Translate this page into:
The impact of different seed dormancy release treatments on seed germination of juniper (Juniperus procera)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Juniper (Juniperus procera) is a common forest tree species in Saudi Arabia. The Juniper forests face frequent episodes of wildfires; therefore, reforestation is necessary to maintain optimum forest cover in the country. However, Juniper seeds are extremely dormant and germinating them is a tough task. This study assessed the potential of different seed dormancy release treatments in improving the seed germination of Juniper.
Methods
Eight different seed dormancy-release treatments, i.e., boiling seeds for 2, 4 and 6 min, chemical scarification with concentrated sulfuric acid for 2, 4 and 6 min, stratification at 4 °C for 8 weeks and mechanical scarification with sandpaper were tested. A control treatment without any seed dormancy-release treatment was included in the experiment for comparison. The experiments were conducted under three different light:dark regimes, i.e., continuous dark, continuous light and alternating light and dark period of 12 h. Furthermore, the impact of four different potassium nitrate (KNO3) levels, i.e., 0, 2.5, 5 and 10 Mm was tested on seedling traits. Data related to seed germination was recorded at 4, 6, 8 and 10 weeks after initiation of the experiment.
Results
The seeds were highly dormant and those in control treatment failed to germinate after 4 weeks. The highest seed germination was recorded for mechanical scarification under all light dark periods (37, 33 and 41 % for continuous dark, continuous light and alternating light and dark, respectively). Overall, the improvement in seed germination by mechanical scarification was 47, 25 and 34 % under continuous dark, continuous light and alternating light and dark, respectively compared to control treatment after 10 weeks. Seedling traits were significantly improved by the application of 5 Mm KNO3 compared to control treatment and higher concentration proved toxic.
Conclusion
It is concluded that mechanical scarification can be used to releases seed dormancy of Juniper seeds. Furthermore, 5 Mm KNO3 could be utilized to improve the early seedling growth.
Keywords
Seed dormancy
Seed germination
Seedling growth
Dormancy-release treatments
1 Introduction
Juniper (Juniperus procera) forests are the predominant vegetation in the highlands situated > 1600 m (Chaudhary, 1997). Saudi Arabia has unique natural forests on the southwestern side, and these are mostly dominated by Juniper forests (Khalofah et al., 2022). These forests provide valuable ecosystem services. The Juniperus L. is a Cupressaceae genus and among the predominant evergreen shrubs represented by ∼ 67 species (Seneta, 1987). The 95 % of the southwestern forests of Saudi Arabia are composed of Juniper trees (Abo-Hassan et al., 1984).
The most important ecosystem service provided by Juniper forests is the prevention of soil erosion (Hernández and Clemente, 1994). The residents of the southwestern areas of Saudi Arabia benefit from other services provided by Juniper tree, including construction materials, firewood, grazing and beekeeping etc. (Abo-Hassan et al., 1984). The Juniper forests harbor significant amount of natural fauna and flora; therefore, important for the ecological balance. The carbon storage is another unique and important service provide by Juniper forests globally as well as in Saudi Arabia. Overall, the Juniper vegetation are drought tolerant and can endure adverse environmental conditions (Ahani et al., 2013; Helmersson and Von Arnold, 2009). Lead pencils are produced from Juniper wood. Similarly, Juniper trees provide timber wood for buildings and outdoor structures etc. (Cantos et al., 1998; Mamo et al., 2011).
However, several biotic and abiotic factors are causing decline in the area under cultivation of Juniper forests. Low natural regeneration is among the most important reasons responsible for the decline of Juniper Forest area. El-Juhany (2009) reported that low regeneration capacity of forest species is the major reason resulting in the global decline of forest area. Recently, Khalofah et al. (2022) reported that balanced application of NPK could improve the growth and seedling establishment of Juniper trees. However, the study did not consider the seed dormancy and germination. Both are the first transition steps in the life cycle of plants and retarded seed germination due to dormancy results in failed stands.
Aref and El-Juhany (2004) suggested drought stress, increased recreational activities, over-grazing and slow growth of Juniper trees is the major hurdle in the reforestation efforts. The low natural regeneration and pest infestation are the other major reasons of failed reforestation efforts (Hajar et al., 1991). Several studies have suggested that low regeneration capacity is the main reason of failed reforestation efforts of Juniper trees (Aref and El-Juhany, 2004; El-Juhany et al., 2008; FAO, 2021; Hajar et al., 1991). Therefore, improving regeneration capacity could aid success to reforestation efforts.
Facilitation of natural regeneration is the most effective option to restore degraded lands (International Tropical Timber Organization, 2002). Restricting the entry of grazing animals in newly planted forest areas could aid in improving regeneration capacity (Buttoud and Yunusova, 2000; Wassie et al., 2009). Junipers are considered the priority species for restoration of degraded lands (El-Juhany et al., 2008). However, limited work has been done on improving the regeneration capacity of Juniper trees. Improving seed germination and early seedling growth of the species being planted are considered as the most important steps in improving their regeneration capacity. However, the seeds reach dormant conditions if suitable conditions are not found. Therefore, releasing seed dormancy becomes a prerequisite for improving seed germination and subsequently regeneration.
Seeds produced by different plant species undergo various fates after they are detached from the mother plant (Walck et al., 2005). Seed dormancy is an important trait exploited by plant species to persist and continue their generation (Barreto et al., 2016; Zhang et al., 2019). The seeds become non-dormant once the environmental conditions becomes suitable (Baskin and Baskin, 2014; Farooq et al., 2021a; Mahmood et al., 2016). However, the seeds must be released from dormancy which are being planted for the restoration of degraded lands. Seed germination is dependent on the level of seed dormancy in plant species produced by seeds (Vidigal et al., 2016; Zhang et al., 2019).
Different Juniper species exhibit various forms of dormancy according to seed dormancy classification of Baskin and Baskin (2014). Seed dormancy creates severe hurdles in the sexual propagation of Junipers. For example, J. phoenicea seeds exhibit physiological dormancy, whereas those of J. polycarpos has morpho-physiological dormancy (Daneshvar et al., 2016; Ezz AL-Dein et al., 2012). Juniper seeds are difficult to germinate, and germination is delayed even for two years if seed dormancy release treatments are not opted under natural conditions. The outer layer of the seeds results in physical dormancy, while chemical components in the embryo cause physiological dormancy both of which prevent seed germination (Tilki, 2007; Tylkowski, 2011, 2009).
Different biotic or abiotic factors (external or internal to seed coat) are responsible for seed dormancy (Batlla and Luis Benech-Arnold, 2007). The dormancy is a dynamic trait and varies depending upon the environmental conditions and maturity of the seeds (Batlla and Benech-Arnold, 2010). Therefore, seed dormancy knowledge is important to improve seedling establishment (Farooq et al., 2021b; Gioria and Pyšek, 2017; Onen et al., 2016; Önen et al., 2018; Ozaslan et al., 2016). Different techniques such as cold stratification, treating seeds with different compounds, smoke and high temperature have been used to release seed dormancy of many plant species (Bailly, 2004; Bethke et al., 2006; Flematti et al., 2004). Juniper seeds are highly dormant; therefore, seed dormancy must be released before planting them. However, limited knowledge is available on seed-dormancy release treatments to overcome dormancy in Juniper seeds.
The current study was conducted to infer the role of various seed dormancy-release treatments in improving seed germination of Juniper. Inferring the role of potassium nitrate n improving seedling growth/traits was the other major objective of the study. It was hypothesized that different seed dormancy-release treatments would differ in their ability to release seed dormancy. It was further hypothesized that potassium nitrate would improve seedling traits. The results would help to improve the regeneration capacity of Juniper trees.
2 Materials and methods
2.1 Experiment site
Seed germination and seedling growth experiments were conducted at King Khalid University (KKU), Saudi Arabia, in the Research Center of Advanced Materials (RCAMS) during 2020–2021.
2.2 Seed collection
Seeds were collected from an established Juniper Forest at Ghulamah mountain in Asir region, (Tanomah) Saudi Arabia. Cones were collected from twenty randomly selected mother trees. The cones were brought to the laboratory, where their fleshy parts were removed, and resin was cleaned for seed extraction. Since large amount of Juniper seeds in the cones are empty, insect-infested, and dead seeds were first removed following the incubation, drying and separation protocol developed by Daneshvar et al. (2016).
Viability of the obtained seeds was tested by topographical tetrazolium (TTC) test (ISTA, 2017) which was 82 %. The seeds were then packed in plastic bags, sealed, and stored in the refrigerator at 5 °C until the experiments were conduct after one week.
2.3 Seed dormancy release treatments
A total eight different seed dormancy treatment along with untreated control were used in the study. Seeds were mechanically scarified with sandpaper through gentle rubbing to make the seed permeable for water imbibition. Similarly, concentrated sulfuric acid (98 %) was used for chemical scarification. The seeds were placed in concentrated sulfuric acid for 2, 4 or 6 min followed by washing with distilled water. Seeds were boiled for 2, 4 or 6 min, cooled, washed with distilled water, and then used in experiments. Similarly, seeds were placed in moistened filter papers for 8 weeks at 4 °C for stratification. Afterwards, seeds were rinsed and used in the experiments.
2.4 Experimental procedure
Seeds obtained after each seed dormancy-release treatment were placed on moistened filter paper in 9.5 mm Petri dishes (25 seed for each dish). The dishes were sealed with paraffin film to avoid moisture loss. Three different experiments, i.e., continuous light, continuous dark and alternating light and dark were conducted where the impact of all seed dormancy release treatments was tested on seed germination. Each treatment had five replications and each replication consisted of 5 dishes. The incubators were maintained at room temperature (24 °C) for 10 weeks. The seeds were checked at each data collection date, i.e., 4, 6, 8 and 10 weeks after the initiation of treatments and moistened to avoid the impacts of osmotic stress.
2.5 Data collection
The Petri dishes were observed at 4, 6, 8 and 10 weeks after the initiation of experiments and number of germinated seeds were counted and removed from the dishes. Seed germination percentage was counted by dividing the number of seeds germinated to the total number of seeds in each Petri dish and expressed in percentage. The seeds which were not germinated at the end of 10 weeks were tested for viability by TTC test as described above. The final germination of each week was tehn adjusted as viability adjusted germination. Viability adjusted germination was calculated by the formula of Weller et al. (2016) as under; where Ngerm is total number of germinated seeds, and Nviable_non_germ is total number of viable non-germinated seeds.
2.6 Seedling growth experiment
Mechanical scarification with sandpaper proved better seed dormancy-release treatment; therefore, dormancy was released by this technique for using seeds in seedling growth experiment. The seeds were planted in 20 cm plastic pots (Farooq et al., 2017; Onen et al., 2017; Özaslan et al., 2016) filled with a mixture of sand and sterilized peat moss at a rate of 1:1 (v/v). The pots were kept in greenhouse and irrigated with distillated water for four weeks. Then, seedlings were treated with potassium nitrate solution at concentrations of 0, 2.5, 5 and 10 mM as a growth enhancer through irrigation for 8 weeks. Seedlings, root length, plumule length, fresh and dry weight of both plumule and root were measured 8 weeks after treatment. The plants were taken off the pots, washed carefully and lengths of different parts were measured with the help of measuring tape. Each treatment had five replications and each replication contained 3 pots. Three seeds were planted in each pot and reduced to one after seedlings appeared.
2.7 Statical analysis
The collected data were analyzed by Analysis of Variance (ANOVA). The data were tested for normality first, which indicated a normal distribution (Shapiro and Wilk, 1965). Therefore, one-way ANOVA was used to infer the significance in seed germination and seedling growth data (Steel et al., 1980). Least significant difference post hoc test was used to compare means where ANOVA indicated significant differences. The statistical analysis was done on SPSS statistical software (IBM and IBM SPSS Inc., 2012).
3 Results
Seed germination was significantly altered by different seed dormancy-release treatments included in the study under all light:dark regimes (Table 1). Here, source = source of variation in the response variable, DF = degree of freedom, * = significant.
Source
DF
Sum of squares
Mean squares
F value
P value
Continuous light
Treatments (T)
8
1738.96
217.37
22.57
< 0.0001*
Weeks (W)
3
8174.22
2724.74
282.95
< 0.0001*
T × W
24
847.11
35.30
3.67
< 0.0001*
Continuous dark
Treatments (T)
8
3319.41
414.93
41.80
< 0.0001*
Weeks (W)
3
6209.63
2069.88
208.53
< 0.0001*
T × W
24
1103.70
45.99
4.63
< 0.0001*
Alternating light/dark
Treatments (T)
8
3347.85
418.48
55.39
< 0.0001*
Weeks (W)
3
8868.15
2956.05
391.24
< 0,0001*
T × W
24
1539.85
64.16
8.49
< 0.0001*
Under continuous dark conditions, low seed germination (0–6.66 %) was recorded at the end of 4 weeks after the initiation of experiment under continuous dark. Mechanical scarification and control treatments recorded the highest and the lowest seed germination percentage, respectively at the end of 4 weeks. The germination increased between 1.33 and 21.33 % after 6 weeks, 4.00–25.33 % at the end of 8 weeks and 5.33 to 37.33 % at the end of 10 weeks. Seed germination percentage in control treatment was improved from 0 to 25.33 % at the end of 10 weeks incubation period. However, chemical scarification -could not improve seed germination even comparable to control treatment (Table 2). Regarding overall performance of the seed dormancy-release treatments, the highest and the lowest seed germination was recorded for mechanical scarification and chemical scarification for 6 min, respectively (Fig. 1). Here, control = no seed dormancy release treatment applied, boiling 2, 4 and 6 = seeds soaked in boiling water for 2, 4 and 6 min, scar-2, 4 and 6 represents chemical scarification with concentrated sulfuric acid for 2, 4 and 6 min, respectively, sandpaper = mechanical scarifications with sand paper and start = stratification of seeds at 4 °C for 8 weeks. Means followed by different letters within a column or a row are significantly different from each other (p < 0.05).
Weeks
4
6
8
10
Control
0.00 k
1.33 jk
8.00 gh
25.33 bc
Boiling 2
1.33 jk
8.00 gh
16.00 e
25.33 bc
Boiling 4
2.66 ijk
6.66 ghi
17.33 de
29.33b
Boiling 6
1.33 jk
9.33 fg
14.66 e
28.00b
Sandpaper
6.66 ghi
21.33 cd
25.33 bc
37.33 a
Scar-2
1.33 jk
4.00 hijk
6.66 ghi
16.00 e
Scar-4
1.33 jk
2.66 ijk
5.33 ghij
13.33 ef
Scar-6
0.00 k
1.33 jk
4.00 hijk
5.33 ghij
Strat-1
4.00 hijk
9.33 fg
16.00 e
22.66c
LSD 5 %
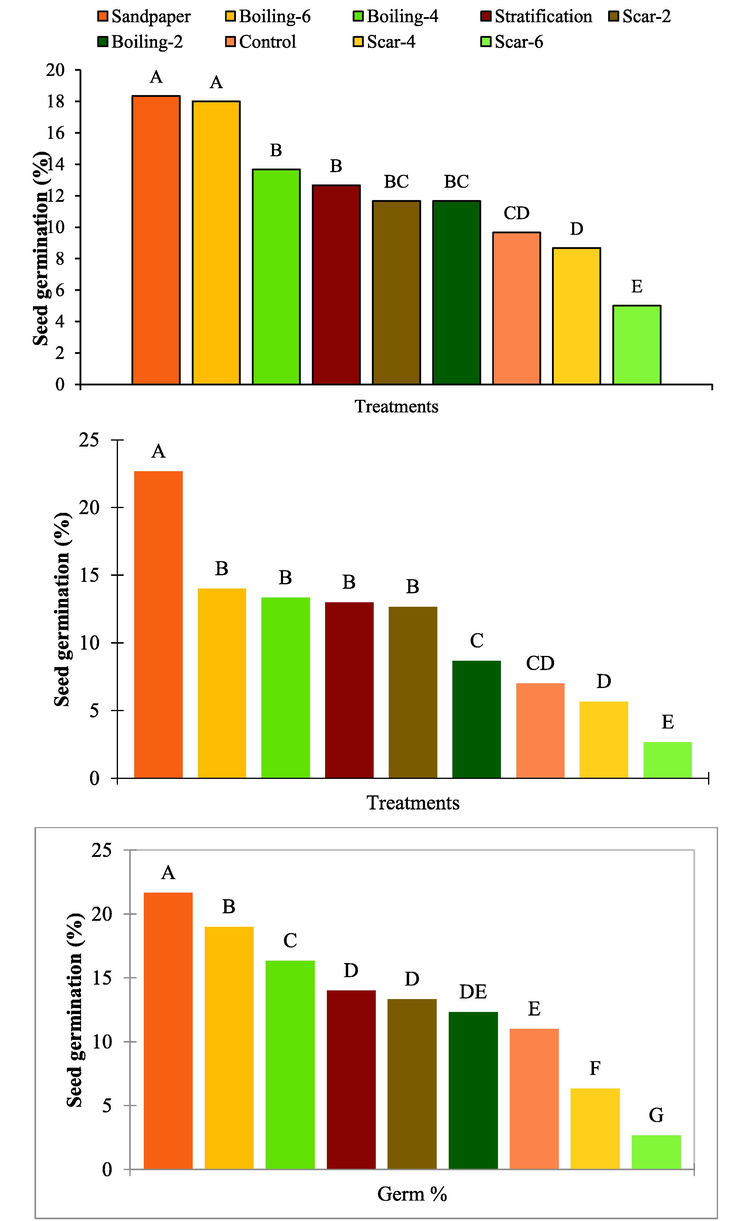
The impact of different seed dormancy release treatments on seed germination percentage of Juniper procera under continuous light (a), continuous dark (b) and alternating light and dark conditions (c).
Under continuous light conditions, low seed germination (0–6.66 %) was recorded at the end of 4 weeks after the initiation of seed dormancy release treatments. Mechanical scarification and control treatment recorded the highest and the lowest seed germination percentage, respectively at the end of 4 weeks. Germination increased between 2.66 and 12.00 % after 6 weeks, 5.33–22.66 % at the end of 8 weeks and 10.66 to 33.33 % at the end of 10 weeks. Seed germination percentage in control treatment was improved from 0 to 26.66 % at the end of 10 weeks incubation period. However, chemical scarification with sulfuric acid could not improve seed germination even comparable to control treatment (Table 3). Regarding overall performance of the seed dormancy-release treatments, the highest and the lowest seed germination was recorded for mechanical scarification and chemical scarification for 6 min, respectively (Fig. 1). Here, control = no seed dormancy release treatment applied, boiling 2, 4 and 6 = seeds soaked in boiling water for 2, 4 and 6 min, scar-2, 4 and 6 represents chemical scarification with concentrated sulfuric acid for 2, 4 and 6 min, respectively, sandpaper = mechanical scarifications with sand paper and start = stratification of seeds at 4 °C for 8 weeks. Means followed by different letters within a column or a row are significantly different from each other (p < 0.05).
Weeks
Treatments
4
6
8
10
Control
0.00o
2.66 mno
9.33 hijk
26.66 cd
Boiling 2
1.33 no
4.00 lmno
13.33 gh
28.00c
Boiling 4
2.66 mno
8.00 ijkl
13.33 gh
30.66 abc
Boiling 6
5.33 klmn
12.00 ghi
20.00 e
34.66 a
Sandpaper
6.66 jklm
10.66 ghij
22.66 de
33.33 ab
Scar-2
5.33 klmn
8.00 ijkl
14.66 fg
18.66 ef
Scar-4
1.33 no
4.00 lmno
10.66 ghij
18.66 ef
Scar-6
0.00o
4.00 lmno
5.33 klmn
10.66 ghij
Strat
2.66 mno
5.33 klmn
13.33 gh
29.33 bc
LSD 5 %
Under alternating light:dark conditions, low seed germination (0–5.33 %) was recorded at the end of 4 weeks after the initiation of experiment. Mechanical scarification with sandpaper and control treatment recorded the highest and the lowest seed germination percentage, respectively at the end of 4 weeks. The germination increased between 1.33 and 14.66 % after 6 weeks, 4.00–25.33 % at the end of 8 weeks and 5.33 to 41.33 % at the end of 10 weeks. Seed germination percentage in control treatment was improved from 0 to 26.66 % at the end of 10 weeks incubation period. However, chemical scarification with sulfuric acid could not improve the seed germination even comparable to control treatment (Table 4). Regarding overall performance of the seed dormancy-release treatments, the highest and the lowest seed germination was recorded for mechanical scarification and chemical scarification for 6 min, respectively (Fig. 1). Here, control = no seed dormancy release treatment applied, boiling 2, 4 and 6 = seeds soaked in boiling water for 2, 4 and 6 min, scar-2, 4 and 6 represents chemical scarification with concentrated sulfuric acid for 2, 4 and 6 min, respectively, sandpaper = mechanical scarifications with sand paper and start = stratification of seeds at 4 °C for 8 weeks. Means followed by different letters within a column or a row are significantly different from each other (p < 0.05).
Weeks
4
6
8
10
Control
2.66 nop
5.33 lmno
10.66 hijk
30.66c
Boiling-2
2.66 nop
5.33 lmno
14.66 fgh
30.66c
Boiling 4
4.00 mnop
10.66 hijk
18.66 ef
32.00 bc
Boiling 6
1.33 op
6.66 klmn
16.00 fg
32.00 bc
Sandpaper
5.33 lmno
14.66 fgh
25.33 d
41.33 a
Scar-2
4.00 mnop
9.33 ijkl
12.00 ghij
18.66 ef
Scar-4
0.00p
2.66 nop
8.00 jklm
14.66 fgh
Scar-6
0.00p
1.33 op
4.00 mnop
5.33 lmno
Stratification
4.00 mnop
13.33 ghi
22.66 de
36.00b
LSD 5 %
3.1 Seedling growth
Different seedling traits, i.e., seedling height, root length, root fresh and dry weight, plumule length and plumule fresh and dry weight were significantly altered by different concentrations of potassium nitrate (Table 5). Here, source = source of variation in the response variable, DF = degree of freedom, * = significant.
Source
DF
Sum of squares
Mean squares
F value
P value
Seedling height
KNO3 concentrations
3
106.70
35.56
71.37
< 0.0001*
Root length
KNO3 concentrations
3
31.20
10.40
33.19
< 0.0001*
Root fresh weight
KNO3 concentrations
3
331.83
110.61
47.30
< 0.0001*
Root dry weight
KNO3 concentrations
3
262.31
87.43
239.24
< 0.0001*
Plumule length
KNO3 concentrations
3
30.66
10.22
98.14
< 0.0001*
Plumule fresh weight
KNO3 concentrations
3
229.37
76.45
82.84
< 0.0001*
Plumule dry weight
KNO3 concentrations
3
57.14
19.04
4.58
0.038*
Seedling height, root length, root fresh and dry weight, plumule length and plumule fresh and dry weight were significantly improved by increasing concentration of potassium nitrate up to 5 Mm and then a slight decline in these traits was noted. Overall, the highest values of seedling height, root length, root fresh and dry weight, plumule length and plumule fresh and dry weight were recorded for 5 Mm potassium nitrate, whereas 0 Mm recorded the lowest values of these traits (Table 6). Means followed by different letters within a column or a row are significantly different from each other (p < 0.05).
Seedling traits
KNO3 concentrations (Mm)
0
2.5
5
10
Seedling length (cm)
10.13c
14.23b
17.83 a
11.26c
Root length (cm)
5.53c
8.06b
9.96 a
7.03b
Plumule length (cm)
3.63c
6.60b
7.30 a
3.96c
Fresh weight of root (g)
58.80c
62.21b
73.00 a
63.70b
Fresh weight of plumule (mg)
26.81c
33.11b
39.17 a
32.86b
Dry weight of root (g)
11.51 d
18.29b
24.21 a
15.04c
Dry weight of plumule (mg)
8.92b
11.50 ab
15.04 a
11.38 ab
4 Discussion
Different seed dormancy release treatments, as hypothesized, significantly altered seed germination of Juniper. The seeds remained highly dormant until 4 weeks and then germination increased in control treatments. The results revealed that seeds were slightly photoblastic as they exhibited higher seed germination under alternating light and dark compared to complete light or complete dark conditions. Therefore, a light dark cycle is necessary to overcome the seed dormancy of Juniper. The 25 % of seeds lose their seed dormancy 10 weeks after sowing, while the remaining 75 % remain dormant. Mechanical scarification with sandpaper reduced the seed dormancy to 59 % indicating that it has significant potential to improve seed germination and resultantly regeneration efforts. Physical dormancy due to seed coat was released by mechanical scarification, whereas physiological dormancy due to embryo remained unreleased. Chemical scarification failed to improve seed germination and even resulted in lower germination compared to control treatment of the study. The higher seed germination in mechanical scarification can be linked to the reason that it made seed coat permeable; thus, seeds imbibed more water compared to the rest of the treatments. Contrastingly, lower seed germination in chemical scarification can be linked to damaged caused by sulfuric acid to seed coat and food reserves which resulted in lower seed germination.
After being separated from their parent plant, seeds of different species travel their own ways (Walck et al., 2005). Seed dormancy is a crucial adaptation that helps plant species endure and perpetuate themselves over successive generations (Barreto et al., 2016; Zhang et al., 2019). The seeds become non-dormant once the environmental conditions become suitable (Baskin and Baskin, 2014; Farooq et al., 2021a; Onen et al., 2016; Ozaslan et al., 2016). However, the seeds that will be sown to revive degraded soils must first be relieved from dormancy. The degree of seed dormancy in plants determines how easily their seeds will germinate (Vidigal et al., 2016; Zhang et al., 2019).
Seed dormancy is caused by a wide variety of biotic and abiotic stimuli (both exterior to the seed coat and inside the seed itself) (Batlla and Luis Benech-Arnold, 2007). Dormancy is a dynamic feature that changes in intensity as a result of differences in environmental circumstances and the seeds' stages of development (Batlla et al., 2004). Consequently, understanding seed dormancy is crucial for enhancing the success of plant reproduction (Gioria and Pyšek, 2017). Many plant species' seed dormancy has been broken using a variety of methods, including cold stratification, seed treatment with various chemicals, smoke, and high temperature (Bailly, 2004; Bethke et al., 2006; Flematti et al., 2004).
Low seed germination rates in tree species may be attributed to their dormant seeds (Li and Min, 2020). Therefore, to improve seed germination and boost regeneration, it is required to relieve seed dormancy. Many seed treatments have the ability to break seed dormancy, and the administration of these substances causes seeds of many plant species to react favorably (Renata and Agnieszka, 2006). The most common chemical employed to break the dormancy of diverse plant species' seeds is gibberellin (Linkies and Leubner-Metzger, 2012; Matilla and Matilla-Vázquez, 2008). Chemical scarification with sulfuric acid did not work to break the dormancy in the present investigation. The study's findings corroborate a number of previous reports that increased concentrations of sulfuric acid reduce the germination rate of many plant species (Foley and Chao, 2008; Wei et al., 2010).
Seedling traits were significantly improved by increasing concentration of potassium nitrate and higher concentration witnessed a decline in these traits. The dual action of potassium and nitrogen could be the reason of improved seedling traits of Juniper. Both nitrogen and potassium play a significant role in improving plant growth. Thus, potassium nitrate could be effectively used to improve the early growth of juniper tree. Khalofah et al. (2022) also reported that application of NPK improved the seedling traits of Juniper tree. Thus, results of the current study are in accordance with their findings.
5 Conclusions
The results of the current study indicated that Juniper seeds were highly dormant and none of the applied treatment could release 100 % seed dormancy. Nevertheless, mechanical scarification with sandpaper proved better among the seed dormancy release treatments opted in the study. Chemical scarification was unable to improve seed germination; rather, decreased it compared to control. The application of 5 Mm potassium nitrate significantly improved early seedling growth of Juniper. It is recommended that releasing seed dormancy with mechanical scarification and applying 5 Mm potassium nitrate during seedling stage could improve the regeneration success of Juniper trees.
Acknowledgement
This work was supported by Institute of Research and consulting Studies, King Khalid University, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Natural forests in the Kingdom of Saudi Arabia and the possibility of exploiting them economically. Natl. Cent. Sci. Technol. (Now King Abdulaziz City Sci. Technol. B. 1984
- [Google Scholar]
- Reproduction of juniper (Juniperus polycarpos) in Khorasan Razavi. Iran. For. Sci. Pract.. 2013;15(3):231-237.
- [Google Scholar]
- Planting Juniperus procera trees in the natural forests of Saudi Arabia: the first trial. In: The Second Conference of Development and Environment in Arab World. Egypt: Assiut University; 2004. p. :23-25.
- [Google Scholar]
- Active oxygen species and antioxidants in seed biology. Seed Sci. Res.. 2004;14(2):93-107.
- [Google Scholar]
- Seed dormancy in Stachytarpheta species (Verbenaceae) from high-altitude sites in south-eastern Brazil. Flora Morphol. Distrib. Funct. Ecol. Plants. 2016;225:37-44.
- [CrossRef] [Google Scholar]
- Seeds: ecology, biogeography, and evolution of dormancy and germination. Seeds 2014
- [CrossRef] [Google Scholar]
- Predicting changes in dormancy level in natural seed soil banks. Plant Mol. Biol.. 2010;73:3-13.
- [CrossRef] [Google Scholar]
- Batlla, D., Kruk, B.C., Benech-Arnold, R.L., 2004. Modelling changes in dormancy in weed soil seed banks: Implications for the prediction of weed emergence, Handbook of seed physiology: Applications to agriculture.
- Predicting changes in dormancy level in weed seed soil banks: Implications for weed management. Crop Prot 2007
- [CrossRef] [Google Scholar]
- Bethke, P.C., Libourel, I.G.L., Jones, R.L., 2006. Nitric oxide reduces seed dormancy in Arabidopsis. J. Exp. Bot. 10.1093/jxb/erj060.
- Buttoud, G., Yunusova, I., 2000. Present issues for a multi-purpose sustainable management of Artcha forests in the south of Kyrgyzstan, in: Proceedings of the International Symposium: Problems of Juniper Forests: Looking for Solutions, Methods, Techniques. Osh: National Academy of Science (Kyrgyzs-Tan)-Forest and Walnut Research Institute.
- Embryo rescue and development of Juniperus oxycedrus subsp. oxycedrus and macrocarpa. International Seed Testing Association; 1998.
- Chaudhary, S.A., 1997. Flora of the Kingdom of Saudi Arabia, 1st ed. National Agriculture and Water Research Centre, Ministry of Agriculture, Saudi Arabia.
- Stimulation of germination in dormant seeds of Juniperus polycarpos by stratification and hormone treatments. New For.. 2016;47(5):751-761.
- [Google Scholar]
- Forestland degradation and potential rehabilitation in southwest Saudi Arabia. Aust. J. Basic Appl. Sci.. 2009;3:2677-2696.
- [Google Scholar]
- The possibility of ameliorating the regeneration of juniper trees in the natural forests of Saudi Arabia. Res. J. Agric. Biol. Sci.. 2008;4:126-133.
- [Google Scholar]
- Propagation physiology of Juniperus phoenicea L. from Jordan using seeds and in vitro culture techniques: Baseline information for a conservation perspective. AFRICAN. J. Biotechnol.. 2012;11
- [CrossRef] [Google Scholar]
- FAO, 2021. Food and Agriculture Organisation of the United Nations pistachio statistics [WWW Document].
- Farooq, S., Onen, H., Tad, S., Ozaslan, C., Mahmoud, S.F., Brestic, M., Zivcak, M., Skalicky, M., El-Shehawi, A.M., 2021b. The influence of environmental factors on seed germination of Polygonum perfoliatum L.: Implications for management. Agronomy. 10.3390/agronomy11061123.
- Range expansion potential of two co-occurring invasive vines to marginal habitats in Turkey. Acta Oeocologica. 2017;84:23-33.
- [CrossRef] [Google Scholar]
- Characteristics and methods to release seed dormancy of two ground cherry (Physalis) species. J. Appl. Res. Med. Aromat. Plants. 2021
- [CrossRef] [Google Scholar]
- Growth Regulators and Chemicals Stimulate Germination of Leafy Spurge (Euphorbia esula) Seeds. Weed Sci. 2008
- [CrossRef] [Google Scholar]
- Early bird catches the worm: germination as a critical step in plant invasion. Biol. Invasions. 2017;19:1055-1080.
- [CrossRef] [Google Scholar]
- Impact of biological stress on Juniperus excelsa M. Bieb. in south-western Saudi Arabia: insect stress. J. Arid Environ.. 1991;21:327-330.
- [Google Scholar]
- Embryogenic cell lines of Juniperus communis; easy establishment and embryo maturation, limited germination. Plant Cell, Tissue Organ Cult.. 2009;96:211-217.
- [Google Scholar]
- Hernández, J.E., Clemente, M., 1994. Protección de la Flora en Andalucía [Protection of the Andalusian Flora]. Agencia Medio Ambient. Junta Andalucía Sevilla, Spain.
- IBM, C., IBM SPSS Inc., 2012. SPSS Statistics for Windows. IBM Corp. Released 2012 Version 20, 1–8.
- ISTA, InternasionaI Seed Testing Association, 2017. International Rules for Seed Testing 2017. Int. Seed Test. Assoc. Switz, ISTA.
- The impact of NPK fertilizer on growth and nutrient accumulation in juniper (Juniperus procera) trees grown on fire-damaged and intact soils. PLoS One. 2022;17:e0262685.
- [Google Scholar]
- Dormancy characteristics and germination requirements of Phoebe bournei seed. Sci. Hortic. (Amsterdam). 2020
- [CrossRef] [Google Scholar]
- Beyond gibberellins and abscisic acid: How ethylene and jasmonates control seed germination. Plant Cell Rep. 2012
- [CrossRef] [Google Scholar]
- Influence of various environmental factors on seed germination and seedling emergence of a noxious environmental weed: green galenia (Galenia pubescens) Weed Sci.. 2016;64:486-494.
- [Google Scholar]
- Longevity of Juniperus procera seed lots under different storage conditions: implications for ex situ conservation in seed banks. J. For. Res.. 2011;22:453-459.
- [Google Scholar]
- Onen, H., Akyol, N., Farooq, S., Ozaslan, C., 2016. Seed dormancy differences among common ragweed populations, in: Proceedings of VII International Scientific Agriculture Symposium “Agrosym 2016.” Jahorina, pp. 643.
- Higher Tolerance to Abiotic Stresses and Soil Types May Accelerate Common Ragweed (Ambrosia artemisiifolia) Invasion. Weed Sci.. 2017;65:115-127.
- [CrossRef] [Google Scholar]
- The Influence of Environmental Factors on Germination of Burcucumber (Sicyos angulatus) Seeds: Implications for Range Expansion and Management. Weed Sci.. 2018;66:494-501.
- [CrossRef] [Google Scholar]
- Organization, I.T.T., 2002. ITTO guidelines for the restoration, management and rehabilitation of degraded and secondary tropical forests. Internaitonal Tropical Timber Organization.
- Ozaslan, C., Tad, S., Onen, H., Farooq, S., 2016. Do bur cucumber populations exhibit differences in seed dormancy?, in: VII International Scientific Agriculture Symposium,“ Agrosym 2016”, 6-9 October 2016, Jahorina, Bosnia and Herzegovina. Proceedings. University of East Sarajevo, Faculty of Agriculture, pp. 1682–1687.
- Invasion Potential of Two Tropical Physalis Species in Arid and Semi-Arid Climates: Effect of Water-Salinity Stress and Soil Types on Growth and Fecundity. PLoS One. 2016;11:1-23.
- [CrossRef] [Google Scholar]
- Nitric oxide and HCN reduce deep dormancy of apple seeds. Acta Physiol. Plant. 2006
- [CrossRef] [Google Scholar]
- Seneta, W., 1987. Drzewa i krzewy iglaste. Państwowe Wydawnictwo Naukowe.
- An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591-611.
- [Google Scholar]
- Steel, R.G.D., Torrie, J.H., Dickey, D., 1980. Principles and Procedures of Statistics: a Biometrical Approach, New York.
- Tilki, F., 2007. Preliminary results on the effects of various pre-treatments on seed germination of Juniperus oxycedrus L. Seed Sci. Technol. 35, 765–770. 10.15258/sst.2007.35.3.25.
- Improving seed germination and seedling emergence in the Juniperus communis. Dendrobiology. 2009;61:47-53.
- [Google Scholar]
- Dormancy breaking in Savin juniper (Juniperus sabina L.) seeds. Acta Soc. Bot. Pol.. 2011;79:27-29.
- [CrossRef] [Google Scholar]
- Altitudinal and climatic associations of seed dormancy and flowering traits evidence adaptation of annual life cycle timing in Arabidopsis thaliana. Plant Cell Environ.. 2016;39:1737-1748.
- [CrossRef] [Google Scholar]
- Defining transient and persistent seed banks in species with pronounced seasonal dormancy and germination patterns. Seed Sci. Res.. 2005;15:189-196.
- [Google Scholar]
- Tree regeneration in church forests of Ethiopia: effects of microsites and management. Biotropica. 2009;41:110-119.
- [Google Scholar]
- Rapid and Effective Methods for Breaking Seed Dormancy in Buffalobur (Solanum rostratum) Weed Sci. 2010
- [CrossRef] [Google Scholar]
- An investigation of the effects of stage of ensilage on Nassella neesiana seeds, for reducing seed viability and injury to livestock. Sci. Rep.. 2016;6:22345.
- [CrossRef] [Google Scholar]
- Non-deep simple morphophysiological dormancy in seeds of Angelica keiskei (Apiaceae) Sci. Hortic. (Amsterdam). 2019;255:202-208.
- [CrossRef] [Google Scholar]







