Translate this page into:
The potential effects of Indigofera coerulea extract on THP-1 human cell line
⁎Corresponding authors at: Department of Zoology, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia (Saad Alkahtani). Department of Infection and Immunity, Research Center, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia and Department of Microbiology and Immunology, College of Medicine, Alfaisal University, Riyadh, Saudi Arabia (Ahmed A. Al-Qahtani). salkahtani@ksu.edu.sa (Saad Alkahtani), aqahtani@kfshrc.edu.sa (Ahmed A. Al-Qahtani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The purpose of this study was to detect the immunomodulatory activity of Indigofera coerulea on the cytokine's expression in the THP-1 human cell line. THP-1 cell lines were differentiated into real macrophages. Cell's viability was assessed by MTT assay. The qRT-PCR assay was used to determine the effects of the I. coerulea extract on mRNA expression levels in THP-1 cells. The effects of extracts on proteins production were analyzed by Western blot assay and protein array. The ELISA was used to determine the effect of the plant extract on cytokine expression. Finally, apoptosis, phagocytosis, and cell migration assays were performed to investigate the effects of the extract on macrophage functions. The obtained results illustrated that I. coerulea extract possesses anti-oxidant and anti-inflammatory activities. In addition, current study reported that I. coerulea extract significantly reduced the proliferation of THP-1 cells at all-time points. Moreover, I. coerulea extract showed significant immunomodulatory activity compared with control macrophages by influencing tumor necrosis factor-α (TNF-α), IL-1β, IL-6, CCL22, CXCL10/IP-10, CXCL8/IL-8, ERK5, BAX, BcL2, Cyclin D1, ERK1, P-IκB-α, P-NF-κB, and P-p38 proteins and the signaling pathways of NF-κB, p38 MAPK, ERK1/2, and IL-6/JAK/STAT3. In conclusion, this study is the first to underline the anti-proliferative, anti-inflammatory, anti-phagocytic, anti-apoptotic, and anti-migratory properties of the studied plant extract in human monocytic THP-1 cells.
Keywords
Cytokines
Human THP-1
Indigofera coerulea
Immunology
Macrophage
- CCL2/MCP-1
-
C–C Motif Chemokine Ligand 2/ Monocyte Chemoattractant Protein1
- CCl4
-
Carbon Tetrachloride
- CCL5
-
C–C Motif Chemokine Ligand 5
- CCL22
-
C–C Motif Chemokine 22
- CXCL10/ IP-10
-
Interferon Γ-Induced Protein 10
- CX3CL1
-
C-X3-C Motif Chemokine Ligand 1
- ERK1
-
Extracellular Signal-Regulated Kinases 1&2
- GM-CSF
-
Granulocyte-Macrophage Colony-Stimulating Factor
- LPS
-
Lipopolysaccharides
- MAP
-
Mitogen-Activated Protein
- M−CSF
-
Macrophage Colony-Stimulating Factor
- NF-κB
-
Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-κB)
- PMA
-
Phorbol-12-Myrstate-13-Acetate
- p-p38
-
Phosphorylated P38
- P-I kB-α
-
Phospho-Nuclear Factor of Kappa Light Polypeptide Gene Enhancer In B-Cells Inhibitor Alpha
- THP1
-
Human Monocytic Leukemia Cell Line
Abbreviations
1 Introduction
Macrophages are immune cells that an essential in tissue development and stimulate cytokines (Carlos, 2015). However, the major functions of macrophages are; eliciting adaptive immunity, antigen presentation, and stimulation lymphocytes, maintain tissue homeostasis (Wynn et al., 2013; Linehan and Fitzgerald, 2015). Phagocytic receptors on pathogens are recognized by specific receptors. These pathogenic components, like lipopolysaccharides (LPS), do not have high mutation rates due to their intrinsic biological role in the invading organism. Phagocytosis occurs relying on the recognition mechanisms. Cellular receptors recognize bacterial surface components and integrins (Aderem and Underhill 1999). During phagocytose pathogens, an inflammatory response occurs and anti-inflammatory response is initiated by macrophages (Stuart and Ezekowitz, 2005; Chung et al., 2007; Al-Qahtani et al., 2021). Exposure to bacterial products, such as LPS, results in macrophages with altered phenotypic and triggered its ability to produce IL-12 (Denkers 2007). However, the deactivated phenotype can be enhanced by exposure to a number of anti-inflammatory cytokines, apoptotic bodies (Denkers 2007).
Human THP-1 cells possess specialized regulatory proteins that activate the process of inflammation upon (LPS) stimulation (Sullivan et al. 2018). Research studies on the signaling pathways of inflammation have facilitated the exploitation of targets for drugs development and have explored the role of medicinal plant extracts in diverse cell-based assays (Bremner et al., 2009; Siriwatanametanon et al., 2010). Medicinal plants have been reported to be adjuvants to traditional remedy for promoting the immune response (Varma et al., 2016; Zhang et al., 2018). A previous study on the liver protection used F. parviflora extract against nimesulide-induced apoptosis in vitro. In addition, it was illustrated that C. epigaeu has a high antioxidants levels (Jeyaseelan et al. 2014). Also, many plant extracts can modulate macrophage functions such as production of inflammatory/anti-inflammatory components (Albrahim et al., 2020). They exert this effect through intracellular signaling pathways such as; mitogen-activated protein (MAP) kinase, nuclear factor kappa-B (NF-kB) protein. Thus, many plant extracts from Saudi Arabia have been used to modulate immune responses in different experimental models including innate and adaptive immune responses. Strong evidence has revealed that some plants have the ability to improve the function of macrophages (Albrahim et al., 2020).
I. caerulea has antimicrobial and antioxidant properties (Guruvaiah et al., 2012; Ponmari et al., 2014) and antigrowth activities (Natarajan et al., 2010). Furthermore, it was reported that I. coerulea plant possesses anti-hepatitis B virus (HBV) infection property (Arbab et al., 2017). In addition, this plant prevents the release of pro-inflammatory cytokines IL-1β and TNF-α (Ponmari et al., 2014). The hepatoprotective properties of I. coerulea phytochemicals and their inhibitory activity against carbon tetrachloride (CCl4)-triggered hepatic injury have also been demonstrated in rat models. I. coerulea extracts not only attenuate the NF-κB pathway, but also block the release of IL-1β and TNF-α (Lopes et al., 2011; Al-Shaebi et al., 2017; Chen et al., 2018). The role of I. coerulea plant extract on human peripheral blood monocytes or THP-1 cell line still unknown, thus current study aim to investigate the anti-proliferative, anti-inflammatory, anti-phagocytic, anti-apoptotic, and anti-migratory properties of the I. coerulea extract in human monocytic THP-1 cells.
2 Materials and methods
2.1 Preparation of plant extracts
The plant was collected from southern part of Saudi Arabia and classified by the taxonomist at King Saud University. Briefly, dried plant was ground to powder and extracted with 80% ethanol followed by filtering and was concentrated using a rotary evaporator (Buchi Labortechnik AG, Flawil, Switzerland) under low pressure at 4 °C. Extract was dissolved in (DMSO; Sigma-Aldrich, Merck KGaA), and the stock (100 mg/ml) was stored at − 20 °C until subsequent use (Arbab et al., 2017).
2.2 Cell culture and differentiation of THP-1 cell line
THP-1 cells were obtained from the acute monocytic leukemia (AML) patient. Cells were cultured in (RPMI-1640) medium accompanied with 10% complement-inactivated fetal bovine serum (FBS), 100 IU/mL penicillin, 100 μg/mL streptomycin, and 3.7 g sodium bicarbonate/L (Sigma-Aldrich, St. Louis, MO, USA). In order to activate THP-1 cells to differentiate, cells were cultured in 96-multi-well culture plates, then 100 ng/mL (162 nM) of phorbol 12-myristate 13-acetate was added, as suggested in previous research (Starr et al. 2018). Cells were washed with RPMI-1640 serum-free medium prior to each experiment to remove undifferentiated cells. For activation of macrophage-like cells, cells were then treated with LPS (100 ng/mL) from E. coli O55:B5 (L2880 Sigma) for 24 h.
2.3 Analysis of cell viability (MTT assay)
Cell viability was measured by MTT assay following manufacturer’s instructions (MTT Assay Kit ab211091, Abcam, city, country). THP-1 cells were exposure to different concentrations (50 or 100 µg/mL) of crude extract of I. caerulea at different time points. Briefly, media was replaced with 50 µL/well serum-free media and 50 µL/well MTT reagents. Cells were cultured in 96-well plates and incubated for three hours. Optical density (OD) was measured at 590 nm using a microplate reader (SpectraMax® MiniMax™300 Imaging cytometer). All experiments were performed in triplicate.
2.4 Gene expression by RT-PCR
THP-1 cells were cultured in a 6-well plate and were incubated with 100 ng/mL LPS only, 100 ng/mL LPS with 100 µg/mL plant extracts, and 100 µg/mL of plant extract only for four hours. RNA was isolated using QlAamp® RNA Blood Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. A total of 10 μg of RNA was used for cDNA synthesis using SuperMix (Biotool, Houston, TX, USA). qRT-PCR was performed using target-specific primers through StepOne RT-PCR system (Applied Biosystems, Foster City, CA, USA). Relative gene expression data were analyzed using the ΔCt method. The data are presented as mean ± standard deviation (SD).
2.5 Western blot analysis
The differentiated cells were treated for four hours with either 100 ng/mL LPS only, 100 ng/mL LPS with 100 µg/mL plant extract, or 100 µg/mL plant extracts only. Western blot was used to assess the expressions of targeted proteins in treated cells. Cells were lysed in RIPA buffer, followed by protein separation on 12% SDS- PAGE. Then proteins were blotted on PVDF membranes. The membranes were then incubated specific primary antibodies overnight at 4 °C, washed, and incubated with HRP-conjugated secondary antibodies. The proteins were detected using Super Signal West Pico16 Chemiluminescent substrate (Thermo Fisher Scientific, Waltham, MA, USA), and visualized on a GE Amersham Imager 600, and were quantified using Image J software (National Institutes of Health, Bethesda, MD, USA).
2.6 Protein array assay
Protein array was performed using the Proteome Profiler™ Array–Human Cytokine Array Kit (R&D Systems). Differentiated THP-1 cells were incubated for four hours with the following combinations: 100 ng/mL LPS only, 100 ng/mL LPS with 100 μg/mL I. caerulea, and I. caerulea only. Cytokines were collected and the membranes were placed into the wells and incubated for one hour. The samples were prepared by mixing 1 mL of each sample with 0.5 mL blocking buffer, 15 μL of reconstituted cytokine array detection antibody cocktail, and then incubated for one hour. Then the prepared sample/antibody mix was added and incubated overnight at 4 °C. Each membrane was then placed individually in 20 mL 1 × wash buffer. We added 2 mL of diluted streptavidin-HRP to each well. The membranes were then returned to the well and incubated at room temperature for 30 min. Finally, 1 mL of the prepared chemiluminescent reagent mix was added to each membrane. The cytokines were detected using an Amersham Imager 600 (GE Healthcare).
2.7 Phagocytosis assay
The phagocytosis activity was carried out on resting M1 and M2 polarized macrophage-like cells using Phagocytosis Assay Zymosan Substrate Kit (Abcam, Cambridge, U.K.). 96 well plates were filled with THP1 cells per well, and incubated with 10 µg/ml PMA for 48 h. The, media was then changed, and the cells were rested for 24 h to differentiate. Activated THP-1 cells were incubated for 75 min with a Zymosan suspension. To block external particles, 100 µL of fixation solution was added to each well. Afterwards, 100 µL of 1X blocking reagent was added to each well, and the plate was incubated for 60 min. Then, cells were washed with a RPMI serum-free medium followed by the addition of 100 µL fixation solution to each well. The absorbance was measured using a SpectraMax® MiniMax™300 Imaging cytometer (Molecular Devices, CA, USA) at OD 405 nm.
2.8 Apoptosis assay
To determine the activation of apoptotic pathways in treated cells, caspase-3 activation assay (Sigma-Aldrich, St. Louis, MO, USA) was performed according to the manufacturer’s protocol. Briefly, cells were cultured in 6 well plates, and incubated for four hours with the treatments, washed with PBS, and incubated with 5 µL Annexin V-FITC conjugated with rabbit anti-human active caspase-3 for 20 min. After that, cells were then washed, incubated with 10 µL of propidium iodide (PI) for 10 min. The fluorescence of the cells was determined using flow cytometry (Becton Dickinson, Franklin Lakes, NJ, USA).
2.9 Enzyme-linked immunosorbent assay (ELISA)
The ELISA was performed to assess cytokines into the cell culture media using a DuoSet® ELISA kit according to the manufacturer’s protocol. In brief, PMA-treated cells were seeded in a 6-well plate and incubated for six hours in different combinations: 100 ng/mL LPS only; 100 ng/mL LPS + 100 µg/mL plants extract; 100 µg/mL plant extracts only. 100 µL of cell culture supernatant was added to each well, and then was incubated for two hours. Cells were treated with primary antibodies, incubated for two hours. 100 μL of streptavidin-HRP-conjugate solution was added to wells and incubated for 20 min. 100 μL of chromogenic substrate was added to each well and the plates were incubated for an additional 30 min. 100 μL of stop solution was added to terminate the reaction. Absorbance was read at 450 nm using a microplate reader and the concentration of each protein was measured using Gen5 software (BioTek Instruments).
2.10 Flow cytometry
To assess the response of THP-1 cells to whether macrophage differentiation factor (PMA) was able to trigger CD-14 expression, a biomarker of monocyte lineage, THP-1 cells were scattered into 96-well culture plates, as explained above in the cell culture and differentiation of the human monocytic THP-1 cell line. Using TrypLE (Invitrogen), cells were removed from the wells at 37 °C after incubating for 10 min. Cells were then placed on ice and all the steps that followed were completed at 4 °C. FACS buffer containing 0.1% azide, 2% fetal calf serum in PBS, and 11 μg/mL of IgG (Jackson) was used to pellet and re-suspend cells. Then, cells were stained with eFluor 450 anti-human CD-14 antibodies (eBioscience, CD14 eFlour 450) and analyzed through flow cytometry and FlowJo software (TreeStar, Inc., vX.0.7 and v9.5.2).
2.11 Statistical analysis
Obtained data are expressed as mean ± standard error of mean. To compare the multiple cell groups, the ANOVA test was used followed by Bonferroni's test. A p-value was considered significant if<0.05.
3 Results
3.1 Effects of I. Caerulea plant extract on proliferation of THP-1 cells
The effect of I. caerulea extract on the proliferation of treated THP-1 cells was detected by MTT assay. Cells were treated with 50 and 100 µg/mL of I. caerulea extract for 1–6 days. THP-1 cells were also treated with vehicle control (DMSO). Fig. 1 shows the percentage of absorbance at 490 nm that induced by DMSO, 50 µg/mL, and 100 µg/mL plant extract relative to the control at 0 days. THP-1 cells treated with both 50 and 100 µg/mL plant extract demonstrated a sustained dampened proliferative response in contrast to DMSO at all-time intervals, indicating proliferation arrest on contact with I. coerulea extract.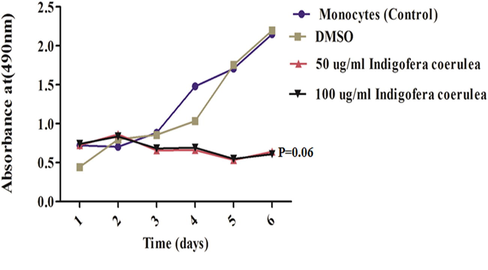
Effects of I. coerulea on proliferation of monocyte cells after incubation with (50 and 100 µg/mL). Each point is the mean of 3 replicates; both 50 and 100 µg/mL I. coerulea reduced cell proliferation.
3.2 Extract of I. Coerulea induces G0/G1 and G2/M arrest
A cell synchronization experiment was performed to test the mechanistic behavior of THP-1 cells upon treatment with the plant extract. The results of cell cycle regulation after treatment with I. coerulea extract are shown in Fig. 2. A combination of monocyte cells with 100 ng/mL nocodazole, 100 ng/mL LPS, ad 100 µg/mL I. coerulea extract showed significantly higher G0/G1. Slight apoptotic activity was also witnessed with all forms of treatment of THP-1 cells. Overall, in agreement with the cell proliferation annexation seen in Fig. 9, cell synchronization analysis through flow cytometry showed that the plant extract caused the THP-1 cells to arrest at the G0/G1 and G2/M cell cycle phases.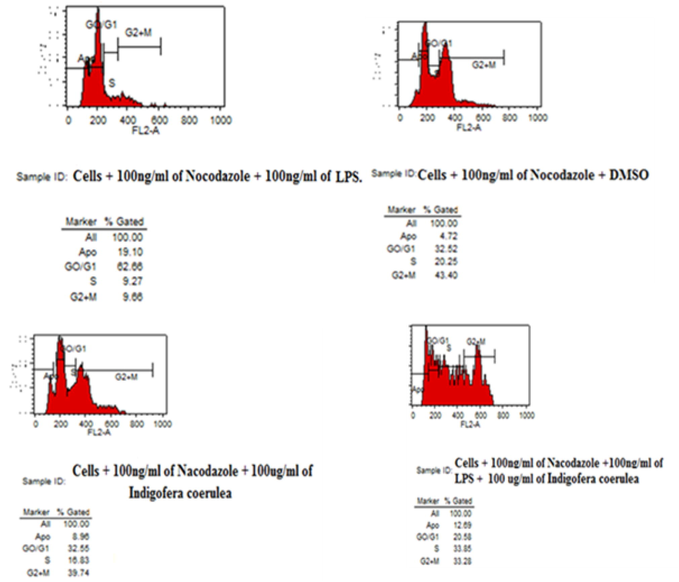
Cell synchronization assay was showed treated human monocytic THP-1 cells with I. coerulea plant extract induced G0/G1 and G2/M arrest in human monocytic THP-1 cells (n = 3).
3.3 Effects of plant extract on mRNA expression
The levels of mRNAs expression were measured in the treated cells. The THP-1 cells were challenged with 100 ng/mL LPS, 100 ng/ml of LPS with 50 µg/mL I. coerulea extract, 100 ng/mL of LPS with 100 µg/mL I. coerulea extract, and 100 µg/mL of I. coerulea alone for the duration of 4 and 6 h. As depicted in Fig. 3A–D, statistically significant increases in the expressions of TNF, IL-1β, and IL-18 mRNA were observed in THP-1 cells compared to their control macrophages. The mRNA expression of TNF was significantly higher (P < 0.0001) in THP-1 cells treated with 100 µg/mL I. coerulea extract alone compared to control macrophage cells at 4 h of incubation (Fig. 3A). The mRNA expression of TNF was significantly higher in THP-1 cells treated with 100 µg/mL I. coerulea extract alone (P < 0.0001; Fig. 3A) in relation to control macrophage cells at 4 h of incubation. The mRNA IL-1β expression was also evidently elevated in THP-1 cells in comparison with control macrophages when treated with 100 ng/mL LPS with 100 µg/mL I. coerulea extract (P < 0.0001) at 4 h and 100 ng/mL LPS with 100 µg/mL I. coerulea extract (P < 0.0001) at 6 h (Fig. 3B). Upon exposure to 100 ng/mL LPS with 100 µg/mL I. coerulea plant extract, IL-18 mRNA expression was significantly increased at 4 h of incubation (P < 0.0001; Fig. 3C). In contrast to the above results, the only mRNA expression that statistically decreased was that of CCL5 with all forms of treatment compared to control macrophages (P < 0.0001; Fig. 3D).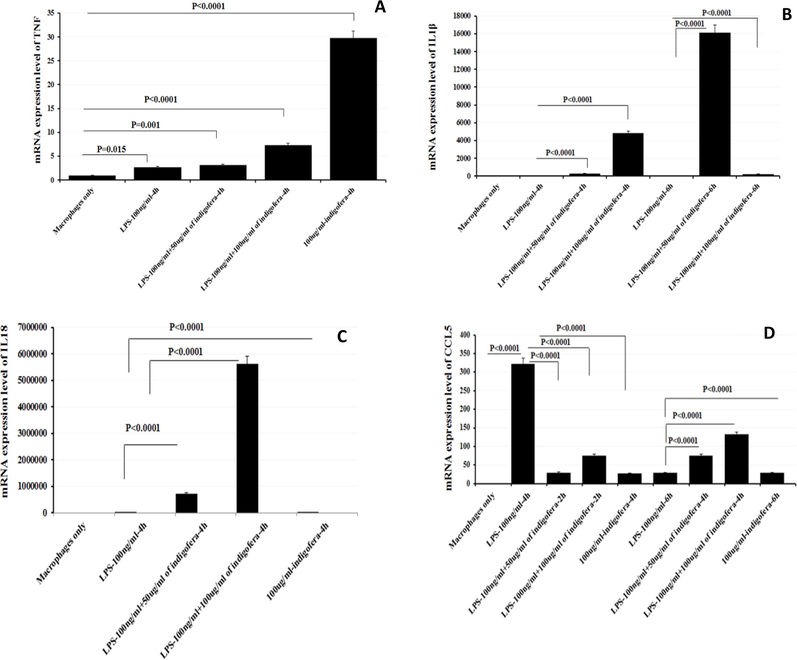
The effects of I. coerulea plant extract on mRNA expression levels of TNF (A), IL-1β (B), IL-18 (C), and CCL5 (D) in THP-1 cells. Result shows significant increase in the mRNA expression of TNF, IL-1β and IL-18 were observed in THP-1 cells compared to their control macrophages counterparts. In contrast to above, the only mRNA expression statistically decreased was that of CCL5 on all forms of treatment compared to control macrophages.
3.4 Effects of I. Coerulea plant extract on the proteins level
The production of targeted proteins was assessed for phorbol-12-myristate 13-acetate (PMA)-differentiated THP-1 cells. To evaluate, we exposed 100 ng/mL of LPS-stimulated and LPS-unstimulated THP-1 cells to 100 µg/mL I. coerulea extract, and protein levels were measured by Western blot analysis. The proteins resolved by SDS-PAGE are displayed in Fig. 4A, B. The expression levels of only ERK5 and P-NF-κB proteins substantially increased, while BAX, BcL2, ERK1, P-IκB-α, and P-p38 proteins were noticeably lowered in LPS-stimulated THP-1 cells exposed to 100 µg/mL I. coerulea extract as compared to controls. The expression of Cyclin D1 wasn’t detected. However, when LPS-unstimulated THP-1 cells were treated with 100 µg/mL I. coerulea extract, the expression of proteins ERK5, BAX, and P-IκB-α augmented, whereas BcL2, Cyclin D1, ERK1, P-NF-κB, and P-p38 proteins expression reduced compared to non-exposed THP-1 cells (See Fig. 5).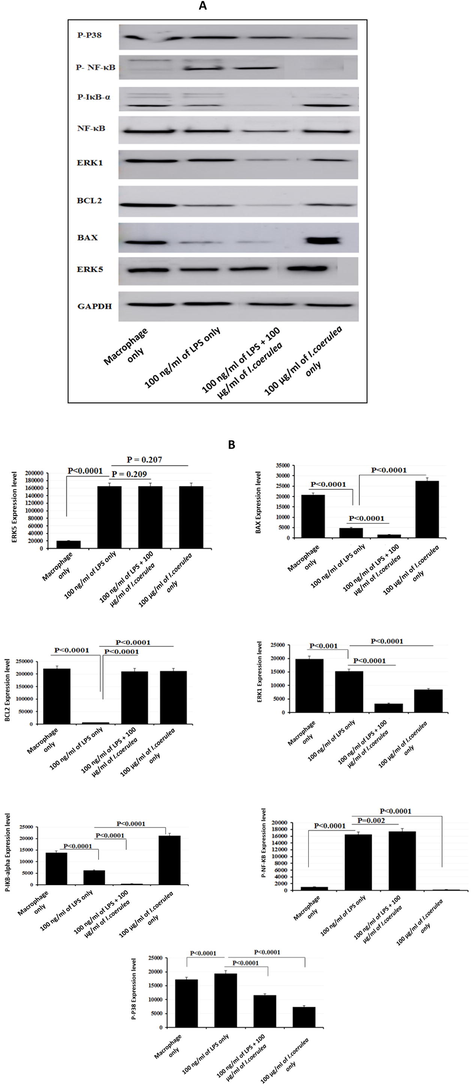
(A) Expression of ERK5, BAX, BCL2, ERK1, NF-KB, P-IKB-α, P-NF-KB, and P-P38 in THP-1 cells treatment with lipopolysaccharide (LPS) and I. coerulea. Proteins were separated on 12% SDS-PAGE. Lane 1: THP-1 cells, Lane 2: Cells treated with 100 ng/mL LPS, Lane 3: Cells treated with 100 ng/mL LPS + 100 µg/mL I. coerulea, Lane 4: Cells treated with 100 µg/mL I. coerulea. Significantly different at p-value < 0.05. (B) Expression of ERK5, BAX, BcL2, ERK1, NF-KB, P-IKB-α, P-NF-KB, and P-P38 in THP-1 cells treatment with LPS and I. coerulea.
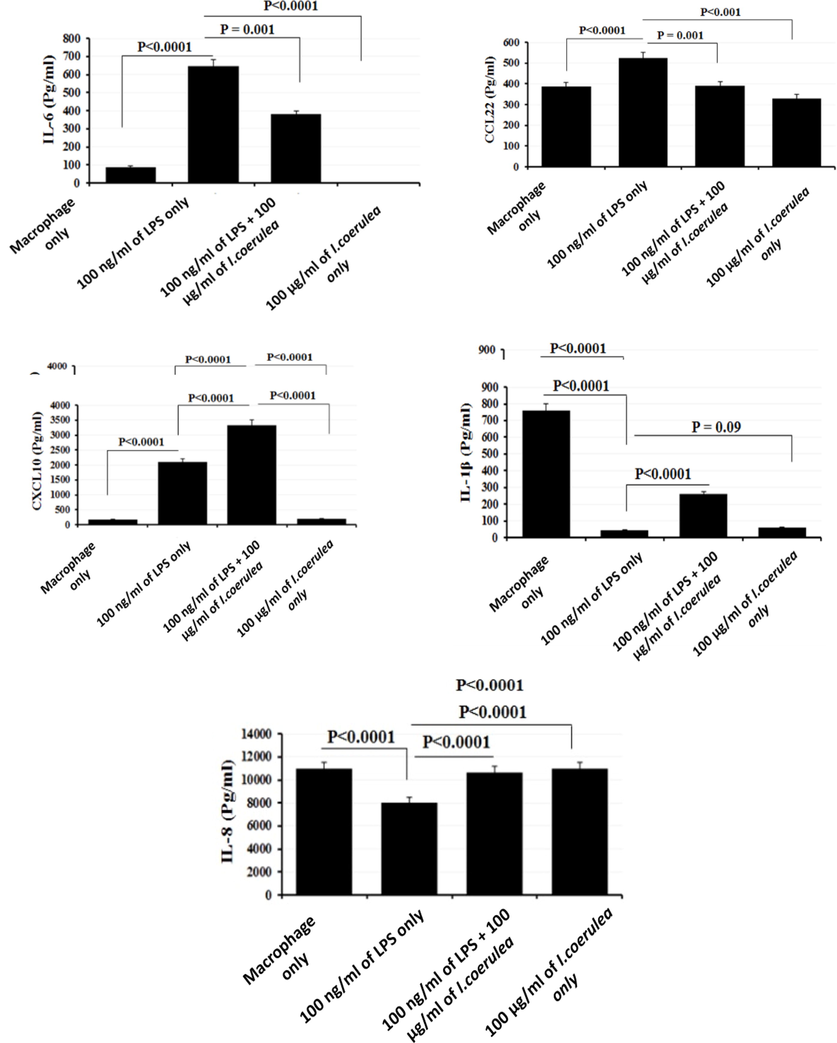
ELISA results for cell culture supernatant of macrophages after stimulation with 100 ng/mL LPS and 100 µg/mL I. coerulea plant extract. Supernatant of culture was harvested 6 h after stimulation. Error bars indicate SEM. All samples were processed in triplicate.
3.5 Effects of plant extract on the expression of inflammatory markers
The inflammatory cytokines produced by PMA-differentiated THP-1 macrophages were analyzed by ELISA to detect the effect of plant extract on their cytokines levels. The data showed that 100 µg/mL plant extract with 100 ng/mL LPS significantly increased the expression of cytokines IL-6 and CXCL10/IP-10. Conversely, 100 ng/mL LPS with 100 µg/mL plant extract reduced the CCL22 and IL-1β expressions in THP-1 compared to controls. CXCL8/IL-8 expression was equal to that of the controls upon exposure to 100 ng/mL LPS combined with 100 µg/mL plant extract. However, 100 µg/mL of plant extract alone caused a slight increase in CXCL8/IL-8 cytokine expression. Conversely, treatment with 100 µg/mL plant extract alone resulted in decreased cytokine expressions of IL-6, CCL22, CXCL10/IP-10, and IL-1β (P = 0.09) compared to control.
3.6 Effects of I. Coerulea extract on protein expressions
The expression levels of proteins were investigated in LPS-stimulated and LPS-unstimulated THP-1 cells with or without 100 µg/mL I. coerulea extract (Figs. 6 and 7). LPS-unstimulated THP-1 cells showed statistically reductions in MIF, IL-1RA/IL-1F3, and CXCL1/GROa proteins when treated with 100 µg/mL I. coerulea extract compared to LPS-stimulated-only THP-1 cells. MIF remained statistically low upon 100 ng/mL LPS and 100 µg/mL in contrast to LPS-only THP-1 cells, while CCL1 protein significantly increased.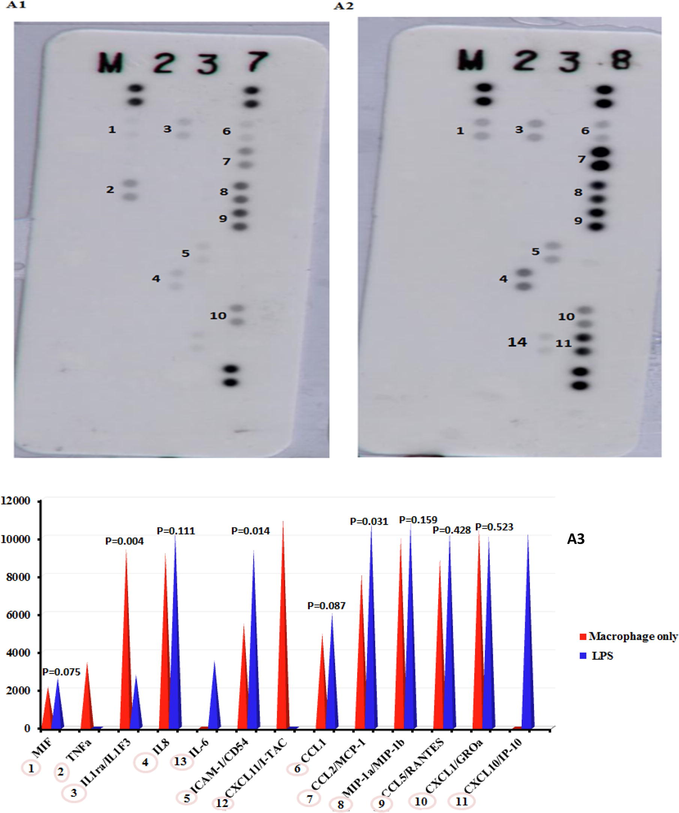
Protein array expression: (A1) macrophages; (A2) cells treated with 100 ng/mL LPS; (A3) histograms comparing the protein array results from macrophages and cells treated with LPS.
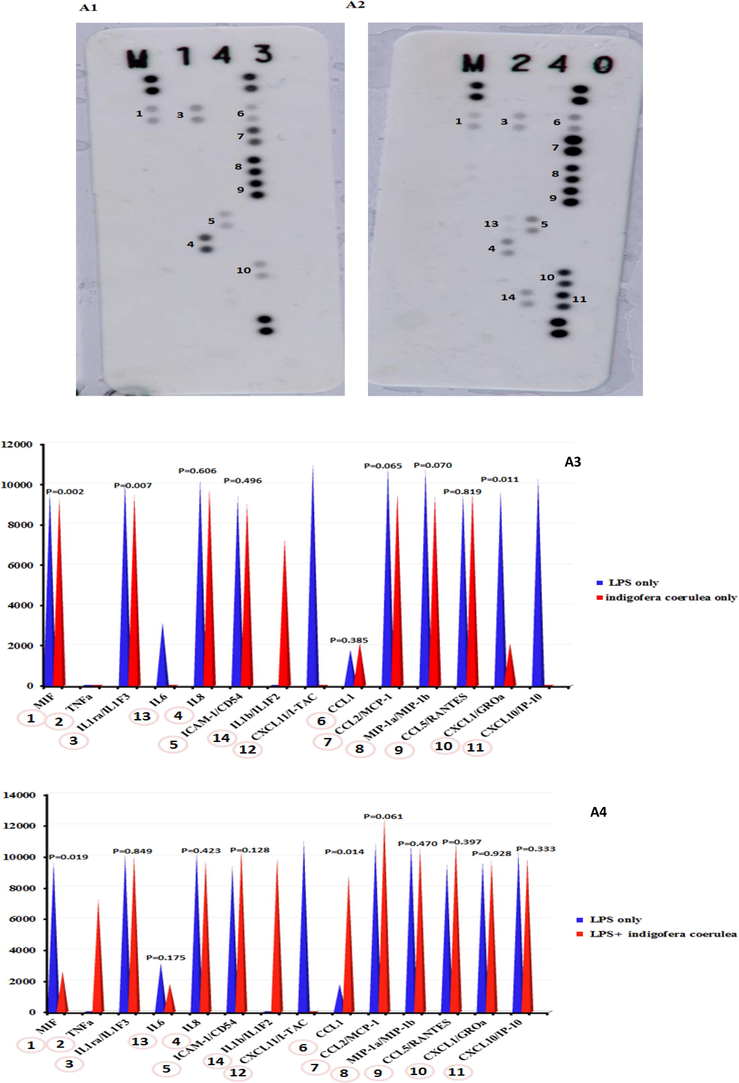
Protein array expression. (A1): I. coerulea; (A2): cells treated with 100 ng/mL LPS + 100 µg/mL of I. coerulea; (A3): histograms comparing the protein array results from LPS and cells treated with I. coerulea; (A4) histograms comparing the protein array results from LPS and cells treated with LPS + I. coerulea.
3.7 Effects of I. Coerulea extract on phagocytosis activity of THP-1 cells
The phagocytosis activity of THP-1 cells treated with 100 ng/mL of LPS and 100 µg/mL of I. coerulea extract was detected through Zymosan particles (Fig. 8). Cytochalasin D was also used before introducing Zymosan particles to inhibit phagocytosis activity. After treatment cells with 100 ng/mL LPS, the phagocytosis response statistically diminished compared with untreated cells. In addition, compared to control THP-1 cells, a modest increase in phagocytosis activity was reported when combination of 100 ng/mL LPS and 100 µg/mL were used to trigger THP-1 cells. Likewise, phagocytosis function remained the same upon THP-1 cells stimulation with 100 µg/mL I. coerulea extract only.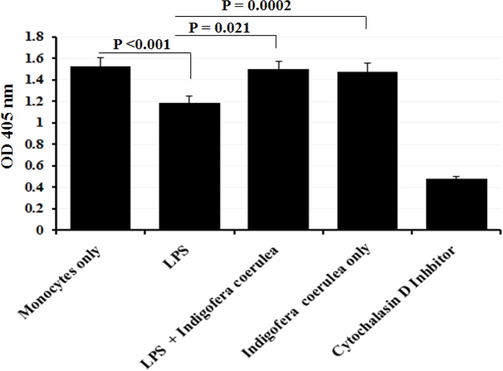
Measurement of phagocytosis using Zymosan particles for the cells treated with 100 ng/mL LPS and 100 µg/mL I. coerulea extract. Phagocytosis response was statistically diminished When THP-1 cells were treated with 100 ng/ml of LPS. Modest increase in phagocytosis activity was reported after treatment with 100 ng/ml of LPS and 100 µg/ml together; (n = 3).
3.8 Effects of I. Coerulea extract on apoptosis
Apoptosis and necrosis are two types of cell death. Annexin-V/PI staining was performed and flow cytometry analysis to detect both apoptotic and necrotic markers that triggered by plant extract. Fig. 9 showed; normal cells and different apoptotic stages. The major proportion of THP-1 cells remained survive; however, apoptotic and late apoptotic cells were recorded. Treatment with 100 ng/mL LPS alone slightly induced the apoptotic THP-1 cells. Conversely, treatment with 100 ng/mL LPS along with 100 µg/mL I. coerulea extract drastically reduced the apoptotic rate and repressed the late apoptosis response by THP-1 cells. Lastly, treatment with 100 µg/mL I. coerulea extract alone also prevent apoptosis. The degree of apoptosis antagonism in THP-1 cells was comparatively lower with stimulation by 100 ng/mL LPS and 100 µg/mL I. coerulea extract combined. These data showed that I. coerulea extract significantly inhibited apoptosis activity in THP-1 cells.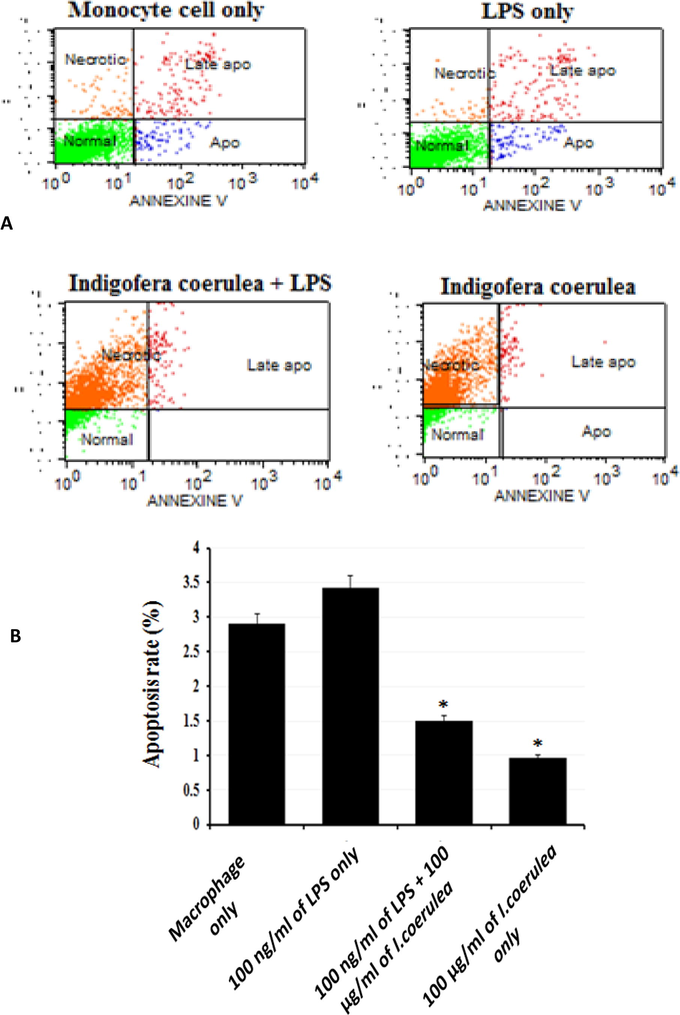
Annexin V/PI staining to analyze apoptosis in monocyte cells. (A) Analysis of apoptosis in monocyte cells treated with LPS + I. coerulea extract; (B) differences in the percentage of apoptotic between the four groups; *p < 0.05 compared to the monocyte cells and LPS groups. 100 ng/ml of LPS treatment alone slightly raised the apoptotic rate in THP-1 cells. Conversely, treatment with 100 ng/ml of LPS along with 100 µg/ml I. coerulea plant extract drastically suppressed the apoptotic activity indicating that I. coerulea plant extract significantly diminishes apoptosis activity in the human monocytic THP-1 cells.
3.9 Effects of I. Coerulea extract on migration
The response of THP-1 cells migration was measured after treatment with and without 100 µg/mL I. coerulea extract and 100 ng/mL LPS, both measured alone and in combination (Fig. 10). The results showed the rate of THP-1 cells migration was negatively affected by 100 ng/mL LPS treatment. Upon addition of 100 µg/mL I. coerulea extract to 100 ng/mL LPS solution, THP-1 cells’ migratory activity was significantly reduced. Similarly, 100 µg/mL I. coerulea extract alone didn’t induce THP-1 cells’ migration, which remained statistically low compared to untreated THP-1 cells.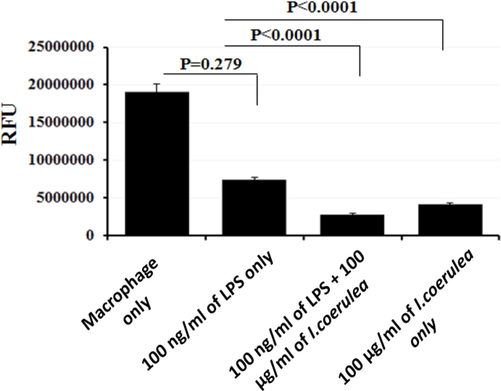
Human monocytic THP-1 and cells treated with 100 ng/mL LPS and 100 µg/mL I. coerulea chemotaxis. We used 200,000 cells in each assay. Migratory cells were quantified by CyQuant® GR Dye. THP-1 cells migratory was negatively affected by 100 ng/ml of LPS induction. 100 µg/ml of I. coerulea and 100 ng/ml of LPS reduced the activity of THP-1 cells migration. Similarly, 100 µg/ml of plant extract alone did not improve THP-1 cells migration and remained statistically low compared to untreated THP-1 cells.
4 Discussion
Macrophages are the main immune cells that orchestrate inflammation through the processes of phagocytosis, recruitment of inflammatory cytokines and activating the adaptive immune system (Parihar et al. 2010). The immune system are inherently conditioned to produce and discharge many cytokines, which trigger both immune and non-immune cells (Varma et al. 2016). Plant-derived compounds have been screened for immunomodulation activity and used to treat immune-triggered disorders (Varma et al., 2016; Francisco et al., 2012). Our findings illustrated that treatment with both 50 and 100 µg/mL I. coerulea extract can powerfully halt the cellular proliferation of THP-1 cells differentiated by PMA. The proliferation arrest was further confirmed by cell synchronization analysis, indicating that I. coerulea extract induces G0/G1 and G2/M arrest in THP-1 cells. The ERK5 protein is necessary for the change from G1 to S phase for successful cell cycle by activating cyclin-dependent protein kinases (CDK) (Gomez et al. 2016). Current results showed significantly enlarged expression of ERK5 in THP-1 cells exposed with 100 ng/mL LPS alone, 100 µg/mL I. coerulea extract alone, and 100 ng/mL LPS with 100 µg/mL I. coerulea extract combined as compared with untreated macrophage cells. The exposure to 50 or 100 µg/mL I. coerulea extract in 100 ng/mL LPS-stimulated PMA-differentiated THP-1 cells resulted in up-regulation of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-18 mRNA at 4 and 6 h compared to control. Conversely, mRNA CCL5 expression was significantly decreased among THP-1 cells treated with 50 or 100 µg/mL I. coerulea plant extract in combination with 100 ng/mL LPS compared to LPS-stimulated cells only at 4 or 6 h. Current results are consistent with those showed increased levels of ERK5, NF-κB signaling pathway activation (high levels of P-IκB-α and P-NF-κB), and IL-8 expression upon stimulation with I. coerulea extract (Pereira et al., 2019). The increased level of ERK5 is limited with the simultaneous up-regulation of the signaling of NF-κB and the subsequently more IL-8 cytokine levels (Park et al. 2016). From the current data, we inferred that despite low P-p38, the NF-κB signaling pathway is highly activated through indirect mechanisms in THP-1 cells treated with I. coerulea extract, suggested by the high levels of P-IκB-α and P-NF-κB proteins. Another MAPK signaling pathway that can potentially activate the NF-κB cascade and translocate NF-κB into the nucleus is extracellular-signal-regulated kinase 1/2 (ERK 1/2) (Sullivan et al. 2018). Inhibition of the ERK 1/2 signaling pathway could prevent diseases linked with unrestrained inflammation. The abnormal activation of the ERK 1/2 signaling pathway has been found to be related with colon cancer cells’ proliferation (Mao et al. 2014). Here, we found reduced expression of ERK1 protein upon exposure to LPS-stimulated and -unstimulated THP-1 cells to 100 µg/mL I. coerulea extract. Previously, phosphorylation of ERK 1/2 signaling pathway was shown to trigger the NF-κB signaling cascade (Winkler et al. 2017). Likewise, earlier studies reported ERK signaling pathway inhibitors to be associated with diminished NF-κB signaling (Vuong et al., 2015; Dilly et al., 2015; Dong et al., 2015). The current findings suggested IL-6 levels to be significantly lower when LPS-stimulated and -unstimulated THP-1 cells were exposed to 100 µg/mL I. coerulea plant extract (Tanaka et al., 2014; Wang and Sun, 2014; Redell et al., 2011). The obtained results showed IL-6 levels to be significantly higher when PMA-differentiated THP-1 cells were treated with 100 ng/mL LPS. The I. coerulea extract might be immensely important in contemplating future immunomodulatory and cancer drugs targeting the IL-6/JAK/STAT3 signaling pathway in inflammation-driven diseases. Also, we observed diminished expression of IL-1 receptor antagonist (IL-1RA) in THP-1 cells upon treatment with I. coerulea extract. IL-1RA, also known as IL-1F3, inhibits the activities of IL-1α and IL-1β and therefore acts as an anti-inflammatory protein (He et al. 2015). For migratory activity analysis, the ELISA results confirmed the decreased expression of CCL22 in LPS-stimulated and LPS-unstimulated THP-1 cells when treated with 100 µg/mL I. coerulea extract compared to LPS stimulation alone. These findings highlighted that I. coerulea extract inhibits THP-1 cells migration activity by upsetting the release of CCL22 through the NF-κB or p38-MAPK transcriptional pathways.
The cells treated with I. coerulea extract showed an enhanced phagocytosis response in THP-1 cells. Bcl-2 protein expression was elevated in LPS-stimulated and unstimulated THP-1 cells when treated with 100 µg/mL I. coerulea extract in comparison with THP-1 cells exposed to 100 ng/mL LPS alone. Interestingly, I. coerulea plant extract alone showed strong expression of BAX protein. However, combined treatment with LPS and I. coerulea extract substantially down-regulated BAX proteins. Several signaling pathways have been shown to play role in the expression of the CXCL10 chemokine (Liu et al. 2011). The increased release of CXCL10 is directly related to the high levels of TNF-α via activation of p38 and the downstream NF-κB signaling pathway in HIV-associated encephalitis (Williams et al. 2009). Rabies virus was reported to induce the expression of CXCL10 in macrophages through activation of the ERK1/2 pathway (Nakamichi et al. 2004). Up-regulation of CXCL10 has been reported in cancer to be facilitated through the p38/MAPK and NF-κB signaling cascades, which stimulate cell proliferation (Liu et al. 2011). We also found CXCL10 to be significantly elevated in the ELISA analysis upon exposure to 100 µg/mL I. coerulea plant extract in LPS-stimulated THP-1 cells, which could be the result of a heightened response from the NF-κB signaling pathway. However, we found decreased levels of p38 protein in LPS-stimulated and unstimulated THP-1 cells treated with 100 µg/mL I. coerulea plant. Zhang et al., in a study on non-alcoholic steatohepatitis (NASH) patients, found that CXCL10 is related with the generation of TNF-α, IL-1β, and CCL2/MCP-1 (C–C motif chemokine ligand 2/monocyte chemoattractant protein 1), and induction of the NF-κB signaling pathway (Zhang et al. 2014).
5 Conclusions
Present findings demonstrated that I. coerulea extract has significant pro-inflammatory, anti-inflammatory, anti-proliferative, pro-phagocytic, anti-apoptotic, and anti-migratory properties in PMA-differentiated THP-1 cells. The immunomodulatory activity of the phytochemicals of I. coerulea extract was evidenced through regulation of investigated proteins and molecular signaling pathways. This findings offer a roadmap for future studies to confirm and elaborate upon the potential role of I. coerulea extract in drug development for chronic diseases. Future studies should investigate these mechanisms in animal models with chronic inflammatory diseases.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No. (RG- 1441-018).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In vitro studies on the immunomodulatory effects of Pulicaria crispa extract on human THP-1 monocytes. Oxid. Med. Cell Longev. 2020
- [CrossRef] [Google Scholar]
- Indigofera oblongifolia leaf extract regulates spleen macrophage response during Plasmodium chabaudi infection. Saudi J. Biol. Sci.. 2017;24(7):1663-1666.
- [Google Scholar]
- Al-Qahtani, A. l-Ahdal, M. Alkahtani, S. (2021). Complement protein C1q binds soluble antigens of Leishmania major (SLA) via the globular head region, activates the classical pathway, and modulates macrophage immune response. J. King Saud Univers. – Sci. 33: 101365.
- In vitro evaluation of novel antiviral activities of 60 medicinal plants extracts against hepatitis B virus. Exp. Ther. Med.. 2017;14(1):626-634.
- [Google Scholar]
- Assessing medicinal plants from South-Eastern Spain for potential anti-inflammatory effects targeting nuclear factor-Kappa B and other pro-inflammatory mediators. J. Ethnopharmacol.. 2009;124(2):295-305.
- [Google Scholar]
- Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204-7218.
- [Google Scholar]
- Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity. 2007;27(6):952-964.
- [Google Scholar]
- Protozoans in Macrophages. CRC Press; 2007.
- Mitogen-activated protein kinase inhibition reduces mucin 2 production and mucinous tumor growth. Transl. Res. J. Laborat. Clin. Med.. 2015;166(4):344-354.
- [Google Scholar]
- CFTR-regulated MAPK/NF-kappaB signaling in pulmonary inflammation in thermal inhalation injury. Sci. Rep.. 2015;5:15946.
- [Google Scholar]
- Immunostimulant activity of Uncaria Tomentosa and its tannins. Planta Med. 2012;78(11):PD9.
- [Google Scholar]
- ERK5 and Cell Proliferation: Nuclear Localization Is What Matters. Front. Cell Dev. Biol.. 2016;4:105.
- [Google Scholar]
- Evaluation of phytochemical constituents and antioxidant activities of successive solvent extracts of leaves of Indigofera caerulea Roxb using various in vitro antioxidant assay systems. Asian Pacific J. Trop. Disease. 2012;2:S118-S123.
- [Google Scholar]
- Association between Polymorphism of Interleukin-1beta and Interleukin-1 Receptor Antagonist Gene and Asthma Risk: A Meta-Analysis. Scient. World J.. 2015;2015:9.
- [CrossRef] [Google Scholar]
- Evaluation of antioxidant and antiinflammatory activities of Corallocarpus epigaeus (Hook. F) rhizomes. Int. J. Pharm. Biomed. Res.. 2014;591:18-24.
- [Google Scholar]
- Ageing and the immune system: focus on macrophages. Europ. J. Microbiol. Immunol.. 2015;5(1):14-24.
- [Google Scholar]
- CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytok. Growth Factor Rev.. 2011;22(3):121-130.
- [Google Scholar]
- Immunostimulatory and cytotoxic activities of Indigofera suffruticosa (Fabaceae) Nat. Prod. Res.. 2011;25(19):1796-1806.
- [Google Scholar]
- Role of ERK-MAPK signaling pathway in pentagastrin-regulated growth of large intestinal carcinoma. World J. Gastroenterol.. 2014;20(35):12542-12550.
- [Google Scholar]
- Rabies virus stimulates nitric oxide production and CXC chemokine ligand 10 expression in macrophages through activation of extracellular signal-regulated kinases 1 and 2. J. Virol.. 2004;78(17):9376-9388.
- [Google Scholar]
- Screening for antibacterial, phytochemical and pharmacognostical properties of Indigofera caerulea Roxb. J. Med. Plants Res.. 2010;4(15):1561-1565.
- [Google Scholar]
- Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J. Innate Immun.. 2010;2(3):204-215.
- [Google Scholar]
- Cis- and Trans-gnetin H from Paeonia suffruticosa suppress inhibitor kappa B kinase phosphorylation in LPS-stimulated human THP-1 cells. J. Ethnopharmacol.. 2016;189:202-209.
- [Google Scholar]
- MEK5/ERK5 activation regulates colon cancer stem-like cell properties. Cell Death Discovery. 2019;5(1):68.
- [Google Scholar]
- NF-κB activation and proinflammatory cytokines mediated protective effect of Indigofera caerulea Roxb. on CCl4 induced liver damage in rats. Int. Immunopharmacol.. 2014;23(2):672-680.
- [Google Scholar]
- Stat3 signaling in acute myeloid leukemia: ligand-dependent and -independent activation and induction of apoptosis by a novel small-molecule Stat3 inhibitor. Blood. 2011;117(21):5701-5709.
- [Google Scholar]
- Traditionally used Thai medicinal plants: in vitro anti-inflammatory, anticancer and antioxidant activities. J. Ethnopharmacol.. 2010;130(2):196-207.
- [Google Scholar]
- Starr T, Bauler TJ, Malik-Kale P, Steele-Mortimer O (2018) The phorbol 12-myristate-13-acetate differentiation protocol is critical to the interaction of THP-1 macrophages with Salmonella Typhimurium. 13 (3):e0193601.
- Kafirin from Sorghum bicolor inhibition of inflammation in THP-1 human macrophages is associated with reduction of intracellular reactive oxygen species. Food Chem. Toxicol. Int. J. Publish. Br. Indust. Biol. Res. Associat.. 2018;111:503-510.
- [Google Scholar]
- IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect. Biol.. 2014;6(10):a016295.
- [Google Scholar]
- IM-133N modulates cytokine secretion by RAW264.7 and THP-1 cells. J. Immunotoxicol.. 2016;13(2):217-225.
- [Google Scholar]
- NF-kappaB transcriptional activation by TNFalpha requires phospholipase C, extracellular signal-regulated kinase 2 and poly(ADP-ribose) polymerase-1. J. Neuroinflammat.. 2015;12:229.
- [Google Scholar]
- The IL-6/JAK/STAT3 pathway: potential therapeutic strategies in treating colorectal cancer (Review) Int. J. Oncol.. 2014;44(4):1032-1040.
- [Google Scholar]
- HIV-1 Tat co-operates with IFN-gamma and TNF-alpha to increase CXCL10 in human astrocytes. PLoS ONE. 2009;4(5):e5709
- [Google Scholar]
- Lipopolysaccharide induced Interleukin-6 production is mediated through activation of ERK 1/2, p38 MAPK, MEK, and NFkappaB in chicken thrombocytes. Dev. Comp. Immunol.. 2017;73:124-130.
- [Google Scholar]
- Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445-455.
- [Google Scholar]
- Virosecurinine induces apoptosis in human leukemia THP-1 cells and other underlying molecular mechanisms. Oncol. Lett.. 2018;15(1):849-854.
- [Google Scholar]
- CXCL10 plays a key role as an inflammatory mediator and a non-invasive biomarker of non-alcoholic steatohepatitis. J. Hepatol.. 2014;61(6):1365-1375.
- [Google Scholar]







