Translate this page into:
The growing menace of drug resistant pathogens and recent strategies to overcome drug resistance: A review
⁎Corresponding author. kkb@vit.ac.in (Kannabiran Krishanan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The wonder drug penicillin was popularised widely in the 1940 s and resistance to it too developed in the same decade. Antibiotic resistance or antimicrobial resistance (AMR) is a grave problem facing the 21st century. It is predicted that nearly 10 million people will die from AMR in 2050. The drug resistance mechanism is categorized as intrinsic drug resistance and extrinsic drug resistance, wherein intrinsic changes arise due to molecular changes in the organism and extrinsic resistance arises due to externally acquired stress. Also, both gram-negative and gram-positive organisms have different drug resistance mechanisms. Many strategies like the use of narrow-spectrum antibiotics, combinatorial therapy, and antibiotic stewardship have been used to combat this menace, but the high adaptability of microbes and the lack of effective new drugs in the recent past have hampered all our efforts. Phage therapy, antimicrobial peptides, nano-emulsions, and quorum sensing inhibitors are some of the new promising methods for combating AMR. Alternatively, host-directed therapies (HDTs), including restoring microbiota by probiotics, prebiotics, and synbiotics approach, have become an attractive option for potential therapeutics for the effective management of AMR. Celebration of the antibiotic week and other mass awareness drives worldwide will help to spread awareness on the use of antibiotics. More research on identifying the new and novel antibiotics from natural sources would serve as an effective means of controlling AMR. All these approaches if followed cautiously and meticulously can have a huge impact on the problem of AMR.
Keywords
Drug-resistant pathogens
Antimicrobial resistance
Phage therapy
Antimicrobial peptides
Nano-emulsions
Quorum sensing inhibitors
Host-directed therapies
1 Introduction
Sir Alexander Fleming, who discovered the wonder drug Penicillin, had predicted the menace of antibiotic resistance, 75 years ago in the year 1945 and today it is a hard truth that the world is facing. Due to the extensive use of antibiotics for common infections trivial pathogens have found a new escape mechanism known as ‘antibiotic resistance. The problem has grown to such an alarming extent that some of the infectious diseases thought to have been eradicated with the help of antibiotics have started to crop up all over the world again. They are spreading at alarming rates. Pathogens are showing high resistance to antibiotics. A few highly resistant strains are showing resistance to even the latest antibiotics such as colistin and carbapenems. Multidrug-resistant bacteria have started to evolve as a common pathogen in recent times, due to which their treatment has become more treacherous, often resulting in patient’s death. It is predicted that 10 million people are going to die from AMR by the year 2050 (O’Neill, 2016). This will bring down the Gross Domestic Product (GDP) by 2–3.5% and a fall in livestock by 8%, which wi cost the world 10 trillion USD (Taneja and Sharma, 2019).
As most of the antibiotics in use today are becoming less effective against bacterial pathogens, new alternatives need to be identified or developed to manage drug resistance effectively. Natural products from plant and microbial origins have been tried in the recent past. Marine microbes were extensively explored for bioactive molecules and most of the antibiotics (80%) available in the market today are from marine actinomycetes (Valli et.al, 2012). They are known for producing clinically important secondary metabolites. Selman Waksman discovered the antibiotic streptomycin from actinomycetes (Woodruff, 2014). Actinomycetes are considered treasure troves for novel biologically active compounds and antibiotics. Extensive research is going on all over the world to isolate novel compounds from actinomycetes. Actinomycetes have been isolated from almost all places like agricultural lands, river beds, mangroves, Sea-beds, etc. Since much work has been done on soil actinomycetes, there is a chance of re-isolating the same compounds from the same organisms over and over again. Hence, scientists have now turned to marine actinomycetes as a potential source of novel secondary metabolite-producing organisms (Subramani and Sipkema, 2019). This review aims to provide an overview of the common mechanisms by which the bacterial pathogens try to resist the drugs and also throw light on various alternative approaches for the management of drug resistance and the potential of marine actinomycetes derived secondary metabolites as antimicrobials against MDR and quorum sensing inhibitors (QSI) in combatting the issue of drug resistance. Drawbacks of the current strategies in dealing with drug resistance and some of the novel screening methods for resistance genes are also discussed.
2 Drug-resistance mechanism of bacterial pathogens
World Health Organization (WHO) defines AMR or drug resistance as the resistance exhibited by bacterial, viral, parasitic, and fungal pathogens to antimicrobial medicines. It occurs naturally over the years but is aggravated by the inappropriate use of antimicrobial medicines in humans, animals, food production, agriculture, and aquaculture sectors; lack of access to health services such as diagnostics and laboratory services; and mixing of antimicrobial residues through the food chain in soil, crops, and water (WHO, 2017). Multi-drug resistance occurs when an organism becomes resistant to more than one antimicrobial compound. Antimicrobial resistance is a broad term that comprises different layers of complexities.
Pathogens have devised various mechanisms to grow in presence of compounds that were toxic to them once through evolution and as a result, they have become resistant to most antimicrobials. Most pathogens build up an internal resistance to antimicrobials making them intrinsically resistant. On the other hand, some pathogens acquire resistant genes from other bacteria or their surroundings. Not all members of a bacterial species or group are necessarily resistant to all antimicrobials. The ability to resist or inhibit a drug can be naturally present in their genetic makeup or it may be a biochemical attribute of the organism. Mutations can also bring about certain changes in the organism resulting in the development of resistance mechanisms (intrinsic resistance). Two major reasons for drug resistance in bacteria genetically are mutations in genes and the acquisition of foreign DNA (Munita and Aria, 2016).
Mutations result in the development of a new pathogenic phenotype or enhance its virulence. These mutations are due to an evolutionary natural process called pathoadaptive mutations. These can enhance or confer bacterial pathogenicity without Horizontal Gene Transfer. Horizontal gene transfer(HGT) is another mechanism by which bacteria acquire resistance. Resistance genes are transferred between related genes under stress conditions. Bacteria typically acquire extra foreign genetic material through transformation, transduction, and conjugation (Peterson and Kaur, 2018). Transformation is the simplest method with less efficiency among the three. Conjugation is the biggest contributor to the dissemination of ARGs(Antibiotic Resistance Genes). Transformation and transduction were not deemed important in the past but recent studies prove they have a bigger role to play than previously thought.ll HGTs contribute significantly to the formation of resistomes(ARG reservoirs) (Wintersdorff et al.,2016). Resistance can be measured using the concept of minimum inhibitory concentration (MIC). It measures the susceptibility and resistance of a pathogen to a drug (Wanda, 2016).
2.1 Limiting the uptake of drug
Drug uptake can be limited due to the difference in cell structures in different bacteria. Gram-negative bacteria are inherently resistant to antimicrobials due to the presence of an outer cell wall (Wanda, 2016). This mechanism is not seen in gram-positive bacteria due to the lack of cell walls. Hydrophobicity and hydrophilicity also have a part in limiting the uptake of drugs. Porins determine which compounds are allowed to enter the cell through the cell wall based on their hydrophilicity and hydrophobicity. Usually, large porins allow hydrophilic molecules to enter while hydrophobic molecules are stopped (Blair et al., 2014).
2.2 Modification of drug target sites
This is one of the most common mechanisms adopted by pathogens. By modifying the target site, the drug fails to interact with the target site properly or may not recognize the site at all. This is achieved through target protection and target modification (Munita and Arias, 2016). Target modifications can be achieved through point mutations in gene coding sites, enzyme-mediated alterations in the binding site, and replacement or bypass of the original target.
2.3 Inactivation of drug
It is achieved through complete drug molecule destruction or the addition of chemical groups to the drug. β-Lactamases are the most common example of drug destruction. They destroy the drug by hydrolyzing a site in the ring structure of β-lactam drugs (Wanda, 2016). In the case of the transfer of chemical groups, the methyl group is transferred to the drug. Acetyl, adenyl, and phosphoryl groups are some of the most commonly transferred chemical groups and acetylation is the most common reaction. It offers resistance against a wide range of antibacterials like aminoglycosides, chloramphenicol, and streptogramins (Wanda, 2016).
2.4 Efflux pumps
These are complex pieces of machinery capable of flushing out toxic compounds from the cell (Munita and Arias, 2016). They were first described in E.coli in the 1980 s (McMurry et al., 1980). Both gram-negative and gram-positive pathogens make use of this mechanism. Five types of efflux pumps have been described so far and they are major facilitator superfamily, small multidrug resistance family, Resistance Nodulation Cell Divison family(RND), ATP binding cassette family (ABC), and multidrug and toxic compound extrusion family (MATE). Another type of efflux pump has been added recently to the list making the total six. The sixth efflux pump is proteobacterial antimicrobial compound efflux (PACE). The ABC family derives its energy for transport by utilizing ATP. The other five utilize electrochemical energy trapped in transmembrane ion gradients and they are secondary active transporters(Du et al., 2018). The mechanism of antibiotic resistance by pathogens is shown in Fig. 1.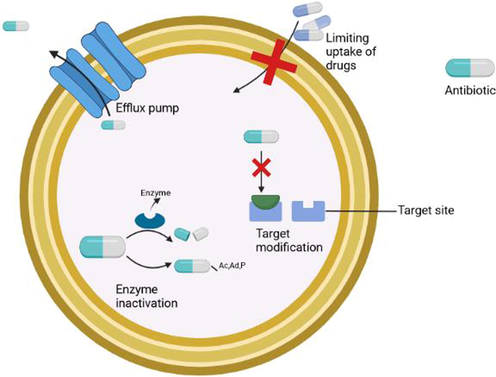
Mechanism of antibiotic resistance in pathogens.
2.5 Mechanism of drug resistance in gram-negative and gram-positive bacteria
The differences in the mechanism of drug resistance in these bacteria primarily depend on their cell structure. The presence of a thick cell wall in gram-negative bacteria helps them in preventing entry of drugs through mechanisms like limited uptake of drugs (Wanda, 2016). On the other hand, gram-positive bacteria do not have this mechanism due to the lack of a cell wall. The thick cell wall in gram-negative bacteria makes it inherently resistant compared to gram-positive bacteria which lack cell walls (Breijyeh and Karaman, 2020). While gram-positive bacteria are naturally resistant to cell wall synthesis inhibiting β- lactamases. Enzymatic degradation of antibiotics by the production of β lactamases and decreasing the susceptibility and affinity to their target binding site, known as penicillin-binding protein (PBP), by the acquisition of exogenous DNA or by native changes in PBP are the two major mechanisms conferring resistance to gram-positive pathogens (Breijyeh et al., 2020) But in recent times, many specialized mechanisms have been described in different bacteria, which depends on their structure, surroundings, etc. In most cases, these mechanisms are variations of the efflux pumps due to mutations in their genes.
3 Current strategies used in combating drug resistance
Antibiotic resistance by infectious bacterial pathogens to more and more classes of antibiotics is a serious issue worldwide. The development of newer antibiotics is unable to go in tandem with the emerging microbial resistance. The increasing emergence of new drug-resistant pathogens like MRSA, NDM-1 positive Enterobacteriaceae, X-DRTB among others is making the challenge all the more complicated. One of the most advised and advocated methods of combatting AMR is antibiotic stewardship. This curbs the problem of AMR to an extent. Antibiotic stewardship brings down the use of antibiotics by avoiding unnecessary antibiotic prescriptions. This ensures that antibiotics are used in a controlled manner, preventing pathogens from gaining continuous exposure to antibiotics and in turn becoming resistant. These programs educate healthcare professionals about the consequences of the overuse of antibiotics and aid them in decreasing the incidences of overtreatment and over-prescription (Gerber et al.,2021). The use of antibiotics may lead to the evolution of resistance genes even in harmless bacteria. This can in turn be transferred to other pathogens through horizontal gene transfer (Andrew, 2014). Horizontal gene transfer predates even the use of antibiotics. The soil is already a rich reservoir of ARGs which makes it the perfect environment for the transfer of resistance genes from one organism to another through HGT. Bacteria in the soil acquire resistance genes through HGT. Also since they are in stressed conditions these too can lead to the development of resistance genes through processes like co-selection(Wintersdorff et al.,2016).
3.1 Narrow spectrum antibiotics
The use of narrow-spectrum antibiotics rather than broad-spectrum is another strategy used by clinicians these days to target AMR or MDR pathogens (Andrew, 2014). Combinatorial drug use has shown better results in the management of AMR. A pathogen might have developed resistance to the mode of action of a particular drug, but it is highly unlikely that it might have gained resistance to two different types of drugs with totally unrelated modes of action (Nosten and others, 2000). The need for combining antibiotics stemmed from the fact that no single antibiotic is effective against all infections universally. Many of the combinations at the beginning were opportunistic and had little data backing their efficacy or molecular mechanism. The advantages of antibiotic combinations were reported in the past. The combination of streptomycin with penicillin was reported in 1950 and trimethoprim with sulfonamides in 1968. These combinations had improved spectrum and efficacy compared to them being used individually. Combination drug therapy backed with clinical and epidemiological data are now a major clinical practice. Synergy and antagonism are the two factors on which drug combinations are built. They are calculated using the Fractional Inhibitory concentration Index (FICI) method in microbiological labs (Tyers and Wright,2019). These are some of the most common strategies adopted by clinicians all over the world to combat AMR under the guidance of WHO, CDC, and the rest.
Apart from these several national and international action plans have been put forward by several countries to control AMR. Some of the noted programs and agencies to combat AMR are National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS) in the USA, Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) in Canada, Observatoire National de l'Epidémiologie de la Résistance Bactérienne aux Antibiotiques (ONERBA) in France, and Danish Integrated Antimicrobial Resistance Monitoring and Research Programme (DANMAP) in Denmark. Celebration of AMR week as ‘World Antibiotic Awareness Week’ in November is also being done globally every year to create more awareness among the public. This also helps and motivates health care workers and policymakers to adopt and engage in best practices to tackle and prevent further antibiotic resistance.
4 Drawbacks in current strategies
Antibiotic resistance is an ever-growing problem worldwide. According to the Centre for Disease Control (CDC) reports, in the U.S alone nearly 2.8 million people get an antibiotic-resistant infection and more than 35,000 people die annually. Antibiotic stewardship has been the most popular and much familiar approach against AMR. It has its shortcomings and the main problem is while the overuse of one drug was regulated only the incidence of resistance to that particular drug was brought down, while the number of resistant organisms to other drugs increased considerably. This effect is called the ‘squeezing the balloon’ phenomenon, wherein the decreased use of one class of antibiotics results in increased use of another (Doron and Davidson, 2011). Pathogens have already become resistant to broad-spectrum antibiotics like extended-spectrum β-lactamases (ESBL) inhibitors and carbapenems which are considered as last-sort antibiotics and there are no more alternative drugs to use. Colistin has been reintroduced as a last-sort drug due to increasing cases of Carbapenem-resistant pathogen infections. This resulted in resistance to this particular drug which is caused by the mobile colistin-resistant (MCR) genes. Multiple MCR gene variants have been reported around the world. These genes have been isolated from poultry, pigs, and cattle and also from various environmental sources like rivers, seas, and hospital sewage (Eldbediwi et al.,2019). Pathogens are exposed to antibiotics not only through hosts but also in the environment. There is an abundance of ARGs in soil. The fate of the antibiotic residue that enters the soil is decided by sorption to organic particles and by degradation or transformation. Antibiotic residues present in the soil affect the microbes by altering their enzyme activity and ability to metabolize various carbon sources. Agricultural and human activities affect the presence of ARGs in soil. Pathways that release resistance-conferring chemicals like antimicrobials, biocides, and heavy metals can be monitored effectively through environmental regulators. Environmental regulators should be considered while devising AMR action plans as they can contribute significantly in devising ways to curb AMR. There is a gap in the existing knowledge in this regard (Cycon et al.,2019) This gap can result in failure to control infections, and in severe resistant cases and ICU patients, it can even be fatal. The other problem associated with antibiotic stewardship is monitoring. Proper implementation is a relative process to ensure 100% effectiveness for which all the hospitals should be interconnected and rigorous evaluation is needed. Huge amounts of data need to be collected periodically regarding which drugs are being used in a controlled fashion, data of resistance towards other drugs, resistance pattern, new outbreak, etc. This needs to be coordinated at both the national and international levels. Proper guidelines have to be laid down for all organizations in the health sector, and a dedicated organization working exclusively for implementation and review should be there and it should function independently. Developed countries might be able to ensure the implementation of antibiotic stewardship but it still is a challenging task for many of the developing countries.
Though the use of narrow-spectrum antibiotics is considered an effective method, this can put high selective pressure on a class of bacteria and may lead to serious complications as well. It might even lead to the transfer of genes from these resistant pathogens to other closely related non-resistant pathogens making them more difficult to treat. Combinatorial drug therapy is one of the most prevalent practices in use now. Though it has been showing great results, there are several concerns associated with it including antagonism. Also, it is comparatively costly because more than one drug is used at a time. Even though it has proved to be a very effective and reliable clinical strategy for the control of gram-positive bacteria, it was not very effective in the case of gram-negative pathogens. The lack of new potent antibiotic discovery is also hampering the fight against drug resistance. According to CDC reports after 2000, the discovery of new drugs has dropped drastically. One of the main reasons for this is that the pharma companies are not taking up research or production of new drugs. This is due to the time scale it takes for a new drug to be developed, go for clinical trials, get necessary approvals for production, and the cost involved. It takes a minimum of 10 years or more for a drug to reach the market. Hence, companies prefer drugs like anti-depressants, pain killers that have already been established, maintain a steady income, and play it safe. The other reason is the rigorous and time-consuming clinical trials and FDA approval. The WHO report in 2017 shows that 51 new antibiotics and biological compounds were listed out of which only 8 were truly innovative. Among these, considering a success rate of 14% from the phase one clinical trial for approval, only 10 are expected to reach the market within the next 10 years of which only 1 or 2 are potentially active against gram-negative bacteria. No new drugs for gram-negative bacteria are being discovered and their structural specialties make them inherently resistant to most drugs. Though many new scientific research papers have been put forward against the treatment of gram-negative pathogens (Bassetti et al., 2011; Bassetti, et al., 2019) such as E. coli, Klebsiella among others their success rate in real-time situations is still debatable.
The extensive use of antibiotics in livestock management, agriculture, and the food industry has only aggravated the situation. Antimicrobials are used for both zoonotic and human pathogenic infections. The increased use of antimicrobials in animals can contribute to the fixation of AMR genes in bacteria. They may be capable of transferring these resistant genes to human pathogens or human gut microbiota either through direct contact or through food exposed to biocides, antiseptics and the environment. So zoonotic pathogens can act as a pool of AMR genes (Argudin et al.,2017). Another shocking discovery is the co-selection for antibiotic resistance among bacteria. Co– selection adaptations can result in resistance across various classes of antimicrobial agents, mobilization of genetic elements, or mutagenesis. Since zoonotic pathogens have high chances of getting exposed to these agents, this may lead to the development of drug resistance in them (Wales and Davies, 2015). Humans can come into direct contact with these pathogens by interacting with animals and biological substances such as blood, urine, feces, milk, saliva, and semen. This makes possible the dissemination of resistant bacteria from one host to another smoothly. Indirect exposure can occur via the consumption of contaminated food such as meat, egg, dairy, and the rest. Indirect exposure occurs as part of the food chain and is a very complex pathway. Antibiotic administration disturbs the gut microbiome in humans leading to poor expression of proteins, lack of richness of taxa, diversity, and evenness. An affected gut microbiome can lead to faulty metabolism resulting in diseases like type-I diabetes and compromised immune systems. Surprisingly the ARG reservoirs are not only found in adults but even in infants and children. It is shocking that ARGs can be found in even one-month-old babies, even in their meconium(first deposition of newborns)(Francino,2016; Founou et al.,2016). Availability of over-the-counter (OTC) antibiotics is the other major concern. This is the culminating effect of lack of proper surveillance by clinicians and doctors, poor knowledge of AMR, and lack of public awareness. Hidden agendas of pharma companies are also a drawback, as doctors are lured by pharma companies with rewards into prescribing antibiotics for longer periods than necessary and also at higher doses again increasing selection pressure on the pathogens.
Due to a lack of innovative methods, the fight against AMR is stagnated. Rather than focussing on drugs affecting a particular metabolic pathway, new targets for drugs like signal molecules, cell wall structure disruptors; osmotic imbalance, and others. can be targeted. Since bacteria are known to have high mutation rates, if a particular pathway is targeted, they easily find an alternative to it or evade it altogether. It is appropriate to target osmotic balance, membrane integrity, receptor molecules, and biofilms. (Roy et al., 2018).
5 Alternative approaches for the management of drug resistance
Several approaches for the management of MDR have been followed. The summaries of recent approaches, which are more promising for the management of MDR are given in Table 1.
Actinomycetes
Antimicrobial peptides
References
Actinomyces ruminicola
Sugrue et al., 2020,
Streptomyces roseosporus
Roseocin
Singh et al.,2020
Marinactinospore
Mathermycin
Chen et al.,2017
Streptomyces sp.
Salinamide
Hassan et al.,2015
Streptomyces parvus
Arylomycin
Rao et al.,2013
Nocardiopsis lucentensis
LucentamycinsA-D
(1–4)
Cho et al., 2007
Planomonospora sp.
Planosporicin
Castiglione et al.,2007
5.1 Antimicrobial peptides
Anti-microbial peptides (AMPs) or host defense particles are produced by all life forms from bacteria to higher organisms. The discovery of AMPs occurred during the extraction of an antimicrobial agent gramicidin from a Bacillus strain isolated from a soil sample. They have a broad spectrum of action, even against bacteria, fungi, and protozoa. Their amphipathic nature facilitates their insertion and interaction into cell walls. Even though their mode of action is through causing damage to the cellular membrane, they can also be employed to target different proteins like regulatory enzymes, DNA, and RNA. Since the chance of developing resistance to AMPs once they are introduced into clinical practice is high, more molecular level studies need to be done to understand their mode of action and resistance to these compounds to go ahead with AMPs as an alternative to antibiotics (Aslam et al., 2018).
5.2 Phage therapy
Phages are used against resistant bacterial pathogens. It is emerging as a very promising method against bacterial AMR. Phage therapy was introduced as a new therapeutic approach when bacteria like E. faecium, S. aureus, and K. pneumoniae started becoming resistant to the existing antibiotic compounds. The major advantages of phage therapy are i) inherent toxicity, ii) lack of cross-resistance with antibiotic classes, iii) they are strain-specific unlike the broad-spectrum antibiotics, and iv) they are effective against both gram-negative and gram-positive bacteria (Principi et al., 2019).
The demerits associated with phage therapy is the chance of treating them as foreign bodies by the immune system, they may also carry antibiotic resistance genes in them. Extensive studies are required to understand the genome of the phages and their properties for manipulating effectively depending upon our requirements.
5.3 Nanoparticles
Nanoparticles (NPs) falling under the 1–100 nm range are used for the treatment of antibiotic-resistant infections (Baptista, 2018). The advantage of the use of NPs in AMR treatment, it increases the drug stability and solubility inside the body. NPs are more target-specific and their small size makes their delivery into the system much easier. NPs also tackle the problem of poor membrane permeability of antibiotics. The use of NPs as an effective agent to tackle the problem of drug resistance cannot be immediately put into practice due to health concerns associated with it. NPs although a promising technique, have been reported to aid in the horizontal gene transfer of Antibiotic Resistance Genes (ARGs). Their excessive use can result in their introduction into the environment subsequently aiding in the transfer of ARGs from one bacteria to another (Su et al.,2019).
5.4 Plant secondary metabolites
Plants are rich in medicinal properties, making them ideal candidates for treating bacterial pathogens. Plants include a wide range of phytochemicals with antibacterial properties such as saponins, alkaloids, tannins, flavonoids, and others (Gupta and Birdi, 2017). Plant phytochemicals are a valuable source of bioactive compounds, have antimicrobial properties, and can reverse antibiotic resistance (Khameneh et al., 2019). Some of the plant secondary metabolites interfere with molecular targets like cell signaling(Gupta and Birdi, 2017).
5.5 Emulsions
It is used for the delivery of a particular drug or compound. Usually, they are administered as oil–water emulsions in the form of droplets. Essential oils have been known to have antibacterial properties and these are most commonly employed. (Franklyne et al. 2016). Among the 3000 known Essential Oils (EOs), 300are are commercially important. EOs have certain drawbacks, they are sensitive to oxygen, light, and moisture making them undesirable for use in pharmaceutical industries. These problems are addressed by nano-encapsulation. Nano-emulsions of EOs can be produced by this process which can increase the activity of these bioactive compounds and also increase their dispensability by increasing their cell permeability through the cell wall of pathogens(Hosna et al.,2019).
5.6 Biosurfactants and quorum sensing inhibitors
They are biologically active surface-acting molecules produced by microbes. Biosurfactants due to their structural specialties have a detergent-like effect on the cell membrane of bacterial pathogens. They can avoid bacterial colonization by acting as anti-adhesive agents and not letting bacteria stick to the surface thereby preventing colonization (Rivardo et al, 2009). Antimicrobial activity of biosurfactants from Bacillus subtillis multidrug-resistant (MDR) bacteria has already been reported (Fernandes et al, 2007).
Quorum sensing (QS) is a surveillance mechanism used by bacteria for colonization and infection. QS is done with help of certain signal molecules produced by the bacteria themselves, which accumulate and get recognized by a receptor on the bacterial surface (Miller and Bassler, 2001). QS is a mode of bacterial communication by breaking the QS by appropriate quorum sensing inhibitors, the process of bacterial colonization can be prevented. It depends on the density of the bacterial population. Once the signal molecules produced by bacteria passes the threshold level the respective receptor will get activated and bind to the signal molecule inducing various bacterial behavior such as biofilm formation, bioluminescence, virulence factor production, and others (Ni et al., 2021). Both gram-positive and gram-negative bacteria have different kinds of quorum sensing signals. In gram-negative bacteria, the Qs system is mediated by the signal molecule Acylhomoserine lactone (AI-1) and in gram-positive bacteria, it is Auto-inducing peptides (AIPs) and Furanosyl Borate diesters in both gram-positive and gram-negative bacteria. (Jiang et al, 2019). QS inhibitors do not kill the pathogen rather weaken their pathogenicity by inhibiting various processes like biofilm formation, production of virulence factors, and expression of pathogenic genes (Ni, et al., 2021). Quorum sensing inhibitors can be used with existing antibiotics as combinatorial therapy against resistant pathogens Breijeh and Karaman,2020).
5.7 Plasmid curing
Often the antimicrobial resistance genes (AGRs) are located on the plasmids. These are mobile and aids in the transfer of ARGs from one cell to another. This type of transmission is responsible for the global dissemination of MDR bacteria. They are typically found in gram-negative bacteria and code for genes such as ESBL, carbapenemases, and others. (Holmes et.al, 2016). Plasmids show a high degree of plasticity (Kado, 2014). Plasmid curing is a process wherein, a bacterium is made devoid of its plasmids which contain most of the resistance genes. This technique removes the resistance leaving the bacterial population intact and making the pathogens more susceptible to antibiotics or antimicrobials. This is advantageous to revive the gut microbiome as most of the antibiotics wipe them out along with the pathogens in the body that might have ended up in the body through the food chain. Plasmids curing agents can be administered to people who are traveling abroad to prevent the spread of AMR globally. It can also be given to patients before surgery to avoid contracting the risk of nosocomial infections. Since not many options are available at present, this technique can be exploited and marketed (Buckner et.al, 2018).
5.8 Reverse vaccinology
It utilizes the sequencing of whole genomes of pathogenic bacteria to discover genome encoded antigens which were impossible in the two centuries of vaccine history. Vaccines could be designed and developed with data from the computer instead of starting from culturing the microbes in labs. Four component Meningococcus B vaccine, which was recently licensed has proven that reverse vaccinology is an efficient way to select antigens that can confer resistance against antigenically variable bacterial pathogens. Antigens are selected based on their sequence conservation and predicted surface exposure. Then expressed as recombinant proteins and tested out in pre-clinical models for eliciting functional antibodies. The promising candidates are then combined in the final step to achieve a vaccine with the best possible spectrum coverage based on molecular epidemiology data. 4CMen B was recently introduced in the U.K in all infants and showed an effectiveness of 82.9% against all Meningococcus B strains (Parikh et.al, 2016).
5.9 Potentiation approaches
In this technique, the developing of efflux pump inhibitors and prioritizing potentiator mechanisms that affect targets on the outer surface of the bacteria rather than penetrating to the cytoplasm have been tried. The need for co-development of a potentiator along with the antibiotic has also been tried as an alternative approach. This method tries to circumvent the poor penetrability of antimicrobials due to the highly complex nature of bacterial cell walls and pumps incorporated in them. It is a rather attractive approach as an effective efflux inhibitor can broaden the spectrum of a gram-positive agent by making it susceptible to gram-negative bacteria(Baker et al. 2018). Potentiation of gram-positive selective antibiotics against gram-negative pathogens reported previously depended on the disruption of the outer membrane (Martin et al., 2019). Adjuvants are used to potentiate antibiotics. They are compounds with very little to no antibiotic activity but when combined with a drug it enhances the activity of the drug considerably. Adjuvants also bring down the demand for the immediate discovery of new drugs as they can revive existing antibiotics against resistant pathogens (Breijyeh and Karaman, 2020).
5.10 Active uptake approaches
In this technique, the antibiotic influx is increased through bacterial membrane transporters. Various bacterial membrane transporters like iron transporters and sugar are exploited to increase the influx into the cells. The antibiotic fosfomycin was tested by using the bacterial sugar transporters to enter the cells (Baker et al. 2018).
5.11 Dendrimers
Dendrimers, dendrons, and dendritic polymers are attractive due to their controlled synthesis and monodispersity. Dendrimers range in size from 1 to 10 nm and they can cross cell membrane due to their controllable size and with the help of lipophilicity of the skeleton. It can encapsulate drugs and deliver to target tissues not entangle at the higher molecular weight. These properties make them very useful to inhibit biofilm formation. In P. aeruginosa they prevent the Lec B- fucose interaction thereby preventing biofilm formation. They can also disperse formed structures and act as antimicrobial nano-carriers (Ortega et.al, 2020).
5.12 Targeting drug-resistant pathogens by actinomycete secondary metabolites
Actinobacteria have been a hot topic among researchers for a long time and this is mainly due to its potential to produce diverse active secondary metabolites. Actinobacterial secondary metabolites have been known to be useful against not only pathogens but also proved to be useful in the agriculture sector and many other industries. Approximately 20,000 biologically active secondary metabolites have been discovered so far of which approximately 13,700 are reported as biologically active compounds and nearly 10,000 are antimicrobials which include antibacterial, antiviral, and antitumor compounds (Berdy, 2012). Of the actinobacterial species, the species Streptomyces alone contributes nearly 7600 compounds with the potential to suppress multi-drug resistant pathogens.
Anti- Quorum sensing or inhibition is the newest and most promising aspect of actinobacteria. It prevents biofilm formation in pathogens (Miao et.al, 2017). These are compounds that can inhibit the expression of virulence factors through the production of enzymes capable of breaking down signaling molecules and quorum sensing peptides (Betancur et.al, 2017). Diverse compounds derived from actinomycetes are effective against MDR pathogens.
Antimicrobial peptides are another promising secondary metabolite from the actinomycetes. They are oligopeptides with a varying number of amino acids. It was first described in 1939. Bacteriocin is a potent peptide isolated from Actinomycetes. They have many immunological activities including antimicrobial activity (Richard et.al, 2015). There have been several reports of antimicrobial peptides from actinomycetes in recent years. Antimicrobial peptides derived from actinomycetes are given in Table1.
5.13 Host-directed therapies (HDTs)
It enhances protective immune signatures, reduces exacerbated inflammation, or balances immune reactivity at the infection site. HDTs also exploit the repurposing of drugs commonly used in therapy. Under HTDs selective pressure on the microbe is avoided and, hence, the risk of developing resistance to treatment is also less thereby it paves way for new therapy (Puccetti et al., 2020).
Some other approaches which include supplementation of micronutrients, immune-modulators, and antimicrobial peptide inducers are suggested to be beneficial. Prolonged intake of antibiotics interferes with the human microbiome thereby weakening the immune system and the host becomes susceptible to pathogens. Hence, restoring microbiota by probiotics, prebiotics, and synbiotics approaches has become an attractive option for potential therapeutics (Puccetti et al., 2020).
5.14 Postbiotics
Deals with the role of microbiota-derived metabolites, high-throughput sequencing, and metabolomics studies lead to the identification of a series of postbiotic compounds that can contribute to directly and specifically manipulate the microbiota and host functions. It includes lipids, carbohydrates, proteins, organic acids, vitamins/co-factors, peptidoglycan-derived muropeptides, or lipoteichoic acids, or immunomodulators, anti-inflammatory, hypocholesterolemic, anti-obesogenic, anti-hypertensive, anti-proliferative, and antioxidants (Roger and Dragsted, 2019). The pros and cons of alternative approaches for the treatment of MDR pathogens are given in Table 2.
Approaches
Advantages
Disadvantages
1. AMPs
Broad-spectrum
Amphipathic in nature
Can target regulatory proteins, DNA, RNAChances of developing resistance to AMPs once they are introduced in regular clinical use is high
2. Phage therapy
Lack of cross-resistance with antibiotic classes
Strain-specific
Effective against both gram-positive and gram-negativeThe immune system may treat them as foreign bodies
The phages themselves may carry antibiotic resistance genes in them
3. Nano-Particles
Increases drug stability
Increases solubility
Small size
Target specificCan cause horizontal gene transfer of antibiotic resistance genes
4. Plant secondary metabolites
Has antimicrobial properties
Can reverse antibiotic resistance
Can interfere with molecular processes like cell signalingThis field needs to be exploited more
5. Emulsions
Have antibacterial properties
They are sensitive to light moisture and oxygen so nano-encapsulation is required
6. Biosurfactants and quorum sensing inhibitors
Prevents colonization of pathogens on a surface
They are effective against both gram-positive and gram-negative pathogensIt is an emerging field with ongoing studies
7. Plasmid curing
They help in reviving gut bacteria
Can be given to people traveling internationally to avoid the spread of AMR globally
Can be given to patients before surgery to avoid nosocomial infectionsAt present not many options are available for this treatment method
8. Reverse vaccinology
An efficient way to select antigens that can offer resistance against antigenically varying pathogens
More studies need to be conducted in this field.
9. Potentiation
Circumvents the problem of poor cell permeability
Targets efflux pumps
The use of adjuvants can revive existing antibiotics against resistant pathogens
Gram-positive drugs can be made susceptible to gram-negative pathogensThere is not enough data available to check the efficiency hence more studies need to be performed
10. Active uptake
Membrane transporters are exploited to increase influx into the cell
11. Dendrimers
Synthesis can be controlled
Monodispersive
Inhibit biofilm formationThis field needs to be exploited more
12. Host Directed Therapy
Selective pressure on microbes can be avoided
Helps in repurposing drugsNeed to be introduced into clinical practice globally
13. Postbiotics
Directly manipulate microbes and host functions
Research is underway and hence not yet popularised globally
5.14.1 Antimicrobial resistance and COVID −19 pandemic
Antibiotic stewardship has been at the helm of the fight against antimicrobial resistance. The current COVID −19 pandemic has severely hampered the regulation of antibiotic usage across the globe. The number of antibiotics being used has increased considerably during this pandemic. WHO has reported that the use of azithromycin along with hydrochloroquinone has shot up considerably across the globe. The huge number of people being admitted to hospitals can also increase the risk of hospital infections. COVID-19 patients often contract secondary bacterial infections and antibiotics are again used for treating these. As part of a single-center independent study done at a tertiary care hospital in Delhi, India, it was observed that there was a 40% increase in AMR bacterial pathogens compared to pre-COVID times. MRSA was one of the isolates obtained among others that proved resistant to antimicrobials. It was concluded that this increase was due to the exaggerated use of antibiotics, which allowed the pathogens to slowly develop resistance mechanisms or acquire them (Saini et al.,2021). This can also lead to the transmission of multi-drug-resistant strains. One of the threats at the time of pandemic arises due to the use of biocidal agents outside hospital settings. Exposure to these agents at low levels can result in the selection of drug-resistant strains and increase the risk of cross-resistance to antibiotics. WHO guidelines advise integrating antibiotic stewardship into pandemic management and not to suggest antibiotics to patients with mild or moderate COVID-19 symptoms unless symptoms of a serious infection. Clinical healthcare workers need to be aware of the consequences and made them more competent. Medical devices and instruments should be well kept and should not be used unnecessarily to avoid healthcare-related infections (Getahun et al., 2020).
6 Conclusion
AMR is a multifaceted problem that needs new, innovative and effective approaches for better control and management. NPs assisted drug delivery, phage therapy, plant-based antimicrobials, and actinomycetes derived secondary metabolites as inhibitors, all appear to be more promising. The Discovery of newer antibiotics and the proper use of existing antibiotics and monitoring of drug abuse are considered to be the need of the hour. Some of the alternate approaches mentioned in this review also help in repurposing existing drugs which will give us a huge edge in the battle against AMR. HDTs, restoring the gut microbiota by probiotics, prebiotics, and synbiotics, and administration of postbiotic compounds to the host are some of the latest and more promising approaches to the effective control and management of drug resistance. Postbiotics and HDTs can open new avenues for the pharma industry and may even help us win the fight against AMR. Rigorous research is required in these fields to gain more in-depth knowledge about the mechanism and molecular basis of each and to exploit these potential approaches commercially. Awareness should be given to every stratum of society to make them understand the dangers of AMR and also how to use antibiotics properly. Coordinated research, effective monitoring, and sharing of information across the world would control the surge of AMR.
Acknowledgements
The authors are thankful to the management of Vellore Institute of technology for the encouragement and for providing the necessary facilities.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antibiotic resistance: a rundown of a global crisis. Infection and drug resistance. 2018;11:1645.
- [Google Scholar]
- Technologies to address antimicrobial resistance. Proceedings of the National Academy of Sciences. 2018;115(51):12887-12895.
- [Google Scholar]
- New treatment options against gram-negative organisms. Crit Care.. 2011;15(2):215.
- [CrossRef] [Google Scholar]
- Multidrug efflux pumps in Gram-negative bacteria and their role in antibiotic resistance. Future microbiology. 2014;9(10):1165-1177.
- [Google Scholar]
- Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25(6):1340.
- [Google Scholar]
- A novel lantibiotic acting on bacterial cell wall synthesis produced by the uncommon actinomycete Planomonospora sp. Biochemistry. 2007;46(20):5884-5895.
- [Google Scholar]
- Mathermycin, a lantibiotic from the marine actinomycete Marinactinospora thermotolerans SCSIO 00652. Applied and environmental microbiology. 2017;83(15)
- [Google Scholar]
- Lucentamycins A− D, cytotoxic peptides from the marine-derived actinomycete Nocardiopsis lucentensis. Journal of natural products. 2007;70(8):1321-1328.
- [Google Scholar]
- Antibiotics in the soil environment degradation and their impact on microbial activity and diversity. Front Microbiol. 2019;10:338.
- [Google Scholar]
- Doron, S. and Davidson, L.E., 2011, November. Antimicrobial stewardship. In Mayo Clinic Proceedings (Vol. 86, No. 11, pp. 1113-1123). Elsevier.
- Multidrug efflux pumps: structure, function and regulation. Nature Reviews Microbiology. 2018;16(9):523-539.
- [Google Scholar]
- Antimicrobial activity of surfactants produced by Bacillus subtilis R14 against multidrug-resistant bacteria. Brazilian Journal of Microbiology. 2007;38(4):704-709.
- [Google Scholar]
- Essential oil micro-and nanoemulsions: promising roles in antimicrobial therapy targeting human pathogens. Letters in Applied Microbiology. 2016;63:322-334.
- [Google Scholar]
- Tackling antimicrobial resistance in the COVID-19 pandemic. Bulletin of the World Health Organization. 2020;98(7):442.
- [Google Scholar]
- Development of botanicals to combat antibiotic resistance. Journal of Ayurveda and integrative medicine. 2017;8(4):266-275.
- [Google Scholar]
- Salinamide F, new depsipeptide antibiotic and inhibitor of bacterial RNA polymerase from a marine-derived Streptomyces sp. The Journal of antibiotics. 2015;68(3):206-209.
- [Google Scholar]
- Jiang, Q., Chen, J., Yang, C., Yin, Y. and Yao, K., 2019. Quorum sensing: a prospective therapeutic target for bacterial diseases. BioMed Research International, 2019.
- Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Resist Infect Control. 2019;8:118.
- [CrossRef] [Google Scholar]
- Small Molecule Potentiation of Gram-positive Selective Antibiotics Against Acinetobacter baumannii. Dis: ACS Infect; 2019.
- Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proceedings of the national academy of sciences. 1980;77(7):3974-3977.
- [Google Scholar]
- Mechanisms of antibiotic resistance. Virulence mechanisms of bacterial pathogens 2016:481-511.
- [Google Scholar]
- Jo ur l P re of. Lett: Chinese Chem; 2021.
- Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Frontiers in microbiology. 2018;9:2928.
- [CrossRef] [Google Scholar]
- Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol.. 2019;10:513.
- [CrossRef] [Google Scholar]
- Postbiotic-Enabled Targeting of the Host-Microbiota-Pathogen Interface: Hints of Antibiotic Decline? Pharmaceutics. 2020;12(7):624.
- [Google Scholar]
- A new antibacterial lipopeptide found by UPLC-MS from an actinomycete Streptomyces sp. HCCB10043. Natural Product Research. 2013;27(23):2190-2195.
- [Google Scholar]
- Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Applied microbiology and biotechnology. 2009;83(3):541-553.
- [Google Scholar]
- Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence.. 2018;9(1):522-554.
- [CrossRef] [Google Scholar]
- Paradigm Shift in Antimicrobial Resistance Pattern of Bacterial Isolates during the COVID-19 Pandemic. Antibiotics. 2021;10(8):954.
- [Google Scholar]
- Roseocin, a novel two-component lantibiotic from an actinomycete. Molecular Microbiology. 2020;113(2):326-337.
- [Google Scholar]
- Marine rare actinomycetes: a promising source of structurally diverse and unique novel natural products. Marine drugs. 2019;17(5):249.
- [Google Scholar]
- Actinomyces produces defensin-like bacteriocins (actifensins) with a highly degenerate structure and broad antimicrobial activity. Journal of bacteriology. 2020;202(4)
- [Google Scholar]
- Metallic nanoparticles induced antibiotic resistance genes attenuation of leachate culturable microbiota: The combined roles of growth inhibition, ion dissolution and oxidative stress. Environment international. 2019;128:407-416.
- [Google Scholar]
- Antimicrobial resistance in the environment: The Indian scenario. The Indian Journal of Medical Research. 2019;149(2):119.
- [Google Scholar]
- Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nature Reviews Microbiology. 2019;17(3):141-155.
- [Google Scholar]
- Co-selection of resistance to antibiotics, biocides and heavy metals, and its relevance to foodborne pathogens. Antibiotics. 2015;4(4):567-604.
- [Google Scholar]
Further reading
- Thoughts and facts about antibiotics: where we are now and where we are heading. The Journal of antibiotics. 2012;65(8):385-395.
- [Google Scholar]
- Marine Actinobacteria as a source of compounds for phytopathogen control: an integrative metabolic-profiling/bioactivity and taxonomical approach. PLoS One. 2017;12(2):e0170148
- [Google Scholar]
- Strategies to combat antimicrobial resistance: anti-plasmid and plasmid curing. FEMS microbiology reviews. 2018;42(6):781-804.
- [Google Scholar]
- Created with BioRender.com.
- Historical events that spawned the field of plasmid biology. Plasmids: Biology and Impact in Biotechnology and Discovery; 2015. p. :1-11.







