Translate this page into:
The potential molecular implications of adiponectin in the evolution of SARS-CoV-2: Inbuilt tendency
⁎Corresponding authors. sbungau@uoradea.ro (Simona Gabriela Bungau), andreiflavius.radu@gmail.com (Andrei-Flavius Radu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
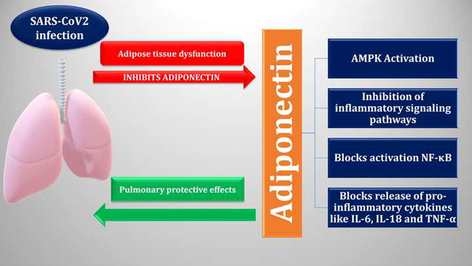
Adiponectin (APN) is an adipokine concerned in the regulation of glucose metabolism, insulin sensitivity and fatty acid oxidation. APN plays a critical role in viral infections by regulating the immune response through its anti-inflammatory/pro-inflammatory axis. Reduction of APN may augment the severity of viral infections because APN inhibits immune cells’ response via suppression of inflammatory signaling pathways and stimulation of adenosine monophosphate protein kinase (AMPK). Moreover, APN inhibits the stimulation of nuclear factor kappa B (NF-κB) and regulates the release of pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukins (IL-18, IL-6). In COVID-19, abnormalities of the fatty tissue due to oxidative stress (OS) and hyperinflammation may inhibit the production and release of APN. APN has lung-protective effect and can prevent SARS-CoV-2-induced acute lung injury (ALI) through the amelioration of endoplasmic reticulum (ER) stress, endothelial dysfunction (ED) and stimulation of peroxisome proliferator-activated receptor-alpha (PPAR-α). It has been established that there is a potential correlation between inflammatory signal transduction pathways and APN that contributes to the development of SARS-CoV-2 infections. Deregulation of these molecular pathways affects the expression of APN and vice versa. In addition, the reduction of APN effect in SARS-CoV-2 infection could be a potential cause of the exacerbation of pro-inflammatory effects which are associated with the disease severity. In this context, exploratory, developmental, and extensive prospective studies are necessary.
Keywords
Adiponectin
COVID-19
Immune response
Acute lung injury
Adipose tissue
1 Introduction
It is observable and anticipated that an increasing proportion of the population is susceptible to a condition of energy surplus that supports exponentially the expansion of obesity-related disorders and other metabolic disorders (i.e., diabetes, gout, etc.) as food intake and unhealthy lifestyles increase with the emergence of modern amenities. During instances of nutritional abundance, white adipocytes deposit energy as triglycerides. However, when its storage load is reached, extra fat is diverted to non-adipose cells and tissues, where it reaches additional non-oxidative mechanisms and stimulates the generation of harmful lipid metabolites in particular organs. Due to it being demonstrated to have pro-metabolic actions via modulating glucose and lipid levels, the involvement of adipocytes or fatty tissues as an endocrine regulator secreting numerous adipokines, in particular adiponectin (APN), has gained significance for the management of metabolic syndrome and types of diabetes (Martínez de Morentin et al., 2010).
APN (considered a polypeptide hormone and also an adipokine produced from adipose tissue, skeletal muscles, placenta, and brain) is intricate in the regulation of insulin sensitivity, glucose metabolism, and fatty acid oxidation, as it is summarized in Fig. 1. This molecule was initially described in 1995 in the differentiated adipocytes by Scherer et al. (Scherer et al., 1995), then it was recognized in mice adipocytes (Maeda et al., 1996), and later on in 2007, it was exposed that APN was involved in the differentiation of adipocytes (Lara-Castro et al., 2007). The APN gene is found on the chromosome region 3q27, which upsurges the predisposition to type 2 diabetes mellitus (T2DM). Thus, reduction of circulating APN level is correlated with the risk of T2DM development (Al-Kuraishy and Al-Gareeb, 2016). APN production from the adipose tissue is contrariwise linked to body mass index (BMI). Therefore, obesity or metabolic syndrome diminish APN. (Al-Kuraishy and Al-Gareeb, 2016).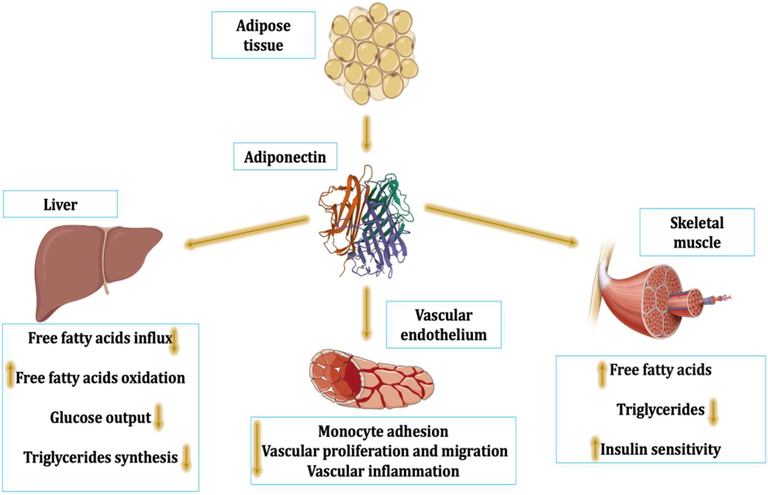
Physiological role of adiponectin (APN): APN improves insulin sensitivity and utilization of free fatty acids with a reduction of triglycerides in the skeletal muscles, promoting the activity of vascular endothelium through inhibition of monocyte adhesion, vascular proliferation, and migration with inhibition of vascular inflammation. In addition, APN effects on the liver inhibit triglyceride synthesis, influx of free fatty acids, and glucose output with improvement of free fatty acids oxidation.
Unlike other adipokines, APN is produced from visceral adipose tissue, mainly from bone marrow adipose tissue (Cawthorn et al., 2014). APN is highly present in plasma compared to other adipokines. APN’s normal level range is 5–10 µg/mL with 2–5 h half-life. It is higher in woman than in men and reduced in obesity and T2DM (Al-Kuraishy and Al-Gareeb, 2016, Bungau et al., 2020). The highest insulin-sensitizing action in the liver cells is upregulated by trimers with reduced molecular mass, which oligomerize to establish hexamers as the intermediate form, which in turn aggregate to generate a high-level configuration of oligomers of approximately 800 kDa. APN disperses in a mixture of these three configurations. Furthermore, the liver and kidneys both play a supporting role in the clearance of circulating APN (Halberg et al., 2009).
APN plasma level is increased during starvation as in anorexia nervosa, due to the release from adipose tissue with bone marrow (Cawthorn et al., 2014). High APN plasma level inhibits adipocyte differentiation and decreases metabolic derangement, which increases the risk of insulin resistance (IR), T2DM, and metabolic syndrome (Liu et al., 2018). Notably, APN enhances insulin sensitivity through inhibition of hepatic glucose production and peripheral fatty acid oxidation (Al-Kuraishy and Al-Gareeb, 2016). High molecular APN is the most active biological form concerning glucose homeostasis (Oh et al., 2007). Likewise, high molecular weight (HMW) APN is positively connected with coronary artery disease (Rizza et al., 2010). APN levels are increased by caloric restriction, aging, estrogen deficiency, and cigarette smoking (Combs et al., 2003). APN acts on precise receptors which are AdipoR1 (mainly in skeletal muscles), AdipoR2 (mainly in the liver) and T-cadherin-CDH13 (mainly expressed in neurons and blood vessels) (Fig. 2) (Yamauchi and Kadowaki, 2013).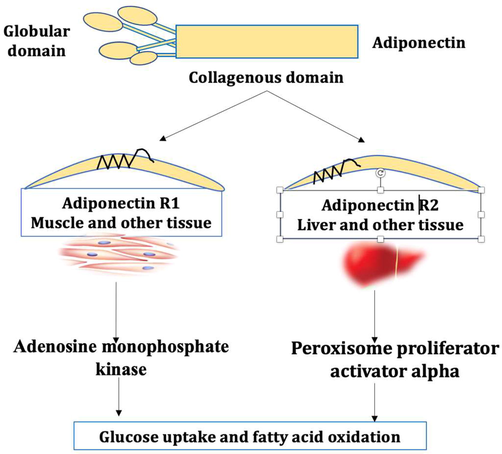
Expression of adiponectin receptors (AdipoRs): APN acts on Adipo1 and Adipo2, which are expressed mainly in the muscle and liver, respectively, leading to induction of adenosine monophosphate kinase and peroxisome proliferator activator alpha with subsequent improvement of glucose uptake and fatty acid oxidation.
It has been shown that APN improves the cognitive function through modulation of synaptic plasticity and memory function. APN acts in conjugation with leptin (Lep) in the regulation of appetite (Forny-Germano et al., 2019). Moreover, APN have antioxidant and anti-inflammatory effects (La Russa et al., 2020) and might be a potential target for diverse drugs and herbal agents that increase APN expression and production, including statins, omega-3 fatty acids, curcumin, and gingerol (Shetty et al., 2009).
On the other hand, different SARS-CoV-2 mutations are the source of coronavirus disease 2019 (COVID-19). The final SARS-CoV-2 mutation that propagated to about 136 nations constituted the final form that the World Health Organization (WHO) formally accepted on November 26, 2021 (Schmidt et al., 2021). Acute inflammation, oxidative stress (OS), and multi-organ damage like acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) are hallmarks of SARS-CoV-2 infections) (Behl et al., 2022). COVID-19 leads to pulmonary and extrapulmonary manifestations with critical complications mainly in severely affected patient (Schmidt et al., 2021).
The scientific topic has been chosen for a variety of reasons: first, it is not sufficiently and clearly described in the literature; moreover, information is dispersed, and, at the same time, we aimed to complete at a highest level our interests for research, our team members having recently published many articles in both areas of interest (obesity/metabolic syndrome markers and COVID-19), but without a connection between the two (APN and COVID-19). The most relevant medical databases were screened, choosing data linked to the present topic, and developing a comprehensive Review type article. The current literature research evaluated the possible function of APN in SARS-CoV-2 infections, considering its antioxidant and anti-inflammatory properties, pathways of action, related factors, and outcomes. The impact of APN activators and APN agonists on this disease and associated impairments have been analyzed, providing important information for clinicians in optimizing the management of severe forms of COVID-19, possibly associated with decreased APN effect.
2 Methodology of research
The present paper centralizes and filters scholar publications on the molecular implications of APN in the evolution of COVID-19, provided by a comprehensive literature search related to immunological and inflammatory processes and biomolecular systems that contribute to the signaling networks, also correlating APN with ALI within COVID-19 evolution. In this regard, medical literature research was conducted by using large and valuable abstract and citation databases that cover a wide range of biomedical topics (e.g., PubMed, ScienceDirect, SpringerLink, and Nature). The search methodology included in the first step an assessment of the topic in the medical literature in terms of research areas, content type, and total number of publications (Fig. 3), while the second search algorithm is based on PRISMA flow diagram criteria that evaluate the flow of information through this review (Fig. 4).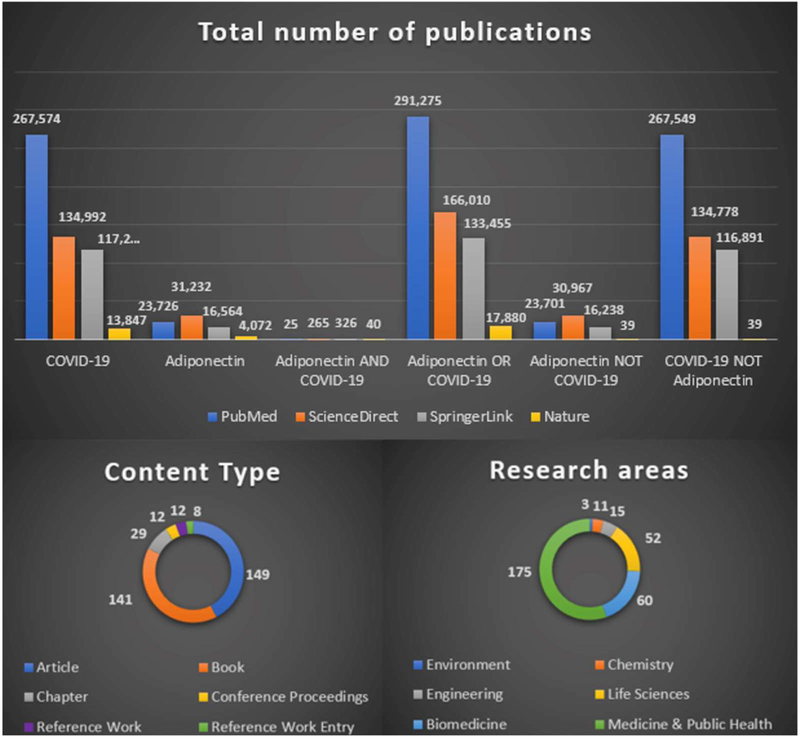
Publications on the COVID-19/APN relationship and related research areas and manuscript types identified in medical databases using boolean search operators.
Literature selection depicted in a PRISMA 2020 type flow diagram.
In the first step, searches were conducted using two essential items (e.g., APN and COVID-19), the correlation between them being analyzed using three basic boolean operators: AND, OR, and NOT. Although the number of studies on APN or COVID-19 per se is numerous, publications evaluating the correlations between the two are fewer, resulting in a need for further research. According to SpringerLink, the main subject areas correlated with types of publications related to APN AND COVID-19 are depicted in Fig. 3 (one publication can be included multiple categories).
Moreover, a comprehensive search of the scientific literature was conducted on all publications connected to the interaction of APN with inflammatory signaling pathways through its regulatory function with regard to the pathophysiology of SARS-CoV-2 infections. Predetermined algorithms were utilized for in-depth searching in the PubMed, ScienceDirect, SpringerLink, and Nature databases. Fig. 4 depicts the PRISMA-based operating processes, along with details on the selection, evaluation, and final inclusion of publications utilized as bibliographic references, which was realized according to Page et al. recommendations (Page et al., 2021).
Publications that were not written in English, were not very informative, or were not article-type have been excluded before screening. A total of 111 bibliographic references between 1995 and 2022 were selected and cited to validate the data in the present paper.
3 APN and inflammation
APN exhibits preventive effects against the onset of numerous illnesses associated with obesity. The inverse correlation involving blood APN concentrations and a variety of inflammation biomarkers has been shown in numerous studies (Fantuzzi, 2008, Popa et al., 2020). By regulating signaling processes in several cell populations, APN reduces inflammatory reactions to a multitude of stressors. One of the main factors contributing to APN's favorable effects on metabolic and cardiovascular diseases, especially IR and atherosclerosis, may be its anti-inflammatory effects. The control of inflammatory and immunological reactions is significantly influenced by APN. The dysregulation of adipose tissue in obesity, T2DM, and metabolic syndrome with the release of various types of pro-inflammatory cytokines may reduce circulating APN (Fantuzzi, 2008, Popa et al., 2020). Of note, adipose tissue-mediated low-grade inflammations inhibits APN production (Fantuzzi, 2008), this manner might explain low APN levels in obesity. Visceral fat accumulation suppresses the production and release of APN, which show important immunological effects, including the inhibition of macrophages activity and the perseveration of endothelial and smooth muscle cells through inhibition of monocyte adhesion (Fig. 5) (Lau et al., 2017).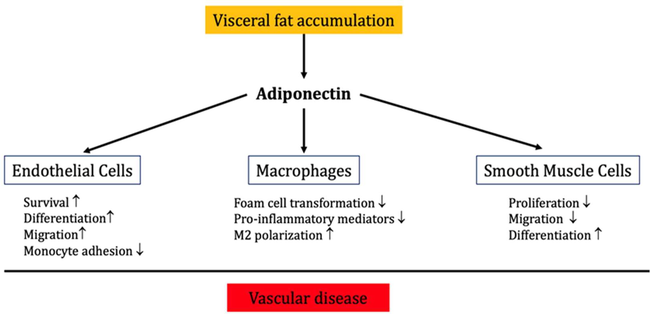
Role of APN in vascular inflammation: APN inhibits vascular inflammation and the development of vascular diseases through improvement of endothelial cell functions, inhibition of smooth muscle proliferation, and macrophage activation.
Principally, HMW APN inhibits immune cells’ response via stimulation of AMP and the suppression of inflammatory signaling pathways. The emission of pro-inflammatory cytokines (i.e., IL-18, IL-6) and tumor necrosis factor alpha (TNF-α) and nuclear factor kappa B (NF-κB) stimulation are inhibited by APN action (Ouchi et al., 2011). The anti-inflammatory effects of APN can reduce vascular inflammation, ALI, and cardiac ischemia, via controlling pro-inflammatory molecules (Shibata et al., 2005). Though, APN has pro-inflammatory effects by increasing the expression of cyclooxygenase-2 (COX2) in the synovial tissues (Lubbeke et al., 2014). Furthermore, APN regulates the innate immunity through sensing of metabolic stress. APN has an identical structure to complement factor C1q, which regulates macrophage polarization and other immune cells by promoting clearance apoptotic cells, thus plummeting the risk of autoimmunity. APN promotes anti-inflammatory M2 macrophage and its biomarkers, including IL-10 or arginase-1. Therefore, it inhibits the macrophage mediated inflammation (Fukushima et al., 2009).
Moreover, AdipoR1, Adipo2 and T-cadherin are highly expressed in monocytes and macrophages. Of interest, AdipoR1 inhibits the expression of NF-κB on the macrophages; AdpR2 induces macrophage polarization, whereas T-cadherin activates APN-induced macrophage proliferation (Mandal et al., 2011). Nevertheless, blocking APNRs has minor effect on APN anti-apoptotic action (Takemura et al., 2007), signifying an unidentified mechanism of APN on the macrophage action. APN can also inhibit macrophage activity through modulation of TLR1-NF-κB signaling pathway (Yamaguchi et al., 2008).
In contradiction of its anti-inflammatory role, by stimulating the NF-κB/ERK axis, APN induces the increase in expression of pro-inflammatory mediators (Park et al., 2007). The pro-inflammatory action of APN during infection is temporary, and its potential function is to neutralize lipopolysaccharide (LPS). Hence, chronic pro-inflammatory effect of APN induces LPS resistance and preclude TNF-α release from macrophages (Park et al., 2007). Likewise, APN induces B cells activation and release of inhibitory peptide on T cell maturations and migrations (Chimen et al., 2015). Certainly, B and T cells expressing AdipoRs are highly inhabitant in the adipose tissues concerned in the induction of IR and T2DM (Costanzo et al., 2015). Therefore, APN diminishes monocyte effects on M1 macrophage to release cytokines that are stimulating the inflammatory response, such as TNF-α or IL-6 and monocyte chemoattractant protein (MCP), but it advances the release of IL-10. APN and inflammatory signaling processes that have been involved in the pathophysiology of SARS-CoV-2 infection also interact among themselves. The expression of APN is impacted by the dysregulation of these inflammatory signaling pathways, and reciprocally. As a result, the intensification of pro-inflammatory effects, which are associated with the severity of COVID-19, might be caused by a decrease in APN function during SARS-CoV-2 exposure (Fig. 6).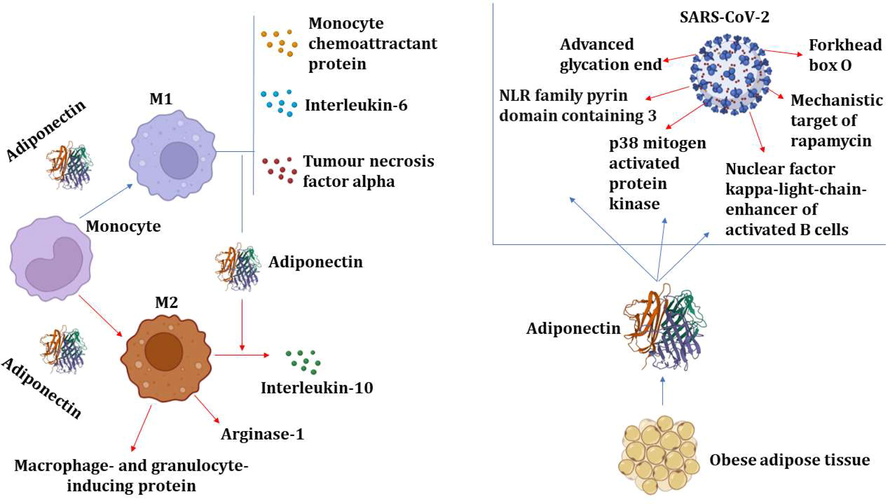
Anti-inflammatory effect of APN: APN inhibits inflammatory signaling pathways, proinflammatory cytokines and the augmentation of anti-inflammatory cytokines. APN promotes macrophage polarization toward anti-inflammatory M2 with the release of IL-10 and arginase. APN inhibits inflammatory signaling pathways in SARS-CoV-2 pathogen.
As part of the molecular mechanism of action of APN on immunological and inflammatory responses, pro-inflammatory mediators are inhibited and anti-inflammatory molecules are stimulated (Wulster-Radcliffe et al., 2004). Likewise, APN can constrain astrocyte-induced inflammation and mitochondrial dysfunction (Wu et al., 2020). Though, APN can't be categorized as either inflammatory or anti-inflammatory, as the net effect of APN is mostly depend on the inflammatory status and immune response (Agarwal and Balasubramanyam, 2015).
4 Role of APN and viral infections
It has been reported that APN is involved in various viral infections during acute and chronic phase. APN plays a critical role in viral infections by regulating the immune response through its anti-inflammatory/pro-inflammatory axis. The reduction of APN may increase the severity of viral infections (Siagris et al., 2007).
APN plasma level is increased in patients with viral hepatitis B and C (HCV), and positively correlated with necro-inflammation stage (Siagris et al., 2007). Liu et al. establish that APN plasma level was linked with viral characteristics of HCV in the induction of IR (Liu et al., 2005). Thus, hyperadiponectinemia reflects the severity of IR and hepatic injury (Liu et al., 2005). As well, APN has a new function in the control of the immune response during HCV infection (Palmer et al., 2008). A prospective study illustrated that HMW APN serum level was reduced in lean patients with HCV infection due to higher anti-HCV antibody. HMW APN improves anti-HCV immune response by induction release of interferon (INF) via T cell activation (Palmer et al., 2008).This verdicts suggests that APN plasma level is inversely correlated with anti-HCV antibody level. Besides, APN has anti-inflammatory and pro-inflammatory role, the inflammatory role being mediated by the activation of mitogen activated protein kinase (MAPK) in regulating INF response during different viral infections (Palmer et al., 2008). As a result, APN is increased in acute HCV infection and reduced after development of anti-HCV antibody. Decreasing of APN plasma level is associated with the progression of steatosis in patients with HCV (Petit et al., 2005).
Moreover, Tsatsanis et al. reported that the reduction of APN plasma level in obese patients could be a potential casual factor for the severity of H1N1 (Tsatsanis et al., 2010). It has been shown that high APN levels can aggravate influenza infection in elderly patients through increase of anti-inflammatory IL-10 (Jiang et al., 2020). Of note, APN is regarded as a negative regulator of T cells during antigenic stimulation (Wilk et al., 2011). Concerning less than 10 % of circulating T cells expressed AdpR1, and most of the T cells store AdpR1 in the intracellular compartment. Following stimulation by antigens, these receptors up-regulated on the surface of T cells and interacted to induce T cell apoptosis and inhibited cytokine production in response to viral antigenic stimulation (Wilk et al., 2011). Of interest, weight gain during respiratory syncytial virus infection may be exacerbated by a deficiency of APN (Kim et al., 2021). Though, APN can improve OS and airway inflammation in experimental obese-induced asthma through modulation of adenosine monophosphate kinase (AMPK) pathway (Zhu et al., 2019).
Concerning the role of APN in HIV infection, different studies reported conflicting results regarding HIV and its therapeutic regimens. Mynarcik et al. establish that the deficiency of APN in HIV infected patients is implicated in the development of lipodystrophy and reduction of insulin sensitivity (Mynarcik et al., 2002). In HIV infected patients, APN level expression is reduced in dystrophic fat. So, APN level in HIV infected men is about 50 % of those without lipodystrophy (Kinlaw and Marsh, 2004). Furthermore, low APN levels in HIV infected patients is associated with the progression of subclinical cardiovascular complications (Fantuzzi, 2008). These findings indicated that low APN level in HIV infected could be a possible factor for long-term complications like T2DM and cardiovascular complications.
It has been observed that adipocyte dysfunction in various viral infections releases abnormal adipocyte defense cell response and molecules that are pro-inflammatory, resulting in inhibition production and release of APN (Agarwal and Balasubramanyam, 2015) (Fig. 7).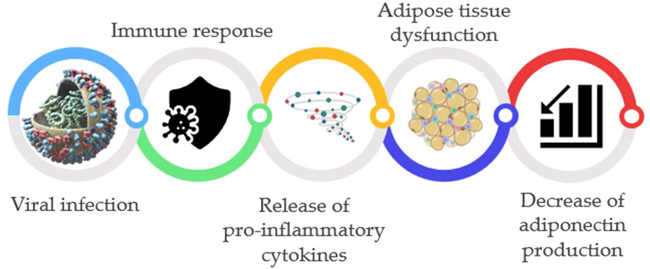
The potential role of APN in viral infections: the immune response in viral infections releases pro-inflammatory proteins which affect expression and APN's release.
5 Role of APN in SARS-CoV-2 infection
SARS-CoV-2 enters infected tissues and cells via the angiotensin converting enzyme 2 (ACE2) receptor. Binding of SARS-CoV-2 with ACE2 triggers deregulation of the renin-angiotensin system (RAS), which is characterized by down-regulation of ACE2, reduction of Ang1-7 with anti-inflammatory potential and elevation of angiotensin II (AngII) as a pro-inflammatory molecule (Yenicesu et al., 2005). High AngII and low Ang1-7 in hypertension and T2DM decrease circulating APN level, hence ACE inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) may augment APN level in patients with hypertension and T2DM (Yenicesu et al., 2005). In addition, ACE2 has a significant contribution in the management of APN generation. Ang1-7 or ACE2 assuage inflammation caused by fatty tissue in the heart by increasing APN function (Patel et al., 2016). According to Liu et al., fosinopril improves hepatic fibrosis through increasing the APN's function via ACE2/Ang1-7 expression (Liu et al., 2012). RAS dysregulation in COVID-19 may therefore be the plausible explanation for the decreased APN concentration.
Remarkably, a case-control study included 29 patients with respiratory failure, 12 COVID-19 patients and 17 non-COVID-19 individuals, indicated that COVID-19 patients with respiratory distress had lower APN concentrations even after alteration for sex, age, and BMI (Kearns et al., 2021). Though, a single center prospective study comprising 60 COVID-19 patients with mild to severe symptoms, showed that APN/Lep ratio was not associated with the severity of disease, because of the compensating rise in APN's capacity to counteract systemic inflammation (Di Filippo et al., 2021). Ho et al. found that aging and comorbidities augment risk of the ARDS in COVID-19, and SARS-CoV-2-induced inflammation can cause IR and APN paradox (Ho et al., 2021). Therefore, inhibition of APN paradox-mediated inflammation could be beneficial in this regard (Ho et al., 2021). In this state, it was clarify that the increase of circulating APN and the development of APN paradox was due to COVID-19-induced IR, and the researchers have not measured APN serum level, but they predicted that APN could be elevated (Ho et al., 2021). This clarification has been incomplete, since APN level is reduced in COVID-19 patients with respiratory failure (Kearns et al., 2021) and increased in mild-moderate COVID-19 patients as a compensatory mechanism against exaggerated immune-inflammatory disorders (Di Filippo et al., 2021).
Consequently, APN could be increased in mild to moderate COVID-19 patients as a compensatory mechanism against SARS-CoV-2-induced inflammation, and reduced in individuals with severe COVID-19 symptoms because of the infection-induced adipose tissue dysfunction with succeeding reduction of APN (Kirino et al., 2012). Furthermore, adipose tissue infection provokes type I interferon response (INF) and activation of innate immune response with reduction of APN and APN/Lep ratio. These changes trigger pancreatic β-cell dysfunction and hyperglycemia, Fig. 8 shows the malfunction of the adipose tissue induced by the viral infection.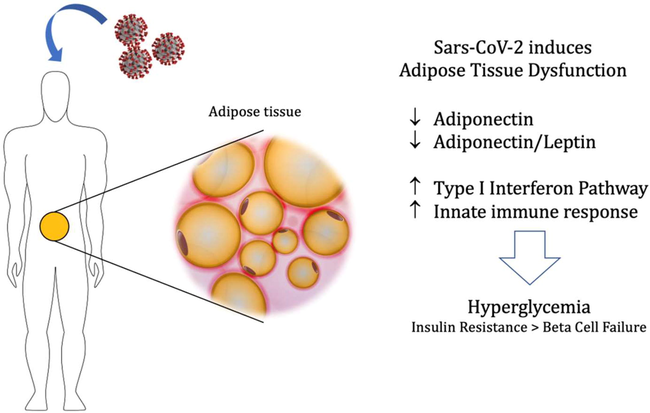
Adipose tissue malfunction after the infection with SARS-CoV-2: viral infection of adipose tissue provokes type I interferon response (INF) and activation of innate immune response with reduction of APN and APN/Lep ratio. These changes trigger pancreatic β-cell dysfunction and hyperglycemia.
In COVID-19, lymphopenia is a marker of SARS-CoV-2-induced inflammatory mechanisms, related to lymphopoiesis suppression and exhaustion of T cells with anti-inflammatory function. Crawford et al. found that APN is generated by lymphocytes and acts as a down-regulator of bone marrow production of granulocytes, with the activation of regulatory T cells (Treg) (Crawford et al., 2010). Lack of APN increases T cells activation with the reduction of Treg that exacerbates the severity of the disease [56]. Furthermore, APN is essential for T cell development in thymus (Zhang et al., 2021). As a result, low APN due to SARS-CoV-2-induced adipose tissue dysfunction and production from T lymphocytes may enhance inflammatory reaction and development of lymphopenia in a vicious cycle.
Dipeptidyl peptidase 4 (DPP4) is overexpressed in fatty tissue in obesity, which lowers the level of APN produced in young individuals (Kirino et al., 2012). A systematic review showed that DPP4 inhibitors like sitagliptin and vildagliptin increased circulating APN (Sahebkar et al., 2016). It was shown that SARS-CoV-2 can bind and activate DPP4, causing systemic inflammation. A prospective study illustrated that the inhibition of DPP4 by sitagliptin improved patient clinical outcomes in severely affected COVID-19 (Al-Kuraishy et al., 2021). Therefore, the activation of DPP4 in adipose tissue could be the possible mechanism for low APN in COVID-19.
Additionally, the production of reactive oxygen species (ROS), activating NADPH oxidase but also decreasing body antioxidant capacity, all contribute to OS being increased in COVID-19 (Chernyak et al., 2020). OS can encourage vasoconstriction, ED, thrombosis and progression of inflammatory disorders (Chernyak et al., 2020). Notably, OS in obesity inhibit APN production and release, since adipocytes are sensitive to the effect of ROS (Soares et al., 2005). In addition, OS attenuates the expression of peroxisome proliferator activator alpha (PPAR-α), which is involved in the activation of APN synthesis (Soares et al., 2005). Essick et al showed that the modulating effect of APN on the OS-induced autophagy in rat cardiomyocytes (Essick et al., 2013). APN has been shown to prevent cardiometabolic complications by inhibiting the induction and progression of OS (Matsuda and Shimomura, 2014). Thus, SARS-CoV-2 infection-induced OS could be the possible mechanism behind reduction of APN in COVID-19.
These observations proposed that higher expression of DDP4, OS and hyperinflammation may be involved in the reduction of APN in COVID-19 patients.
5.1 APN and inflammatory signaling pathways in SARS-CoV-2 infection
The pathophysiology of COVID-19 involves encompasses several inflammatory signaling mechanisms. The ORF7a viral protein substantially stimulates the NF-κB signaling cascade during viral infection, which causes the production of pro-inflammatory mediators (Su et al., 2021). By lowering the function of inflammatory molecules, NF-κB inhibitors play a significant part in the mitigation of COVID-19 evolution (Ma et al., 2020). Ouchi et al. revealed that APN inhibits NF-κB signaling pathway via cyclic adenosine monophosphate dependent pathway (Ouchi et al., 2000). As well, APN blocks the activation of macrophage toll-like receptor 4/NF-κB axis (Yamaguchi et al., 2005). Thus, the reduction of APN in COVID-19 may exacerbate the activation of NF-κB signaling pathway.
The stimulation of natural killer cells and the NF-κB signaling mechanisms that releases interferon gamma are both attributed to the nod like receptor pyrin 3 receptor (NLRP3) inflammasome. NLRP3 inflammasome antagonists can lessen organ harm brought on by a heightened immune reaction and cytokine storm (Rojas et al., 2014). Indeed, APN can inhibit NLRP3 inflammasome activation and OS by regulating AMPK pathway in experimental cerebral ischemia (Liu et al., 2020). Wang et al. confirmed that APN attenuates NLRP3 inflammasome activation via modulation of AMPK pathway and production of ROS (Wang et al., 2018). Moreover, the reduction of APN in viral infections triggers NLRP3 inflammasome activation with succeeding inflammatory complications.
Furthermore, the progression of acute cardiac damage and ALI in infections with the novel coronavirus is linked to the pro-inflammatory cascade of p38 mitogen activated protein kinase (p38MAPK) (Grimes and Grimes, 2020). The up-regulation of p38MAPK in COVID-19 is due to the downregulation of ACE2 and augmentation of AngII level. The virus can rapidly initiate the p38MAPK signaling mechanisms, resulting in systemic inflammation, ED, vasoconstriction, and thrombosis (Grimes and Grimes, 2020). Thus, p38 MAPK inhibitors may attenuate COVID-19 severity and associated complications. APN's anti-inflammatory properties can be achieved by p38MAPK suppression (Wang et al., 2017). An in vitro cell line study demonstrated that APN reduced glucose-mediated apoptosis via inhibition of p38MAPK and activation of AMPK pathways (Wang et al., 2017). Yan et al. establish that APN had anti-lipogenic effect through the inhibition of p38MAPK and activating transcription factor 2 (ATF-2) (Yan et al., 2013). These observations pointed out that activated p38MAPK and associated hyperinflammation in COVID-19 could be a causal factor for the reduction of APN.
The mechanistic target of rapamycin (mTOR) framework, which acts as the primary modulator of cell formation and expansion, multiplication, metabolism, and lifespan, was developed. Moreover, it is part of the kinase family and detects both external and internal cell regulatory stimuli to regulate organelle synthesis, autophagy, and the activation of inflammatory genes (Zheng et al., 2020). Terrazzano et al. hypothesized that throughout the infections with the novel coronavirus, the mTOR biochemical route is active and engaged in viral transcription and mRNA translation. The inhibition of the mTOR pathway by everolimus might limit the multiplication of viral particles, making it a potential molecule with pharmacotherapeutic use in COVID-19 (Terrazzano et al., 2020). As well, APN inhibited vascular calcification and regulated the differentiation of vascular smooth cells via inhibiting mTOR pathway (Zhan et al., 2014). Likewise, AdipR agonists regulated the differentiation of vascular smooth cells by suppressing mTOR pathway via AMPK-independent pathway (Fairaq et al., 2017). Additionally, obesity, which is associated with the activation of the mTOR pathway (Bolourian and Mojtahedi, 2020) and a decrease in APN production (Al-Kuraishy and Al-Gareeb, 2016), is considered to be a potential risk factor for disease severity. These observations concluded that by activating mTOR pathway in COVID-19, the beneficial effect of APN against the viral infection could be blocked.
Additionally, advanced glycation end products (AGE) are associated with diabetic vasculopathy and IR through the attenuation of APN production (Monden et al., 2013). Lee et al. confirmed that APN could be a beneficial therapeutic tool against diabetic nephropathy by inhibiting the production of AGE (Lee et al., 2019). Receptors for advanced glycation end (RAGE) are part of the COVID-19 pathophysiology. Though, soluble RAGE (sRAGE) is effective for mitigating disease severity (Yalcin Kehribar et al., 2021). Therefore, abnormal reactivity and interaction of AGE may reduce APN production in COVID-19.
Through modulating apoptosis, the protein forkhead box O (FoxO) has an essential role in maintaining cell homeostasis, OS, and survival, as well as the maturation of lymphocytes and the production of anti-inflammatory cytokines (Cheema et al., 2021). FoxO possesses anti-inflammatory properties, hence its enhancers might lessen the disease severity (Cheema et al., 2021). The anti-inflammatory effect of FoxO can reduce the severity of illness and longevity by regulating the inflammatory biological environment and cellular balance (Tia et al., 2018). Zhang et al. suggested that systemic anti-inflammatory effect of APN is mediated by the activation of FoxO (Zhang et al., 2014). Consequently, the reduction of APN in COVID-19 may decrease the protective effect of FoxO against SARS-CoV-2 infection.
A published study proposed an interaction between BDNF-derived neurotrophic factor (brain) and APN, which interfered with fat regulation (Jo et al., 2020).
Additionally, it has been shown that there is a strong correlation between the inflammation that is present in COVID-19 and the level of APN, a protein that is primarily synthesized in white adipose tissue. As a result, there is an inversely proportional relationship between APN amount and the identification of distinctive metabolic cardiovascular risk indicators. According to available research, APN levels have risen proportionally with aging. Therefore, it might represent an action taken by the fatty tissue to counteract Sars-CoV-2 inflammatory processes. The BDNF/APN ratio can thus be considered a reliable marker of this result (Minuzzi et al., 2021).
Furthermore, APN/Lep ratio, which is an accurate biomarker of adipose function and metabolic health, was severely decreased (by 10-fold) in infected patients as opposed to ARDS+; it was also reduced by 6 times higher than ARDS matched controls (Reiterer et al., 2021a,b).
Published data also suggested that fatty tissue malfunction is characteristic in infections with the novel coronavirus and might result in hyperglycemia. APN/Lep ratios and APN were significantly decreased in COVID-19 patients. According to investigations, SARS-CoV-2-infected hamsters had significantly lower levels of APN and viral RNA in their fatty tissue (Reiterer et al., 2021a,b). These results suggest that this contagious infection targeting among others fat tissues is one of the underlying mechanisms explaining both IR and adipose tissue dysfunction. However, more studies remain to be conducted, determining which type of adipose tissue cells are infected. As a future perspective, an accurate diagnosis of the adipose dysfunction in COVID-19 can potentiate the clinical medical actions (by assessing circulating adipokine levels), taking into account drugs (e.g., thiazolidinediones), which improve both the insulin sensitivity and adipose function (Reiterer et al., 2021a,b).
Considering their overall effect, the inflammatory signaling mechanisms are augmented in infections with the novel coronavirus, resulting in systemic abnormalities. Fortunately, APN has potential effect in mitigation of hyperinflammation through attenuation expression and release of inflammatory signaling pathways.
5.2 APN and ALI in infections with SARS-CoV-2
ARDS and ALI are commonly developed in severe COVID-19, connected with the advancement of the cytokine storm and direct pulmonary inflammation brought on by the viral infection. An experimental study demonstrated that APN attenuates LPS-induced ALI in mice via the inhibition of endothelial cell activation. Moreover, hypoadiponectimia in obese patients predisposes to the development of ALI by exaggerating inflammatory and immune responses in the pulmonary vascular endothelium (Konter et al., 2012).
Wei et al. illustrated that APN protected obese rats from ALI by inhibiting ER stress (Wei et al., 2020). Disruption homeostasis of ER by various viral infections, including SARS-CoV-2, promotes ER stress, which is correlated with pulmonary ED (Banerjee et al., 2020). A previous study showed that APN mitigate pulmonary ER stress via activation of PPAR-α and AMPK pathways (Liu et al., 2016). Tsuchida et al. demonstrated that PPAR-α activators improve APN level and reduce obesity-mediated inflammation (Tsuchida et al., 2005). Moreover, PPAR-α activators reduced vascular leakage and inflammation in a murine model of ALI (Schaefer et al., 2008). APN can decrease ED through modulation of AMPK and COX-2 pathways, with mitigation of endothelial cells apoptosis and release of pro-inflammatory cytokines (Shah et al., 2017). Furthermore obesity mediated ED increases the risk of ALI as high circulating free fatty acids induce pulmonary endothelial cells ER stress (Rojas et al., 2014). ED is regarded as a global marker of SARS-CoV-2 infection mediated complications, including ALI/ARDS (Froldi and Dorigo, 2020).
As well, in vitro published data revealed the fact that APN inhibits the IL-6 expression as well through murine endothelial pulmonary cells, reducing the lung inflammation induced by bacterial liposaccharides during ARDS (Konter et al., 2012).
A prospective study, a total of 170 critically ill patients (48 without sepsis + 122 with sepsis) were registered in the moment of their admission to an intensive care unit (ICU) and being compared with a group of 60 patients (healthy controls). Patients' rate of survival was followed almost 3 years. APN serum concentrations registered no differences between the two groups mentioned above. Nonetheless, the subjects having decompensated liver cirrhosis manifested significantly increased serum APN levels. Like non-critical patients, ICU patients with pre-existing obesity or diabetes had significantly reduced circulating APN. In addition, inflammatory cytokine values were not correlated with serum APN. The interesting aspect of the study was that, when hospitalized in the ICU, low levels of APN were an independent, positive predictor of both short and overall term survival (Koch et al., 2011). More medical data were brought to the field by an observational, single-center, prospective study assessing COVID-19. Both APN and Lep were determined in 60 subjects with mild (n = 11, outpatients), moderate (n = 25, hospitalized without requiring intensive care) and severe (n = 24, ICU or death) disease. Patients with moderate symptoms presented the most elevated APN/Lep ratio. The ratio APN/Lep was correlated with the systemic inflammation (APN or Lep alone not having the same action). When the patients were divided into APN/Lep tertials, APN was at the highest level, whereas Lep was at the lowest level in the 3rd and maximal tertile. Subjects included in the most elevated APN/Lep tertial revealed significantly reduced rates of obesity, diabetes, and high blood pressure, as well as decreased ratios of admission to ICU/death vs other tertiles. C-reactive protein predicted significantly APN/Lep (p = 0.022, β = 0.291), if considering the linear regression in the entire cohort; meanwhile, diabetes (p = 0.028, β = −0.257), women (p = 0.016, β = −0.289), and hypertension (p = 0.043, β = −239) were negative predictors (Di Filippo et al., 2021).
An observational prospective single-center study included 63 (36 of them were negative controls and 27 were infected with the novel coronavirus) immunocompetent subjects having pneumonia in a severe form; the majority of patients needed critical medical assistance. Their biological/clinical features (i.e., adipokines detected in plasma, glucose metabolism, and cytokine amounts) and final outcomes were assessed. Patients with COVID-19 (having similar initial severity) required a significantly longer period of mechanical ventilation than the negative controls (p = 0.0049). Plasma levels of APN/Lep were correlated with glucose metabolism and BMI (insulinemia and glycemia), being scarcely distinct between the two sets that were evaluated. Adipokines were not linked with the majority of the various inflammatory proteins, duration of mechanical ventilation, or initial severity (SOFA score), although Lep concentrations were inversely linked with IL-1 and IL-6 (Blot et al., 2021).
Therefore, through improving ER function, ED, or activating PPAR-α, APN has a pulmo-protective impact and can prevent ALI secondary to this viral infection. For that reason, the reduction of APN in COVID-19 increases the risk for ALI development (Fig. 9).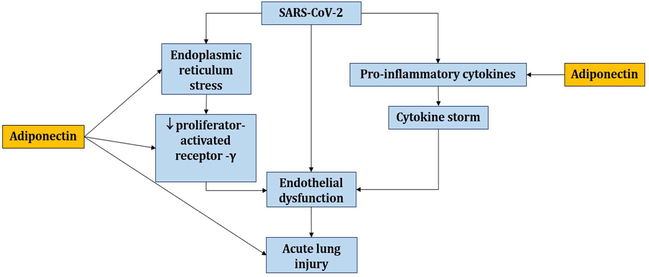
Role of APN against development of acute ALI in SARS-CoV-2 infection: APN inhibits endoplasmic reticulum stress and downregulation of peroxisome proliferator activator, resulting in improved endothelial function and inhibition of pro-inflammatory cytokine release and cytokine storm development.
5.3 APN activators in COVID-19
Interestingly, some of the repurposed drugs in the management of COVID-19 produce their effects through the activation of APN production. Omega-3 fatty acid improves circulating APN in patients with T2DM (Bahreini et al., 2018), sustaining its helpful effect in the management of T2DM through the APN-dependent pathway. A recent systematic review by Rausch et al. discovered that omega-3 fatty acid increased APN level and reduced Lep/APN ratio (Rausch et al., 2021). Moreover, omega-3 fatty acid supplements also improve clinicopathological and biological results in patients with a severe form of infection via modulation of inflammatory milieu and thrombotic disorders (Safaei Ardestani and Rahideh, 2022). Due to their anti-inflammatory properties with improvement of APN levels, omega-3 fatty acids could be a potential therapeutic tool against this viral infection-induced dysregulation of APN expression.
Likewise, PPAR-γ and -α activators, including rosiglitazone and fenofibrate, improves APN production in rats (Choi et al., 2005). Carboni et al. concluded that PPAR-γ agonist pioglitazone might be favorable in treating COVID-19, by elevating anti-inflammatory APN and by diminishing pro-inflammatory cytokines (Carboni et al., 2020). Similarly, the PPAR-agonist fenofibrate reduced the pathological processes of the novel coronavirus infection by improving APN functions or improving pro-inflammatory cytokine release. (Yasmin et al., 2021).
Furthermore, pleiotropic effects of the lipid-lowering medication statins occur, although their influence on circulating APN is contested and contradictory research linked statins to a reduction in APN levels (Singh et al., 2018). However, a meta-analysis and systematic review illustrated that statins enhanced and increased APN levels by modulation of inflammatory cytokines (Chruściel et al., 2016). Moreover, statins have been shown to be effective by reducing mortality in severe forms of infection (Zhang et al., 2020). Statins' potential beneficial impact in COVID-19 is mediated by suppressing the pro-inflammatory cytokines (Zhang et al., 2020) and could be through the increase of APN levels.
Indeed, resveratrol is a polyphenol found in red wine that has a potent anti-inflammatory effect by rising APN production and inhibits the expression of inflammatory genes in mononuclear cells in patients with coronary artery diseases (Tomé-Carneiro et al., 2013). Jimoh et al. showed that resveratrol increased APN levels in rats with experimental hypercholesterolemia (Jimoh et al., 2018). It has been reported that resveratrol, can also mitigate immuno-thrombotic complications in COVID-19 (Giordo et al., 2021).
Furthermore, AdipoR agonists (e.g., AdipRon), which stimulate both AdipoR1 and AdipoR2 ameliorate lipotoxicity and diabetic complications via the reduction of sphingolipids and ceramide subspecies (Choi et al., 2018). AdipoRon can reduce ED and OS in T2DM in experimental mice with diabetic nephropathy (Choi et al., 2018). AdipoRon reduces the expression of pro-inflammatory cytokines and glomerular inflammation in mice with induced obesity through the inhibition of p38MAPK and NF-κB signaling pathway (Lindfors et al., 2021). Wu et al. confirmed in an in vitro study that AdipoRon reduced T2DM-induced peritonitis by enhancing autophagy (Wu et al., 2022). Notoriously, no study evaluated the effect of AdipoRon on SARS-CoV-2 infection. Thus, AdipoRon could be a novel therapeutic strategy against viral infections by modulating pro-inflammatory molecules or inflammatory signaling pathways.
Overall, APN activators and AdipoR agonists might be efficient against the infection with SARS-CoV-2 and related consequences through modulation of the pro-inflammatory/anti-inflammatory axis.
6 Conclusions
The present manuscript provides an updated comprehensive description of the biomolecular implications of APN in COVID-19, highlighting effects due to multiple physiological roles, molecular pathways of action, and associated factors, by presenting the current state of knowledge in this subfield. APN is a relevant adipokine, mainly produced from visceral adipose tissue, and having dual anti-inflammatory and pro-inflammatory role in different inflammatory conditions. It is the most prevalent adipokine in human plasma and has been shown in multiple studies to have both antioxidant and insulin-sensitizing properties. Because of adipose tissue malfunction, it is dysregulated in a variety of viral illnesses, such as the infection with the novel coronavirus. In COVID-19, OS, activation of inflammatory signaling pathways, and hyperinflammation-induced adipose tissue dysfunction may reduce production of APN with subsequent exacerbation of inflammatory disorders. It could additionally suggest that patients with low APN levels are more susceptible to COVID-19 respiratory failure. Adipose tissue impairment is indicated by a decreased APN/Lep ratio. The lowest APN/Lep ratio is found in patients with cardiometabolic disorders, indicating that it may be a contributor to worsening COVID-19 prognosis.
Studies on the potential molecular implications of APN in the development of COVID-19 have been continuously developed in recent years to provide a more accurate assessment of interactions at the biomolecular level in inflammatory, metabolic, and immune pathways. The potential role of APN in COVID-19 has been described by various observational, case-control, and experimental studies, but additional medical research is required in order to thoroughly examine the putative molecular role of APN in COVID-19.
CRediT authorship contribution statement
Hayder M. Al-Kuraishy: Conceptualization, Writing – original draft, Writing – review & editing, Supervision. Ali I. Al-Gareeb: Writing – review & editing. Simona Gabriela Bungau: Writing – review & editing, Supervision, Funding acquisition. Andrei-Flavius Radu: Writing – original draft, Writing – review & editing. Gaber El-Saber Batiha: Writing – review & editing, Supervision.
Acknowledgements
The authors thank the University of Oradea, considering the logistic facilities they used and for supporting the publication of this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Viral mechanisms of adipose dysfunction: lessons from HIV-1 Vpr. Adipocyte. 2015;4(1):55-59.
- [Google Scholar]
- Effects of rosuvastatin alone or in combination with omega-3 fatty acid on adiponectin levels and cardiometabolic profile. J. Basic Clin. Pharm.. 2016;8(1):8.
- [Google Scholar]
- Impact of sitagliptin in non-diabetic covid-19 patients. Curr. Mol. Pharmacol. 2021
- [CrossRef] [Google Scholar]
- The effect of omega-3 on circulating adiponectin in adults with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Can. J. Diabetes. 2018;42(5):553-559.
- [CrossRef] [Google Scholar]
- Crosstalk between endoplasmic reticulum stress and anti-viral activities: A novel therapeutic target for COVID-19. Life Sci.. 2020;255:117842.
- [CrossRef] [Google Scholar]
- CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ.. 2022;808:152072
- [CrossRef] [Google Scholar]
- Are adipokines the missing link between obesity, immune response, and outcomes in severe COVID-19? Int. J. Obes. (Lond).. 2021;45(9):2126-2131.
- [CrossRef] [Google Scholar]
- Obesity and COVID-19: The mTOR pathway as a possible culprit. Obes. Rev.. 2020;21(9):e13084-e.
- [CrossRef] [Google Scholar]
- Interactions between leptin and insulin resistance in patients with prediabetes, with and without NAFLD. Exp. Ther. Med.. 2020;20(6)
- [Google Scholar]
- Can pioglitazone be potentially useful therapeutically in treating patients with COVID-19? Med. Hypotheses. 2020;140:109776.
- [CrossRef] [Google Scholar]
- Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab.. 2014;20(2):368-375.
- [Google Scholar]
- Exploring the therapeutic potential of forkhead box O for outfoxing COVID-19. Open Biol.. 2021;11(6):210069.
- [CrossRef] [Google Scholar]
- COVID-19 and oxidative stress. Biochemistry (Mosc). 2020;85(12):1543-1553.
- [CrossRef] [Google Scholar]
- Homeostatic regulation of T cell trafficking by a B cell–derived peptide is impaired in autoimmune and chronic inflammatory disease. Nat. Med.. 2015;21(5):467-475.
- [Google Scholar]
- Adiponectin receptor agonist AdipoRon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metabolism. 2018;85:348-360.
- [CrossRef] [Google Scholar]
- Effect of PPAR-alpha and -gamma agonist on the expression of visfatin, adiponectin, and TNF-alpha in visceral fat of OLETF rats. Biochem. Biophys. Res. Commun.. 2005;336(3):747-753.
- [CrossRef] [Google Scholar]
- Impact of statin therapy on plasma adiponectin concentrations: A systematic review and meta-analysis of 43 randomized controlled trial arms. Atherosclerosis. 2016;253:194-208.
- [CrossRef] [Google Scholar]
- Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52(2):268-276.
- [Google Scholar]
- Obesity impairs γδ T cell homeostasis and antiviral function in humans. PLoS ONE. 2015;10(3):e0120918.
- [Google Scholar]
- Adiponectin is produced by lymphocytes and is a negative regulator of granulopoiesis. J. Leukoc. Biol.. 2010;88(4):807-811.
- [CrossRef] [Google Scholar]
- Adiponectin to leptin ratio reflects inflammatory burden and survival in COVID-19. Diabetes Metab.. 2021;47(6):101268.
- [CrossRef] [Google Scholar]
- Adiponectin modulates oxidative stress-induced autophagy in cardiomyocytes. PLoS ONE. 2013;8(7):e68697-e.
- [CrossRef] [Google Scholar]
- AdipoRon, an adiponectin receptor agonist, attenuates PDGF-induced VSMC proliferation through inhibition of mTOR signaling independent of AMPK: Implications toward suppression of neointimal hyperplasia. Pharmacol. Res.. 2017;119:289-302.
- [CrossRef] [Google Scholar]
- Adiponectin and inflammation: consensus and controversy. J. Allergy Clin. Immunol.. 2008;121(2):326-330.
- [Google Scholar]
- The role of leptin and adiponectin in obesity-associated cognitive decline and Alzheimer’s disease. Front. Neurosci.. 2019;12:1027.
- [Google Scholar]
- Endothelial dysfunction in Coronavirus disease 2019 (COVID-19): Gender and age influences. Med. Hypotheses. 2020;144:110015
- [CrossRef] [Google Scholar]
- Adiponectin prevents progression of steatohepatitis in mice by regulating oxidative stress and Kupffer cell phenotype polarization. Hepatol. Res.. 2009;39(7):724-738.
- [CrossRef] [Google Scholar]
- Therapeutic potential of resveratrol in COVID-19-associated hemostatic disorders. Molecules. 2021;26(4)
- [CrossRef] [Google Scholar]
- p38 MAPK inhibition: A promising therapeutic approach for COVID-19. J. Mol. Cell. Cardiol.. 2020;144:63-65.
- [CrossRef] [Google Scholar]
- Systemic fate of the adipocyte-derived factor adiponectin. Diabetes. 2009;58(9):1961-1970.
- [Google Scholar]
- Diabetes, inflammation, and the adiponectin paradox: Therapeutic targets in SARS-CoV-2. Drug Discov. Today. 2021;26(8):2036-2044.
- [CrossRef] [Google Scholar]
- Adiponectin exacerbates influenza infection in elderly individuals via IL-18. Signal Transd. Target. Ther.. 2020;5(1):1-3.
- [Google Scholar]
- Resveratrol increases serum adiponectin level and decreases leptin and insulin level in an experimental model of hypercholesterolemia. Pathophysiology. 2018;25(4):411-417.
- [CrossRef] [Google Scholar]
- Role of adiponectin and brain derived neurotrophic factor in metabolic regulation involved in adiposity and body fat browning. J Clin Med.. 2020;10(1)
- [CrossRef] [Google Scholar]
- Reduced adiponectin levels in patients with COVID-19 acute respiratory failure: A case-control study. Physiol. Rep.. 2021;9(7):e14843.
- [CrossRef] [Google Scholar]
- Rapid weight gain in early life is associated with severity of respiratory syncytial virus (RSV) bronchiolitis in children. Allergol. Immunopathol.. 2021;49(2):23-30.
- [Google Scholar]
- Adiponectin and HIV-lipodystrophy: taking HAART. Endocrinology. 2004;145(2):484-486.
- [Google Scholar]
- Plasma dipeptidyl peptidase 4 activity correlates with body mass index and the plasma adiponectin concentration in healthy young people. Endocrinol. J.. 2012;59(10):949-953.
- [CrossRef] [Google Scholar]
- Serum adiponectin upon admission to the intensive care unit may predict mortality in critically ill patients. J. Crit. Care. 2011;26(2):166-174.
- [CrossRef] [Google Scholar]
- Adiponectin attenuates lipopolysaccharide-induced acute lung injury through suppression of endothelial cell activation. J. Immunol.. 2012;188(2):854-863.
- [CrossRef] [Google Scholar]
- Antioxidant/anti-Inflammatory effects of caloric restriction in an aged and obese rat model: the role of adiponectin. Biomedicines. 2020;8(12):532.
- [Google Scholar]
- Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr. Opin. Lipidol.. 2007;18(3):263-270.
- [Google Scholar]
- Role of adipokines in cardiovascular disease. Circ. J.. 2017;81(7):920-928.
- [CrossRef] [Google Scholar]
- Adiponectin for the treatment of diabetic nephropathy. Korean J. Intern. Med.. 2019;34(3):480-491.
- [CrossRef] [Google Scholar]
- Adiponectin receptor agonist AdipoRon ameliorates renal inflammation in diet-induced obese mice and endotoxin-treated human glomeruli ex vivo. Diabetologia. 2021;64(8):1866-1879.
- [CrossRef] [Google Scholar]
- Serum adiponectin correlates with viral characteristics but not histologic features in patients with chronic hepatitis C. J. Hepatol.. 2005;43(2):235-242.
- [Google Scholar]
- Adiponectin reduces ER stress-induced apoptosis through PPARα transcriptional regulation of ATF2 in mouse adipose. Cell Death Dis.. 2016;7(11):e2487.
- [Google Scholar]
- Meta-analysis of adiponectin as a biomarker for the detection of metabolic syndrome. Front. Physiol.. 2018;9:1238.
- [Google Scholar]
- Fosinopril improves liver fibrosis by upregulating ACE2/Angiotensin-(1–7) axis activation in rats with nonalcoholic steatohepatitis. Lat. Am. J. Pharm. 2012
- [Google Scholar]
- Adiponectin peptide alleviates oxidative stress and NLRP3 inflammasome activation after cerebral ischemia-reperfusion injury by regulating AMPK/GSK-3β. Exp. Neurol.. 2020;329:113302
- [CrossRef] [Google Scholar]
- Adipokines correlate with pain and function in lower limb osteoarthritis: different associations in hip and knee. Osteoarthr. Cartil.. 2014;22:S403-S404.
- [Google Scholar]
- Liu Shen capsule shows antiviral and anti-inflammatory abilities against novel coronavirus SARS-CoV-2 via suppression of NF-κB signaling pathway. Pharmacol. Res.. 2020;158:104850.
- [CrossRef] [Google Scholar]
- cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPoseMost abundant Gene transcript 1) Biochem. Biophys. Res. Commun.. 1996;221(2):286-289.
- [Google Scholar]
- Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J. Biol. Chem.. 2011;286(15):13460-13469.
- [Google Scholar]
- Hypothalamic lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta (BBA) – Mol. Cell Biol. Lipids. 2010;1801(3):350-361.
- [CrossRef] [Google Scholar]
- Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev. Endocr. Metab. Disord.. 2014;15(1):1-10.
- [CrossRef] [Google Scholar]
- COVID-19 outcome relates with circulating BDNF, according to patient adiposity and age. Journal. 2021;8:784429
- [CrossRef] [Google Scholar]
- Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: involvement of Toll-like receptor 2. Diabetes. 2013;62(2):478-489.
- [CrossRef] [Google Scholar]
- Mynarcik, D. C., T. Combs, M. A. McNurlan, et al., 2002. Adiponectin and leptin levels in HIV-infected subjects with insulin resistance and body fat redistribution. J. Acquired Immune Def. Syndr. (1999). 31 (5) 514-520.
- Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102(11):1296-1301.
- [CrossRef] [Google Scholar]
- Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol.. 2011;11(2):85-97.
- [Google Scholar]
- The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. (Engl. Ed.). 2021;74(9):790-799.
- [CrossRef] [Google Scholar]
- A novel role for adiponectin in regulating the immune responses in chronic hepatitis C virus infection. Hepatology. 2008;48(2):374-384.
- [Google Scholar]
- Short-term treatment of RAW264. 7 macrophages with adiponectin increases tumor necrosis factor-α (TNF-α) expression via ERK1/2 activation and Egr-1 expression: role of TNF-α in adiponectin-stimulated interleukin-10 production. J. Biol. Chem.. 2007;282(30):21695-21703.
- [Google Scholar]
- ACE2/Ang 1–7 axis: A critical regulator of epicardial adipose tissue inflammation and cardiac dysfunction in obesity. Adipocyte. 2016;5(3):306-311.
- [CrossRef] [Google Scholar]
- Decreased plasma adiponectin concentrations are closely related to steatosis in hepatitis C virus-infected patients. J. Clin. Endocrinol. Metab.. 2005;90(4):2240-2243.
- [Google Scholar]
- Risk factors for adiposity in the urban population and influence on the prevalence of overweight and obesity. Exp. Therap. Med.. 2020;20(1):129-133.
- [CrossRef] [Google Scholar]
- Systematic review of marine-derived omega-3 fatty acid supplementation effects on leptin, adiponectin, and the leptin-to-adiponectin ratio. Nutr. Res.. 2021;85:135-152.
- [CrossRef] [Google Scholar]
- Reiterer, M., M. Rajan, N. Gómez-Banoy, et al., 2021. Hyperglycemia in acute COVID-19 is characterized by adipose tissue dysfunction and insulin resistance. Journal. 2021.2003.2021.21254072. https://doi.org/10.1101/2021.03.21.21254072.
- Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab.. 2021;33(11):2174-2188.
- [CrossRef] [Google Scholar]
- Adiponectin isoforms in elderly patients with or without coronary artery disease. J. Am. Geriatr. Soc.. 2010;58(4):702-706.
- [Google Scholar]
- The role of adiponectin in endothelial dysfunction and hypertension. Curr. Hypertens. Rep.. 2014;16(8):463.
- [CrossRef] [Google Scholar]
- The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: a randomized clinical trial. J. Transl. Med.. 2022;20(1):32.
- [CrossRef] [Google Scholar]
- Effect of dipeptidyl peptidase-4 inhibitors on plasma adiponectin: A systematic review and meta-analysis of randomized controlled trials. Curr. Med. Chem.. 2016;23(13):1356-1369.
- [CrossRef] [Google Scholar]
- Peroxisome proliferator-activated receptor-alpha reduces inflammation and vascular leakage in a murine model of acute lung injury. Eur. Respir. J.. 2008;32(5):1344-1353.
- [CrossRef] [Google Scholar]
- A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem.. 1995;270(45):26746-26749.
- [Google Scholar]
- Obesity-induced endoplasmic reticulum stress causes lung endothelial dysfunction and promotes acute lung injury. Am. J. Respir. Cell Mol. Biol.. 2017;57(2):204-215.
- [CrossRef] [Google Scholar]
- Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol. Sci.. 2009;30(5):234-239.
- [Google Scholar]
- Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2–dependent mechanisms. Nat. Med.. 2005;11(10):1096-1103.
- [CrossRef] [Google Scholar]
- Serum adiponectin in chronic hepatitis C and B. J. Viral Hepatitis. 2007;14(8):577-583.
- [Google Scholar]
- Statins decrease leptin expression in human white adipocytes. Physiol. Rep.. 2018;6(2)
- [CrossRef] [Google Scholar]
- Effects of oxidative stress on adiponectin secretion and lactate production in 3T3-L1 adipocytes. Free Radical Biol. Med.. 2005;38(7):882-889.
- [CrossRef] [Google Scholar]
- Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci. Rep.. 2021;11(1):13464.
- [CrossRef] [Google Scholar]
- Adiponectin modulates inflammatory reactions via calreticulin receptor–dependent clearance of early apoptotic bodies. J. Clin. Invest.. 2007;117(2):375-386.
- [Google Scholar]
- An open question: is it rational to inhibit the mTor-dependent pathway as COVID-19 therapy? Front. Pharmacol.. 2020;11
- [CrossRef] [Google Scholar]
- Role of Forkhead Box O (FOXO) transcription factor in aging and diseases. Gene. 2018;648:97-105.
- [CrossRef] [Google Scholar]
- Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: a triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther.. 2013;27(1):37-48.
- [CrossRef] [Google Scholar]
- Association between H1N1 infection severity and obesity—adiponectin as a potential etiologic factor. J. Infect. Dis.. 2010;202(3):459-460.
- [Google Scholar]
- Peroxisome proliferator-activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes. 2005;54(12):3358-3370.
- [CrossRef] [Google Scholar]
- Adiponectin inhibits NLRP3 inflammasome by modulating the AMPK-ROS pathway. Int. J. Clin. Exp. Pathol.. 2018;11(7):3338-3347.
- [Google Scholar]
- Adiponectin attenuates high glucose-induced apoptosis through the AMPK/p38 MAPK signaling pathway in NRK-52E cells. PLoS ONE. 2017;12(5):e0178215.
- [CrossRef] [Google Scholar]
- Adiponectin protects obese rats from aggravated acute lung injury via suppression of endoplasmic reticulum stress. Diabetes Metab. Syndr. Obes.. 2020;13:4179-4190.
- [CrossRef] [Google Scholar]
- Adiponectin is a negative regulator of antigen-activated T cells. Eur. J. Immunol.. 2011;41(8):2323-2332.
- [Google Scholar]
- Recombinant adiponectin peptide ameliorates brain injury following intracerebral hemorrhage by suppressing astrocyte-derived inflammation via the inhibition of Drp1-mediated mitochondrial fission. Transl. Stroke Res.. 2020;11(5):924-939.
- [CrossRef] [Google Scholar]
- A novel adiponectin receptor agonist (AdipoAI) ameliorates type 2 diabetes-associated periodontitis by enhancing autophagy in osteoclasts. J. Periodontal Res. 2022
- [CrossRef] [Google Scholar]
- Adiponectin differentially regulates cytokines in porcine macrophages. Biochem. Biophys. Res. Commun.. 2004;316(3):924-929.
- [CrossRef] [Google Scholar]
- The receptor for advanced glycation end product (RAGE) pathway in COVID-19. Biomarkers. 2021;26(2):114-118.
- [CrossRef] [Google Scholar]
- Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett.. 2005;579(30):6821-6826.
- [CrossRef] [Google Scholar]
- Adiponectin inhibits induction of TNF-α/RANKL-stimulated NFATc1 via the AMPK signaling. FEBS Lett.. 2008;582(3):451-456.
- [Google Scholar]
- Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab.. 2013;17(2):185-196.
- [Google Scholar]
- Adiponectin decreases lipids deposition by p38 MAPK/ATF2 signaling pathway in muscle of broilers. Mol. Biol. Rep.. 2013;40(12):7017-7025.
- [CrossRef] [Google Scholar]
- The role of fenofibrate in the treatment of COVID-19. Ann. Med. Surg. (Lond.) 2021:102974.
- [CrossRef] [Google Scholar]
- Blockade of the renin-angiotensin system increases plasma adiponectin levels in type-2 diabetic patients with proteinuria. Nephron Clin. Pract.. 2005;99(4):c115-c121.
- [CrossRef] [Google Scholar]
- Adiponectin attenuates the osteoblastic differentiation of vascular smooth muscle cells through the AMPK/mTOR pathway. Exp. Cell Res.. 2014;323(2):352-358.
- [CrossRef] [Google Scholar]
- Adiponectin-expressing Treg facilitate T lymphocyte development in thymic nurse cell complexes. Commun. Biol.. 2021;4(1):344.
- [CrossRef] [Google Scholar]
- Adiponectin ameliorates experimental periodontitis in diet-induced obesity mice. PLoS ONE. 2014;9(5):e97824.
- [CrossRef] [Google Scholar]
- In-Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID-19. Cell Metab.. 2020;32(2):176-187.e174.
- [CrossRef] [Google Scholar]
- Immunoregulation with mTOR inhibitors to prevent COVID-19 severity: A novel intervention strategy beyond vaccines and specific antiviral medicines. J. Med. Virol.. 2020;92(9):1495-1500.
- [CrossRef] [Google Scholar]
- Adiponectin alleviates exacerbation of airway inflammation and oxidative stress in obesity-related asthma mice partly through AMPK signaling pathway. Int. Immunopharmacol.. 2019;67:396-407.
- [Google Scholar]







