The protective effect of Ammi visnaga extract against human hepatic cancer
⁎Corresponding author at: Department of Zoology, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia. salkahtani@ksu.edu.sa (Saad Alkahtani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cancer is the most serious disease globally and it’s the second in mortality rate after heart failure. Hepatic cancer is a common cancer disease worldwide. This investigation was designed to assess the cytotoxic and apoptotic effects of Ammi visnaga (A. visnaga) seed extract on human liver cancer cell line (HuH-7). Based on IC50 values, three doses (500, 1000, 1500 μg/ml) were selected. Treated cells with MTT assay reduced cell viability at 1000 μg/ml and higher after 48hrs. NRU test demonstrated significant increase at (1500 and 2000 μg/ml) in dose-dependent manner indicating lysosomal toxicity. The level of oxidative stress-mediated cytotoxicity was assessed using SOD, glutathione (GSH), catalase and ROS assays. The intracellular ROS was highly increased and loss of mitochondrial membrane potential (MMP) confirmed the damage of mitochondrial membrane in HuH-7 cells. The level of GSH significantly increased after treatment. In contrast, the levels of SOD, and catalase activities significantly decreased after treatment. The effects of MMP significantly decreased after treatment compared to control. Gene expression data reported significantly upregulation of apoptotic genes. In conclusion, this finding revealed that A. visnaga seeds extract has hepatoprotective potential against HuH-7 cells, signifying their promising clinical applications as a potential therapeutic agent for liver diseases.
Keywords
Apoptosis
Ammi visnaga
Cytotoxicity
HuH-7 cells
Liver
Cancer
- Bax
-
Bcl-2 associated X
- Bcl-2
-
B-cell lymphoma-2
- Caspase
-
Cysteine dependent aspartate-specific proteases
- Cas-3
-
Caspase-3
- Cdk
-
Cyclin-dependent kinases
- DISC
-
Death-inducing signaling complex
- FADD
-
Fas associated via death domain
- GSH
-
Glutathione
- MMP
-
Mitochondrial membrane potential
- NRU
-
Neutral red uptake
- p53
-
Tumor suppressor gene
- OD
-
Optical density
- ROS
-
Reactive oxygen species
- SOD
-
Super oxide dismutase
- TNF
-
Tumor necrosis factor
Abbreviations
1 Introduction
Cancer comprises a huge number of diseases worldwide. Cancer cells may possess the ability to spread whole body organs. Hepatic cancer is one of the most common cancers globally. In Saudi Arabia, hepatic cancer increased three times in the years between 1990 and 2016 (Althubiti and Eldein, 2018). Plant products play an important role in traditional and pharmaceutical medicine. However, generally, liver cancer doesn't have a good prognosis, curative treatments can be presented to almost 30% of patients, but are complicated by a high rate of recurrence with patients (Mokdad et al., 2015). Understanding the treatment of hepatic carcinoma is essential for its prevention (Imawari, 2002). More than 80% of the population in the world counts on plant-based medicine nowadays for their primary healthcare (Majeed, 2017).
Plants phytochemical studies are becoming popular because of their anticancer effects and natural source for promoting human health. More than 80% of the world's population relies on plant-based medicine for primary healthcare, a system that developed over time by dynamic interactions between people and their environment. Currently, plants and their products are important in traditional, botanical and pharmaceutical medicine (Albrahim et al., 2020). The Ammi visnaga plant is endogenous in the Middle East regions. This plant has a slight aromatic odour and a very bitter taste. The fruits of A. visnaga have been used in folk medicine for many years ago to relief kidney stones. In addition, A. visnaga extract showed marked antimicrobial activity against gram-positive (Beltagy and Beltagy, 2015) and used in the treatment of coronary diseases and bronchial asthma, reduces blood pressure and has anti-inflammatory effect in microglial cells (Kwon et al., 2010; Lee et al., 2010). Because of significant lack of cytotoxicity data of A. visnaga, the current study is designed to investigate the toxicity of A. visnaga in human liver cancer cell line (HuH-7), focusing on cells viability, oxidative stress-mediated cytotoxicity, evaluation of the genotoxic effects, and apoptosis.
2 Materials and methods
2.1 Preparation of aqueous plant extracts
The seeds of A. visnaga were crushed into fine powder using mortar and pestle, regular blender, and electric sieve system. After pulverization, the powder of seeds was stored at −20 °C. Briefly, 30 g of the crushed material was soaked for 24 h in 300 ml distilled water (10% w/v) at room temperature. The soaked material was macerated with 50 ml distilled water (10% w/v) in a conical flask and kept in an orbital shaker (250 rpm at 45 °C overnight). The extract was then concentrated and dried under reduced pressure at 40 °C using a rotary evaporator (Rotavapor® R-215, BUCHI). All the filtered extract was preserved aseptically in glass bottles at 4 °C until further use. The aqueous extract was prepared using sterile aqueous extracts and with distilled water (10% w/v). The reconstituted aqueous extract was passed through 0.45 μM bacterial filter papers (Millipore Inc., Riyadh, Saudi Arabia) before using them (Fatimah et al., 2019).
2.2 Cytotoxicity assessment by MTT assay
The HuH-7 cells were obtained from (ATCC, USA). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) Sigma-Aldrich (St Louis, MO, USA) added to 10% Fetal Bovine Serum (FBS) (Sigma-Aldrich (St Louis, MO, USA) and 1% penicillin–streptomycin (Invitrogen (Carlsbad, CA, USA). In brief, HuH-7 cells were seeded in plates at a density of 1 × 104 cells per well and allowed to grow up to 90–95% confluence at 37 °C humidified cabinet of 5% CO2. Then, in order to determine the IC50, cells were exposed to various concentrations (375, 500, 750, 1000, 1500, 2000 and 3000 μg/ml) of A. visnaga seeds extract for 48 hrs. The MTT assay was performed as described by Mosmann (1983). The formazan crystal dissolved in dimethylsulphoxide, and measured at 595 nm (multi-mode Microplate Reader-Gen5™, BioTek Cytation 5™, USA).
2.3 Neutral red uptake (NRU) test
Neutral red uptake (NRU) test was performed according to the method described Ali et al. (2011). In brief, (2 × 104) cells/well in 96-well plates and kept in the 5% CO2 incubator for 24 h at 37 °C prior to experiment for the proper growth of the cells. After incubation the cells were exposed to A. visnaga extract for different time points. The cells were incubated for 3 h in complete medium containing neutral red dye (50 µg/ml). The accumulated dye was extracted with 50% ethanol containing 1% (v⁄v) acetic acid and incubated 20 min at 37 °C. finally, plates were read at 540 nm (Microplate Reader-Gen5™ BioTek Cytation 5™, USA).
2.4 Oxidative stress biomarkers
The levels of oxidative stress biomarkers in HuH-7 cells were measured. In brief, HuH-7 cells (6 × 106) were cultured and treated with different doses of A. visnaga (500, 1000, and 1500 μg/ml) for 48hr. After respective exposure cells were washed with PBS and scraped. The scraped cells were incubated in lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM Na2 EDTA, 1% Triton, and 2.5 mM sodium pyrophosphate). HuH-7 cells were centrifuged at 15,000 rpm at 4 °C for 15 min. The supernatant was kept at −80 °C in freezer to measure the oxidative stress biomarkers such as glutathione (GSH), superoxide dismutase (SOD), and catalase. GSH was measured by using a GSH kit (item no. 703002, Cayman chemical). SOD was measured by using a SOD kit (item no. 706002, Cayman chemical), and catalase was measured by using a catalase kit (item no. 707002, Cayman chemical).
2.5 Reactive oxygen species (ROS) generation
In brief, cells were exposed to seeds extract for 48 h. Culture media was aspirated off and carefully add ∼150 µl of included cell-based assay buffer. Cell-based assay buffer carefully aspirates, leaving a small amount (20 µl) of liquid in the well. 130 µl of ROS staining buffer was added to each well. 10 µl N-acetyl cysteine assay reagent was added to negative control wells. The plate was covered and incubated in dark place for 30 min at 37 °C. Plate was incubated for 30 min in 10 µl of the antimycin. Working reagent was added to positive control wells and incubated for an additional one hour at 37 °C in the dark. The ROS Staining Buffer was aspirated and 100 µl of cell-based assay buffer was aspirated. Assay plate placed on fluorescent plate reader and measured the fluorescence using an excitation wavelength between 480 and 520 nm and an emission wavelength between 570 and 600 nm. The fluorescence of cells was analyzed using a fluorescence microscope (Olympus).
2.6 Mitochondrial membrane potential (MMP)
Cells were cultured in 96-well black plate at a density of 5 × 104 cells/well in 100 µl culture medium and incubated in 5% CO2 incubator overnight at 37 °C. Control and treated cells were washed twice with PBS. Then the cells were further treated with 10 mg/ml of Rhodamine-123 fluorescent dye for 15–30 min at 37 °C in dark. The plate was centrifuged at 400xg for 5 min and supernatant was discarded. Then assay buffer (200 µl) was added to each well and quantified by fluorescent Microplate Reader-Gen5™ (BioTek Cytation 5™, USA). Also, HuH-7 cells were evaluated by Rhodamine-123 fluorescence dye. The fluorescence of Rhodamine-123 intensity was determined by fluorescence microscope by grabbing the images at 20X magnification.
2.7 Quantitative assay of apoptotic genes by RT-PCR
HuH-7 cells were grown in 6-well plates and exposed with seeds extract. RNA was isolated using a RNeasy Mini Kit (Cat No./ID: 74104). The purity and concentration of RNA were measured using a Nanodrop (DS-11; Bio-Rad Laboratories Inc., Hercules, California). The purity of RNA (A260/A280 ratio) sample was ∼2. We have synthesized cDNA from RNA (250 ng) using a cDNA reverse transcription kit (Revert Aid First Strand cDNA Synthesis Kit; Thermo Fisher Scientific). Analysis of Bax, Bcl-2, caspase-3 and p53 mRNA levels was done by RT-PCR (PE Applied Biosystems, Foster City, California). The primer sequences targeting apoptotic genes. The RT-PCR was performed; 5 min at 95 °C for initial denaturing, followed by 30 cycles, involving denaturation at 95 °C for 15 s, annealing at 60 °C for 20 s and elongation at 72 °C for 20 s. The cycle threshold (Ct) values were standardized to the housekeeping gene (GAPDH), and data were analyzed using the comparative the ΔΔCt method23 and the expression of target genes was normalized to GAPDH. Each experiment was performed with three replicates.
2.8 Statistical analysis
The present data were analyzed by one-way analysis of variance (ANOVA), and the significant value was set at p < 0.05, p < 0.01 and p < 0.001.
3 Results
3.1 Cytotoxicity assessment by MTT and NRU assays
In the MTT assay, results showed that A. visnaga was exhibited cytotoxicity. The cells viability decreased in a dose and time dependent manner. HuH7 cells significantly (p < 0.05, p < 0.01, p < 0.001) decreased after treatment with 1000, 1500, 2000 and 3000 μg/ml by 75%, 62%, 49% and 34% respectively, compared to the control (Fig. 1A). HuH-7 treated with A. visnaga shown cell toxicity by the NRU test. Lysosomal toxicity significantly (p < 0.05, p < 0.01) increased after treatment with 1500 and 2000 μg/ml compared to the control (Fig. 1B). The reduction in cell survival was clear in both MTT and NRU results.
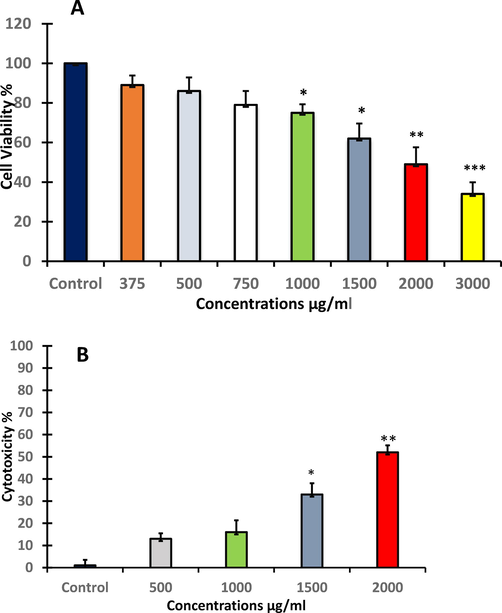
- The effect of A. visnaga seeds extract on treated HuH-7 cells for 48 h as evaluated by A: cell viability (MMT assay), and B: cytotoxicity (NRU assay). Each value represents the mean ±SE of three experiments. n = 3, (*p < 0.05, **p < 0.01, ***p < 0.001) compared with control.
3.2 ROS generation activity and oxidative stress biomarkers
Generation of ROS and levels of GSH, SOD and catalase were examined to show the potential of seeds extract of A. visnaga to increase oxidative stress in HuH-7 cells. The results revealed that HuH-7 cells exposed to seeds extract had higher intracellular ROS generation. The production of (ROS) was exceedingly later exposing various doses (500, 1000 and 1500 μg/ml) of plant extract as stained with fluorescence dye DCFDA. However, the generated ROS was significantly (*p < 0.05) increased at 1500 μg/ml compared with untreated control (Fig. 2A). To confirm ROS activity, treated HuH-7 cells were examined by fluorescence microscope. Images showed the production of ROS was exceeding after treating with different concentration (500, 1000 and 1500 μg/ml) of plant extract as stained with fluorescence dye. The density of fluorescence dye was increased in dose dependent manner (Fig. 2B); (A): control. (B): HuH7 cells at 500 μg/ml. (C): HuH7 cells at 1000 μg/ml. (D): HuH7 cells at 1500 μg/ml.
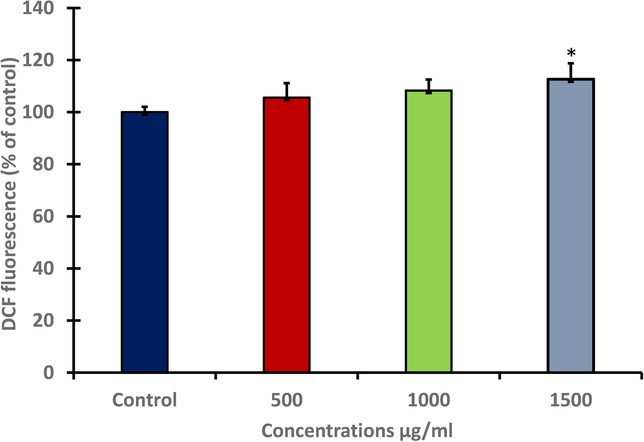
- Generation of (ROS) levels in HuH-7 after treating with different concentration of A. visnaga seeds extract for 48 h. Data represents the mean ±SE of three experiments. n = 3, (*p < 0.05) compared with untreated control.
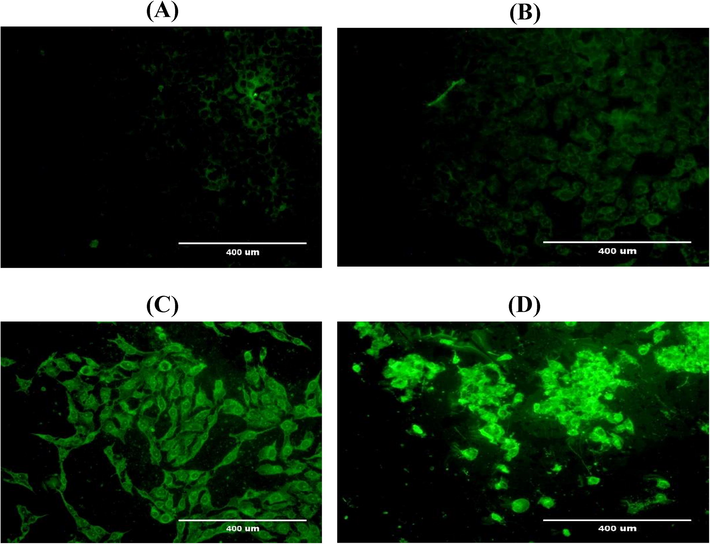
- Shows the production of (ROS) was exceeding after treating with different concentration (500, 1000 and 1500 μg/ml) of plant extract as stained with fluorescence dye. (A): control. (B): HuH7 cells at 500 μg/ml. (C): HuH7 cells at 1000 μg/ml. (D): HuH7 cells at 1500 μg/ml.
The level of GSH in HuH-7 after treating with different concentration of A. visnaga extract for 48 h significantly (*p < 0.05 and **p < 0.01) increased at (500 and 1000 μg/ml) compared with untreated control (Fig. 3A). In contrast, current results showed a significantly (p < 0.05, p < 0.01) depletion in the levels of SOD at (500, 1000 and 1500 μg/ml) compared with untreated control (Fig. 3B). For catalase activity the results revealed clear and strong significant (p < 0.05, p < 0.01 and p < 0.001) depletion in enzyme concentration at three doses and this reduction was found in a concentration-dependent manner (Fig. 3C).
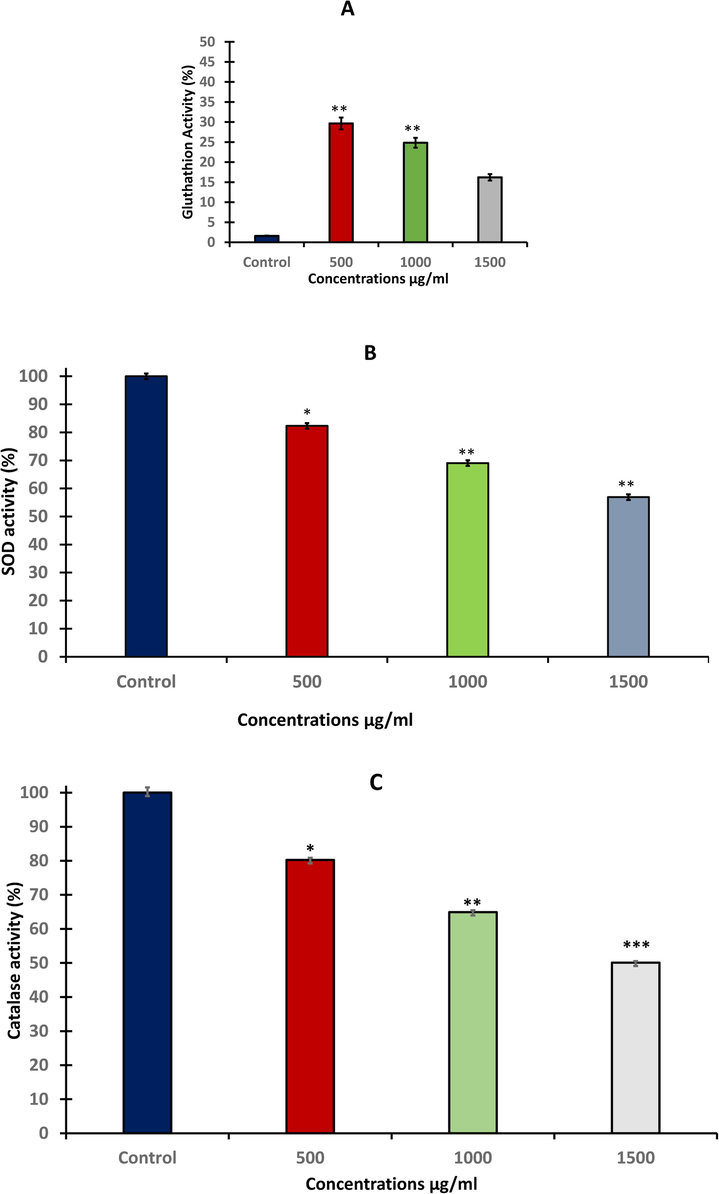
- Oxidative Stress Biomarkers after treating HuH-7 cell line with different concentration of A. visnaga seeds extract for 48 h. A: level of GSH. B: SOD activity, and C: level of catalase. Data represents the mean ±SE of three experiments. n = 3, (**p < 0.01) compared with untreated control.
3.3 Determination of MMP (ΔΨm).
The effect of A. visnaga exposure on MMP was evaluated in HuH-7 cells by JC-1 dye as shown in (Fig. 4A). The level of MMP was significantly (p < 0.05, p < 0.01) decreased as concentration increased. For reconfirm the result, the intact MMP was also evaluated by rhodamine fluorescence dye. Result showed the intact MMP was found in control cells as rhodamine dye was penetrated to the cells and caused dye aggregates, with deep red fluorescence. In contrast, treated cells showed significant loss of MMP (Fig. 4B).
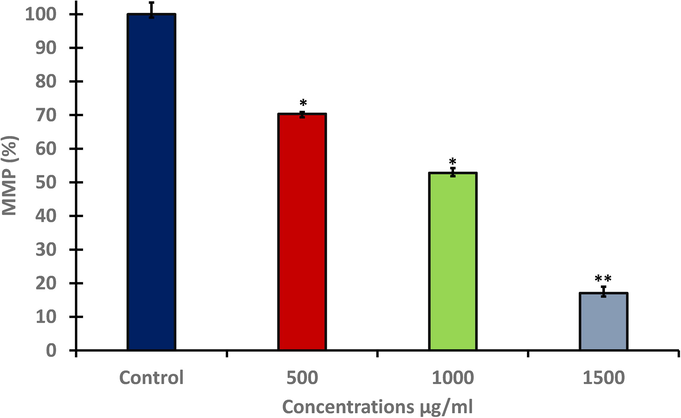
- Level of MMP in HuH-7 after treating with different concentration of A. visnaga seeds extract for 48 h. Data represents the mean ±SE of three experiments. n = 3, (*p < 0.05 and **p < 0.01) compared with untreated control.
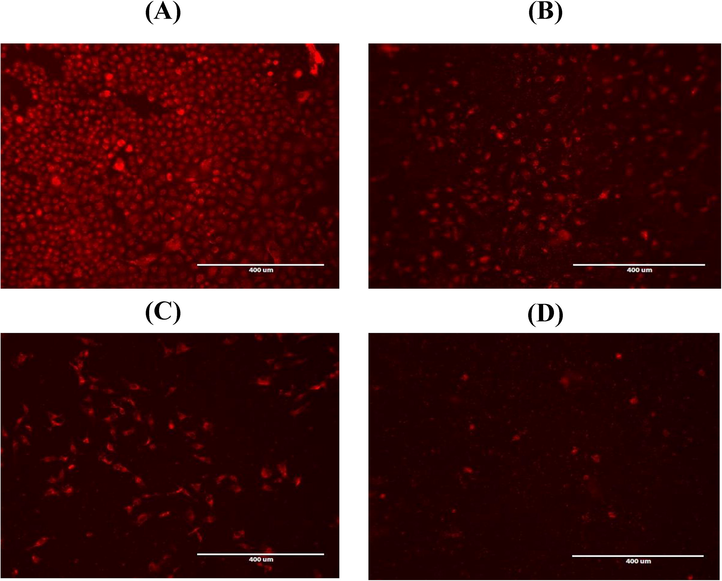
- MMP as evaluated by rhodamine stain the strong MMP was found in control cells as dye penetrate to cells and produced aggregated (deep red fluorescence). Cells at 1500 μg/mL of A.visnaga exposure decreased their MMP and showed low density stain.
3.4 Quantitative real time PCR (qRT-PCR) of apoptotic marker genes
After exposure to A. visnaga quantitative real-time PCR was used to detect changes in mRNA levels of different apoptotic-related genes (Bax, Bcl-2, Cas-3, and p53) in treated HuH-7 cells. The level of mRNA expression for Bax as a pro-apoptotic gene was significantly (p < 0.05) up-regulated after 48 h (Fig. 5A). Also, treated HuH-7 cells showed significantly (p < 0.05 and p < 0.01) increasing in the level of Cas-3 as an executioner gene at three doses compared with untreated cells (Fig. 5B). In contrast, the anti-apoptotic gene Bcl-2 as an anti-apoptotic gene was significantly (p < 0.05) increased only at 1000 μg/ml (Fig. 5C). Tumor suppressor gene is one of the most important gene that has ability to activate apoptosis. The level of p53 was measured after exposing to A. visnaga seeds extract. Current study illustrated that treated HuH-7 cells were significantly (p < 0.05) increased the level of p53 after 48hrs at all tested doses Fig. 5D).
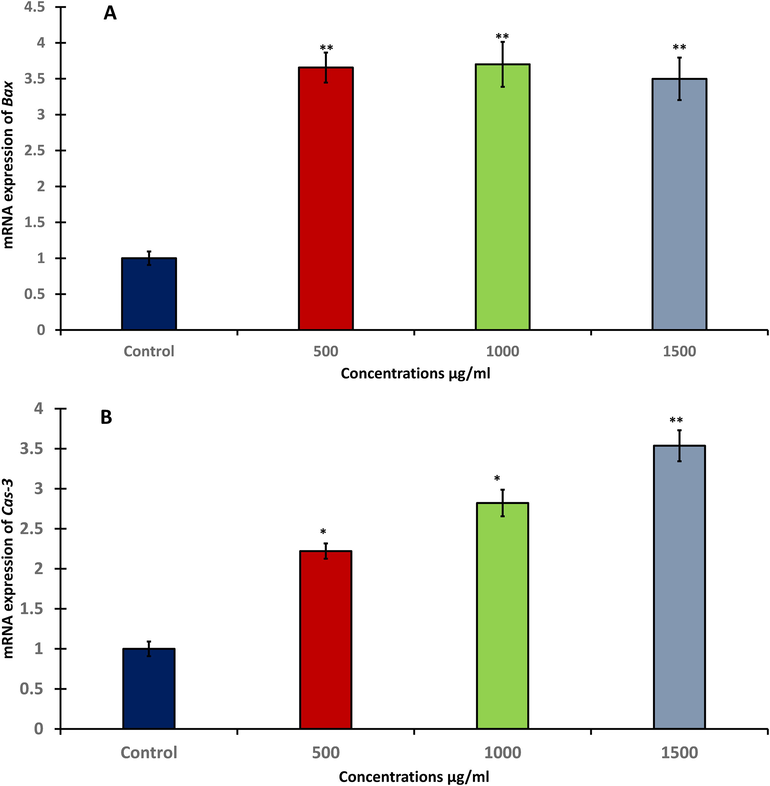
- RT-PCR of apoptotic genes in HuH-7 cells after treating with different concentration of A. visnaga seeds extract for 48h; A: Bax gene expression, B: Cas-3 gene expression, C: Bcl-2 gene expression, D: p53 gene expression. Data represents the mean ±SE of three experiments. n = 3, (**p < 0.01) compared with control.
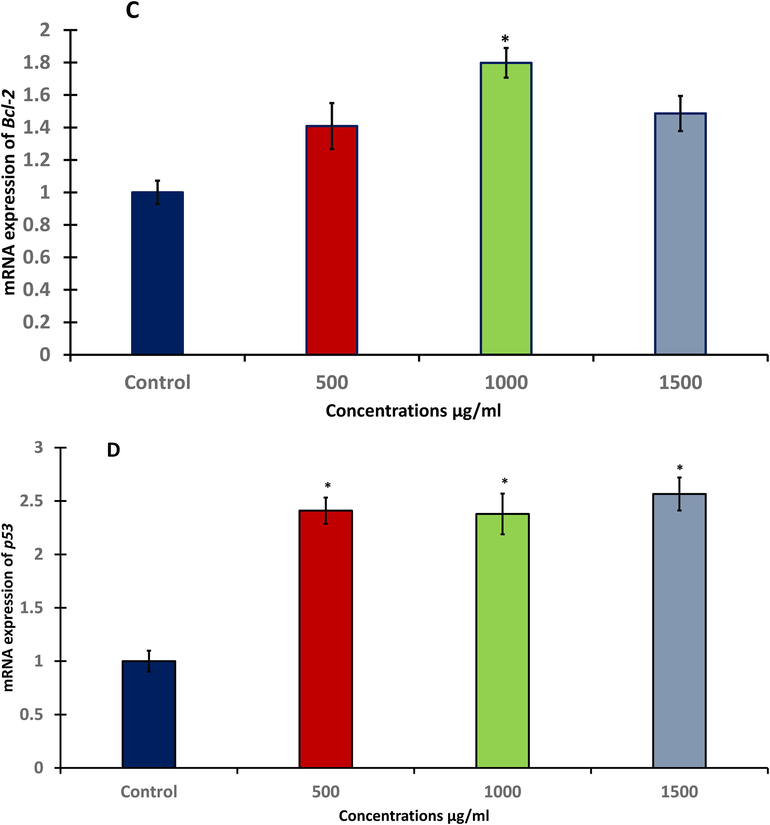
- RT-PCR of apoptotic genes in HuH-7 cells after treating with different concentration of A. visnaga seeds extract for 48h; A: Bax gene expression, B: Cas-3 gene expression, C: Bcl-2 gene expression, D: p53 gene expression. Data represents the mean ±SE of three experiments. n = 3, (**p < 0.01) compared with control.
4 Discussion
Liver cancer became a common cancer disease globally. In Saudi Arabia, hepatic cancer increased three times in the years between 1990 and 2016 (Althubiti and Eldein, 2018). However, researcher continually investigate the effective remedy including plant products (Mokdad et al., 2015). Recently, medicinal plants have attracted research worldwide due to their unique and fascinating properties. Wang et al., (2018a) has reported that flavonoids have used as anti-aging, anti-viral/bacterial and cardio protective compounds. These agents protect cells by acting as antioxidants to neutralize the ROS. In healthy cells antioxidant enhance cell signaling to activate defense system of cells against oxidative stress and regulate the proliferation and apoptotic rate (Tait and Green, 2013; Ali and Ali, 2014; Halliwell and Gutteridge, 2015).
Current study was designed to assess the cell toxic and apoptotic effects of A. visnaga seeds extract on HuH-7 cells. The result of MTT test exposed that A. visnaga decreased the viability HuH-7 cells. In addition, NRU test showed the toxic effects of plant extract in the treated cells. These findings may be attributed to the evident lysosomal damage. However, destabilization of lysosomes is considered to be an earlier sign of mitochondrial injury (Zhao et al., 2003). For further investigation, fluorescence microscopic analyze was done to confirm the loss of MMP. Fluorescence enhancement is related with function of the mitochondria, which swells during apoptosis and generate cellular ROS (Murphy, 2009). The most common pathway revealed that once mitochondrial functions affected, a reaction cascade enhanced by the release of cytochrome c and some proteins which produce the apoptosome complex, activates caspases (Garrido et al., 2006) as a consequence it promotes the generation of intracellular free radicals. These oxidant factors affect cell organelles and cellular compounds (Lobo et al., 2010). Furthermore, ROS promote the caspase-3 activity which in turn results in chromatin condensation in treated cell lines. Furthermore, changes in the structure of lysosomes and mitochondria membranes increase their permeability, resulting in a release of their contents (Mathiasen and Jäättelä, 2002).
Superoxide dismutase SOD plays a dynamic role against ROS by converting antioxidant to harmless molecules. This enzyme mainly exists in the cytosol with different forms but also it is localized in peroxisomes (Wang et al., 2018b). The expression of catalase enzyme varies in tissues; this variation reflects on the cellular stress (He et al., 2019). Downregulation of catalase may enhance oncogenesis by elevating ROS levels in transformed cells to take place and further, in metastatic tumor cells. Isolated cancer cells from circulating blood and secondary tumor sites have displayed greater cytoplasmic and mitochondrial-derived ROS than those from their primary tumors (Glorieux and Calderon, 2018). These results indicate that the increased levels of SOD are dynamic responses to ROS and/or oxidative damage and may relate to a defensive response to elevated intracellular H2O2. However, the regulation of catalase expression under oxidative stress is not predictable (Choi et al., 2009). The action mechanisms of A. visnaga for the prevention of cell damage has not fully understood (Vanachayangkul et al., 2010). Our result showed that the lower and medium concentrations of A. visnaga induced a significant raise in the oxidative stress and GSH enzyme at 48 hr (Zalewska-Ziob et al., 2019). However, the high expression level of SOD was connected with survival in various types of cancerous cell lines (Robbins and Zhao, 2014). In contrast, it showed that reduced SOD expression is related with mortality of hepatocellular carcinoma (Wang et al., 2016). Our results demonstrated A. visnaga extract induced apoptosis and oxidative stress, this result support previous study by Aydoğmuş-Öztürk et al, 2019 that revealed visnagin has the ability to induce ROS (Aydoğmuş-Öztürk et al., 2019).
Apoptotic genes are an essential marker to monitor cytotoxicity and genotoxicity that may have existed as a result of oxidative stress. Bcl-2 gene plays a regulatory role as an anti-apoptotic factor, which maintain cell against apoptosis and allow DNA repair mechanisms to save cell life. Previous study showed that the low level of Bcl-2 protein was involved in the apoptotic pathways of GCD II PCFs disturbs the engulfment of ROS. On the other hand, upregulation of catalase caused an increase in Bcl-2 expression. Thus, ROS and Bcl-2 levels have a reciprocal relationship where an increase in ROS correlates with a decrease in Bcl-2 levels and vice versa. This finding suggests that increased ROS may reduce Bcl-2 levels (Choi et al., 2009). In addition, it has been shown that ceramide treatments induce oxidative damage through proteolytic cleavage of catalase by activated Cas-3. Activated Cas-3 reduced the levels of catalase protein because of its proteolysis, not because of inhibition of mRNA levels (Choi et al., 2009). The loss of mitochondria membrane potential has considered as the specific intrinsic mitochondrial pathway of in apoptosis as consequence the release of cytochrome c, in that way activating Cas-9 followed by Cas-3, in the process of apoptosis (Tait and Green, 2013;Ali and Ali, 2014; Abdullah et al., 2018).
Current study reported the dysfunction of MMP due to A. visnaga extract that has been influenced by the upregulation of Bax and Bcl-2 at transcriptional level (Wang et al., 2019). Moreover, the Cas-3 was upregulated in a time- and dose-dependent manner. Previously, it was reported that p53 promote the upregulation of Bax. Thus, it presumed that p53 plays a crucial role in Bax upregulation by incorporating Bax into the mitochondrial membrane, and thus activates apoptotic pathways. However, in some types of cancer Bcl-2 expression was negligible, and the rate of apoptosis was markedly higher than expected. In contrast Bax, has apoptosis-promoting activity and can counter the anti-apoptotic effects of Bcl-2. The p53 is the tumor suppressor gene and the essential regulator of DNA repair, once p53 activated, the homo-oligomerize of Bak and Bax occurs and leads to a different event like; mitochondrial outer membrane permeabilization (MOMP) (Sheikh and Fornace, 2000). Also, current study showed the p53 gene was upregulated compared to the control as shown in the results section, this illustrated both Cas-3 and p53 were activated and induced apoptosis. The activation of p53 arises due to cellular stress that eventually led to DNA damage which in turn induces pre-apoptotic gene expression on the mitochondrial membrane and induces caspase enzyme to promote death of cell (Sheikh and Fornace, 2000). Moreover, the p53 turns to add various stress into different anti-proliferative responses such as its capability to induce apoptosis, and interrupt promote tumor growth. Apparently p53 induce apoptosis by transcription dependent and nondependent mechanisms.
5 Conclusion
The A. visnaga seeds extract has showed anti-cancerous properties and significantly decreased viability of HuH-7 cells. Moreover, toxicity of lysosomal was seen, indicating the toxicity of plant extract on treated cells. The plant extract also significantly increased the level of oxidative stress and altered the mitochondrial function, which lead to the onset of apoptosis in the HuH-7 cells. Upregulation of apoptotic; Bax, Bcl-2, Cas-3 and p53 genes expression was reported. Taken together, current findings revealed that A. visnaga seeds extract have hepato-protective potential against human hepatic cancer cell line HuH-7, signifying their promising clinical applications as a potential therapeutic agent for liver diseases.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RG-1441-018).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cytotoxicity and genotoxicity of cypermethrin in hepatocarcinoma cells: a dose- and time-dependent study. Dose Response. 2018;16(2) 1559325818760880
- [Google Scholar]
- Assessment of DNA damage and cytotoxicity of palmatine on human skin epithelial carcinoma cells. Toxicol. Environ. Chem.. 2014;96(6):941-950.
- [Google Scholar]
- UVB-induced apoptosis and DNA damaging potential of chrysene via reactive oxygen species in human keratinocytes. Toxicol. Lett.. 2011;204(2-3):199-207.
- [Google Scholar]
- Trends in the incidence and mortality of cancer in Saudi Arabia. Saudi Med. J.. 2018;39(12):1259-1262.
- [Google Scholar]
- The anticancer activity of visnagin, isolated from Ammi visnaga L., against the human malignant melanoma cell lines, HT 144. Mol. Biol. Rep.. 2019;46(2):1709-1714.
- [Google Scholar]
- Chemical composition of Ammi visnaga L. And new cytotoxic activity of its constituents khellin and visnagin. J. Pharm. Sci. Res.. 2015;7:285-291.
- [Google Scholar]
- Decreased catalase expression and increased susceptibility to oxidative stress in primary cultured corneal fibroblasts from patients with granular corneal dystrophy type II. Am. J. Pathol.. 2009;175(1):248-261.
- [Google Scholar]
- Fatimah, A.-O., Alharbi, R.I., Albasher, G., Almeer, R., Alsaggabi, N.S., 2019. Antifungal Potential of Aqueous Extract of Boswellia carteri. J. Pure Appl. Microbiol., 2019, 13 (4): 2375–2381.
- Mechanisms of cytochrome c release from mitochondria. Cell Death Differ.. 2006;13(9):1423-1433.
- [Google Scholar]
- Catalase down-regulation in cancer cells exposed to arsenic trioxide is involved in their increased sensitivity to a pro-oxidant treatment. Cancer Cell Int.. 2018;18:1-9.
- [Google Scholar]
- Free Radicals in Biology and Medicine. USA: Oxford niversity Press; 2015.
- A catalase-like metal-organic framework nanohybrid for O2 evolving synergistic chemoradiotherapy. Angew. Chemie. 2019;131(26):8844-8848.
- [Google Scholar]
- Neuroprotective effect of visnagin on kainic acid-induced neuronal cell death in the mice hippocampus. Korean J. Physiol. Pharmacol.. 2010;14(5):257.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effect of visnagin in lipopolysaccharide-stimulated BV-2 microglial cells. Arch. Pharm. Res.. 2010;33(11):1843-1850.
- [Google Scholar]
- Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev.. 2010;4(8):118.
- [Google Scholar]
- Evidence-based medicinal plant products for the health care of world population. Ann. Phytomed.. 2017;VI(I):1-4.
- [Google Scholar]
- Triggering caspase-independent cell death to combat cancer. Trends Mol. Med.. 2002;8(5):212-220.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1-2):55-63.
- [Google Scholar]
- Manganese superoxide dismutase in cancer prevention. Antioxid. Redox Signal.. 2014;20(10):1628-1645.
- [Google Scholar]
- Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol.. 2013;5(9):a008706
- [Google Scholar]
- Potential anti-inflammatory and anti-apoptotic effect of Coccinia grandis plant extract in LPS stimulated-THP-1 cells. Environ. Sci. Pollut. Res.. 2020;27:21892-21904.
- [Google Scholar]
- An aqueous extract of Ammi visnaga fruits and its constituents khellin and visnagin prevent cell damage caused by oxalate in renal epithelial cells. Phytomedicine. 2010;17(8-9):653-658.
- [Google Scholar]
- Reduced SOD2 expression is associated with mortality of hepatocellular carcinoma patients in a mutant p53-dependent manner. Aging (Albany NY). 2016;8(6):1184-1200.
- [Google Scholar]
- Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci.. 2018;13(1):12-23.
- [Google Scholar]
- Psoralen inhibits malignant proliferation and induces apoptosis through triggering endoplasmic reticulum stress in human SMMC7721 hepatoma cells. Biol. Res.. 2019;52:34.
- [Google Scholar]
- Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol.. 2018;217:1915-1928.
- [Google Scholar]
- Activity of antioxidant enzymes in the tumor and adjacent noncancerous tissues of non-small-cell lung cancer. Oxid. Med. Cell. Longev. 2019
- [Google Scholar]
- Lysosomal enzymes promote mitochondrial oxidant production, cytochrome c release and apoptosis. Eur. J. Biochem.. 2003;270(18):3778-3786.
- [Google Scholar]







