Translate this page into:
The hydrophilic/hydrophobic ratio vs. dissolved organics removal by coagulation – A review
*Tel./fax: +213 25 43 36 31 djamel_andalus@yahoo.fr (Djamel Ghernaout) djamel.ghernaout@univ-blida.dz (Djamel Ghernaout)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 27 September 2013
Peer review under responsibility of King Saud University.

Abstract
This review discusses the hydrophilic/hydrophobic ratio as a function of the hydrophilic and hydrophobic contents removal by coagulation process. It is well established that coagulation process could bring a reduction in dissolved organic carbon of around 30–60% by increasing the coagulant dose and optimising reaction pH, in which large organic molecules with hydrophobic property was removed preferentially. Furthermore, the literature affirmed that the greater removal of UV-absorbing substances indicates that alum coagulation preferentially removed the hydrophobic fraction of the total organic carbon. For the hydrophobic fraction, it needs to be removed entirely without its transformation into hydrophilic fractions by coagulation process avoiding pre-chlorination/pre-oxidation due to the risk of organic molecules fragmentation. Determining the exact numerical values of the hydrophilic/hydrophobic ratio for raw water and treated water at different stages of the treatment processes in a water treatment plant, as for the DCO/DBO5 ratio in the case of wastewater treatment, would help on more focusing on OM control and removal.
Keywords
Hydrophilic/hydrophobic ratio
Disinfection by-products (DBPs)
Dissolved organics
Cyanotoxins
Natural organic matter (NOM)
Coagulation
1 Introduction
It is well known now that natural organic matter (NOM) in the aquatic environment consists of a wide variety of organic compounds that are primarily derived from the decomposition of plant and animal residues (Chen et al., 2002; Joseph et al., 2012). Further, the presence of NOM in various water sources is a major concern for environmental scientists and engineers, specifically in water treatment (Ghernaout et al., 2011; Liu et al., 2012). There is no doubt here that NOM often contributes to offensive taste and odours in potential drinking water sources and acts as a carrier for metals and various harmful organic chemicals (Ghernaout et al., 2011). Moreover, NOM can disrupt various processes in a conventional water treatment facility (Baghoth et al., 2011). Indeed, NOM is considered to be a precursor for carcinogenic disinfection by-products (DBPs) such as trihalomethanes (THMs) and haloacetic acids (HAAs) that can form during chlorination (Chowdhury et al., 2009; Rook, 1974, 1977; Rice and Gomez-Taylor, 1986; Gang et al., 2005; Roccaro et al., 2008; Kristiana et al., 2013) and contribute to bacterial re-growth and biofilm formation in drinking water distribution systems (Ghernaout et al., 2011).
On the other hand, it is well established that coagulation/flocculation in water/wastewater treatment involves the addition of chemicals to alter the physical state of dissolved and suspended solids and facilitate their removal by sedimentation (Dentel, 1991; Cheng, 2002; Duan and Gregory, 2003; Matilainen et al., 2010; Sáez et al., 2010; Alexander et al., 2012; Kimura et al., 2013). As chemical products, coagulants react with the suspended and colloidal particles in the water, causing them to bind together and thus allowing for their removal in the subsequent treatment processes (Jiang, 2001; Li et al., 2006; Jiang and Wang, 2009). The aggregation mechanisms through which particles and colloids are removed include a combination of charge neutralisation, entrapment, adsorption and complexation with coagulant ions into insoluble masses (Duan and Gregory, 2003; Zhou et al., 2005; Zhang and Wang, 2009; Cheng et al., 2010; Matilainen et al., 2010; Verma et al., 2012; Ghernaout and Ghernaout, 2012).
As an integral part of the conventional water treatment scheme, coagulation treatment has been employed to decrease turbidity and colour and to remove pathogens (Hai et al., 2007; Matilainen et al., 2010; Verma et al., 2012). Coagulation can remove efficiently the hydrophobic and high molar mass fractions of NOM (Matilainen et al., 2010; Mao et al., 2013). Moreover, coagulation/flocculation/precipitation processes have been intensively used for decolourising wastewater (Hai et al., 2007; Verma et al., 2012) and coagulation is often applied to augment biological phosphorous removal in activated sludge processes (Nguyen et al., 2010).
It is well established through the long and large literature that the coagulation process efficiency is highly dependent on hydrophilic and hydrophobic properties (Table 1) of NOM and dissolved organics (Edzwald, 1993; Jiang and Graham, 1996; Ghernaout et al., 2009; Weng et al., 2012; Zhao and Zhang, 2011; Wu et al., 2011). This review aims to discuss the influence of these fundamental characteristics on the coagulation performance. Several and different examples from the pertinent selected references treating coagulation of NOM and dissolved organics are given in the following sections. The main objective here is to find or establish a hydrophilic/hydrophobic ratio to well understand and optimise the coagulation process. In order to explain why the hydrophilic/hydrophobic ratio would be introduced, the following sections discuss coagulation of trace organic contaminants, organics in bio-treated textile wastewater, cyanotoxins, and AOP-pretreated samples. Coagulation diagram and changes during coagulation in NOM reactivity are also treated before discussing the hydrophilic/hydrophobic ratio.
Fraction
Chemical groups
Hydrophobic
Acids
Strong
Humic and fulvic acids, high MW alkyl monocarboxylic and dicarboxylic acids, aromatic acids
Weak
Phenols, tannins, intermediate MW alkyl monocarboxylic and dicarboxylic acids
Bases
Proteins, aromatic amines, high MW alkyl amines
Neutrals
Hydrocarbons, aldehydes, high MW methyl ketones and alkyl alcohols, ethers, furans, pyrrole
Hydrophilic
Acids
Hydroxy acids, sugars, sulfonics, low MW alkyl monocarboxylic and dicarboxylic acids
Bases
Amino acids, purines, pyrimidine, low MW alkyl amines
Neutrals
Polysaccharides, low MW alky alcohols, aldehydes, and ketones
2 Coagulation for trace organic contaminant removal
The term trace organic contaminants refer to contaminants present in water and wastewater at very low concentrations. Types of trace organic contaminants include endocrine disrupting compounds, pharmaceutical and personal care products, and disinfection by-products (DBPs) (Alexander et al., 2012). Even if coagulation, followed by flocculation and sedimentation, has been proved to be effective for bulk NOM removal from water, it has traditionally been found to be inefficient in removing trace organic contaminants (Boyd et al., 2003; Choi et al., 2006; Dempsey and O’Melia, 1984; Bodzek and Dudziak, 2006; Huerta-Fontela et al., 2011; Kim et al., 2007; Le-Minh et al., 2010; Snoeyink and Chen, 1985; Vieno et al., 2006; Sharma, 2012). Advanced water treatment processes, on the other hand, such as adsorption (Bundy et al., 2007; Choi et al., 2006) and reverse osmosis/nanofiltration processes have been found to be more effective in the removal of trace organic contaminants from water (Al-Rifai et al., 2011; Comerton et al., 2008; Nghiem et al., 2004).
Alexander et al. (2012) summarised the advantages and disadvantages of the available techniques for trace organic contaminant removal from water/wastewater. They indicated that all methods listed have of course some advantages as well as some drawbacks, and a universal standalone process applicable for majority of the trace organic contaminants is yet to be developed. The advanced processes are generally more energy intensive and complex in operation than conventional treatment processes. For example, membrane bioreactor presents an excellent removal of significantly hydrophobic trace organics in a single-step compact biological process and on the other hand it shows an inefficient removal of hydrophilic and persistent trace organics requiring a polishing step.
Since coagulation and flocculation are cost-competitive and common methods, it is obvious to investigate ways to improve their performance of dissolved organics removal (Bond et al., 2011). Huerta-Fontela et al. (2011) achieved an effective removal of some pharmaceutical compounds, with the exception of hydrochlorothiazide; this may be the result of a removal phenomenon known as partitioning, given their relative hydrophobicity. During coagulation and flocculation, Suarez et al. (2009) removed some musk compounds such as tonalide, galaxolide, and celestolide from hospital wastewater by 83%, 79%, and 78%, respectively with the high degree of removal possibly attributed to the hydrophobic nature of these compounds (Suarez et al., 2009). Recently, Alexander et al. (2012) carried out an analysis of the removal data for a long list of compounds covered in their review according to their relative hydrophobicity. They showed that although hydrophobicity can explain the high removal of certain compounds, there was no discernable correlation between hydrophobicity and removal, suggesting that hydrophobicity is not the sole factor governing removal by coagulation, even for significantly hydrophobic compounds. This means that the usual method of coagulant selection based on turbidity/suspended solids removal may not always be effective. Moreover, pre-coagulation has been found to enhance the trace organic contaminant removal performance of the advanced treatment processes such as ozonation, GAC adsorption and nanofiltration. They also concluded that the research to date has largely focused on traditional coagulants such as ferric and aluminium coagulants which were not designed for the purpose of removing trace organic contaminants. Consequently, there is the potential to investigate the efficiency of new generation high performance coagulants, such as composite coagulants and inorganic/organic additives, in removing trace organic contaminants (Alexander et al., 2012).
3 Coagulation of organics in bio-treated textile wastewater
Qian et al. (2013) employed a novel hybrid process involving stepwise coagulation and intermediate ozonation in the presence of granular activated carbon (GAC/O3) to remove effluent organic matter (OM) from bio-treated textile wastewater. They evaluated the removal behaviour of effluent OM in different processes, including biodegradability, hydrophobic and hydrophilic property, and apparent molecular weight (MW) distribution. They demonstrated that when the polyaluminium chloride dose of 25 mg L−1 as Al was used in both pre-coagulation (pH 8.0) and post one (pH 5.5), and the ozone dose of 3.1 mg O3 mg−1 COD was applied in GAC/O3 (GAC 10 g L−1) lasting 5 min, the superior removal efficiencies of water quality parameters like turbidity, colour, COD, DOC and UV254 were 95.8%, 97.5%, 88.1%, 68.7% and 90.5%, respectively. They also showed that GAC/O3 not only gave the efficient decolourisation and DOC removal, but also enhanced the treatability of biodegradable and hydrophilic organics of AWM in 1–10 kDa by post-coagulation, likely due to the effective removal of coloured and hydrophobic organics in apparent MW > 1 kDa via pre-coagulation. They proved that the hybrid process applying appropriate operational parameters is an attractive strategy in the wastewater reclamation.
Yan et al. (2007) investigated the transformations of particles, metal elements and NOM in a pilot-scale water treatment plant and found that pre-ozonation and mid-ozonation could oxidise high MW DOC into lower MW DOC and change hydrophobic acid and electrical hydrophilic DOC into hydrophobic base, hydrophobic neutral, and weakly hydrophobic acid, which could improve the removal efficiencies of following coagulation and GAC adsorption (Sam et al., 2010).
4 Cyanotoxins coagulation
Coagulation can be an efficient method for eliminating cyanobacterial cells from water; but soluble cyanotoxins, which are hydrophilic, are not efficiently removed by this method (Huang et al., 2009; Yang et al., 2011; Ma et al., 2012; Pantelić et al., 2013). It is obvious that the efficiency of coagulation process is dependent on pH values and chemical doses (Cañizares et al., 2009; Xiao et al., 2009; Ghernaout et al., 2010). This treatment may, however, cause lysing of cyanobacterial cells and releasing of toxins in water, what presents the major problem in application of coagulation process in removal of cyanotoxins from the water (Ghernaout et al., 2010). Researchers such as Xagoraraki (2007) reported that coagulation has up to 90% reported removals for intracellular microcystin, however, sludge containing toxic cyanobacteria should be isolated from the treatment process as cells contained in sludge can break down rapidly and release dissolved toxin (Chow et al., 1999). Extracellular microcystin-LR is practically not removed by coagulation (2.5–7.9% for raw water and 6.8–11.7% for ozonated water) as reported by Teixeira and Rosa (2007). Furthermore, NOM in surface waters may result in lower cyanobacterial removal efficiencies (Ma and Liu, 2002).
Li et al. (2012) focused their study on algae organic matters (AOM), including intracellular organic matters (IOM) and extracellular organic matters (EOM), which are causing numerous water quality issues, among which is the formation of DBPs and odour & taste (O&T) compounds. They comprehensively characterised physiochemical properties of IOM and EOM of Microcystic aeruginosa under an exponential growth phase (2.01 × 1011 L−1). They also quantified the yields of DBPs during AOM disinfection and O&T-causing compounds. They found that hydrophilic OMs accounted for 86% and 63% of DOC in IOM and EOM, respectively. They also found that MW fractions of IOM in <1, 40–800, and >800 kDa were 27%, 42%, and 31% of DOC, respectively, while EOM primarily contained 1–100 kDa molecules. Besides, a low specific ultraviolet absorbance (SUVA) (0.84 L mg−1 m−1) and the specific fluorescence spectra suggested that AOM (especially IOM) was principally comprised of protein-like substances, instead of humic-like matters. Moreover, the formation potentials of chloroform, chloroacetic acid, and nitrosodimethylamine were 21.46, 68.29 and 0.0096 μg mg C−1 for IOM, and 32.44, 54.58 and 0.0189 μg mg C−1 for EOM, respectively. Further, the dominant O&T compound produced from EOM and IOM were 2-MIB (68.75 ng mg C−1) and b-cyclocitral (367.59 ng mg C−1), respectively.
Takaara et al. (2010) found that the negatively charged hydrophilic substances with a MW higher than 10 kDa have a significant role in coagulation inhibition.
In a full scale drinking water treatment plant, Zamyadi et al. (2012) studied the fate of cyanobacteria and their associated toxins after the addition of coagulant and powdered activated carbon, post clarification, within the clarifier sludge bed, after filtration and final chlorination. They found that elevated cyanobacterial cell numbers (4.7 × 106 cells mL−1) and total microcystins concentrations (up to 10 mg L−1) accumulated in the clarifiers of the treatment plant. They also observed breakthrough of cells and toxins in filtered water. Also, they measured a total microcystins concentration of 2.47 mg L−1 in chlorinated drinking water. They proved that cyanobacterial cells and toxins from environmental bloom samples were more resistant to chlorination than results obtained using laboratory cultured cells and dissolved standard toxins.
We have concluded, in our previous review (Ghernaout et al., 2010), that since dissolved microcystins are efficiently removed by charge neutralisation more than sweep coagulation, enhanced coagulation would be more convenient for their removal (Ghernaout et al., 2010).
As seen above, the membrane integrity of cells is significant for the safe and effective removal of cyanobacterial cells from drinking water sources. Sun et al. (2012) applied cell density counting, cell viability testing, chlorophyll-a determination, extracellular MC-LR monitoring and PCR-DGGE analysis to assess the effects of coagulant dose, shear and floc storage time on the integrity of Microcystis aeruginosa. They showed that all cells were removed without damage to membrane integrity under the optimum coagulation conditions: coagulant dose 15 mg L−1 AlCl3, rapid mix speed 250r min−1, rapid mix time 1 min, slow mix speed 20r min−1, slow mix time 20 min. They found that the coagulant dose and shear did not cause the lysis of cells and ensuing release of MC-LR, but when the flocs were stacked over 6 days, the cells lysed and the MC-LR concentration increased above the background level. Furthermore, the degree of cell breakage without coagulation was higher than the coagulated cells in flocs. Consequently, they concluded that keeping the flocs safely treated or disposed of on time as well as keeping the cyanobacterial cells integrally removed plays an important part in controlling the harm of blooms to drinking water production.
In their review, Srinivasan and Sorial (2011) concluded that conventional treatment processes in water treatment plants, such as coagulation, sedimentation and chlorination have been found to be ineffective for removal of 2-methyl isoborneol and geosmin. They also concluded, however, that powdered activated carbon, ozonation and bio-filtration were proved to be effective in treatment of these two compounds.
Henderson et al. (2008) investigated the coagulation and flotation of four species of algae. They measured zeta potential at optimum removal and they observed that when the zeta potential was reduced to between −8 and +2 mV, removal of algae and associated organic material was optimised, irrespective of the coagulant dose or pH. They concluded that process control using zeta potential is a viable tool for algae removal.
5 Treatability of AOP-pretreated samples by coagulation
As a very good indicator of raw water treatability by coagulation/flocculation, the value of SUVA is used for providing information about the distribution of NOM as hydrophobic/hydrophilic, low/high MW and low/high charge density properties (Chu et al., 2011; Tubić et al., 2011; Ziylan and Ince, 2013; Bose and Reckhow, 2007; Shi et al., 2007; Choi et al., 2008; Kim and Kang, 2008). For example, as reported by Ziylan and Ince (2013), a low value such as 2.16 L mg−1 m−1 [see Ziylan and Ince (2013)’s Table 2 which presents properties of the influent water in water treatment plant (WTP) after aeration and [Edzwald and Tobiason (1999)’s Table 3 for guidelines on nature of NOM removal and expected DOC removals] for the raw water in WTP it shows that NOM is composed of a mixture of aquatic humics and other components that are mostly of hydrophilic and low MW character, 25% or a little greater of which is expected to be eliminated as DOC by coagulation with alum and ferric, respectively (Edzwald and Tobiason, 1999).
Parameter
Value
pH
7.16
Alkalinity (mg L−1 CaCO3)
99.0
Hardness (mg L−1 CaCO3)
91.0
Turbidity (NTU)
3.71
Conductivity (μS cm−1)
235.8
TDS (mg L−1)
457.0
TOC (mg L−1)
5.22
DOC (mg L−1)
4.64
UV254 (cm−1)
0.10
UV436 (cm−1)
0.003
SUVAa (L mg−1 m−1)
2.16
Fluoride (μg L−1)
66.70
Bromide (μg L−1)
410.0
Chloride (mg L−1)
23.23
NH3-N (mg L−1)
0.055
NO3 (mg L−1)
1.46
(mg L−1)
31.99
Total Fe (mg L−1)
0.037
SUVA
Composition
Coagulation
DOC removals
∼4 or greater
Mostly aquatic humics, high hydropphobicity, high MW
NOM controls, good DOC removals
>50% for alum, little greater for ferric
2–4
Mixture of aquatic humics and other NOM, mixture of hydrophobic and hydrophilic NOM, mixture of MWs
NOM influences, DOC removals should be fair to good
25–50% for alum, little greater for ferric
<2
Mostly non-humics, low hydrophobicity, low MW
NOM has little influence, poor DOC removals
<25% for alum, little greater for ferric
Ziylan and Ince (2013) found that the selected pre-treatment operations led to significant enhancements in the expected removal of DOC by coagulation as depicted in Fig. 1. The obtained maximum efficiency at the coagulation basin of the WTP at the same conditions but with no pre-treatment was 34% with alum and 41% with FeSO4 (slightly higher than predicted); whereas values as high as 63–65% were attained by pre-treatment of the influent with O3/UV/ultrasound (US) or O3/US/Fe2+ prior to coagulation. They noted that a higher efficiency of coagulation in samples pretreated with O3/US/Fe2+ (than those coagulated with alum) is due to a larger adsorptive capacity of Fe(OH)3(s) (than Al(OH)3(s)) as was also reported in the literature (Julien et al., 1994). They also noted that coagulation without pretreatment (regardless of the coagulant type and dosage) was unable to remove diclofenac-Na (less than 0.5%), whereas complete removal was achieved when it followed an advanced pretreatment option such as P4, P5 and P6 (Fig. 1).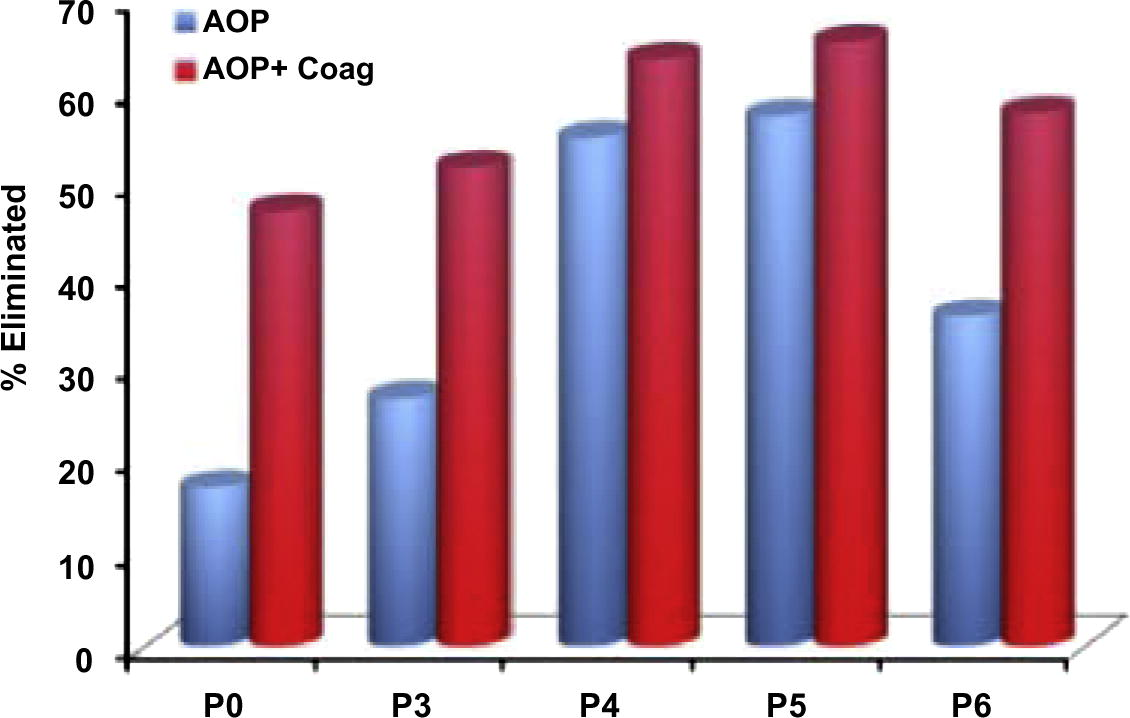
The impact of AOP-pretreatment on the efficiency of DOC elimination by coagulation. P0 = O3; P3 = O3/US; P4 = O3/UV/US; P5 = O3/US/FeSO4; P6 = US/UV (Ziylan and Ince, 2013).
Walsh et al. (2009) worked on three source waters which had high SUVA values (i.e. >4.0 L mg−1 m−1) indicating the presence of primarily aquatic humic substances with high hydrophobicity and high MW compounds. It is well known that the removal of DOC via coagulation processes is generally expected for source water with SUVA values greater than 4.0 L mg−1 m−1 (Edzwald, 1993; Edzwald and Tobiason, 1999). Teixeira et al. (2010) reported that the specific coagulant demand was severely affected by NOM hydrophobicity: hydrophobic NOM (with a SUVA above 4 L mg−1 m−1) requiring ca. triple of hydrophilic NOM (SUVA below 3 L mg−1 m−1), i.e. 0.7 vs. 0.2–0.3 mg Al2O3 mg DOC−1.
An interesting approach was presented by Zhang et al. (2008) who explored the synergistic combination of coagulation with biological processes for drinking water treatment on the basis of on-site biofilters and jar tests. Their experimental results indicated that biological processes can result in a significant change of zeta potential of water flow, making the zeta potential of effluent less negative than that of raw water. Their observation of biofilm morphology with scanning electronic microscope showed that the biofilm is in the shape of a cobweb and can hold a large number of turbidity particles. Zhang et al. (2008) proved that the combination of biotreatment with coagulation can enhance process efficiency and save coagulant significantly. However, they suggested that further explorations are needed to determine the best support medium for biofilm growth and the best combination of coagulation with bio-treatment in different situations. However, Bond et al. (2009) concluded that the biological treatment was effective in removing amino acids but also moderately increased the HAA formation potential of hydrophilic compounds.
Chang et al. (2001) investigated the MW distribution and chemical composition of precursors and their relationship with DBPs. They found that most of the OMs responsible for the major DBP precursors in the Pan-Hsin water are small compounds with a MW less than 1 kDa. They also found that the hydrophobic acids display the greatest ability to produce DBP. They concluded that an effective removal of small molecules or hydrophobic acid organics prior to disinfection process will significantly reduce the DBP concentration in the finished water. They also concluded that although the coagulation process is effective in removing large organic precursors and the removal efficiencies of CHCl3 formation potential and organic carbon increase proportionally to the MW of the precursors, the conventional treatment methods have limited efficiency in eliminating small precursors. The same conclusion was reached by Chiang et al. (2002).
Badawy et al. (2012) indicated that using pre-ozonation/enhanced coagulation/activated carbon filtration treatment train appears to be the most effective method for reducing DBP precursors in drinking water treatment. However, Can and Gurol (2003) confirmed that ozonation caused almost an immediate formation of formaldehyde, which reached a peak value, and then started to decrease with continued ozonation. Furthermore, ozonation of aqueous fulvic acid produced higher concentrations of formaldehyde compared to other types of humic material. More importantly, formaldehyde formation was suppressed by high bicarbonate levels, and enhanced at higher pH and formaldehyde accumulation was more dramatic at low ozone dosages (Can and Gurol, 2003). Similar conclusions were obtained by several authors such as Van Breemen et al. (1979), Amy et al. (1991), Schechter and Singer (1995), and Bose and Reckhow (2007). For Chen et al. (2008), the pre-chlorination and filtration process had a negative effect on DBP or precursor removal.
6 Changes during coagulation in NOM reactivity towards DBPs formation
Tubić et al. (2013) investigated the role of individual NOM fractions on changes in THM and HAA formation during coagulation with iron chloride (FeCl3) and a combination of polyaluminium chloride and iron chloride (FeCl3/polyaluminium chloride). Based on their hydrophobicity, these authors fractionated into four fractions the dissolved organic carbon (DOC) in the raw water and after coagulation. Their fractionation showed that most of the DOC (68%) in the raw water comes from the fulvic acid fraction, yielding 41% of the total THM precursors and 21% of the total HAA precursors. Moreover, both coagulants remove the humic acid fraction, but result in different changes to the reactivity of the remaining NOM fractions towards THM and HAA formation, indicating that coagulation occurs by different pathways, depending upon the type of coagulant used. In particular, significant changes in the reactivities of the hydrophilic acidic and non-acidic fractions were observed (Tubić et al., 2013).
Another interesting work was performed by Diemert et al. (2013) since they investigated the link between NOM characteristics and halo-benzoquinones (HBQs) – which have been previously detected as DBPs in chlorinated drinking water – formation during bench-scale coagulation of raw water. Three source waters were subjected to jar testing using alum followed by chlorination. They identified one HBQ, 2,6-dichloro-(1,4) benzoquinone (2,6-DCBQ) in all waters after chlorination, and appeared to decrease with increased applied alum dose. They demonstrated that 2,6-DCBQ exhibited high correlations with some humic NOM indicators: humic substance concentration (in two waters), UV absorbance at 254 nm, UV absorbance at 254 nm of the humic peak, and SUVA of humics. With their data pooled from the three waters, the biopolymer fraction of NOM was most strongly correlated with 2,6-DCBQ formation (R2 = 0.78, p < 0.001); they attributed this to the co-removal of biopolymers with HBQ precursors during coagulation. They concluded that their results indicate that coagulation processes can be effective for reduction, but not elimination, of HBQ precursors.
On the other hand, Lou et al. (2012)affirmed that the disadvantage of using some polymeric salts is that the preformed species are stable and cannot be further hydrolysed during coagulation, and may not be efficient in removing a highly hydrophobic NOM (Lou et al., 2012).
7 Coagulation diagram
Researchers such as Kim et al. (2001), Rigobello et al. (2011), and Xiao et al. (2013) reminded us that a coagulation diagram still exits. A coagulation diagram, as a definition, is a figure in which coagulant metal concentration-pH coordinates are used to outline the regions of different coagulation mechanisms. In order to select the appropriate coagulant product and coagulant dosage for a given application and to interpret coagulation results from both jar tests and plant studies, the coagulation diagram was first used by Amirtharajah and Mills (1982).
Indeed Xiao et al. (2013) delineated removal areas of polar compounds, perfluorooctane sulphonate (PFOS) and perfluorooctanoate (PFOA) which are persistent organic pollutants that have been found to be ubiquitous in the environment, on a coagulation diagram (Fig. 2). Xiao et al. (2013) considered some key variables such as solution pH, coagulant dosage, coagulants (alum and ferric chloride), NOM, initial turbidity, and flocculation time. Their jar-test results show that conventional coagulation (alum dosage of 10–60 mg L−1 and final pH of 6.5–8.0) removed ⩽20% of PFOS and PFOA which tended to be removed better by enhanced coagulation at higher coagulant dosages (>60 mg L−1) and (thus) lower final pH (4.5–6.5). In their coagulation diagram (Fig. 2), Xiao et al. (2013) defined the coagulant dosage and solution pH for PFOS/PFOA removal. Their results suggest that the primary PFOS/PFOA removal mechanism is adsorption to fine Al hydroxide flocs freshly formed during the initial stage of coagulation; and increasing flocculation time from 2 to 90 min could not further improve PFOS and PFOA removals. Xiao et al. (2013) concluded that the removal of PFOS/PFOA by coagulation appears to be controlled by both electrostatic and hydrophobic effects: indeed, (1) the coagulant dosage and solution pH are two dominant factors affecting the removal rate of PFOS/PFOA and increasing coagulant dosage can increase the available surface area for adsorption/enmeshment; (2) and the resulting decrease in pH (from the increase in coagulant dosage) can increase the number of protonated adsorption sites. Furthermore, Xiao et al. (2013) obtained different fates of PFOS/PFOA and turbidity particles during coagulation indicating that these chemicals were not co-removed with turbidity particles and require a different removal approach than what is required for reducing turbidity.![Average removals of PFOS and PFOA presented on an alum coagulation diagram (pAl = log[Al3+]; Al13: Al 13 O 4 ( OH ) 24 7 + (Xiao et al., 2013).](/content/185/2014/26/3/img/10.1016_j.jksus.2013.09.005-fig2.png)
Average removals of PFOS and PFOA presented on an alum coagulation diagram (pAl = log[Al3+]; Al13:
(Xiao et al., 2013).
8 The hydrophilic/hydrophobic ratio
In this research in the recent literature, the first authors who mentioned the hydrophilic/hydrophobic ratio were Chiang et al. (2002) and Yang et al. (2013). They briefly mentioned the hydrophilic/hydrophobic ratio or distributions, respectively, as a characteristic of OM in finished water in one place of their research paper. Lyon et al. (2012) mentioned that some workers have observed an increase in the ratio of hydrophilic to hydrophobic OM in filtered surface water after UV irradiation.
For Gang et al. (2005), the relative distribution of HAAs and THMs is believed to be influenced by the hydrophobic/hydrophilic distribution of NOM in the waters being chlorinated. They affirmed that alum coagulation has been reported to remove more hydrophobic organic carbon than hydrophilic organic carbon compounds, resulting in a shift in the hydrophobic/hydrophilic (i.e., the inverse of the hydrophilic/hydrophobic ratio) distribution of NOM, and hence the relative concentrations of HAAs and THMs upon chlorination (White et al., 1997; Lee and Westerhoff, 2006; Shi et al., 2007).
On the other hand, under the pH conditions of most natural waters, humic substances occur as negatively charged macromolecules due to the presence of functional groups, i.e. carboxyl and phenol (Edwards and Amirtharajah, 1985). It is well established that pH level affects the configuration of the molecule (i.e., expanded vs. coiled), as well as charge density (Rigobello et al. (2011)). Generally, the fulvic acids have a higher acidity and correspondingly higher charge density than humic acids derived from the same source (Sharp et al., 2006). As proved by some authors (Kaleta and Elektorowicz, 2009), both pH and hardness have a significant effect on the effectiveness of humic substances removal from aqueous solutions by the coagulation process. They confirmed that the coagulation was mostly effective between pH values of 5 and 6. However, when pH is comprised between 7 and 9, the effectiveness of humic substances removal steadily decreased, and increased slightly again at pH 10.
Furthermore, there is no obvious trend in chemical fractionation associated with the type of water system since each water system may have multiple sources of natural dissolved OM and different organic carbon fraction contents (Rigobello et al. (2011)). For water systems containing terrestrial materials as a major dissolved OM input, they generally tend to have higher hydrophobic fraction content or humic substances. The hydrophobic content typically varies between 50% and 60% of the total DOC for highly coloured surface waters (Sharp et al., 2006). On the other hand, low hydrophobic content has been observed in the South Platte River in Colorado and Clinton Lake in Kansas, composing 34% and 33% of the dissolved OM, respectively (Rigobello et al. (2011)). Consequently, let us consider that a medium value of the hydrophobic fraction is around 45% (the arithmetic medium of 50%, 60%, 34% and 33%) and the hydrophilic fraction represents the remaining portion [some authors such as Kim (2009) and Kim and Kang (2008) reported another fraction, i.e. transphilic]. In other words, considering that the dissolved OM is mainly composed of hydrophilic fraction and hydrophobic fraction, the hydrophilic/hydrophobic ratio may be calculated as follows:
The literature has mainly proposed two mechanisms to explain the removal of humic substances by coagulation: (1) charge neutralisation/precipitation at acidic pH, and (2) adsorption and/or sweep coagulation in a hydroxide precipitate at alkaline pH (Kim et al., 2001; Pernitsky and Edzwald, 2006; Duan and Gregory, 2003; Siéliéchi et al., 2008).
Rigobello et al. (2011) concluded that the higher the percentage of aquatic fulvic acids in water samples containing larger numbers of negatively charged groups (oxygenated groups) and aliphatic carbon atoms, the stronger the influence on coagulation. In order to achieve the same degree of colour removal, the water samples with smaller apparent molecular sizes required higher doses of both aluminium sulphate and ferric chloride. Chow et al. (2009) confirmed that polysaccharides and their derivatives are recalcitrant to removal with alum coagulation.
Following the topics of this review, a very interesting work was done by Xing et al. (2012). They used reverse phase high performance liquid chromatography (RPHPLC) as a rapid assessment of the hydrophobicity/hydrophilicity of NOM. They found that the reduction of total RPHPLC peak area correlated well with DOC and UV absorbance at 254 nm (UV254) removal efficiency. After resolving the RPHPLC profile using a peak fitting technique, Xing et al. (2012) affirmed that the ratio between hydrophobic and hydrophilic peak area can be used to quantify the treatability of NOM (particularly DOC removal). Further, their statistical analysis grouped the resolved peaks into three groups (two groups of hydrophilic and one group of hydrophobic) and established an expression to link peak area with UV254 and DOC removal. They further compared their characterisation results by the traditional resin fractionation technique using DAX-8 and XAD-4 resins (combined). They confirmed that despite the definition that hydrophobic and hydrophilic components measured by both methods could be different, both methods confirmed that the hydrophilic fraction was recalcitrant after coagulation and is of small MW.
Uyguner et al. (2007) concluded that considering that enhanced coagulation generally removes large molecular size components expressing different hydrophobicity/hydrophilicity characteristics, the treatment efficiencies are also expected to be altered. Xiao et al. (2010) concluded that enhanced coagulation showed a strong ability in controlling DBPs formation (in comparison to conventional coagulation), but the remaining OM after enhanced coagulation still contributed a considerable amount of halogenated DBPs when a high chlorine dosage was applied. Their results imply that the fraction of NOM that is not readily removed by (enhanced) coagulation may have low reactivity towards chlorine.
At this stage of this review, the following question arises: Why the hydrophilic and hydrophobic characteristics are important in dissolved organics coagulation process? The hydrophilic adjective describes the character of a molecule or atomic group that has an affinity for water and the hydrophobic adjective describes the character of a molecule or atomic group that is insoluble in water, or resistant to wetting or hydration. The main difficulty of hydrophilic organics removal is their passage from the dissolved/soluble state to the insoluble state. On the other hand, colloids are categorised as hydrophobic or hydrophilic. Obviously, hydrophobic colloids do not react with water and most natural clays are hydrophobic, and hydrophilic colloids react with water and organics causing colour are hydrophilic. As discussed previously, hydrophilic colloids may chemically react with coagulants used in the treatment process but they require more coagulant than hydrophobic colloids (Al-Malack et al., 1999; Uyak and Toroz, 2006; Ghernaout et al., 2009; Zhan et al., 2010; Matilainen et al., 2010).
For Bond et al. (2010), the principal characterisation is related to hydrophobicity where water is fractionated into hydrophobic and hydrophilic components by use of resins. While there is a perception that hydrophobic NOM is the major source of DBP precursors, previous research has shown how other NOM groups also contain significant levels of precursors (Bond et al., 2010). For example, in NOM from the South Platte River (USA), THM formation potential for the hydrophobic acid and hydrophilic acid fractions were comparable, at 46 and 35 μg CHCl3 mg C−1, respectively (Bond et al., 2010). Moreover, there is evidence that since hydrophilic NOM is less treatable by coagulation, it is this group which can determine post-coagulation NOM levels (Yoon et al., 2009; Bond et al., 2010), and in turn final DBP formation, at least where chlorination is the final treatment step. Indeed, Lu et al. (2009) indicated that aromatic moieties are responsible for DBPs formation for both hydrophobic and hydrophilic DOM fractions.
If we return to Eq. (1) for the final state, i.e., after conventional water treatment, and considering that the hydrophilic fraction remains constant and the hydrophobic fraction is reduced to 45%, the ratio r would be:
The ratio r increases from 11/9 to 20/9 because the hydrophilic fraction remains constant and the hydrophobic fraction is reduced by both its removal by adsorption/enmeshment in coagulation process and its transformation into hydrophilic fragments by charge neutralisation – if pre-oxidised, the hydrophobic OMs could be converted to hydrophilic structure (Swietlik, 2004; Iriarte-Velasco et al., 2007) and OM with higher MW oxidised to lower MW material (Li et al., 2009). The charge neutralisation may result in organic molecules fragmentation due the metal cationic species reaction forming metal–organic complexes which are more soluble in water (Jekel, 1986; Gregor et al., 1997; Lu et al., 1999; Ghernaout et al., 2009) before their transformation into insoluble complexes (Van Benschoten and Edzwald, 1990; Huang and Shiu, 1996; Vrijenhoek et al., 1998; Shi et al., 2007) which are removed by adsorption and sweep coagulation (Jung et al., 2005; Siéliéchi et al., 2008; Yan et al., 2008; Liu et al., 2009; Xiao et al., 2009; Ghernaout and Ghernaout, 2012). In other words, charge neutralisation mechanism as a chemical reaction would not be totally achieved.
Furthermore, pH also affects the physicochemical properties of humic acid in water (Yang et al., 2010). Even if humic acids may be considered less hydrophilic and more hydrophobic (fulvic acids are more hydrophilic), the protonation at lower pH (i.e., pH = 5–6) makes humic acids easier to be charge-neutralised and destabilised (Xu et al., 2010; Yan et al., 2008; Rizzo et al., 2008; Zhang et al., 2008). Moreover, pH could affect the balance between the reactions of organic functional groups with hydrogen ions and Al hydrolysis products (Yang et al., 2010). In other words, at lower pH, hydrogen ions could out compete with the metal hydrolysis products for organic ligands; consequently, the amount of unsatisfied organic ligands is decreased and then humic acids could be removed more efficiently by coagulation process (Yang et al., 2010).
Kazpard et al. (2006) suggested that the functional groups involved in the aggregation of humic substances change with pH and coagulant concentration, i.e., at low aluminium concentration, hydrolysed aluminium species bind selectively to carboxylic groups at pH 6, and to phenolic moieties at pH 8.
Wandruszka et al. (1997) have shown that aqueous solutions of soil humic acids respond to the addition of cations by forming intramolecular and intermolecular aggregates, compact structures with relatively hydrophobic interiors and hydrophilic surfaces. As explained by Anđelković et al. (2004), when cations are added humic acids macromolecules tend to shrink or contract; mutual repulsion among negatively charged carboxyl groups is minimised and they fold forming intramolecular and intermolecular aggregates. This is may be due to two mechanisms: charge neutralisation and functional group bridging. Further, functional group bridging enhances this effect, especially with multivalent cations, by drawing together various groups on the humic acid chain (Anđelković et al., 2004).
The main question which may be addressed here is what should be the configuration of the conventional water treatment to achieve r (see Eq. (2)) equals 0? In 1986, Rice and Gomez-Taylor (1986) concluded that to minimise the presence of oxidation by-products in drinking water, the concentrations of oxidisable organic/inorganic impurities should be lowered before any oxidising agent is added. The same conclusion was achieved by Ji et al. (2008).
On the other hand, we must keep in mind that, as discussed by Bond et al. (2009), there is conflicting literature regarding which NOM types are predominant as precursors of THMs and HAAs. Some authors reported that hydrophilic/polar NOM is more prevalent in the formation of HAAs than THMs (Bond et al., 2009), whereas others implicated hydrophobic/non-polar NOM (Liang and Singer, 2003; Kim and Yu, 2005).
9 Conclusions
NOM in raw water can contribute in many ways to the poor quality of drinking water, including the formation of DBPs such as THM and HAA during disinfection. Consequently, the NOM removal in conventional water treatment by coagulation process is a vital preoccupation for water treatment engineers. Based on the literature reviewed, the following points may be drawn:
-
If we consider the hydrophilic/hydrophobic ratio for the raw water and also for the treated water, i.e. after coagulation and/or pre-chlorination: (1) In the case of coagulation without pre-chlorination and if we admit that the hydrophilic fraction is less reduced (i.e., it remains approximately constant) than the hydrophobic one (which decreases since it is removed by charge neutralisation/sweep coagulation), the hydrophilic/hydrophobic ratio may increase. (2) In the case of pre-chlorination followed by coagulation and if we suppose that the hydrophobic fraction is transformed into hydrophilic fragments by pre-oxidation, the hydrophilic fraction increases due to the hydrophobic decomposition before coagulation, the hydrophilic/hydrophobic ratio may greatly increase more than in the case of coagulation alone (because the hydrophobic fraction is not removed by coagulation as in the case of coagulation alone but it remains in solution as more dissolved OM, i.e. hydrophilic).
-
As it was established by the literature, enhanced coagulation is well-convenient for the case of coagulation without pre-chlorination. Enhanced coagulation acts on both fractions hydrophilic and hydrophobic by charge neutralisation more than sweep coagulation (the H+ action on C⚌C and C⚌O bonds is more rapid than the action of Al3+/Fe3+), the hydrophilic fraction is more reduced by charge neutralisation more than sweep coagulation comparatively to the case of coagulation alone and the hydrophobic fraction is also well removed by both charge neutralisation (the hydrophobic fraction is more macromolecular than the hydrophilic fraction, the C⚌C and C⚌O bonds are easily exposed to the H+ attack in the case of the hydrophilic fraction) and sweep coagulation (especially when the metal salt dosage is increased) comparatively to the case of coagulation alone.
-
Representing NOM concentration in the raw water, the hydrophilic/hydrophobic ratio is well reduced in the case of enhanced coagulation more than in the case of coagulation alone and also more than in the case of pre-chlorination followed by coagulation.
-
Determining the exact numerical values of the hydrophilic/hydrophobic ratio for raw water and treated water at different stages of the treatment processes in a WTP, as for the DCO/DBO5 ratio in the case of wastewater treatment, would help on focusing more on OM control and removal.
References
- Chemical coagulation-based processes for trace organic contaminant removal: current state and future potential. J. Environ. Manage.. 2012;111:195-207.
- [Google Scholar]
- Coagulation of polymeric wastewater discharged by a chemical factory. Water Res.. 1999;33:521-529.
- [Google Scholar]
- Removal of pharmaceuticals and endocrine disrupting compounds in a water recycling process using reverse osmosis systems. Sep. Purif. Technol.. 2011;77:60-67.
- [Google Scholar]
- Rapid-mix design for mechanisms of alum coagulation. J. Am. Water Works Assoc.. 1982;74(4):210-216.
- [Google Scholar]
- The effects of ozonation and activated carbon adsorption on trihalomethane speciation. Water Res.. 1991;25:191-202.
- [Google Scholar]
- Destabilization and aggregation of aqueous humic acids solution by metal ions. Phys. Chem. Technol.. 2004;3:79-85.
- [Google Scholar]
- Minimization of the formation of disinfection by-products. Chemosphere. 2012;89:235-240.
- [Google Scholar]
- Tracking natural organic matter (NOM) in a drinking water treatment plant using fluorescence excitation-emission matrices and PARAFAC. Water Res.. 2011;45:797-809.
- [Google Scholar]
- Elimination of steroidal sex hormones by conventional water treatment and membrane processes. Desalination. 2006;198:24-32.
- [Google Scholar]
- Chemical and biological oxidation of NOM surrogates and effect on HAA formation. Water Res.. 2009;43:2615-2622.
- [Google Scholar]
- Disinfection by-product formation of natural organic matter surrogates and treatment by coagulation, MIEX(R) and nanofiltration. Water Res.. 2010;44:1645-1653.
- [Google Scholar]
- The effect of ozonation on natural organic matter removal by alum coagulation. Water Res.. 2007;41:1516-1524.
- [Google Scholar]
- Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario. Can. Sci. Total Environ.. 2003;311:135-149.
- [Google Scholar]
- Removal of pharmaceuticals and related compounds by a bench-scale drinking water treatment system. J. Water Supply: Res. Technol. – AQUA. 2007;56:105-115.
- [Google Scholar]
- Formaldehyde formation during ozonation of drinking water. Ozone: Sci. Eng.. 2003;25:41-51.
- [Google Scholar]
- The pH as a key parameter in the choice between coagulation and electrocoagulation for the treatment of wastewaters. J. Hazard. Mater.. 2009;163(2009):158-164.
- [Google Scholar]
- Characteristics of organic precursors and their relationship with disinfection by-products. Chemosphere. 2001;44:1231-1236.
- [Google Scholar]
- Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere. 2002;48:59-68.
- [Google Scholar]
- Disinfection by-products and their precursors in a water treatment plant in North China: seasonal changes and fraction analysis. Sci. Total Environ.. 2008;397:140-147.
- [Google Scholar]
- Coagulation mechanisms of iron salt and salicylic acid. Sep. Sci. Technol.. 2002;37:2113-2127.
- [Google Scholar]
- Water coagulation using electrostatic patch coagulation (EPC) mechanism. Drying Technol.. 2010;28:850-857.
- [Google Scholar]
- NOM characteristics and treat abilities of ozonation processes. Chemosphere. 2002;46:929-936.
- [Google Scholar]
- Removal efficiencies of endocrine disrupting chemicals by coagulation-flocculation, ozonation, powdered granular activated carbon adsorption, and chlorination. Korean J. Chem. Eng.. 2006;23:399-408.
- [Google Scholar]
- Role of hydrophobic natural organic matter flocs on the fouling in coagulation-membrane processes. Sep. Purif. Technol.. 2008;62:529-534.
- [Google Scholar]
- The impact of conventional water treatment processes on cells of the cyanobacterium Microcystis aeruginosa. Water Res.. 1999;33:3253-3262.
- [Google Scholar]
- Optimised coagulation using aluminium sulfate for the removal of dissolved organic carbon. Desalination. 2009;245:120-134.
- [Google Scholar]
- Models for predicting disinfection byproduct (DBP) formation in drinking waters: a chronological review. Sci. Total Environ.. 2009;407:4189-4206.
- [Google Scholar]
- Impacts of drinking water pretreatments on the formation of nitrogenous disinfection by-products. Bioresour. Technol.. 2011;102:11161-11166.
- [Google Scholar]
- The rejection of endocrine disrupting and pharmaceutically active compounds by NF and RO membranes as a function of compound and water matrix properties. J. Membr. Sci.. 2008;313:323-335.
- [Google Scholar]
- Removal of naturally occurring compounds by coagulation and sedimentation. Crit. Rev. Environ. Control. 1984;14:311-331.
- [Google Scholar]
- Removal of halo-benzoquinone (emerging disinfection by-product) precursor material from three surface waters using coagulation. Water Res.. 2013;47:1773-1782.
- [Google Scholar]
- Coagulation by hydrolysing metal salts. Adv. Colloid Interface Sci.. 2003;100–102:475-502.
- [Google Scholar]
- Coagulation in drinking water treatment: particles, organics and coagulants. Water Sci. Technol.. 1993;27:21-35.
- [Google Scholar]
- Enhanced coagulation: US requirements and a broader view. Water Sci. Technol.. 1999;40:63-70.
- [Google Scholar]
- Effects of alum coagulation on speciation and distribution of trihalomethanes (THMs) and haloacetic acids (HAAs) J. Environ. Sci. Health. 2005;40:521-534.
- [Google Scholar]
- Sweep flocculation as a second form of charge neutralisation – a review. Desalin. Water Treat.. 2012;44:15-28.
- [Google Scholar]
- Natural organic matter removal and enhanced coagulation as a link between coagulation and electrocoagulation. Desalin. Water Treat.. 2009;2:203-222.
- [Google Scholar]
- Algae and cyanotoxins removal by coagulation/flocculation: a review. Desalin. Water Treat.. 2010;20:133-143.
- [Google Scholar]
- Embodying the chemical water treatment in the green chemistry – a review. Desalination. 2011;271:1-10.
- [Google Scholar]
- Optimising natural organic matter removal from low turbidity waters by controlled pH adjustment of aluminium coagulation. Water Res.. 1997;31:2949-2958.
- [Google Scholar]
- Hybrid treatment systems for dye waste water. Crit. Rev. Environ. Sci. Technol.. 2007;37:315-377.
- [Google Scholar]
- Successful removal of algae through the control of zeta potential. Sep. Sci. Technol.. 2008;43:1653-1666.
- [Google Scholar]
- Interactions between alum and organics in coagulation. Colloids Surf., A. 1996;113:155-163.
- [Google Scholar]
- A comparison of the role of two blue–green algae in THM and HAA formation. Water Res.. 2009;43:3009-3018.
- [Google Scholar]
- Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res.. 2011;45:1432-1442.
- [Google Scholar]
- Removal and structural changes in natural organic matter in a Spanish water treatment plant using nascent chlorine. Sep. Purif. Technol.. 2007;57:152-160.
- [Google Scholar]
- Interactions of humic acids and aluminum salts in the flocculation process. Water Res.. 1986;20:1535-1542.
- [Google Scholar]
- Removal of disinfection by-products precursors by polyaluminum chloride coagulation coupled with chlorination. Sep. Purif. Technol.. 2008;62:464-469.
- [Google Scholar]
- Development of coagulation theory and pre-polymerized coagulants for water treatment. Sep. Purif. Methods. 2001;30:127-141.
- [Google Scholar]
- Enhanced coagulation using Al/Fe(IIII) coagulants: Effect of coagulant chemistry on the removal of colour-causing NOM. Environ. Technol.. 1996;17:937-950.
- [Google Scholar]
- Comparative coagulant demand of polyferric chloride and ferric chloride for the removal of humic acid. Sep. Sci. Technol.. 2009;44:386-397.
- [Google Scholar]
- Removal of natural organic matter from potential drinking water sources by combined coagulation and adsorption using carbon nanomaterials. Sep. Purif. Technol.. 2012;95:64-72.
- [Google Scholar]
- Comparison of organic compounds removal by coagulation-flocculation and by adsorption onto performed hydroxide floc. Water Res.. 1994;28:2567-2574.
- [Google Scholar]
- Coagulation of humic substances and dissolved organic matter with a ferric salt: an electron energy loss spectroscopy investigation. Water Res.. 2005;39:3849-3862.
- [Google Scholar]
- Removal of humic substances from aqueous solutions by the coagulation process. Environ. Technol.. 2009;30:119-127.
- [Google Scholar]
- Fate of coagulant species and conformational effects during the aggregation of a model of a humic substance with Al13 polycations. Water Res.. 2006;40:1965-1974.
- [Google Scholar]
- Characterization of natural organic matter in conventional water treatment processes for selection of treatment processes focused on DBPs control. Water Res.. 2005;39:4779-4789.
- [Google Scholar]
- Effects of pH and dosage on pollutant removal and floc structure during coagulation. Microchem. J.. 2001;68:197-203.
- [Google Scholar]
- Occurrence and removal of pharmaceuticals and endocrine disruptors in south Korean surface, drinking, and waste waters. Water Res.. 2007;41:1013-1021.
- [Google Scholar]
- Minimizing residual aluminum concentration in treated water by tailoring properties of polyaluminum coagulants. Water Res.. 2013;47:2075-2084.
- [Google Scholar]
- Formation of N-nitrosamines from chlorination and chloramination of molecular weight fractions of natural organic matter. Water Res.. 2013;47:535-546.
- [Google Scholar]
- Dissolved organic nitrogen removal during water treatment by aluminum sulfate and cationic polymer coagulation. Water Res.. 2006;40:3767-3774.
- [Google Scholar]
- Fate of antibiotics during municipal water recycling treatment processes. Water Res.. 2010;44:4295-4323.
- [Google Scholar]
- Characterization of floc size, strength and structure under various coagulation mechanisms. Powder Technol.. 2006;168:104-110.
- [Google Scholar]
- Impact of preozonation on the performance of coagulated flocs. Chemosphere. 2009;75:187-192.
- [Google Scholar]
- Characterization of intracellular AND extracellular algae organic matters (AOM) of Microcystic aeruginosa and formation of AOM-associated disinfection byproducts and odor AND taste compounds. Water Res.. 2012;46:1233-1240.
- [Google Scholar]
- Factors influencing the formation and relative distribution of haloacetic acids and trihalomethanes in drinking water. Environ. Sci. Technol.. 2003;37:2920-2928.
- [Google Scholar]
- Coagulation of humic acid by PACl with high content of Al13: the role of aluminum speciation. Sep. Purif. Technol.. 2009;70:225-230.
- [Google Scholar]
- Removal of natural organic matter for controlling disinfection by-products formation by enhanced coagulation: a case study. Sep. Purif. Technol.. 2012;84:41-45.
- [Google Scholar]
- Coagulation optimization for low temperature and low turbidity source water using combined coagulants: a case study. Desalin. Water Treat.. 2012;46:107-114.
- [Google Scholar]
- Spectroscopic study of aluminium speciation in removing humic substances by Al coagulation. Water Res.. 1999;33:3271-3280.
- [Google Scholar]
- Evaluation of disinfection by-products formation during chlorination and chloramination of dissolved natural organic matter fractions isolated from a filtered river water. J. Hazard. Mater.. 2009;162:140-145.
- [Google Scholar]
- The effect of inorganic precursors on disinfection byproduct formation during UV-chlorine/chloramine drinking water treatment. Water Res.. 2012;46:4653-4664.
- [Google Scholar]
- Effectiveness and mechanism of potassium ferrate (VI) preoxidation for algae removal by coagulation. Water Res.. 2002;26:871-878.
- [Google Scholar]
- Effects and mechanisms of pre-chlorination on Microcystis aeruginosa removal by alum coagulation: significance of the released intracellular organic matter. Sep. Purif. Technol.. 2012;86:19-25.
- [Google Scholar]
- Impact of enhanced coagulation ways on flocs properties and membrane fouling: increasing dosage and applying new composite coagulant. Desalination. 2013;314:161-168.
- [Google Scholar]
- Natural organic matter removal by coagulation during drinking water treatment: a review. Adv. Colloid Interface Sci.. 2010;159:189-197.
- [Google Scholar]
- Estrogenic hormone removal from wastewater using NF/RO membranes. J. Membr. Sci.. 2004;242:37-45.
- [Google Scholar]
- A new combined inorganic–organic flocculant (CIOF) as a performance enhancer for aerated submerged membrane bioreactor. Sep. Purif. Technol.. 2010;75:204-209.
- [Google Scholar]
- Cyanotoxins: characteristics, production and degradation routes in drinking water treatment with reference to the situation in Serbia. Chemosphere. 2013;91:421-441.
- [Google Scholar]
- Selection of alum and polyaluminum coagulants: principles and applications. J. Water Supply Res. Technol. AQUA. 2006;55:121-141.
- [Google Scholar]
- Removal characteristics of organics in bio-treated textile wastewater reclamation by a stepwise coagulation and intermediate GAC/O3 oxidation process. Chem. Eng. J.. 2013;214:112-118.
- [Google Scholar]
- Occurence of by-products of strong oxidants reacting with drinking water contaminants – scope of the problem. Environ. Health Perspect.. 1986;69:31-44.
- [Google Scholar]
- Influence of the apparent molecular size of aquatic humic substances on colour removal by coagulation and filtration. Environ. Technol.. 2011;32:1767-1777.
- [Google Scholar]
- Coagulation/chlorination of surface water: a comparison between chitosan and metal salts. Sep. Purif. Technol.. 2008;62:79-85.
- [Google Scholar]
- Differential absorbance study of effects of temperature on chlorine consumption and formation of disinfection by-products in chlorinated water. Water Res.. 2008;42:1879-1888.
- [Google Scholar]
- Formation of haloforms during the chlorination of natural water. Water Treat. Exam.. 1974;23:234-243.
- [Google Scholar]
- Chlorination reactions of fulvic acids in natural waters. Environ. Sci. Technol.. 1977;11:478-482.
- [Google Scholar]
- Improving the efficiencies of batch coagulation processes with small modifications in the pH. Sep. Sci. Technol.. 2010;45:1411-1417.
- [Google Scholar]
- The effect of ozone on the reversibility of floc breakage: Suspensions with high humic acid content. Ozone: Sci. Eng.. 2010;32:435-443.
- [Google Scholar]
- Kinetics and mechanism of formation and destruction of N-nitrosodimethylamine in water – a review. Sep. Purif. Technol.. 2012;88:1-10.
- [Google Scholar]
- Seasonal variations in natural organic matter and its impact on coagulation in water treatment. Sci. Total Environ.. 2006;363:183-194.
- [Google Scholar]
- Coagulation of humic acid: the performance of preformed and non-preformed Al species. Colloids Surf., A. 2007;296:141-148.
- [Google Scholar]
- Changes in humic acid conformation during coagulation with ferric chloride: implications for drinking water treatment. Water Res.. 2008;42:2111-2123.
- [Google Scholar]
- Removal of organic micropollutants by coagulation and adsorption. Sci. Total Environ.. 1985;47:155-167.
- [Google Scholar]
- Treatment of taste and odor causing compounds 2-methyl isoborneol and geosmin in drinking water: a critical review. J. Environ. Sci.. 2011;23:1-13.
- [Google Scholar]
- Pre-treatment of hospital wastewater by coagulation/flocculation and flotation. Bioresour. Technol.. 2009;100:2138-2146.
- [Google Scholar]
- The lysis of Microcystis aeruginosa in AlCl3 coagulation and sedimentation processes. Chem. Eng. J.. 2012;193–194:196-202.
- [Google Scholar]
- Reactivity of natural organic matter fractions with chlorine dioxide and ozone. Water Res.. 2004;38:547-558.
- [Google Scholar]
- Surface-retained organic matter of Microcystis aeruginosa inhibiting coagulation with polyaluminum chloride in drinking water treatment. Water Res.. 2010;44:3781-3786.
- [Google Scholar]
- Comparing dissolved air flotation and conventional sedimentation to remove cyanobacterial cells of Microcystis aeruginosa. Part II. The effect of water background organics. Sep. Purif. Technol.. 2007;53:126-134.
- [Google Scholar]
- Investigating dissolved air flotation performance with cyanobacterial cells and filaments. Water Res.. 2010;44:3337-3344.
- [Google Scholar]
- Removal of natural organic matter from groundwater using advanced oxidation processes at a pilot scale drinking water treatment plant in the Central Banat Region (Serbia) Ozone: Sci. Eng.: J. Int. Ozone Assoc.. 2011;33:267-278.
- [Google Scholar]
- Insight into changes during coagulation in NOM reactivity for trihalomethanes and haloacetic acids formation. J. Environ. Manage.. 2013;118:153-160.
- [Google Scholar]
- Modeling the formation of chlorination by-products during enhanced coagulation. Environ. Monit. Assess.. 2006;121:503-517.
- [Google Scholar]
- A comparative approach to the application of a physico-chemical and advanced oxidation combined system to natural water samples. Sep. Sci. Technol.. 2007;42:1405-1419.
- [Google Scholar]
- Chemical aspects of coagulation using aluminum salts-II. Coagulation of fulvic acid using alum and polyaluminum chloride. Water Res.. 1990;24:1527-1535.
- [Google Scholar]
- A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manage.. 2012;93:154-168.
- [Google Scholar]
- Removal of pharmaceuticals in drinking water treatment: effect of chemical coagulation. Environ. Technol.. 2006;27:183-192.
- [Google Scholar]
- Removing particles and THM precursors by enhanced coagulation. J. Am. Water Works Assoc.. 1998;90:139-150.
- [Google Scholar]
- Effect of coagulation and flocculation conditions on water quality in an immersed ultrafiltration process. Environ. Technol.. 2009;30:927-938.
- [Google Scholar]
- The role of selected cations in the fomation of pseudomicelles in aqueous humic acid. Talanta. 1997;44:805-809.
- [Google Scholar]
- Effect of hydrophobicity of humic substances on electro-ultrafiltration. Desalination. 2012;284:128-134.
- [Google Scholar]
- Evaluating criteria for enhanced coagulation compliance. J. Am. Water Works Assoc.. 1997;89:64-77.
- [Google Scholar]
- Enhanced coagulation for treating slightly polluted algae-containing surface water combining polyaluminum chloride (PAC) with diatomite. Desalination. 2011;279:140-145.
- [Google Scholar]
- Fate of pharmaceuticals during water chlorination.Water Quality Technology Conference. Charlotte, NC: AWWA; 2007.
- Indecisiveness of electrophoretic mobility determination in evaluating Fe(III) coagulation performance. Sep. Purif. Technol.. 2009;68:273-278.
- [Google Scholar]
- Effects of enhanced coagulation on polar halogenated disinfection byproducts in drinking water. Sep. Purif. Technol.. 2010;76:26-32.
- [Google Scholar]
- Mechanisms for removal of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) from drinking water by conventional and enhanced coagulation. Water Res.. 2013;47:49-56.
- [Google Scholar]
- Characterization of organic matter in alum treated drinking water using high performance liquid chromatography and resin fractionation. Chem. Eng. J.. 2012;192:186-191.
- [Google Scholar]
- Effect of shear force and solution pH on flocs breakage and re-growth formed by nano-Al13 polymer. Water Res.. 2010;44:1893-1899.
- [Google Scholar]
- Transformations of particles, metal elements and natural organic matter in different water treatment processes. J. Environ. Sci.. 2007;19:271-277.
- [Google Scholar]
- Mechanism of natural organic matter removal by polyaluminum chloride: effect of coagulant particle size and hydrolysis kinetics. Water Res.. 2008;42:3361-3370.
- [Google Scholar]
- Effect of pH on the coagulation performance of Al-based coagulants and residual aluminum speciation during the treatment of humic acid-kaolin synthetic water. J. Hazard. Mater.. 2010;178:596-603.
- [Google Scholar]
- Formation of disinfection byproducts from chlor(am)ination of algal organic matter. J. Hazard. Mater.. 2011;197:378-388.
- [Google Scholar]
- Relationship between residual Al species, floc operational parameters and coagulation performance during reservoir water treatment by PAC-PDMDAAC. Sep. Purif. Technol.. 2013;102:147-156.
- [Google Scholar]
- Characteristics of dissolved organic matter after treatment by clinoptilolite-amended activated sludge in association with coagulation processes. Desalination. 2009;243:229-239.
- [Google Scholar]
- Toxic cyanobacterial breakthrough and accumulation in a drinking water plant: a monitoring and treatment challenge. Water Res.. 2012;46:1511-1523.
- [Google Scholar]
- Coagulation behavior of polyferric chloride for removing NOM from surface water with low concentration of organic matter and its effect on chlorine decay model. Sep. Purif. Technol.. 2010;75:61-68.
- [Google Scholar]
- Removal of dissolved organic matter and ophthalmic acid esters from landfill leachate through a complexation-flocculation process. Waste Manage.. 2009;29:110-116.
- [Google Scholar]
- Coagulation characteristics of polyaluminum chlorides PAC-Al30 on humic acid removal from water. Sep. Purif. Technol.. 2008;63:642-647.
- [Google Scholar]
- Algae-removing and algicidal efficiencies of polydiallyldimethylammonium chloride composite coagulants in enhanced coagulation treatment of algae-containing raw water. Chem. Eng. J.. 2011;173:164-170.
- [Google Scholar]
- Competitive complexation of metal ions with humic substances. Chemosphere. 2005;58:1327-1337.
- [Google Scholar]
- Ozonation-based advanced oxidation for pre-treatment of water with residuals of anti-inflammatory medication. Chem. Eng. J.. 2013;220:151-160.
- [Google Scholar]







