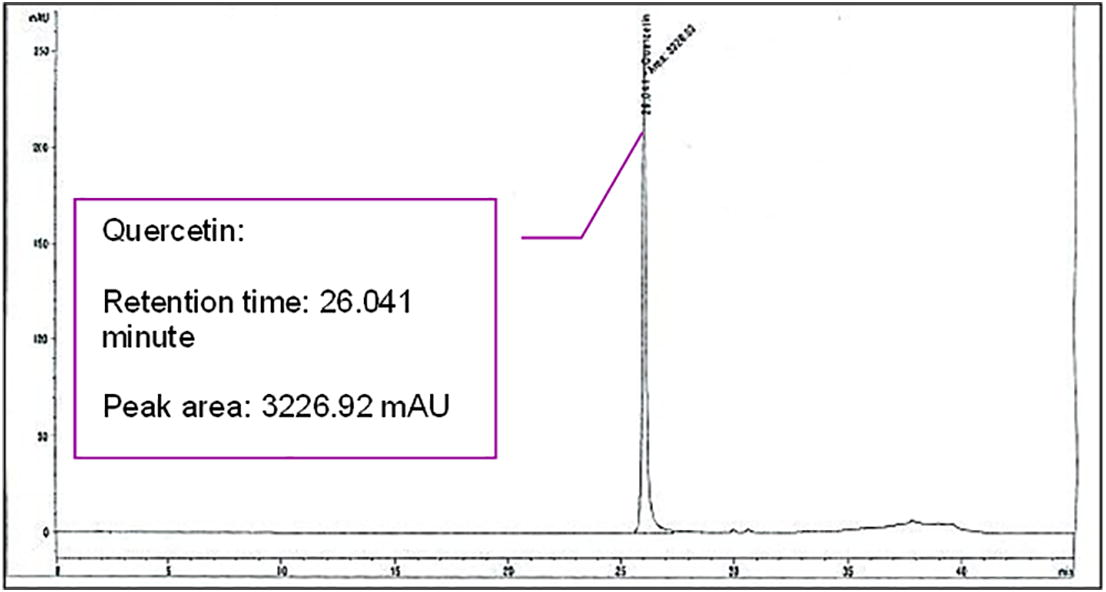
The Chromatogram of the Quercetin Standard.
The effects of the treatment with A. excelsa and quercetin towards the FBG and body weight of the STZ-induced diabetic rats are presented in Table 2. At the end of the experimental period, treatments with metformin, A. excelsa and quercetin revealed a spectacular reduction of FBG levels, which were by 32.08 ± 7.14%, 22.21 ± 9.95% and 14.55 ± 8.38%, respectively from the baseline. On the other hand, DC group showed a significant (p < 0.05) increased of FBG after the treatment period ends, which as much as 52.96 ± 24.24% as compared to the baseline. In addition, DC group had demonstrated a significantly (p < 0.05) lower body weight as compared to NC group. From the data, the percentages of body weight changes at the beginning and at the end of the treatment period were obtained. The highest percentage of increment in body weight can be seen in DQ group which was 18.67 ± 6.47%. This was followed by DAE group which was 18.07 ± 16.63%. On the other hand, the DC and DMET groups experienced as much as 11.36 ± 9.43% and 4.63 ± 4.85% reduction of body weight, respectively. Paired t-test conducted to compare the FBG and body weight at the onset and the end of the treatment period showed no significant differences (p > 0.001). Superscripts ashowed the significant difference (p < 0.05) between the NC group and bshowed the significant difference (p < 0.05) between the DC group for each of the parameters tested, where NC (normal control), DC (diabetic control), DMET (diabetic treated with metformin, DAE (diabetic treated with A. excelsa extract) and DQ (diabetic treated with quercetin).
Groups
FBG (mmol/L)
Body weight (g)
Onset of the study
End of the study
Onset of the study
End of the study
NC
4.80 ± 0.17b
4.93 ± 0.21b
402.00 ± 38.20
432.33 ± 44.14b
DC
20.00 ± 1.87a
30.13 ± 2.63a
296.33 ± 24.03
261.67 ± 32.27a
DMET
29.30 ± 2.14ab
19.83 ± 3.75ab
270.33 ± 44.64
254.00 ± 29.48a
DAE
30.33 ± 1.56ab
23.33 ± 3.79ab
268.00 ± 43.58
300.33 ± 37.30a
DQ
28.87 ± 2.62ab
24.33 ± 2.47ab
302.33 ± 25.96
305.67 ± 23.31a
Table 3 showed the concentrations of brain amylin and insulin in all groups after the eight weeks of the treatment period. A. excelsa treated group showed a significant (p < 0.05) improvement in the brain amylin (47.80 ± 1.15 ng/ml) and insulin (62.31 ± 0.26 pg/ml) concentration as compared to DC with 33.34 ± 1.19 ng/ml amylin and 44.93 ± 3.45 pg/ml insulin. Superscripts ashowed the significant difference (p < 0.05) between NC group, bshowed the significant difference (p < 0.05) between DC group, while cshowed the significant difference (p < 0.05) between DMET group for each of the parameter tested, where NC (normal control), DC (diabetic control), DMET (diabetic treated with metformin, DAE (diabetic treated with A. excelsa extract) and DQ (diabetic treated with quercetin).
Groups
Brain amylin (ng/ml)
Brain insulin (pg/ml)
NC
53.34 ± 6.73b
63.15 ± 0.38b
DC
33.34 ± 1.19a
44.93 ± 3.45a
DMET
36.23 ± 3.57a
43.04 ± 3.87a
DAE
47.80 ± 1.15
62.31 ± 0.26bc
DQ
35.48 ± 3.59a
38.38 ± 1.66a
Fig. 2 showed the results obtained in five days of MWM test which includes EL, DT and SS. On Day 1, all rats showed no significant differences in EL and DT. However, DQ group showed significantly (p < 0.05) higher SS (0.36 ± 0.06 m/s) as compared to DC and DMET groups (0.20 ± 0.05 m/s and 0.18 ± 0.03 m/s, respectively). The DMET group showed the alleviation in EL on Days 2, 4 and 5 (24.41 ± 9.39 s, 17.26 ± 10.21 s and 6.72 ± 2.44 s, respectively) as compared with Day 1 (37.43 ± 4.92 s) and Day 3 (45.13 ± 8.69 s). Similarly, the DAE group had shown a significant improvement (p < 0.05) in EL on Days 2, 4 and 5 (37.38 ± 8.66 s, 17.77 ± 6.67 s, 8.46 ± 2.64 s, respectively) as compared with Day 1 (55.69 ± 2.49 s) and Day 3 (49.37 ± 4.06 s). In addition, DQ group showed a significant reduction (p < 0.05) of the EL from Days 2 to 5 (19.22 ± 10.13 s, 12.29 ± 5.62 s, 10.21 ± 3.17 s and 12.17 ± 2.04 s, respectively) as compared to Day 1 (37.34 ± 5.34 s).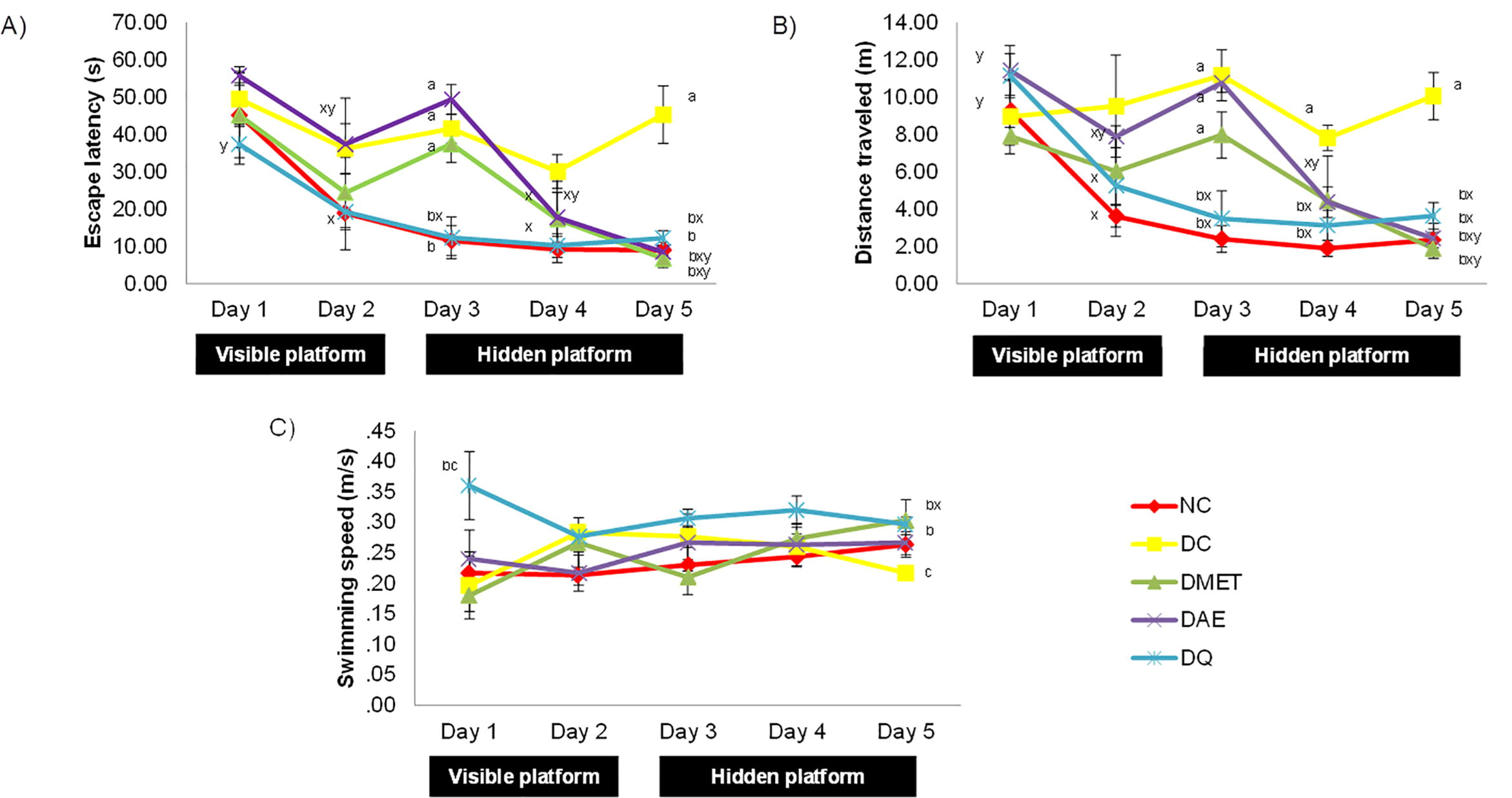
(A) Escape Latency, (B) Mean Path Length and (C) Swimming Speed of Rats in All Groups for 5 Days Learning Test. Superscripts ashowed the significant difference (p < 0.05) between NC group, bshowed the significant difference (p < 0.05) between DC group, cshowed the significant difference (p < 0.05) between DMET group, xshowed the significant difference (p < 0.05) between Day 1, yshowed the significant difference (p < 0.05) between Day 3 for each of the parameters tested. NC (normal control), DC (diabetic control), DMET (diabetic treated with metformin, DAE (diabetic treated with A. excelsa extract) and DQ (diabetic treated with quercetin).
A similar pattern of improvement in EL can be observed in DT. The DMET group had shown the improvement in DT on Days 2, 4 and 5 (6.03 ± 1.78 m, 4.46 ± 2.39 m and 1.88 ± 0.52 m, respectively) as compared with Day 1 (7.90 ± 0.48 m) and Day 3 (7.97 ± 1.23 m). Similarly, the DAE group also showed a significant alleviation (p < 0.05) in DT on Days 2, 4 and 5 (7.88 ± 0.59 m, 4.37 ± 0.82 m and 2.42 ± 0.81 m, respectively) as compared with Day 1 (11.43 ± 1.34 m) and Day 3 (10.76 ± 0.51 m). In addition, the significant improvement (p < 0.05) of DT can also be seen in the DQ group from Days 2 to 5 (5.25 ± 2.72 m, 3.48 ± 1.49 m, 3.12 ± 0.82 m and 3.63 ± 0.72 m, respectively) as compared to Day 1 (11.15 ± 1.19 m).
When vertical analysis was conducted, it can be clearly seen that on Day 3 when the hidden platform paradigm started, all groups except NC and DQ groups showed a significant increment in EL and DT. The EL for DC, DMET and DAE groups were 41.55 ± 3.90 s, 37.43 ± 4.92 s and 49.37 ± 4.06 s, respectively, while the NC and DQ groups were 11.51 ± 4.03 s and 12.29 ± 5.62 s, respectively. On the other hand, the DT for DC, DMET and DAE groups were 11.17 ± 1.36 m, 7.97 ± 1.23 m and 10.76 ± 0.51 m, respectively, while the NC and DQ groups were 2.39 ± 0.72 m and 3.48 ± 1.49 m, respectively. Surprisingly, on the 5th day of the test, NC, DMET, DAE and DQ groups showed significantly (p < 0.05) lower EL (8.97 ± 1.30 s, 6.72 ± 2.44 s, 17.70 ± 6.74 s and 12.17 ± 2.04 s, respectively) as compared to DC group (45.30 ± 7.71 s). In addition, they also showed a significant (p < 0.05) lower DT (2.34 ± 0.40 m, 1.88 ± 0.52 m, 4.12 ± 0.97 m and 3.63 ± 0.72 m, respectively) as compared to DC group (10.06 ± 1.27 m).
It was clearly shown in Table 4 that DC group had the significantly (p < 0.05) highest EL, longer DT and lowest%TQ (42.57 ± 1.49 s, 8.79 ± 0.19 m and 14.90 ± 1.59%, respectively). On the other hand, DAE and DQ groups showed a significantly (p < 0.05) lower EL, shorter DT and higher%TQ (23.20 ± 7.81 s, 3.52 ± 0.55 m and 27.01 ± 2.42%, respectively, for DAE group, while 12.60 ± 4.23 s, 2.82 ± 0.81 m and 36.10 ± 5.26%, respectively for DQ group) as compared to DC. However, treatment with metformin only showed a significant (p < 0.05) improvement in EL and DT (11.27 ± 0.82 s and 3.25 ± 0.65 m, respectively) as compared to DC group. There was no significant difference (p > 0.05) in SS of treated groups as compared with DC group. Superscripts ashowed the significant difference (p < 0.05) between NC group, bshowed the significant difference (p < 0.05) between DC group, while cshowed the significant difference (p < 0.05) between DMET group for each of the parameters tested, where NC (normal control), DC (diabetic control), DMET (diabetic treated with metformin, DAE (diabetic treated with A. excelsa extract) and DQ (diabetic treated with quercetin).
Group
EL (s)
DT (m)
SS (m/s)
%TQ (%)
NC
7.70 ± 0.61bc
1.84 ± 0.27b
0.27 ± 0.01
37.81 ± 3.12bc
DC
42.57 ± 1.49ac
8.79 ± 0.19a
0.21 ± 0.00
14.90 ± 1.59a
DMET
11.27 ± 0.82b
3.25 ± 0.65b
0.30 ± 0.03
21.97 ± 2.79a
DAE
23.20 ± 7.81ab
3.52 ± 0.55b
0.24 ± 0.06
27.01 ± 2.42ab
DQ
12.60 ± 4.23b
2.82 ± 0.81b
0.22 ± 0.02
36.10 ± 5.26bc
Pearson’s correlation showed a negative correlation between brain insulin concentration and the EL (r = −0.228; r2 = 0.052) as well as DT (r = −0.227; r2 = 0.051) on Day 5 of the training. In addition, it also showed the negative correlation between the brain insulin concentration and the EL (r = −0.161; r2 = 0.026) as well as DT (r = −0.351; r2 = 0.123), but positive correlation between brain insulin level and the%TQ (r = 0.343; r2 = 0.118) in memory consolidation test. The relationship between brain amylin level with the learning and memory consolidation parameters also showed the similar pattern. It was found that, the brain amylin level had a negative correlation with EL (r = −0.420; r2 = 0.176) and DT (r = −0.459; r2 = 0.211) in learning test. Interestingly, brain amylin concentration manifested a significant correlation with the%TQ (r = 0.648; r2 = 0.420; p = 0.009) and DT (r = −0.585; r2 = 0.343; p = 0.022) in the memory consolidation test. The significant correlation between brain amylin and%TQ as well as DT are illustrated in Fig. 3, while others are illustrated in the Appendices 1 and 2.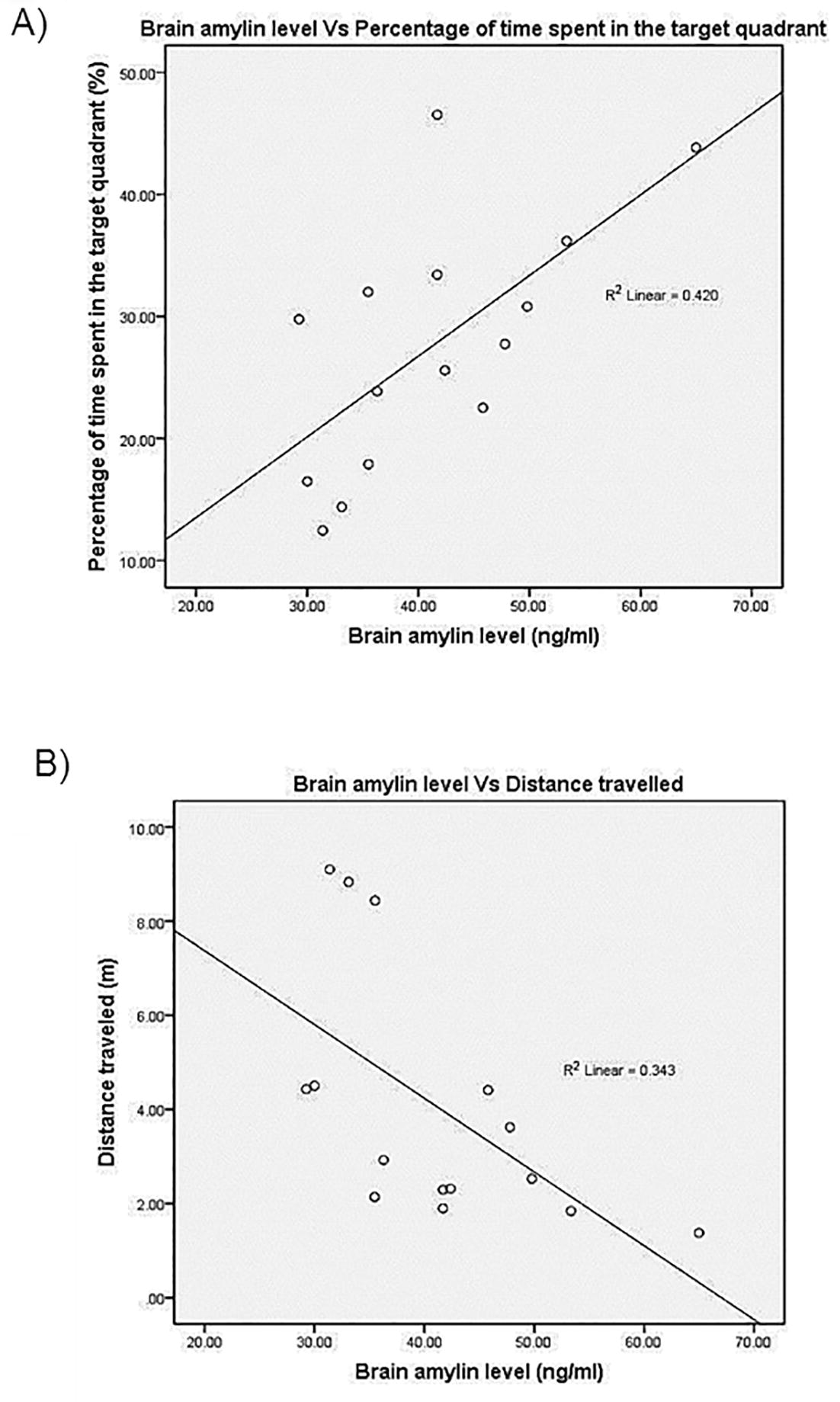
Pearson’s Correlation and Regression Between the Brain Amylin and the (A) Percentage of Time Spent in the Target Quadrant and (B) Distance Travelled During the Memory Test.
4 Discussion
In this study, the effect of A. excelsa treatment towards hyperglycemia and its complication in the learning and memory decline were ascertained. It was incorporated with quercetin as the highly antioxidant content active compound that presence in a wide scope of plant kingdom. The HPLC analysis confirmed the presence of quercetin in the ethanolic extract of A. excelsa and this is in line with the previous studies conducted by Sithisarn et al. (2007). Thus, it is hypothesised that quercetin might be one of the contributing factors for the protective properties of A. excelsa against learning and memory impairment due to diabetes.
At the onset of the study, the DC group showed a significantly lower FBG as compared with DMET, DAE and DQ. This was to ensure that the DC rats could sustain until the end of the experiment since they were not receiving any treatment. The significant difference of FBG at the onset of the experiment between the diabetic control group and the diabetic-treated groups had also been demonstrated previously by Oyedemi et al. (2011). The hypoglycemic effect of 250 mg/kg bwt A. excelsa as well as 40 mg/kg bwt quercetin could be clearly seen when both concentrations had significantly reduced the FBG among STZ-induced diabetic rats. This might be given by their antioxidant properties in reducing oxidative stress in the pancreas caused by the STZ induction effect (Szkudelski, 2001), hence ameliorates the glucose metabolism in the diabetic rats. The no significant difference of both A. excelsa and quercetin as compared to 1000 mg/kg bwt metformin treatment suggests that the two dosages were more robust than the effect of metformin in treating hyperglycemia. The ability of A. excelsa and quercetin in reducing FBG is in line with previous studies (Bhutada et al., 2010; Nurdiana et al., 2013).
The STZ induction does exhibit a significantly lower body weight in the diabetic control group as compared to the normal control. This is corroborated by the previous studies by Rasineni et al. (2010). Surprisingly, metformin was not able to significantly prevent body weight loss in diabetic rats, and it is in agreement with previous studies by Erejuwa et al. (2011), Pournaghi et al. (2012) and Kamboj et al. (2013). According to Malin and Kashyap (2014), the consumption of metformin causes the elevation of 5′ adenosine monophosphate-activated protein kinase (AMPK) in the adipose tissue which leads to the inhibition of body weight gain. On the other hand, treatment with A. excelsa and quercetin caused a significant improvement in body weight as compared to the diabetic control rats. Restoration of the body weight loss in diabetic rats by quercetin had also been revealed by Buthada et al. (2010). Furthermore, the distinct enhancement can also be evaluated in the consumption of traditional medicine and phytochemical as been indicated previously using Abrus precatorius leaf extract (Umamahesh and Veeresham, 2016) and gallic acid (Latha and Daisy, 2011), respectively. The consumption of both A. excelsa and quercetin might manifest a different action in glucose metabolism and fat homeostasis in diabetes from metformin.
Furthermore, this study also revealed that amylin and insulin deficiencies could contribute to the learning and memory impairment. STZ–induced diabetic rats had significantly reduced both amylin and insulin concentrations in the brain as compared to the normal control rats. Amylin is a 37 amino acid peptide that is synthesized and co-secreted with insulin by the pancreatic β-cells (Kahn et al., 1990). The presence of insulin in the brain remains controversial either it is synthesized in the brain or fully transported from the pancreas. However, it is clear that insulin does have the ability to cross the blood brain barrier and is abundantly detected in the brain (Liu et al., 2015). Meanwhile, it was previously proven that the amylin presence in the brain is derived from the circulation, as no amylin mRNA detected in the brain (Banks et al., 1995). Thus, this suggested that both insulin and amylin, are mainly exist in the brain via the transport from the blood across the blood brain barrier (Banks and Kastin, 1998). With the reduction of pancreatic insulin and amylin production as STZ caused the destruction of pancreatic β-cells (Horcajada-Molteni et al., 2001), the transport of both hormones to the brain might also be affected. In this study, treatment with metformin did not show any significant improvement in the brain insulin, while only small insignificant improvement in the brain amylin concentration. This might be explained by the inability of metformin to directly stimulate the insulin secretion. Instead, the capability of metformin in improving blood glucose might be contributed by the reduction in the insulin-mediated hepatic glucose production as well as increases the peripheral glucose disposal (Sebastiao et al., 2014). Plus, the regeneration of the Islet of Langerhans by metformin in STZ-induced diabetic rats might not reflect its improvement in β-cells function as metformin alone did not manifest a well preservation against the DNA damages as well as fibrosis and shrinkage cells as compared to its combination with Clover honey (Abou El-Soud et al., 2016). Since STZ had destructed the ability of pancreatic β-cells to secrete insulin and amylin, this simultaneously caused the reduction of both hormones in the brain. Treatment with metformin alone was not powerful enough to alleviate this impact. Surprisingly, treatment with A. excelsa was able to restore the concentration of both brain amylin and insulin in this group, but not quercetin. This suggests that 40 mg/kg bwt quercetin might not enough to facilitate the transportation of both hormones due to diabetic complications. There could also be a synergistic effect of other active compounds that present in the A. excelsa ethanolic extract as well.
The improvement in the FBG level, brain amylin and insulin in A. excelsa treated rats consequently improved spatial learning ability and memory consolidation of the diabetic rats. In the MWM test, the visible platform tested the changes of the sensorimotor function affected by the STZ-induction (Bhutada et al., 2010). All rats seemed to have a poor performance on the first day. This might be due to the adaptation period of the rats. On Day 2, the rats seemed to have a better performance, which indicated that STZ-induction did not manifest a marked sensorimotor changes among the diabetic rats. However, it can be seen that there was a slight increased in EL on Day 3 in DC, DMET and DAE groups. This might be due to the changes in the test phase, which was from visible platform to hidden platform. Meanwhile, DQ group seemed to not be affected by the changes. This might suggest that the treatment with quercetin not only manifested a better sensorimotor function, but tend to retain a search memory faster as compared to the other treatments, which directly facilitate the learning progress. This outstanding performance of quercetin treated diabetic rats was also demonstrated previously by Bhutada et al. (2010). At the end of the five consecutive days of learning test, all the treated groups showed a significant reduction in EL and DT as compared to DC group. This showed that the diabetic rats had poorer performance in searching for the platform using the brightly colored cues. The deprivation in the learning ability of diabetic rats had also been reported earlier (Kuhad et al., 2008; Xia et al., 2014). In addition, it was clearly shown that DC group had a poorer memory consolidation performance as compared to the NC and other treated groups during the probe test. The reduction in memory consolidation among the DC group had also been suggested by previous studies (Liu et al., 2012b,a). These results demonstrated that all treatments were able to improve memory and learning among diabetic rats within eight weeks of treatment. The ability of quercetin to decrease the EL and increase the%TQ is in line with previous studies (Bhutada et al., 2010). However, the inability of metformin to significantly alleviate the%TQ as the main indicator in determining the degree of memory consolidation marked a weakness of metformin as compared with A. excelsa and quercetin in ameliorating memory impairment.
Based on the Pearson’s correlation coefficient, it can be summarised that the brain insulin and amylin deficiencies caused the impairment in both learning and memory consolidation parameters in the STZ-induced diabetic rats. Weak correlation between the brain insulin and the learning as well as the memory consolidation parameters suggested that insulin might play only a supporting role in the alleviation of the learning and memory impairment in the treated rats. Nevertheless, improvement in both hormones level by A. excelsa leaves extract do caused an improvement in the learning and memory consolidation parameters. The presence of amylin in the brain is important as it helps in improving glucose metabolism, relaxing cerebrovascular structure, modulating inflammatory reaction and enhancing neural regeneration (Qiu et al., 2014). In addition, the maintenance of neuronal insulin signaling contributes to the protection of synaptic plasticity, from the disturbances of oxidative energy metabolism or other metabolic insults (Querfurth and LaFerla, 2010). Even though quercetin did not significantly increased the brain amylin and insulin levels, the improvement in learning and memory consolidation might be contributed by some other factors which include its antioxidant properties that ameliorate the oxidative stress caused by hyperglycemia. Thus, this recommend that not only quercetin that is responsible in the neuroprotective effects of A. excelsa in diabetic rats. Perhaps, it is caused by the synergistic effect between quercetin and other active compounds present in the A. excelsa ethanolic that need to be searched further in the future study.
5 Conclusion
This study found that A. excelsa leaves ethanolic extract gave promising results in ameliorating hyperglycemia, brain amylin and insulin deficiencies as well as memory and learning ability in diabetic rats. Therefore, it is concluded that A. excelsa has a significant therapeutic potential to impede cognitive impairment due to diabetes.
Acknowledgements
This project was financially supported by Fundamental Research Grant Scheme (600-RMI/FRGS 5/3 (5/2014)) and Faculty of Applied Sciences, Universiti Teknologi MARA.
References
- Honey versus metformin: effects on pancreatic beta-cells in streptozotocin induced diabetic rats. Der Pharma Chemica. 2016;8(16):29-39.
- [Google Scholar]
- Neuroprotective effects of the amylin analogue pramlintide on Alzheimer’s disease pathogenesis and cognition. Neurobiol. Aging. 2014;35:793-801.
- [Google Scholar]
- Avoiding hypoglycaemia: a key to success for glucose-lowering therapy in type 2 diabetes. Vasc. Health Risk Manage.. 2013;9:155-163.
- [Google Scholar]
- Progressive reduction in body weight after treatment with amylin analogue pramlintide in obese subjects: A phase 2, randomized, placebo-controlled, dose-escalation study. J. Endocrinol. Metab.. 2007;92(8):2977-2983.
- [Google Scholar]
- Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides. 1998;19(5):883-889.
- [Google Scholar]
- Permeability of the blood-brain barrier to amylin. Life Sci.. 1995;57(22):1993-2001.
- [Google Scholar]
- Neurobiology of learning and memory ameliorative effect of quercetin on memory dysfunction in streptozotocin-induced diabetic rats. Neurobiol. Learn. Mem.. 2010;94(3):293-302.
- [Google Scholar]
- Water maze learning and hippocampal synaptic plasticity in streptozotocin – diabetic rats: Effects of insulin treatment. Brain Res.. 1998;800:125-135.
- [Google Scholar]
- Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis for longitudinal studies. Int. Med. J.. 2012;42(5):484-491.
- [Google Scholar]
- Long-acting intranasal insulin determir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J. Alzheimer’s Dis.. 2015;44:897-906.
- [Google Scholar]
- Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr. Alzheimer Res.. 2012;9(1):35-66.
- [Google Scholar]
- Hyeramylinemia contributes to cardiac dysfunction in obesity and diabetes–a study in humans and rats. Circ. Res.. 2012;110(4):598-608.
- [Google Scholar]
- Glibenclamide and metformin combined with honey improves glycemic control in streptozotocin-induced diabetic rats. Int. J. Biol. Sci.. 2011;7(2):244-252.
- [Google Scholar]
- Salidroside amelioratescognitive impairment in a D-galactose-induced rat model of Alzheimer’s disease. Behav. Brain Res.. 2015;293(2015):27-33.
- [Google Scholar]
- An old test for new neurons: refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front. Neurosci.. 2013;7:63.
- [CrossRef] [Google Scholar]
- Genetic variation of six Azadirachta excelsa (Jacks) Jacob populations. Int. J. Biol.. 2009;1(2):34-40.
- [Google Scholar]
- Amylin and bone metabolism in streptozotocin-induced diabetic rats. J. Bone Miner. Res.. 2001;16(5):958-965.
- [Google Scholar]
- Hypoglycemic and antidiabetic activity of flavonoids: Boswellic acid, ellagic acid, quercetin, rutin on streptozotocin-nicotinamide induced type 2 diabetic rats. Int. J. Pharmaceutical Sci.. 2012;4(2):251-256.
- [Google Scholar]
- Evidence of cosecretion of islet amyloid polypeptide and insulin by β-cells. Diabetes. 1990;39:634-638.
- [Google Scholar]
- Evaluation of antidiabetic activity of hydroalcoholic extract of Cestrum nocturnum leaves in streptozotocin-induced diabetic rats. Adv. Pharmacol. Sci.. 2013;2013:1-4.
- [Google Scholar]
- Ameliorative effects quercetin against impaired motor nerve function, inflammatory mediators and apoptosis in neonatal streptozotocin-induced diabetic neuropathy in rats. Biomed. Aging Pathol.. 2012;2:173-186.
- [Google Scholar]
- Khanam, Z., Kong, H.S., Nur Hazerra, M.Z., Chua, H.C., Irshad Ul Haq, B., 2015. Determination of polyphenolic content, HPLC analyses and DNA cleavage activity of Malaysian Averrho carambola L. fruit extracts. J. King Saud Univ.-Sci. 27(4) 331–337.
- Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr. Res. Practice. 2011;5(2):107-111.
- [Google Scholar]
- Lycopene attenuates diabetes-associated cognitive decline in rats. Life Sci.. 2008;83(3–4):128-134.
- [Google Scholar]
- Insulin-secretagogue, antihyperlipidemia and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. in streptozotocin-induced diabetic rats. Chem. Biol. Interact.. 2011;189:112-118.
- [Google Scholar]
- Neuronal LRP1 regulates glucose metabolism and insulin signaling in the brain. Neurobiol. Dis.. 2015;35(14):5851-5859.
- [Google Scholar]
- Ginsenoside re attenuates diabetes-associated cognitive deficits in rats. Pharmacol. Biochem. Behav.. 2012;101(1):93-98.
- [Google Scholar]
- Total saponins from Rhizoma anemarrhenae ameliorate diabetes-associated cognitive decline in rats: Involvement of amyloid-beta decrease in brain. J. Ethnopharmacol.. 2012;139(1):194-200.
- [Google Scholar]
- Increased amyloid β-peptide (1–40) level in brain of streptozotocin-induced diabetic rats. Neuroscince. 2008;153:796-802.
- [Google Scholar]
- Effects of metformin on weight loss: Potential mechanisms. Curr. Opin. Endocrinol. Diabetes Obesity. 2014;21(5):323-329.
- [Google Scholar]
- Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681-683.
- [Google Scholar]
- Antioxidant potential of ethanolic extract of Azadirachta excelsa in streptozotocin induced diabetic rats. Int. J. Pharmaceutical Sci.. 2017;43(2):135-138.
- [Google Scholar]
- Antidiabetic activity of Azadirachta excelsa extract on alloxan induced diabetic rats. Open Conf. Proc. J.. 2013;4:49-52.
- [Google Scholar]
- Attenuation of pancreatic histology, hematology and biochemical parameters in type 2 diabetic rats treated with Azadirachta excelsa. Int. J. Med. Health Biomed. Bioeng. Pharmaceutical Eng.. 2014;8(9):613-616.
- [Google Scholar]
- Qualitative phytochemical screening and GC-MS profiling of Azadirachta excelsa leaf extract. Malaysian Appl. Biol.. 2015;44(3):87-92.
- [Google Scholar]
- Orwa, C., Mutua, A., Kindt, R., Jamnadass, R., Anthony, S., 2009. Agroforestree Database:a tree reference and selection guide version 4.0 http://www.worldagroforestry.org/sites/treedbs/treedatabases.asp
- Antidiabetic and hematological effect of aqueous extract of stem bark of Afzelia Africana (Smith) on streptozotocin-induced diabetic Wistar rats. Asian Pacific J. Trop. Biomed.. 2011;1(5):353-358.
- [Google Scholar]
- An investigation on body weight, blood glucose levels and pituitary-gonadal axis hormones in diabetic and metformin-treated diabetic female rats. Vet. Res. Forum. 2012;3(2):79-84.
- [Google Scholar]
- Positive association between plasma amylin and cognition in a homebound elderly population. J. Alzheimer’s Dis.. 2014;42(2):555-563.
- [Google Scholar]
- Mechanism of disease: Alzheimer’s Disease. The New England Journal of Medicine. 2010;362:329-344.
- [Google Scholar]
- Antihyperglycemic activity of Catharantus roseus leaf powder in streptozotocin-induced diabetic rats. Pharmacognosy Res.. 2010;2(3):195-201.
- [Google Scholar]
- Effects of intranasal insulin on cognition in memory-impaired older adults: Modulation by APOE genotype. Neurobiol. Aging. 2006;27:451-458.
- [Google Scholar]
- Intranasal insulin improves cognition and modulates β-amyloid in early AD. Neurology. 2008;70:1-9.
- [Google Scholar]
- Antidiabetic and synergistic effects of anthocyanin fraction from Berberis integerimma fruit on streptozotocin-induced diabetic rats model. Trends Pharmaceutical Sci.. 2016;2(1):43-50.
- [Google Scholar]
- Neurons in the hippocampal CA1 region, but not dentate gyrus, are susceptible to oxidative stress in rats with streptozotocin-induced type 1 diabetes. Neural Regener. Res.. 2015;10(3):451-456.
- [Google Scholar]
- Insulin as a bridge between type 2 diabetes and Alzheimer disease – how anti-diabetics could be a solution for dementia. Front. Endocrinol.. 2014;5(110):1-13.
- [Google Scholar]
- Quercetin vs chrysin: Effect on liver histopathology in diabetic mice. Hum. Exp. Toxicol.. 2013;32(10):1058-1066.
- [Google Scholar]
- Antioxidative effects of leaves from Azadirachta species of different provenience. Food Chem.. 2007;103(2007):1539-1549.
- [Google Scholar]
- Neuroinflammation and neurologic deficits in diabetes linked to brain accumulation of amylin. Mol. Neurodegener.. 2014;9(30):1-12.
- [Google Scholar]
- The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res.. 2001;50:536-546.
- [Google Scholar]
- Hypoglycaemic effects of antidiabetic drugs in streptozotocin-nicotinamide-induced mildly diabetic and streptozotocin-induced severely diabetic rats. Basic Clin. Pharmacol. Toxicol.. 2008;103:560-568.
- [Google Scholar]
- Effect of melatonin and vitamin E on diabetes-induced learning and memory impairment in rats. Eur. J. Pharmacol.. 2006;537:106-110.
- [Google Scholar]
- Antihyperglycemic and insulin secretagogue activities of Abrus precatorius leaf extract. Pharmacognosy Res.. 2016;8(4):303-308.
- [Google Scholar]
- Insulin treatment restores glutamate (α–amino–3–hyroxy–5–methyl–4–isoxazolepropionoc acid) receptor function in the hippocampus of diabetic rats. J. Neurosci. Res.. 2015;93(9):1442-1450.
- [Google Scholar]
- Effects of 20-hydroxyecdysone on improving memory deficits in streptozotocin-induced type 1 diabetes mellitus in rat. Eur. J. Pharmacol.. 2014;740:45-52.
- [Google Scholar]
- Intraperitoneal injection of the pancreatic peptide potently reduces behavioural impairment and brain pathology in murine models of Alzheimer’s disease. Mol. Psychiatry. 2015;20:232-239.
- [Google Scholar]
Appendix A
Appendix 1
See Fig. 4.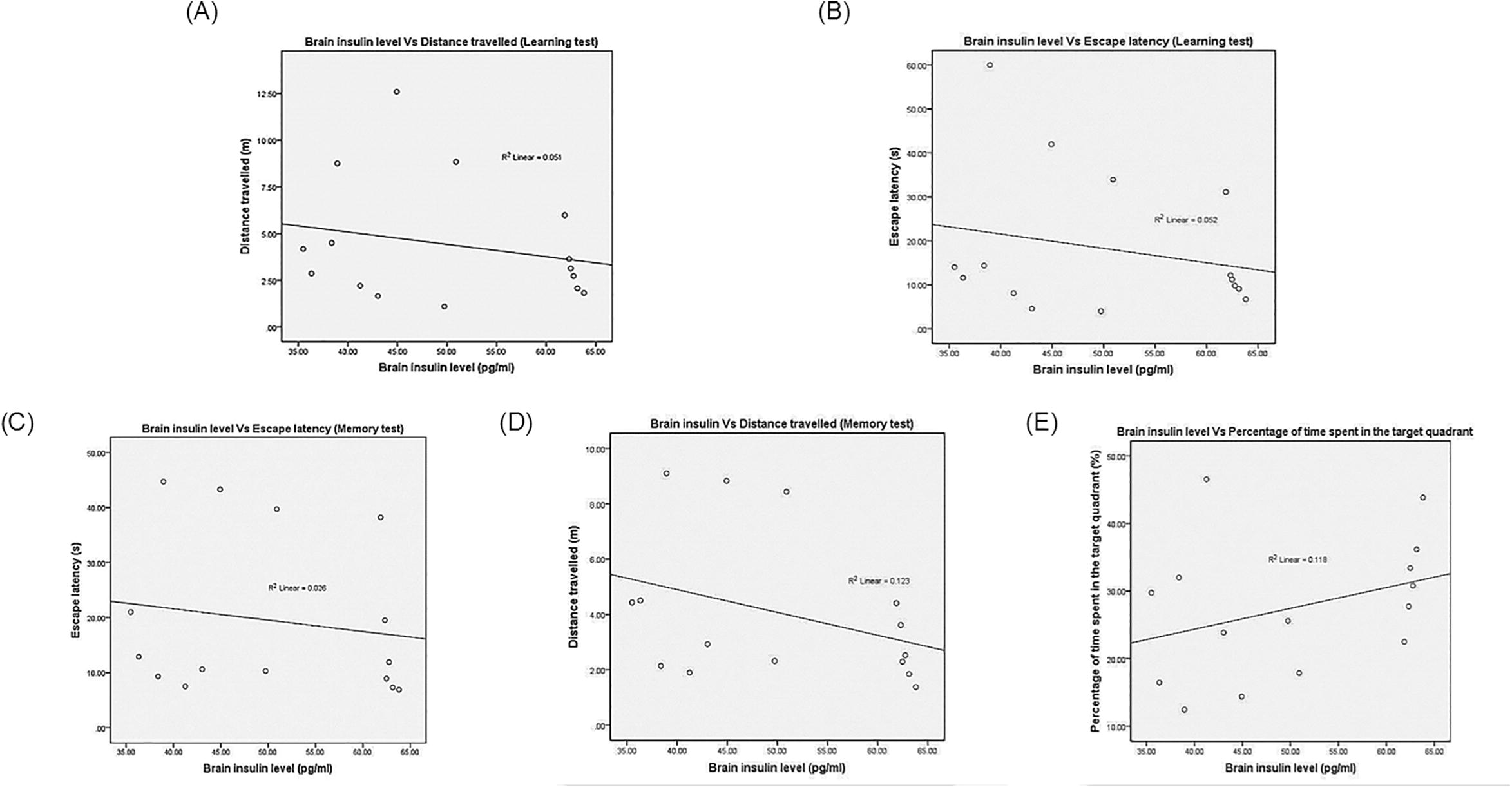
The Pearson’s Correlation and Regression Between the Brain Insulin with (A) Distance Travelled (Learning Test), (B) Escape Latency (Learning Test), (C) Escape Latency (Memory Test), (D) Distance Travelled (Memory Test) And (E) Percentage of Time Spent in the Target Quadrant During the Memory Test.
Appendix B
Appendix 2
See Fig. 5.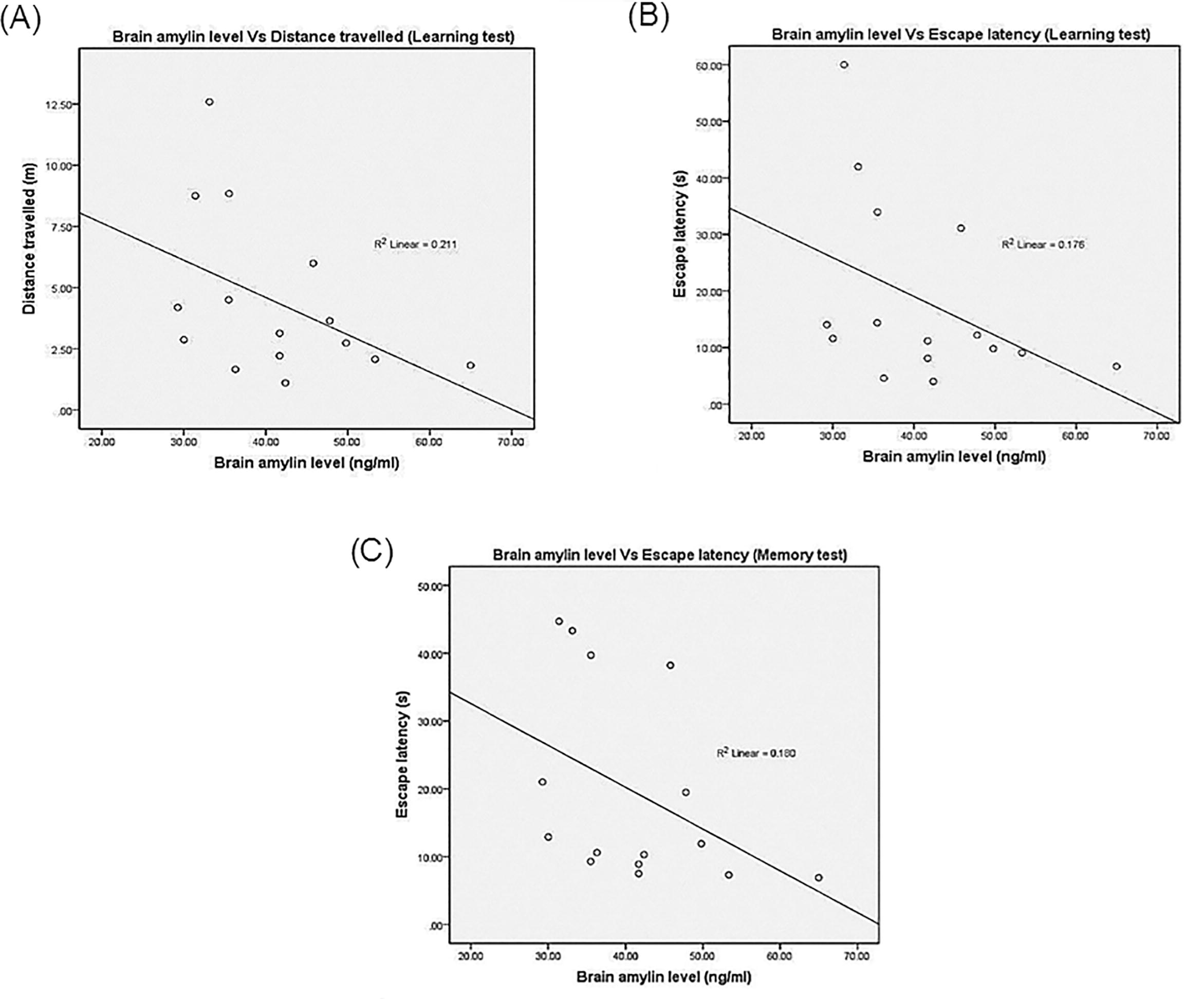
The Pearson’s Correlation and Regression Between the Brain Amylin with (A) Distance Travelled (Learning Test), (B) Escape Latency (Learning Test) and (C) Escape Latency During the Memory Test.







