Translate this page into:
The protecting role of Moringa oleifera in cypermethrin-induced mitochondrial dysfunction and apoptotic events in rats brain
⁎Corresponding author at: Biochemistry Department, Faculty of Science, University of Jeddah, 21589 – P.O box, 80200 Jeddah, Saudi Arabia. mialkhalaf@uj.edu.sa (Maha I. Alkhalaf)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cypermethrin caused mitochondrial dysfunction, so it causes neurotoxicity and genotoxicity. Neuronal diseases are linked to apoptotic markers that are related to mitochondrial dysfunction. Co-treatment with moringa extract ameliorated the mitochondrial dysfunction and AchE. The moringa extract acts as a neuroprotective agent.
Abstract
Cypermethrin (CYP) can cause neurotoxicity and plays a role as an inducer for mitochondrial dysfunction. The defensive capability of Moringa oleifera versus neuronal issues prompted by CYP intoxication in adult male albino rats was investigated. Sixty male rats were divided into six experimental groups; G1: controlled group. G2 and G3 were exposed to CYP at different doses of 26.15 mg/Kg/day (representing 1/10 LD50) and 8.72 mg/kg/day (representing1/30 LD50) respectively. G4 treated with moringa extract at 250 mg/kg/day by gavage. G5 and G6 were given moringa extract by gavage (250 mg/kg/day) and CYP as G2 and G3 respectively. G4, G5, and G6 were given moringa extract by gavage (250 mg/kg/day) for 14 days before starting the experiment and during it (28 days). The results indicated that exposure to CYP resulted in a significant reduction in the NADH dehydrogenase, ATPase in mitochondria, and AchE enzyme activity, while the brain caspase-3 activity and DNA fragmentation were increased significantly compared to the controlled group. The co-treatment of moringa extract alleviated the adverse effect of CYP in the treated groups. Moringa declined mitochondrial dysfunction, apoptotic markers, and enhanced AchE activity. In conclusion, moringa extract has an impact to minimize cypermethrin-induced neurotoxicity and apoptosis in rats’ brains.
Keywords
Neurotoxicity
Cypermethrin
Moringa
Mitochondrial dysfunction
Apoptosis
Caspases-3
- CYP
cypermethrin
- AchE
acetylcholinesterase
Abbreviations
1 Introduction
Pesticides are one of the most commonly used environmental variables in the globe linked with the enhanced danger of neurodegenerative diseases. Cypermethrin (CYP) as a type II pyrethroids class, contains a cyano group in the carboxyl α position which has neurotoxic effects due to its interference with chemical neurotransmission or ion channels (Sharma et al., 2014). Pyrethroids are widely used in all fields of insect control in animals, agriculture, home and garden as potential alternatives to some organophosphate, carbamate or organochlorine insecticides because they are less persistent in the environment. Often used as an ectoparasiticide for animals (Elsawya et al., 2017; Guven et al., 2018).
Human exposure to CYP maybe occurs through inhalation, dermal, and oral by water and food ingestion. Lipophilic properties of CYP is related to its accumulation in adipose tissues. This accumulation causes cardiotoxicity, immunotoxicity, reproductive toxicity, hepatotoxicity, nephrotoxicity (Wang et al., 2019).
Pyrethroids can cause multiple neurotoxic symptoms and due to crossing the brain’s blood barrier and generating free radicals. CYP causes oxidative damage in the nigrostriatal pathway's (Singh et al., 2010).
The functions of the nervous system rely on mitochondria’s function as the brain consumes a large amount of energy. Furthermore, environmental pollutants may affect mitochondrial functions via respiration chain dysfunction leading to neurodegenerative diseases (Federico et al., 2012). Such effects may occur in dopaminergic neurons that have been associated with the impact of pesticides in Parkinson's disease (Wang et al., 2011).
Neurodegeneration has been associated with disruption of macromolecules and energy production where, oxidative stress is one of mechanisms for pesticides toxicity (Ghorzi et al., 2017). Furthermore, it is well documented that reactive oxygen species (ROS) trigger mitochondrial dysfunction caused by disruption of complexes I and III in the electron transport chain induced by environmental pollutants (Zolkipli-Cunningham and Falk, 2017). Complex I disruption has been related to neurodegenerative diseases such as Leber hereditary optic neuropathy (Steve and Neustadt, 2007).
Natural antioxidant compounds of many plants are used as therapeutic agents for neuronal disorders by enhancing alertness; memory and brain function in general. Moringa oleifera called a miracle tree by containing vitamins, minerals, phytochemicals, and essential amino acids in leaves extract. So it is used as a cognitive enhancer and neurological prevention (Mahaman et al., 2018). It has the effect of facilitating memory (Ganguly et al., 2005) and has potential therapeutic values such as an antipyretic, anticancer, anti-inflammatory, antiulcer, antidiabetic, and antimicrobial agent (Kou et al., 2018).
The GC/MS analysis of moringa leaves extract contains retinol, palmitic acid, ascorbic acid, myristic acid, thymol, and linoleic acid (Mansour et al., 2019). Several studies showed that the moringa extract has been alleviated hyperphosphorylation and amyloid-β pathology in Alzheimer's disease (Mahaman et al., 2018) and the hepatotoxicity in diabetic rats induced by alloxan (Abd Eldaim et al., 2017).
The objective of the present research is to evaluate neurotoxicity induced by CYP in the brain of male albino rats by studying mitochondrial dysfunction and apoptosis markers with the protective role of moringa extract to minimize the toxic effect of CYP in neuronal impacts.
2 Materials and methods
2.1 Chemicals
CYP formulation (20% emulsifiable concentrate “EC”) was purchased from Kafer El-zaiat company Egypt. While Moringa oleifera Lam (Moringaceae) leaves (powder) were collected from the farm of the Egyptian Scientific Society of Moringa and were purchased from National Research Centre, Giza, Egypt. All chemicals used in this study were purchased from Sigma (USA), and MP (France).
2.2 Animals and care
Mature male albino rats (Rattus norvegicus) weighted 160 ± 10 g were purchased from local suppliers “New Veterinary Office (experimental animals)” and were kept in plastic cages in hygienic conditions under controlled light and temperature. Also, they fed on a rat pellet diet, these pellets were obtained from an Agricultural-Industrial Integration Company, Giza, and all rats were left in their housing for 2 weeks for adaptation to the environmental condition preceded experiment.
2.3 Preparation of Moringa leaves extract
Moringa leaves powder was extracted with dehydrated ethanol at room temperature for one day with occasional shaking. The extract was filtered through Whatman filter paper and then vacuum dried at 40–50 °C to get a dry powder after that it was dissolved in distilled water for final use (Ganguly et al., 2005).
2.4 Experimental design
Sixty mature male rats were divided randomly into 6 equal groups, 10 rats each, and daily treated by oral gavage for 28 days as follow:
G1: It remained intact as a normal control group that received 1 ml distilled water. G2 and G3 were exposed to CYP at different doses 26.15 mg/Kg/day (representing 1/10 LD50) and 8.72 mg/kg/day (representing1/30 LD50) respectively. G4 treated with moringa extract at 250 mg/kg/day by gavage. G5 and G6 were given moringa extract by gavage (250 mg/kg/day) and CYP as G2 and G3 respectively. G4, G5, and G6 were given moringa extract by gavage (250 mg/kg/day) for 14 days before starting the experiment and during it. The selected doses of CYP based on determined acute LD50 according to the protocol of OECD guideline 401 for testing of chemicals “Acute Oral Toxicity“ (OECD, 1987).
This experiment carried out according to the standard procedures designed by Organization for Economic Cooperation and Development guideline 407 (OECD, 2008) “Repeated Dose 28-Day” Oral Toxicity Study in Rodents. This study was approved by the Ethics Committee for Institutional Animal Care and Use Committee (CU-IACUC) at Cairo University (CU/I/F/ 8/18).
2.5 Samples collection
At the end of the experiment, the rats were weighed and sacrificed for sample collection. The brain of each rat was collected, weighed and fractioned into biochemical and comet assay.
2.6 Isolation of mitochondria from rats’ brain
Mitochondria were isolated according to (Constantini et al., 1995) from the brain of fasted male albino rats for determination of NADH dehydrogenase and ATPase enzymes activity.
2.7 Preparation of brain tissue homogenate
A brain homogenate was prepared in ice-cold 50 mM potassium phosphate buffer, pH 7.4 containing 1 mM EDTA per gram tissue in a ratio of 1 g/10 ml phosphate buffer. Then, tissue was homogenized using a chilled glass-Teflon Potter-Elvehjem tissue homogenizer. The homogenate was centrifuged at 10,000 × g for 15 min at 4 °C, and the supernatant was collected and stored at −20 °C for caspases-3 and AchE enzymes. Protein concentration was estimated in brain homogenates by the dye-binding method of Bradford (1976).
2.8 Biochemical analysis
2.8.1 Enzymes assay in mitochondria extract
2.8.1.1 NADH dehydrogenase activity
The enzyme activity was measured according to the method described by (Galente and Hatefi, 1978) using potassium ferricyanide, as an electron acceptor and following the reduction rate of the substrate NADH at 340 nm spectrophotometrically. Specific activity was expressed as nmol NADH oxidized /min/mg protein.
2.8.1.2 Mitochondrial ATPase activity
The main idea of this method is to measure the amount of inorganic phosphate produced from the hydrolytic reaction of ATP by the ATPase. It was determined colorimetrically as described by (Taussky and Shorr, 1953). The intensity of the color was measured spectrophotometrically at 740 nm. The concentration of Pi was calculated graphically from a standard curve and the specific activity was calculated as μmole Pi/mg protein/min.
2.8.2 Determination of the effect of CYP and moringa extract on brain apoptotic markers
2.8.2.1 Determination of caspase-3 activity by ELISA in brain tissue
Enzymatic activity of caspase 3 was determined by using rat CASP3 ELISA kit by the manufacturer’s instructions (Elabscience Biology Co., Ltd., China). This ELISA kit uses sandwich-ELISA as the method. The micro ELISA plate provided in this kit has been pre-coated with an antibody specific to CASP-3.
2.8.2.2 Determination of brain genomic (DNA fragmentation by comet assay)
The comet assay in the present study was applied under alkaline conditions using ordinary microscope slides as previously described by (Singh et al., 1988).
2.8.3 Determination of acetylcholinesterase (AchE) activity as a neurotransmitter
The acetylcholinesterase (AChE) activity of brain tissue was determined by the method described by (Ellman et al., 1961). This method based on measuring the increase of yellow color produced from the reaction between thiocholine with dithiobisnitrobenzoate ion.
2.9 Statistical analysis
A standard computer program SPSS for Windows, release 21.0 (IBM SPSS Inc, USA) was used to enter and analyze data. Mean values and standard deviation of the mean (mean ± SD) were calculated for each tested group based on values obtained from seven individual rats. For the quantitative variables which are normally distributed, One Way ANOVA followed by Tukey Post-hoc analysis used to declare the significant difference between groups at p < 0.05.
3 Results
3.1 Influence of CYP and moringa extract on mitochondrial dysfunction
3.1.1 The activity of NADH dehydrogenase enzyme
Mitochondrial NADH dehydrogenase activity in rats’ brains in CYP exposed groups G2 and G3 showed a significant reduction (p < 0.05) (69% and 73% respectively) comparing with the control group. It was noted that after treatment with moringa extract, NADH dehydrogenase activity in G5 and G6 treated groups was a significant enhancement (44% and 53% respectively) in comparison with G2 and G3 respectively as shown in Table 1. Each value reflects the average of seven animals ± SD in different experimental groups, G1 (control), G2 (26.15 mg/kg BW CYP), G3 (8.72 mg/kg BW CYP), G4 (250 mg/kg BW moringa extract), G5 (250 mg/kg BW moringa extract + 26.15 mg/kg BW CYP) and G6 (250 mg/kg BW moringa extract + 8.72 mg/kg BW CYP).The same letter implies that there is an insignificant difference between groups by using One Way ANOVA at P < 0.05. The different letter implies that there is a significant difference between groups by using One Way ANOVA at P < 0.05.
Groups
NADH dehydrogenase enzyme activity (nmol/min/mg)
ATPase enzyme activity (µmol pi/mg)
G1
8.28 ± 0.84a
28.45 ± 1.05a
G2
2.58 ± 0.09b
9.67 ± 0.58b
G3
2.25 ± 0.19b
9.18 ± 1.06b
G4
8.16 ± 1.04a
28.39 ± 0.99a
G5
4.61 ± 0.42c
18.67 ± 1.73c
G6
4.82 ± 0.51c
19.58 ± 1.54c
3.1.2 Mitochondrial ATPase enzyme activity
The results presented in Table 1 show a significant (p < 0.05) decline of the mitochondrial ATPase activity in rats brain in CYP exposed groups G2 and G3 (66% and 68% respectively) compared with the control group. While the groups that treated with moringa extract, G5 and G6 revealed a significant improvement (48% and 53% respectively) in ATPase activity compared to G2 and G3 respectively.
3.2 Influence of CYP and Moringa extract on brain apoptotic markers
3.2.1 caspase-3 activity in brain tissue
Table 2 indicates the brain caspase-3 activity (ng/mg protein) in different experimental groups following oral intoxication by CYP and moringa extract separately or combined in rats' brains for 28 successive days. There was a significant increase (p < 0.05) in CYP exposed groups G2 and G3 (113% and 116% respectively) in comparison with the control group, meanwhile the brain caspase-3 activity was an insignificant differed in moringa treated groups G5 and G6 in comparison with the control group. Each value reflects the average of seven animals ± SD in different experimental groups, G1 (control), G2 (26.15 mg/kg BW CYP), G3 (8.72 mg/kg BW CYP), G4 (250 mg/kg BW moringa extract), G5 (250 mg/kg BW moringa extract + 26.15 mg/kg BW CYP) and G6 (250 mg/kg BW moringa extract + 8.72 mg/kg BW CYP).The same letter implies that there is an insignificant difference between groups by using One Way ANOVA at P < 0.05. The different letter implies that there is a significant difference between groups by using One Way ANOVA at P < 0.05.
Groups
caspase-3 activity (ng/mg protein)
AchE activity (µmol/min/mg protein)
G1
0.24 ± 0.023a
87.81 ± 7.95a
G2
0.51 ± 0.062b
42.45 ± 6.38b
G3
0.52 ± 0.083b
41.53 ± 8.55b
G4
0.22 ± 0.041a
87.64 ± 8.64a
G5
0.24 ± 0.041a
82.69 ± 6.21a
G6
0.24 ± 0.048a
83.32 ± 7.40a
3.2.2 Brain genomic (DNA fragmentation by comet assay)
The statistical analysis disclosed a significant increase (p < 0.05) in the percentage of DNA damage level, percentage of DNA in tail and tail moment by comet assay of brain rats in CYP exposed groups G2 and G3 (165% and 188%), (165% and 181%) and (347% and 375%) respectively when compared with the control group, on the other hand, moringa treatment alleviated percentage DNA damage level, percentage DNA in tail and tail moment in treated groups G5 and G6 (28% and 29%), (73% and 61%) and (120% and 124%) respectively in comparison with G2 and G3 groups as shown in Table 3 and Fig. 1. Each value reflects the average of seven animals ± SD in different experimental groups, G1 (control), G2 (26.15 mg/kg BW CYP), G3 (8.72 mg/kg BW CYP), G4 (250 mg/kg BW moringa extract), G5 (250 mg/kg BW moringa extract + 26.15 mg/kg BW CYP) and G6 (250 mg/kg BW moringa extract + 8.72 mg/kg BW CYP).The same letter implies that there is an insignificant difference between groups by using One Way ANOVA at P < 0.05. The different letter implies that there is a significant difference between groups by using One Way ANOVA at P < 0.05.
Groups
%DNA damage
%DNA in tail
Tail moment
G1
6.93 ± 0.66a
4.72 ± 0.68a
0.32 ± 0.05a
G2
18.35 ± 1.92b
12.49 ± 2.71b
1.43 ± 0.33b
G3
19.93 ± 1.42b
13.25 ± 2.58b
1.52 ± 0.23b
G4
6.80 ± 0.93a
4.45 ± 0.72a
0.33 ± 0.07a
G5
14.30 ± 1.20c
7.19 ± 1.06c
0.65 ± 0.12c
G6
15.48 ± 2.42c
8.24 ± 1.14c
0.68 ± 0.14c
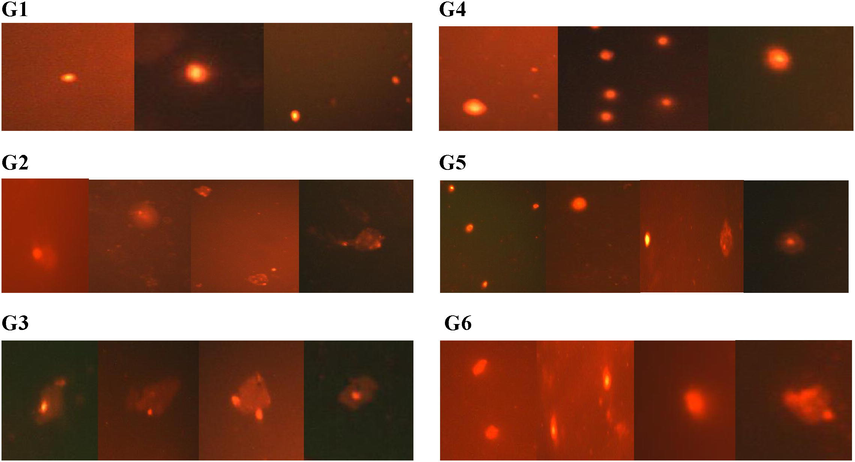
Visual scoring of DNA damage in brain tissue according to comet appearance in different experimental groups, G1 (control), G2 (26.15 mg/kg BW CYP), G3 (8.72 mg/kg BW CYP), G4 (250 mg/kg BW moringa extract), G5 (250 mg/kg BW moringa extract + 26.15 mg/kg BW CYP) and G6 (250 mg/kg BW moringa extract + 8.72 mg/kg BW CYP).
3.3 Influence of CYP and Moringa extract on neurotransmitter “AchE’’ activity
Rats exhibited a significant decrease (p < 0.05) in AchE brain activity in CYP exposed groups G2 and G3 (52% and 53% respectively) in comparison with the control group. Treatment by moringa extract in G5 and G6 groups exhibited a significant enhancement until almost normal activity (Table 2).
4 Discussion
Mitochondria play a fundamental role in the generation and regulation of cellular bioenergetics that produces the ATP molecules through oxidative phosphorylation. These functions are related to neurodevelopment, connectivity, plasticity, and differentiation (Bergman and Ben-Shachar, 2016). In our results, CYP causes mitochondrial dysfunction in brain male rats which confirmed by the inhibition of the mitochondrial NADH dehydrogenase and ATPase enzymes activity. Complex I Inhibition by toxic materials can lead to the etiology of neurodegenerative diseases where, complex I Inhibition can decrease ATP output, ROS elevation, and initiate a self-amplification cycle of occurrences that encourage mitochondrial disorders (Zolkipli-Cunningham and Falk, 2017). NADH dehydrogenase enzyme is the first and the largest complex of the mitochondrial oxidative phosphorylation respiratory chain (complex I). It creates the electrochemical gradient in the oxidative phosphorylation respiratory chain to generate the ATP molecules (Chen et al., 2017). The inhibition of NADH dehydrogenase enzyme (complex I) maybe because of the complex I has more than 40 regions as potential binding sites for pyrethroids for their hydrophobic nature. This illustration was showed by Guven et al. (2018) and also may be related to ROS. Moreover, mitochondrial ATPase is one of the membrane-bound enzymes connected with the lipoprotein of the cell membrane. It has a critical function within the liberation and absorption of the biogenic amines in the central nervous system. The inhibition in mitochondrial ATPase activity induced by CYP may be due to its impact on cell membranes because of its affinity to interact with membrane lipids that cause inhibition of ATPase activity via affecting the enzyme complex. As a result, ATPase used as an effective biomarker for mechanistic toxicity studies of pesticides especially pyrethroids as evidenced by Ksheerasagar et al., (2011). Our data are consistent with other previous studies (Agrawal et al., 2014; Hamed, 2017).
Moringa extract was used in this work as a neuroprotective agent against CYP effects. Our results showed that moringa extract alleviates the toxic effect induced by CYP in brain tissue. Moringa minimizing impaired mitochondria was confirmed by improving the mitochondrial NADH dehydrogenase and ATPase enzyme activity. This protective effect can be because of its antioxidant activity, which contains bioactive polyphenols (catechin, quercetin, and kaempferol) against ROS and prevents oxidative damage. Chen et al., (2015) exhibited that catechin polyphenols can act as antioxidants by eliminating free radicals and chelating surplus metal ions. Furthermore, it contains quercetin, which contains hydroxyphenyl groups with an antioxidant effect with authenticated therapeutic purposes and other flavonoids, which inhibits the production of nitrogen species and reactive oxygen (Omotoso et al., 2018).
Mitochondria are the first place to trigger cell apoptosis across numerous factors such as the cytochromes and the caspases. Where, Agrawal et al. (2014) reported that reduction in mitochondrial complex I (NADH dehydrogenase enzyme) activity may lead to enhance oxidative stress and apoptosis. Caspase-3 is the main effector protein of apoptosis and interacts with caspase-8 and caspase-9. This activation plays an essential role in the execution phase of apoptosis (Jevtić et al., 2016).
In the present work, exposure to CYP resulted in a high-activity of the brain’s caspase-3. This activation triggers rapid DNA fragmentation by activating caspase-activated deoxyribonuclease. Whereas, mitochondrial dysfunction induced by pesticides can increase ROS production and cause disturbance of mitochondrial membrane permeability releasing cytochrome c into cytosol and then activates caspase-9 and caspase-3 as was shown in Zhang et al. (2017).
The data showed that CYP causes DNA damage in the brain as evidenced by an increase in the tail moment. The hydrophobic nature of CYP and its small molecular size aid in crossing the cell membrane and arriving at the nucleus. Besides, there are vinyl and dimethylcyclopropane groups of CYP that, can be oxidized by rearranging the radical and the forming a carbocation into methyl butanol. The resulting active metabolites (methyl butanol and vinyl) may cause DNA damage (Sankar and Manimaran, 2010). These results are in agreement with the previously reported results, which showed that cypermethrin induced apoptosis as elevation caspase-3 and DNA fragmentation (Hussien et al., 2013; Gasmi et al., 2017).
Otherwise, treatment with moringa extract significantly reduces apoptosis markers induced by CYP because it ameliorates mitochondrial functions as moringa extract has Luteolin that has powerful antioxidant activity and a protective ability on DNA. Also, moringa extract has free radical removal and anti-inflammatory capabilities (El-Hawary et al., 2012). Additionally, it contains Vit E (α-tocopherol) which prevents apoptosis programmed cell death via breaking the propagation of free radical chain reactions in the lipid portion of the cell membrane (Roy and Das, 2013). Recently, Khan et al. (2018) reported quercetin in moringa extract may regulate mitochondrial apoptotic pathway by preventing the activation of cytochrome-c and caspases-3.
AchE is a biological marker of neurotoxicity (cholinergic marker) related to several illnesses like neurodegenerative disorders. Exposures to CYP inhibited the AchE activity in brain tissue which may be due to containing aromatic amino acid in the active site of AchE enzyme representing a hydrophobic area that reacts with CYP. Besides, the cyano group of CYP reacts with different amino acids side chains in the active site and stabilized by hydrogen bonding (Sharma et al., 2014). The inhibition of AchE is following the findings Elsawya et al., (2017).
On the other hand, AchE activity was reversed into normal by moringa extract treatment. These alleviations may be due to reducing the formation of free radicals and lipid peroxidation because it contains vitamins E, C, B, and β-carotene. The metabolite of Vit C is dehydroascorbic acid. that cross the blood membrane barrier and has an antioxidant effect by increasing blood flow in the cerebral (Kirisattayakul et al., 2013). Moringa oleifera leaves extract has important roles in memory retention by improving the cholinergic function, reducing neuronal cell death, increasing blood flow in the cerebral cortex, and increasing dopaminergic function where it is multi-target sites. So, it attenuates neuronal degeneration (Pakade et al., 2013). Moringa oleifera components can pass through the blood–brain barrier and may have a direct effect on the vital functions of the intoxicated-rats with CYP (Mahaman et al., 2018). Neuronal protection exhibited moringa extract is in an agreement with results got by Kou et al. (2018).
5 Conclusion
The current findings revealed that CYP exposed animals can cause serious mitochondrial dysfunction of the brain. So CYP may be induced neurodegenerative disorders by activation of caspases rats’ brain through the brain's genomic DNA damage and inhibition of AchE activity. It also caused necrosis cell death and apoptosis. Our outcomes demonstrated that moringa extract has a neuroprotective effect against CYP-induced neuronal disorder by minimizing its adverse impacts on most of the parameters examined. Moringa has a vital role as an antioxidant which increases antioxidant status and reduces the oxidative damage to a nucleic acid of neuronal cells.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Moringa oleifera leaves aqueous extract ameliorates hepatotoxicity in alloxan - induced diabetic rats. Biochem. Cell Biol. 2017
- [CrossRef] [Google Scholar]
- Cypermethrin-induced nigrostriatal dopaminergic neurodegeneration alters the mitochondrial function: a proteomics study. Mol. Neurobiol. 2014
- [CrossRef] [Google Scholar]
- Mitochondrial oxidative phosphorylation system (OXPHOS) deficits in schizophrenia. Can. J. Psychiatry. 2016;61(8):457-469.
- [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248-254.
- [Google Scholar]
- Chen, Y.Z., Lyons, K.E., Pahwa, R., Reddy, M.B., 2015. Green tea consumption reduces oxidative stress in Parkinson’s disease patients. J. Behavioral Brain Sci., 5, 194–202.
- Effects of commonly used pesticides in China on the mitochondria and ubiquitin-proteasome system in Parkinson’s disease. Int. J. Mol. Sci.. 2017;18:2507-2523.
- [Google Scholar]
- On the effect of paraquat on isolated mitochondria. Evidence that paraquat causes opening of the Cycloporin A-sensitive permeability transition pore synergistically with nitric oxide. Toxicology. 1995;99:77-88.
- [Google Scholar]
- Polyphenolics content and biological activity of Plectranthus amboinicus (Lour) Spreng growing in Egypt (Lamiaceae) Pharmacognosy J.. 2012;4(32):45-54.
- [Google Scholar]
- A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol.. 1961;7:88-95.
- [Google Scholar]
- Protective effect ofα-lipoic acid againstα-cypermethrin-induced changes in rat cerebellum. J. Chem. Neuroanat.. 2017;86:52-58.
- [Google Scholar]
- Mitochondria, oxidative stress, and neurodegeneration. J. Neurol. Sci. 2012
- [CrossRef] [Google Scholar]
- Resolution of complex I and isolation of NADH dehydrogenase and an iron-sulfur protein. Methods Enzymol.. 1978;53:15.
- [Google Scholar]
- Effect of Moringa Oleifera in an experimental model of Alzheimer's disease: role of antioxidants. Ann. Neurosci.. 2005;12(3):33-36.
- [Google Scholar]
- Salim Gasmi, Rachid Rouabhi1, Mohamed Kebieche, Samira Boussekine, Aya Salmi, Nadjiba Toualbia, Chahinez Taib, Zina Bouteraa, Hajer Chenikher, Sara Henine and Belgacem Djabri, 2017. Effects of Deltamethrin on the striatum and hippocampus mitochondrial integrity and the protective role of Quercetin in rats. Environ. Sci. Pollut. Res., 24, 16440–16457.
- Long term biochemical changes in offspring of rats fed a diet containing alpha-cypermethrin. Pestic. Biochem. Physiol.. 2017;142:133-140.
- [Google Scholar]
- Celal Guven, Yusuf Sevgiler, Eylem Taskin, 2018. Pyrethroid Insecticides as the Mitochondrial Dysfunction Inducers. Mitochondrial diseases, chapter 12, 293–322.
- Protective effect of quercetin against oxidative stress and mitochondrial bioenergetic deficiency caused by lambda-cyhalothrin. Alexandria Sci. Exchange J.. 2017;38(1):82-88.
- [Google Scholar]
- Cypermethrin induced damage in genomic DNA and histopathological changes in brain and haematotoxicity in rats: the protective effect of sesame oil. Brain Res. Bull. 2013;92:76-83.
- [Google Scholar]
- Mitochondrial impairment, apoptosis, and autophagy in a rat brain as immediate and long-term effects of perinatal phencyclidine treatment influence of restraint stress. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;3(66):87-96.
- [Google Scholar]
- Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain. Front. Pharmacol. 2018
- [CrossRef] [Google Scholar]
- Cerebroprotective effect of Moringa oleifera against focal ischemic stroke induced by middle cerebral artery occlusion. Oxid. Med. Cell. Longevity 2013
- [CrossRef] [Google Scholar]
- Nutraceutical or pharmacological potential of Moringa oleifera Lam. Nutrients. 2018;10:343-355.
- [Google Scholar]
- Impairment of hepatic biochemical contents and enzyme activities during carbosulfan intoxication in albino mice. Int. Multidisciplinary Res. J.. 2011;1(3):6-15.
- [Google Scholar]
- Moringa oleifera alleviates homocysteine-induced Alzheimer’s disease-like pathology and cognitive impairments. J. Alzheimer’s Disease. 2018;63(3):1141-1159.
- [Google Scholar]
- Moringa peregrine leaves extracts induce apoptosis and cell cycle arrest of hepatocellular carcinoma. Biomed Res. Int. 2019
- [CrossRef] [Google Scholar]
- OECD, 1987. OECD Guidelines for Testing Chemicals. Organization for Economic Co-operation and Development, Guidelines 402.
- OECD, 2008. Guidelines for the Testing of Chemicals Repeated Dose 28-Day Oral Toxicity Study in Rodents, Guidelines 407.
- Moringa oleifera phytochemicals protect the brain against experimental nicotine-induced neurobehavioral disturbances and cerebellar degeneration. Pathophysiology. 2018;25(1):57-62.
- [Google Scholar]
- Comparison of antioxidant activity of Moringa oleifera and selected vegetables in South Africa. S Afr. J. Sci.. 2013;109:1-5.
- [Google Scholar]
- Role of Moringa oleifera on brain electrical activity in colchicine induced experimental rat model of Alzheimer's disease: possible involvement of antioxidants. Int. J. Curr. Pharm. Res.. 2013;5(4):40-45.
- [Google Scholar]
- Curcumin protects against cypermethrin-induced genotoxicity in rats. Environ. Toxicol. Pharmacol.. 2010;30:289-291.
- [Google Scholar]
- Neurotoxic effect of cypermethrin and protective role of resveratrol in Wistar rats. Nutr. Pharmacol. Neurol. Dis.. 2014;4:104-111.
- [Google Scholar]
- A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res.. 1988;175:184-191.
- [Google Scholar]
- Effects of cypermethrin on monoamine transporters, xenobiotic-metabolizing enzymes, and lipid peroxidation in the rat nigrostriatal system. Free Radical Res.. 2010;44(12):1416-1424.
- [Google Scholar]
- Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol.. 2007;83:84-92.
- [Google Scholar]
- With technical assistance. Amicrocolorimetric method for the determination of inorganic phosphorus. J. Biol. Chem.. 1953;253:956-964.
- [Google Scholar]
- Parkinson’s disease risk from ambient exposure to pesticides. Eur. J. Epidemiol.. 2011;26(7):547-555.
- [Google Scholar]
- Cypermethrin exposure reduces the ovarian reserve by causing mitochondrial dysfunction in granulosa cells. Toxicol. Appl. Pharmacol. 2019
- [CrossRef] [Google Scholar]
- The potential threat of Chlorpyrifos to human liver cells via the caspase-dependent mitochondrial pathways. Food Agric. Immunol.. 2017;29(1):294-305.
- [Google Scholar]
- Clinical effects of chemical exposures on mitochondrial function. Toxicology. 2017;1(391):90-99.
- [Google Scholar]







