Translate this page into:
The effectiveness of Xanthium strumamrium L. extract and Trichoderma spp. against pomegranate isolated pathogenic fungi in Taif, Saudi Arabia
⁎Corresponding authors. m.khyate@tu.edu.sa (Mohamed M. Hassan), elkazafi.taha@agr.kfs.edu.eg (El-Kazafy A. Taha),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Pomegranate (Punica granatum L.) is frequently affected by fungal infections during the pre-and post-harvesting periods, resulting in severe losses to the farming economy. The common pre-and post-harvest pomegranate pathogens affecting the final yield and quality of the fruits are Botrytis cinerea, Alternaria alternata, Penicillium implicatum, and Aspergillus niger. This study aimed to isolate and identify the pathogens causing pomegranate rot diseases. Also, the values of Xanthium strumarium extracts as eco-friendly control agents and three Trichoderma strains as biological control agents against the pomegranate fungal pathogen were determined.
Methods
The plate dilution method was used to isolate fungi populations from pomegranate plants. The pathogens fungi were identified by the morphological and molecular method by sequenced of internal transcript spacer (ITS) region. The antifungal activities of X. strumarium extracted either by both ethanol or methanol and Trichoderma were investigated.
Results and conclusions
The isolated and identified pathogens were P. implicatum, A. alternata, A. niger, Fusarium oxysporum, and F. chlamydosporum. The obtained sequences of these fungi were deposited in NCBI gene bank with accession numbers OK562113–OK562125. The efficiency of X. strumarium extract against pomegranate fungal pathogens ranged from 40.32 to 69.53% for ethanol extract and from 44.40 to 70.28% for methanol extract compared to 44.43%–85.71% for the antifungal Nystatin. Moreover, the efficiency of Trichoderma was 42.22%–72.50%, 42.30%–70.21%, and 44.54%–72.50% for strains ABSA-16, TSA-17, and ABSA-18, respectively. It could be concluded that the isolated pomegranate's pathogenic fungi in Taif were P. implicatum, A. alternata, A. niger, F. oxysporum, and F. chlamydosporum. The extract of X. strumarium obtained from the Taif region and Trichoderma can successfully assist control the pomegranate's pathogenic fungi at pre-and post-harvest, and hence can be successfully included in the IPM programs.
Keywords
Internal transcript spacer
Pathogens
Pomegranate
Taif
Trichoderma
Xanthium strumarium
1 Introduction
Pomegranate, Punica granatum L. (Myrtales: Lythraceae) is an important crop in Taif governorate, Saudi Arabia. The Taif pomegranate cultivation had a long agricultural history in the Taif governorate of the Kingdom of Saudi Arabia (KSA). It is well adapted to high altitudes and occupies vast cultivation areas in western KSA, such as Taif and Abha (Gaber et al., 2015). Whether eaten fresh or juiced, pomegranate fruits contain necessary nutrients for human health and several mineral elements (Cheurfa et al., 2020; Sorrenti et al., 2019). In addition, pomegranate fruit contains relatively high amounts of pharmacologically critical bioactive compounds that act as antioxidants, antimicrobials, antivirals, anticancer, vascular protective effects, and alleviate inflammatory bowel diseases (Magangana et al., 2020). Pomegranate trees are highly suitable for newly reclaimed areas because the temperatures are moderate in summer and not like the low-altitude regions of Saudi Arabia (Gaber et al., 2015).
The pomegranate trees are susceptible to many fungal and bacterial diseases that infect the whole plant including the fruits during their growth and even after harvest. Among these diseases, rot diseases are the most dangerous diseases in KSA, where rot diseases weaken the trees by diminishing the shoot system of the trees. Recent studies have shown different pathogens cause rot diseases in pomegranate, which include Botrytis cinerea (Nakasugi et al., 2021), B. phaeriadothidea (Rosas-Burgos et al., 2017), P. implicatum, A. niger (Parveen et al., 2017a), A. alternate (Yao, 2017) and Fusarium oxysporium (Mariolatry et al., 2022). Since pomegranate is an important fruit crop in the Taif region, it is necessary to isolate and identify the microbial pathogens that threaten this crop while considering the effective biological methods in combating and preventing these diseases. Many morphological and physiological characteristics have already been employed to identify these pathogens. Furthermore, in recent years, DNA sequencing for molecular identification, which commonly depends on the sequence of the internal transcript spacer (ITS) region of the nuclear ribosomal DNA has been used (Hassan et al., 2019; Mazrou et al., 2020b).
Controlling rot diseases in pomegranates with chemical fungicidal sprays may result in soil and environmental pollution, endangering human health (Nakasugi et al., 2021). On the other hand, biological control is considered one of the safest options for human health and environmental preservation (Mazrou et al., 2020a). The X. strumarium is a traditional medicinal plant in Saudi Arabia that belongs to the Asteraceae family. It has been demonstrated a broad variety of biological actions against bacteria and fungi (Rad et al., 2013; Parveen et al., 2017b). Xanthium extracts have been shown to exhibit antifungal properties against a wide range of plant pathogenic fungi including A. niger, A. flavus, Fusarium spp., and A. alternata (Rad et al., 2013). We may hypothesize that the X. strumarium extract may have a significant impact on some pomegranate pathogenic fungi. Hence, the current study aimed to isolate and identify the pomegranate pathogenic fungi in the Taif region. Moreover, the efficacy of X. strumarium extracts as ecofriendly control agents and three Trichoderma strains as biocontrol agents was evaluated against the pomegranate pathogenic fungi.
2 Materials and methods
2.1 The experimental samples and location
Thirty different pomegranates, P. granatum L. plants and rhizosphere soil samples were collected from four farms in various areas of the Taif (21°16′30.34″N 40°24′22.16″E) governorate, Mecca Province, Saudi Arabia (Fig. 1). Taif Governorate is located at an altitude of 1,879 m (6,165 ft) high in the Hijaz Mountains, which are part of the Sarawat mountain ranges. Taif Governorate enjoys a moderate desert climate, with partly hot summers and mild winters. Temperatures are moderate in summer and not like in the low-altitude regions of Saudi Arabia such as Makkah and Jeddah. The weather is cooler in Taif during the summer than it is in other parts of the Kingdom of Saudi Arabia, especially in the open mountainous areas. Precipitation in moderate amounts throughout the year and increases in the spring and autumn compared to the rest of the year (El-Tarras et al., 2013).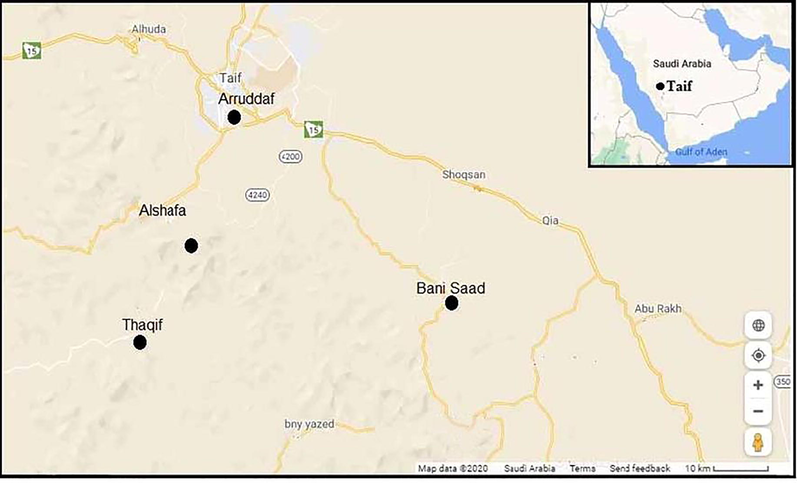
A map shows Taif's geographical location where samples were collected. Farm 1: Alrruddaf, Farm 2: Al-Shafa, Farm 3: Bani-Saaf and Farm 4: Thaqif.
2.2 Pomegranate pathogenic fungi isolation
The plate dilution method was used to isolate fungi populations from plants and soil taken from the rhizosphere (Hassan et al., 2019). For isolation from rhizospheric soil, approximately 1 g of rhizospheric soil was homogenized with 9 ml sterilized distilled water, shaken for 30 min and the fungi were cultured on the potato dextrose agar (PDA) medium and incubated at 28 °C for 5 days. Regarding the isolation from the plant samples, the infected leaves and fruits with fungi were scraped on the PDA medium and incubated at 28 °C for 5 days. The obtained fungi were preliminarily identified based on their morphological, conidial, and cultural properties and microscope with a digital camera using a lactophenol cotton blue-stained slide mounted with a small portion of the mycelium (Gaddeyya et al., 2012).
2.3 DNA extraction
The pathogenic fungi mycelia were inoculated for five days into Czapex Dox broth. Then the simple method for extraction of fungal genomic DNA (Al-Samarrai and Schmid, 2000) was used for genomic DNA extraction.
2.4 Sequence analysis of 5.8S-ITS region
The ITS1 and ITS4 primers were used to amplify the ITS region with the PCR conditions according to Mazrou et al. (2020a). The PCR products were sequenced by Macrogen International Co. Seoul, Korea. Multiple nucleotide alignment of the ITS regions was performed using BioEdit version 7.2.5 software then The BLAST tool was chosen to compare the obtained sequences and related sequences in the NCBI database. The phylogenetic tree was drawn using MEGA software version 7.0.
2.5 Xanthium strumarium L. extraction
In September 2020, fresh leaves of cocklebur, X. strumarium plants were collected from their natural habitat in the Al-Hada (21°22′07″N 40°17′05″E) region near the Taif Governorate, Mecca Province, Saudi Arabia. The fresh leaves were air-dried and ground into a fine powder, then it was extracted with 100 ml ethanol or methanol (95%) at room temperature for 3 days. Each extract (pellets) was dissolved in an aqueous solution of dimethyl sulfoxide 1% (DMSO). Then, the extracts were stored at 4 °C until they were used for the experiments and HPLC analysis.
2.6 HPLC analysis for phenol and flavonoid compounds
The analysis and detection of Phenol and flavonoid compounds for the tested extracts were conducted according to Lu et al. (2011) with minor modifications using Agilent 1260 Infinity HPLC Series (Agilent, USA), equipped with a quaternary pump. Kinetex® 5 µm EVO C18 100 mm × 4.6 mm, (Phenomenex, USA) was used as the column and operated at 30 °C.
2.7 Antifungal activity of X. strumarium extracts
The antifungal activities of X. strumarium extracted by both ethanol and methanol were investigated using the agar disc diffusion method against the isolated pomegranate pathogens including P. implicatum, A. alternata, A. niger, F. oxysporum, and F. chlamydosporum. Seven-day-old fungal culture was cut aseptically with a sterile needle of generally 5 mm diameter and inoculated upside down on the center of the PDA. Three replicates of each extract were incubated for 7 days at concentrations of 100 mg/mL, as well as the positive control Nystatin fungicide (100 µg/mL) at a temperature of 28 ± 1 °C for fungi. Nystatin USP powder was purchased from Medisca Inc. (Montreal, Canada). The fungal growth was measured on the 7th day of incubation (Gaber et al., 2015). The percentage of linear growth reduction of pathogenic fungi compared with control was calculated using the formula given by Khalil and Dababneh (2007).
where R1 = radius of the untreated pathogen and R2 = radius of the treated pathogen.
2.8 Antagonistic activity against some pomegranate pathogens
The biocontrol activities of Trichoderma (Hypocreales: Hypocreaceae) strains (strains ABSA16, TSA17, and ABSA18) with accession numbers MK680282, MK680283, and MK680282, respectively were examined in two sets against the following pomegranate pathogenic fungi: Penicillium implicatum Biourge (Eurotiales: Trichocomaceae), Alternaria alternata (Fr.) Keissl. (Pleosporales: Pleosporaceae), Aspergillus niger van Tieghem (Eurotiales: Trichocomaceae), Fusarium oxysporum Schlecht (Hypocreales: Nectriaceae), and F. chlamydosporum Wollenw. & Reinking by the dual culture technique using PDA medium as described by Fahmi et al. (2012).
2.9 Statistical analysis
The means of Trichoderma antagonistic activity and both extracts of X. strumarium against pomegranate pathogens were analyzed using Pearson’s simple linear correlation coefficient (r) test with SPSS software (SPSS, 2006). The data are shown as means ± standard error, and differences with p < 0.05 were considered to be significant.
3 Results
3.1 Observation of the rot symptoms
Both types of disease symptoms, such as soft and dry rot were noticed on the pomegranates. The soft rot lesions in the fruits had a mushy appearance; with a yellowish-brown coat and dark color at the edge (the blue dotted line indicates the rotten area as shown in Fig. 2a). Black-spotted mildew appeared on the lesion at the late stage of infection (Fig. 2a). In the dry rot case, the lesions were dry and brown, with an irregular shape (Fig. 2b). As shown in Fig. 2c the fruit was infected with Penicillium, Fig. 2d depicted Aspergillus-infected fruit, Fig. 2e depicted dry rot within the fruit, and Fig. 2f depicted soft rot within the fruit.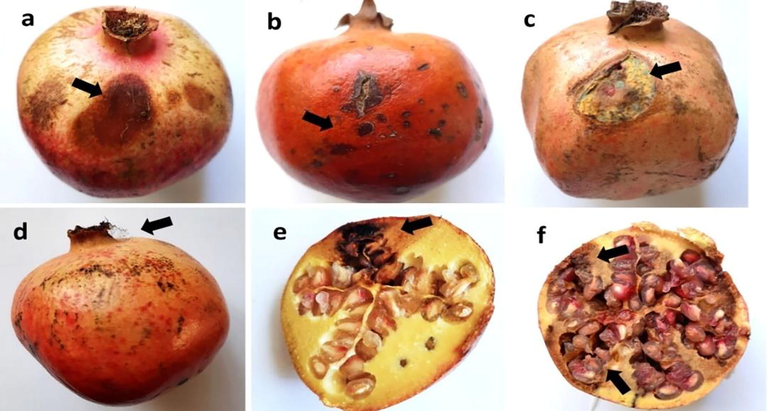
Symptoms of the infected pomegranate fruit: soft rot (a), dry rot (b), infected fruit with Penicillium (c), infected fruit with Aspergillus (d), dry rot inside the fruit (e), and soft rot inside the fruit.
3.2 Morphological identification
A total of 149 fungal isolates were collected from the infected pomegranate plants and the rhizosphere of different regions in Taif, Saudi Arabia (Table 1and Fig. 3). Twenty-two of them were fast-growing in shades of green, sometimes white, with dense conidiophores which identified as Penicillium sp. Twenty-one isolates were identified as Alternaria sp.; their conidia were of muriform shape and light brown. Forty-eight isolates were classified as Aspergillus sp. Their colonies grew on PDA, having initially white floccose mycelium spread rapidly, turning quickly into black color colonies due to producing black spores. Fifteen isolates were identified as Fusarium sp., with dirty white dense mycelia growing in thick concentric rings, and yellow pigmentation at the periphery was also found. Some isolates showed light pink pigmentation in the center. The eighteen isolates, which grown on a PDA media; they formed several concentric rings with green conidia that increased in density in the center and then spread to the extremities, which were known to be Trichoderma spp. Finally, the isolates were numbered sequentially and stored for the next experiment. Whereas, Farm 1: Alrruddaf, Farm 2: Al-Shafa, Farm 3: Bani-Saaf and Farm 4: Thaqif.
Fungi
Place of collection
No. isolates
Location 1
Location 2
Location 3
Location 4
Penicillium sp.
6.00
5.00
4.00
7.00
22.00
Alternaria sp.
3.00
12.00
2.00
4.00
21.00
Aspergillus sp.
12.00
9.00
15.00
12.00
48.00
Fusarium sp.
6.00
4.00
2.00
3.00
15.00
Trichoderma sp.
4.00
6.00
2.00
6.00
18.00
Others
6.00
7.00
5.00
7.00
25.00
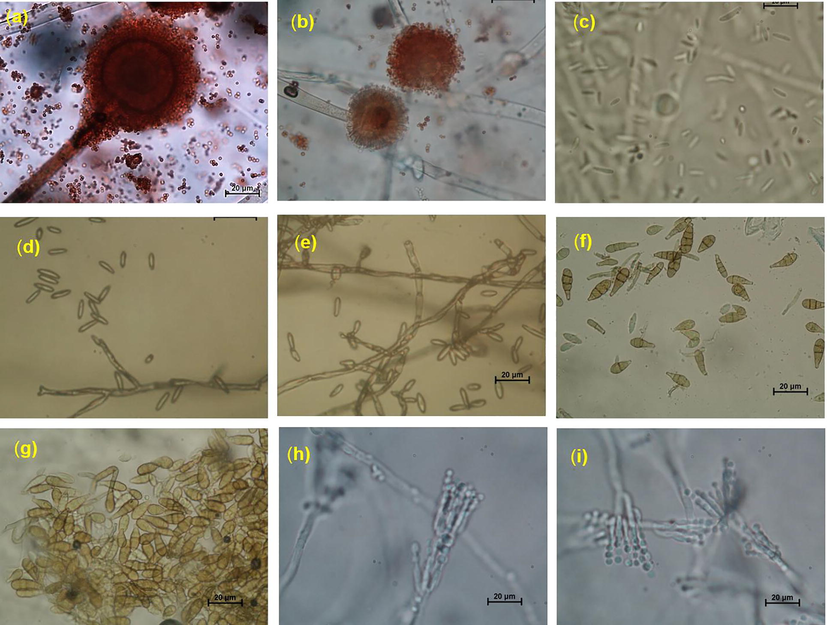
Microscopic image of pomegranate pathogenic fungi, a and b: conidiophore, conidial head and conidia of A. niger, c: conidia of F. oxysporium, d and e: conidiophore and conidia of F. chlamydosporum, f and g: conidia of A. alternata, h and i: conidiophore, philidia and conidia of P. implicatum.
3.3 Fungal isolates and phylogenetic analysis
The ITS region sequencing is a powerful tool for identifying fungal species. Data in Table 2 display the results of NCBI BLAST queries for the 13 pomegranates pathogenic fungus isolates chosen. For the 13 fungi isolates queries, E values were zero, which indicated non-chanced alignments. The identifying percentage with similar species ranged from 96% with P. implicatum TU-1 to 99% with A. niger TU-7. According to the phylogenetic tree (Fig. 4), the three isolates TU-1, TU-2, and TU-3 were identified as P. implicatum. Nucleotide comparisons of ITS regions among P. implicatum strains and other similar strains from NCBI revealed that P. implicatum TU-1, TU-2, and TU-3 strains exhibited 97, 98, and 99% similarity with strain P. implicatum MF687276; 98, 97, and 96% identity between A. niger MW081366 from the GenBank and each of A. niger TU-7, TU-8, and TU-9 strains. Fusarium oxysporum TU-10 and TU-11 showed high genetic similarity with about 99 and 98% with strain F. oxysporum OK087316, whereas F. chlamydosporum TU-12 and TU-13 showed genetic similarity with 99 and 98% with the strain F. chlamydosporum MZ914682. Alternaria alternate TU-4 and TU-5 displayed moderate genetic similarity ranging from 96 and 98% with strain A. alternata MH237643; while A. alternata TU-6 was highly similar to the same strain A. alternata MH237643 from NCBI database.
Isolates
Species
Query
coverage %E
ValueIdent %
Accession number
TU-1
Penicillium implicatum
98.00
0.00
96.00
OK562113
TU-2
P. implicatum
99.00
0.00
97.00
OK562114
TU-3
P. implicatum
99.00
0.00
98.00
OK562115
TU-4
Alternaria alternata
99.00
0.00
98.00
OK562116
TU-5
A. alternata
98.00
0.00
96.00
OK562117
TU-6
A. alternata
99.00
0.00
99.00
OK562118
TU-7
Aspergillus niger
100.00
0.00
99.00
OK562119
TU-8
A. niger
100.00
0.00
98.00
OK562120
TU-9
A. niger
100.00
0.00
97.00
OK562121
TU-10
Fusarium oxysporum
100.00
0.00
99.00
OK562122
TU-11
F. oxysporum
99.00
0.00
98.00
OK562123
TU-12
F. chlamydosporum
100.00
0.00
99.00
OK562124
TU-13
F. chlamydosporum
100.00
0.00
98.00
OK562125
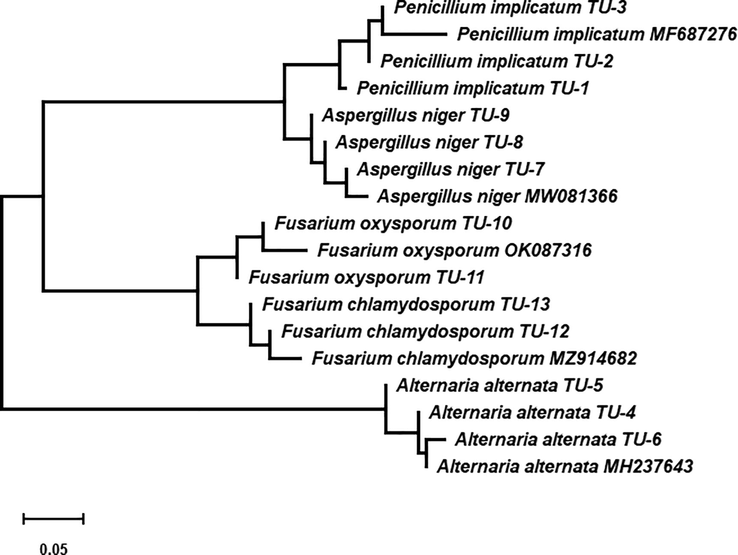
Phylogenetic tree and diversity of 5.8S-ITS region in some pomegranate pathogenic fungi isolated from different regions of Taif, Saudi Arabia.
3.4 Phenol and flavonoid compounds in X. strumarium
The extraction of phenols and flavonoids from two extracts using two polar solvents (Ethanol and Methanol) yielded a variety of chemicals depending on the chemical composition and solvent used. A large amount of kaempferol, followed by resveratrol and myricetin, were extracted by ethanol and methanol. Moderate amounts of rosmarinic acid, ellagic acid cinnamic acid, caffeic acid, o-coumaric acid, and rutin followed by low amounts of catechol, chlorogenic acid, catechin, p-coumaric acid, p-hydroxybenzoic acid, syringic acid, and quinol were found in the extracts (Table 3). On the other hand, naringenin, vanillic acid, gallic acid, 3-hydroxytyrosol, and pyrogallol were non detected in the extracts due to the few ingredients in the tested extracts. The presence of hydroxyl and carboxylic groups, which were bound to the solvents by hydrogen bonds, revealed the high polarity of these compounds in the chemical structures of the extracted components (Table 3). ND = non-detected (less than the instrument sensitivity).
Compounds
Xanthium-ethanol
Xanthium-methanol
Kaempferol
5525.58
7612.25
Resvertol
4060.61
3323.80
Myricetin
1451.58
1421.75
o-Coumaric acid
85.91
123.80
Caffeic acid
74.40
317.70
Quercitin
59.50
1456.90
Rosmarinic acid
57.60
629.50
Cinnamic acid
52.92
112.60
Chlorogenic acid
19.00
75.19
Syringic acid
15.70
45.84
Quinol
12.20
18.20
Benzoic acid
553.30
ND
Ferulic acid
17.25
ND
Ellagic acid
ND
748.35
Rutin
ND
116.03
Catechol
ND
85.05
Catechin
ND
56.13
p-Hydroxy benzoic acid
ND
43.37
p-Coumaric acid
ND
32.58
Vanillic acid
ND
ND
Gallic acid
ND
ND
3-Hydroxytyrosol
ND
ND
Naringenin
ND
ND
Pyrogallol
ND
ND
3.5 Antifungal activity of X. strumarium extracts
Data in Table 4 and Fig. 5 show the average % inhibition of pomegranate pathogen mycelial growth after treatment with ethanol and methanol extracts at concentrations of 100 g/mL, as well as the positive control Nystatin fungicide (100 µg/mL). Both ethanol and methanol extracts could inhibit the growth of pomegranate pathogens mycelia. The reduction percentage in the pathogen's growth varied between 40.32 ± 1.01% and 69.53 ± 1.10% with ethanol extract and between 44.40 ± 0.89% and 74.28 ± 0.85% with methanol extract compared to the Nystatin fungicide that ranged from 44.43 ± 1.07% to 85.71 ± 1.17%. The fungicide treatment showed the highest inhibition percentage (85.71%) against P. implicatum. When compared to the fungicide treatment, the methanol extract inhibited the mycelial growth of P. implicatum much more, with an inhibition percentage of 74.28 ± 0.85%, followed by the ethanol extract (65.56 ± 1.85%) Interestingly, A. alternata was more significantly inhibited (70.40 ± 1.07%) by methanol extract followed by ethanol extract (69.43 ± 0.99%), whereas fungicide treatment was the lowest % inhibition (66.67 ± 0.54%). Fusarium oxysporum and F. chlamydosporum were significantly (50.00 ± 1.16%) inhibited by methanol extract, followed by fungicide treatment (48.89 ± 1.14%), while ethanol extract treatment recorded the lowest inhibition percentage (42.22 ± 0.93%). Moreover, In Trichoderma, the mycelial growth was significantly (79.63 ± 1.11%) inhibited by fungicide treatment followed by methanol extract (59.36 ± 1.08%), while ethanol extract showed the lowest inhibition percentage (53.12 ± 0.91%). Values are the mean ± standard error. The means in each column followed by the same letter are not significantly different at P ≤ 0.05.
Pathogenic fungi
% Inhibition zone
Fungicide (Nystatin)
Ethanol extract
Methanol
extract
Penicillium implicatum – TU-1
85.71 ± 1.17a
65.56 ± 1.85c
74.28 ± 0.85a
P. implicatum – TU-2
82.75 ± 1.03c
59.89 ± 0.99e
68.96 ± 0.94 cd
P. implicatum – TU-3
83.33 ± 0.94b
63.40 ± 0.73d
70.22 ± 1.32b
Alternaria alternata – TU-4
66.67 ± 0.54f
65.78 ± 0.91c
67.20 ± 1.11d
A. alternata – TU-5
64.50 ± 1.03 g
68.55 ± 1.06b
69.43 ± 0.99c
A. alternata – TU-6
65.61 ± 0.87 fg
69.53 ± 1.10a
70.40 ± 1.07b
Aspergillus niger – TU-7
82.50 ± 0.93c
57.89 ± 0.63f
66.43 ± 1.03e
A. niger – TU-8
77.63 ± 1.34e
50.21 ± 0.82 h
69.22 ± 0.67c
A. niger – TU-9
80.40 ± 0.88d
54.10 ± 1.01 g
64.78 ± 0.19f
Fusarium oxysporum – TU-10
46.76 ± 0.69i
44.45 ± 1.21i
47.56 ± 0.97 h
F. oxysporum – TU-11
44.43 ± 1.07j
40.32 ± 1.01 k
44.40 ± 0.89j
F. chlamydosporum – TU-12
48.89 ± 1.14 h
42.22 ± 0.93j
46.67 ± 1.02i
F. chlamydosporum – TU-13
48.70 ± 0.68 h
42.43 ± 0.89j
50.00 ± 1.16 g
Trichoderma – TSA-16
67.76 ± 0.71f
53.12 ± 0.91 g
64.40 ± 0.89f
Trichoderma – TSA-17
77.63 ± 0.84e
56.28 ± 1.33f
69.67 ± 1.24c
Trichoderma – TSA-18
79.63 ± 1.11d
59.36 ± 1.08e
73.69 ± 0.52b
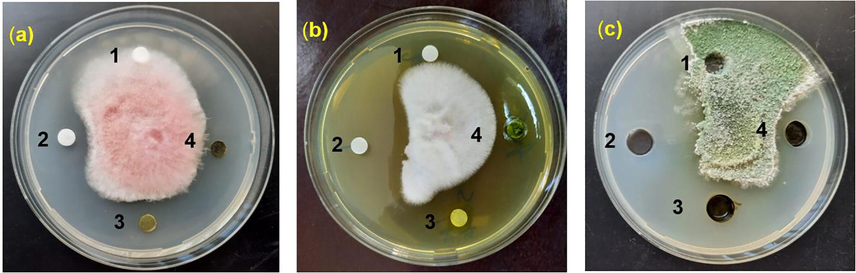
Antifungal activity of Xanthium strumarium extracts against various pomegranate pathogenic fungi, (a) is F. chlamydosporum, (b) is F. oxysporium, and (c) is T. harzianum. Whereas, 1: Negative control (duple distilled water); 2: Positive control (100 µg/ml Nystatin), 3: Xanthium ethanol extract, and 4: Xanthium ethanol extract.
3.6 Antagonistic ability of Trichoderma spp. in dual culture
The antagonistic effects of three Trichoderma strains (TSA-16, TSA-17, and TSA-18) were tested against pomegranate pathogens (P. implicatum, A. alternata, A. niger, F. oxysporum, and F. chlamydosporum) on PDA medium. Only plant pathogen species and Trichoderma isolates were inoculated in the negative control plates. The contact zone was a curve in all the tested dual culture plates, with concavity oriented towards the pomegranate pathogen (Table 5 and Fig. 6). The reduction percentage in the growth of these pathogens varied between 33.33 ± 1.01% and 72.50 ± 1.21%. Among all Trichoderma strains, ABSA-16 strain showed a significantly higher % inhibition of the mycelial growth of P. implicatum with 72.50 ± 1.21% inhibition followed by strain TSA-17 (70.21 ± 0.97%). In comparison, ABSA-18 gave the lowest inhibition percentage (66.67 ± 1.11%) against P. implicatum. Moreover, A. alternata was more significantly (72.50 ± 0.97%) inhibited by ABSA-18 strain followed by TSA-17 strain (69.33 ± 0.89%), whereas ABSA-16 gave the lowest % inhibition (67.50 ± 0.99%). Mycelial growth of A. niger was significantly (56.67 ± 0.96 and 56.67 ± 0.74%) inhibited by ABSA-16 and ABSA-18 strains, followed by TSA-17 strain which showed the lowest inhibition (33.33 ± 1.01%). Fusarium oxysporum and F. chlamydosporum were significantly (65.71 ± 0.91%) inhibited by ABSA-18 strain, followed by ABSA-16 strain (57.77 ± 0.95%), while TSA-17 strain showed the lowest % inhibition (51.35 ± 0.92%). Values are the mean ± standard error. The means in each column followed by the same letter are not significantly different at P ≤ 0.05.
Pathogenic fungi
% Antagonistic potential inhibition
ABSA-16
TSA-17
ABSA-18
Penicillium implicatum – TU-1
72.50 ± 1.21a
70.21 ± 0.97a
65.00 ± 1.08c
P. implicatum – TU-2
72.50 ± 1.07a
70.21 ± 1.10a
63.30 ± 0.82d
P. implicatum – TU-3
68.76 ± 0.89b
65.72 ± 1.02d
66.67 ± 1.11b
Alternaria alternata – TU-4
67.50 ± 0.99b
69.33 ± 0.89b
72.50 ± 0.97a
A. alternata – TU-5
58.80 ± 1.02c
55.46 ± 0.93e
62.10 ± 0.89 g
A. alternata – TU-6
57.61 ± 0.72d
67.53 ± 0.76c
60.00 ± 1.00 h
Aspergillus niger – TU-7
51.11 ± 1.10f
44.62 ± 0.61 g
56.67 ± 0.74i
A. niger – TU-8
56.67 ± 0.96de
33.33 ± 1.01i
52.22 ± 1.04j
A. niger – TU-9
42.22 ± 1.01 h
42.30 ± 1.11 h
44.54 ± 1.13 k
Fusarium oxysporum – TU-10
45.71 ± 0.87 g
55.26 ± 0.78e
65.71 ± 0.91c
F. oxysporum – TU-11
56.75 ± 1.09de
51.35 ± 0.92f
62.16 ± 0.99 g
F. chlamydosporum – TU-12
55.56 ± 1.12e
52.51 ± 0.88f
64.44 ± 0.84e
F. chlamydosporum – TU-13
57.77 ± 0.95d
54.27 ± 1.09ef
62.22 ± 1.18 g
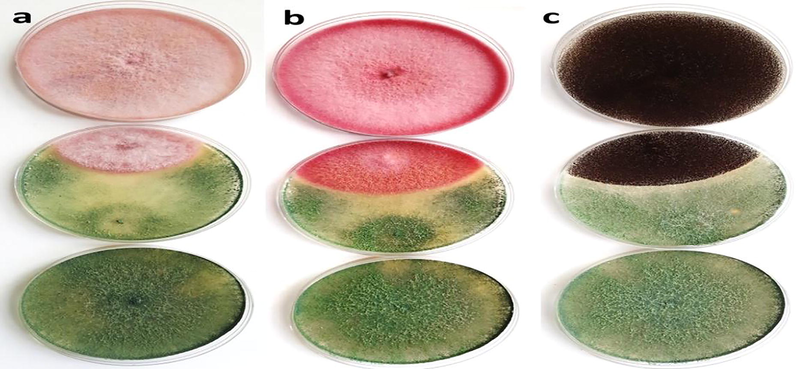
Antagonistic activity (in vitro) of the Trichoderma sp. against (A) F. oxysporum (B) F. chlamydosporum and (C) A. niger on PDA plates. Whereas the top plate is a pathogen, the bottom plate is Trichoderma as biological control, and the plate in the middle is the interaction between pathogen and Trichoderma.
4 Discussion
The ITS region sequencing is a powerful tool for identifying fungal species. It is difficult to precisely identify fungi based just on their morphological characteristics, therefore, in such tasks, integration of morphological and molecular parameters has been employed. DNA coding is a new method for reliable and rapid identification of different fungi at the species level (Hassan et al., 2019). The nrITS region is the global barcode for fungi, and the ITS1 and ITS2 subregions have been applied as metabolic markers (Hassan et al., 2019; Mazrou et al., 2020b). According to recent research, the ITS region sequence is one of the most successful approaches for identifying fungal strains through molecular analysis. Molecular identification to species level of fungi using ITS region sequencing has recently been used for accurate identification of Penicillium species (Visagie et al., 2014), Fusarium (Carvalhais et al., 2019), Trichoderma (Hassan et al., 2019), Alternaria species (Mohammadi and Bahramikia, 2019), and Aspergillus species (Mazrou et al., 2020b).
Due to the similarity of the molecular results with the morphological and microscopic results that helped in the identification of pathogens isolated from pomegranate, scientific approaches were employed in this research to identify distinct pomegranate pathogens (Rosas-Burgos et al., 2017; Nakasugi et al., 2021). By integrating the results of morphological analyzes, such as culture appearance and conidia morphology, with molecular analyzes and determination of the ITS region sequencing, different pathogens that are responsible for the rot diseases of pomegranate fruit were reported previously, such as Botrytis cinerea (Nakasugi et al., 2021), Fusarium spp. (Rosas-Burgos et al., 2017), P. implicatum, A. niger (Parveen et al., 2017a), A. alternate (Yao, 2017) and Fusarium oxysporium (Mariolatry et al., 2022).
Plant extracts and bio-control agents may exert significant biological activity against plant fungal pathogens and thus may be used as bio-fungicidal agents (Romanazzi et al., 2012; Cheurfa et al., 2020). These products offering as a selective to a specific target with shorter shelf life, limited field persistence, and with no residual threats. These offer an important role to play in integrated pest management (IPM) programs (Nuzhat and Vidyasagar, 2013). The extract of X. strumarium is a traditional medicinal plant in KSA, showing a wide range of antifungal effects on different fungi such as A.niger, A. flavus, Fusarium spp., and A. alternata (Parveen et al., 2017b). The extract exhibit greater potentiality to anti-fungal action due to the presence of terpenes (Bisht and Singh, 1978) followed by the compound named ‘acetyl xanthumin’ as it dominates the fungal derivative. The mycelial growth of F. moniliforme can be inhibited by the extract of the plant (Kishore et al., 1982). The extract activity suppresses the action of fluconazole and hexane content (Amerjothy et al., 2007) against A. fumigatus by increasing the activity of the free-chemical group. Furthermore, treatment with X. strumarium extracts inhibited the growth of a wide range of fungal strains including A. niger, A. flavus, F. oxsporum, F. solni, A. alternate, and P. digitatum (Parveen et al., 2017b). Thus, the high concentration of X. strumarium extract gave high antifungal action to protect pomegranate against various pathogenic fruit rot fungi and it is considered a highly effective and cost-effective approach.
Trichoderma species are widely used as biocontrol agents due to their efficient utilization of nutrients, high reproductive capacity, and strong aggressiveness against other pathogenic organisms. The main strategy of Trichoderma spp. to inhibit other pathogens involves mycoparasitism. Krauss et al. (1998) have shown that the Trichoderma spp. can mycoparasitic various pathogens viz., Botryodiplodia theobromae, Colletotrichum spp., Fusarium spp., and Aspergillus sp. The results of paired culture of Trichoderma with Penicillium spp., Alternaria sp., Aspergillus spp., Fusarium spp. inoculated on potato dextrose agar medium showed that the hyphae of the antagonistic Trichoderma inhibited Penicillium spp. mycelia when they came in contact with each other. The death of the pathogen was due to starvation resulting from the competition for limited nutrients and space (Siameto et al., 2011; Hassan et al., 2019). The inhibition of mycelial growth of Penicellium sp. by dual culture could be due to its fast-growing nature. Similarly, Calistru et al. (1997) have shown that the production of volatile substances of T. harzianum and T. viride inhibited the growth of F. moniliforme and A. flavus. Also, the antagonistic properties of Trichoderma sp. against Aspergillus sp. and Penicillium spp. have been reported (Agarwal et al., 2011). Kishore et al. (2001) have demonstrated that in vitro the Trichoderma spp. significantly reduced the radial growth of Aspergillus spp. Besides, Trichoderma isolates significantly inhibited Aspergillus spp. growth (Rao and Sitaramaih, 2000).
5 Conclusions
Rot disease is the main problem for the growth and production of pomegranate trees and fruit that have grown under Taif Governrate. The isolated pathogens were P. implicatum, A. alternata, A. niger, F. oxysporum, and F. chlamydosporum, based on morphological and molecular identification. The X. strumarium extracts and Trichoderma strains showed strong inhibitory effects on most pathogenic fungi. The superior treatments were Trichoderma in most cases. This indicated that the X. strumarium or/and Trichoderma can be used as a natural and safe alternative to synthetic pesticides. Moreover, it is necessary to complete the study of this part to know the exact names of the active substances and their mechanism of action in the X. strumarium extract. Accordingly, the present study demonstrated that locally available botanicals such as X. strumarium and biocontrol agents such as Trichoderma can successfully assist control the pomegranate’s pathogenic fungi at pre-and post-harvest, and hence can be successfully included in IPM programs.
Acknowledgements
The authors express their deep thanks to the Ministry of Education represented by the Agency for Research and Innovation, in Saudi Arabia for its contribution in funding the current research work through Project No. 1-441-128.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In vitro interaction of Trichoderma isolates against Aspergillus niger, Chaetomium sp. and Penicillium sp. Indian J. Fund. Appl. Life Sci.. 2011;1:125-128.
- [Google Scholar]
- A simple method for extraction of fungal genomic DNA. Lett. Appl. Microbiol.. 2000;30(1):53-56.
- [Google Scholar]
- Antimicrobial assay of the leaf extracts of Xanthium indicum Koen. Pharm. Mag.: Short Commun.. 2007;11:197.
- [Google Scholar]
- Chemical Investigation of the leaves of Xanthium indicum L. J. Indian Chem. Soc.. 1978;55:707-708.
- [Google Scholar]
- In Vitro studies on the potential for biological control of Aspergillus flavus and Fusarium moniliforme by Trichoderma species. Mycopathology. 1997;137:115-124.
- [Google Scholar]
- Molecular diagnostics of banana fusarium wilt targeting secreted–in–xylem genes. Front. Plant Sci.. 2019;10:547.
- [Google Scholar]
- Antioxidant and anti-diabetic activity of pomegranate (Punica granatum L.) leaves extracts. Foods Raw Mate.. 2020;8:329-336.
- [Google Scholar]
- Evaluation of the genetic effects of the in vitro antimicrobial activities of Rhazya stricta leaf extract using molecular techniques and SEM. Afr. J. Biotechnol.. 2013;12:3171-3180.
- [Google Scholar]
- Protoplast fusion enhances antagonistic activity in Trichoderma sp. Nat. Sci.. 2012;10:100-106.
- [Google Scholar]
- In vitro Antimicrobial comparison of Taif and Egyptian pomegranate peels and seeds extracts. J. Appl. Biol. Biotechnol.. 2015;3:12-17.
- [Google Scholar]
- Isolation and identification of soil mycoflora in different crop fields at Salur Mandal. Adv. Appl. Sci. Res.. 2012;3:2020-2026.
- [Google Scholar]
- Rapid identification of Trichoderma koningiopsis and Trichoderma longibrachiatumusing sequence characterized amplified region markers. Egypt. J. Biol. Pest.. 2019;29:13.
- [Google Scholar]
- Inhibition of phytopathogenic fungi by extracts from medicinal plants in Jordan. Int. J. Biol. Sci.. 2007;7(3):579-581.
- [Google Scholar]
- Biological control of crown rot groundnut by Trichoderma harzianum and T. viride. Int. Arachis Newslet.. 2001;21:39-40.
- [Google Scholar]
- Fungi toxicity of the leaf extracts of some higher plants against Fusarium moniliforme. Nat. Acad. Sci. Lett.. 1982;5:9-10.
- [Google Scholar]
- Isolation and preliminary evaluation of mycoparasites as biocontrol agents of crown rot of banana. Biol. Cont.. 1998;13(2):111-119.
- [Google Scholar]
- Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res. Int.. 2011;44(2):530-536.
- [Google Scholar]
- Processing factors affecting the phytochemical and nutritional properties of pomegranate (Punica granatum L.) peel waste. Molecules. 2020;25:4690.
- [Google Scholar]
- Antagonistic activity and molecular characterization of biological control agent Trichoderma harzianum from Saudi Arabia. Egypt. J. Biol. Pest Cont.. 2020;30:4.
- [Google Scholar]
- Influence of chitinase production on the antagonistic activity of Trichoderma against plant-pathogenic fungi. J. Environ. Biol.. 2020;41:1501-1510.
- [Google Scholar]
- Molecular identification and genetic variation of Alternaria species isolated from tomatoes using ITS1 sequencing and inter simple sequence repeat methods. Curr. Med. Mycol.. 2019;5:1-8.
- [Google Scholar]
- Antifungal and antiaflatoxigenic activities of thymol and carvacrol against Aspergillus flavus. Saúde Pesqui.. 2021;14:113-123.
- [Google Scholar]
- Antifungal investigations on plant essential oils. A review. Int. J. Pharm. Pharm. Sci.. 2013;5:19-28.
- [Google Scholar]
- Antimycotic potential of some phytoextracts on some pathogenic fungi. J. Biopest.. 2017;10:60-65.
- [Google Scholar]
- Chemical composition and antifungal activity of essential oil from Xanthium strumarium L. leaves. Indian J. Pharm. Sci.. 2017;79:316-321.
- [Google Scholar]
- In-vitro antioxidant and antibacterial activities of Xanthium strumarium L. extracts on methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Ancient Sci. Life.. 2013;33:107-111.
- [Google Scholar]
- Management of collar rot disease (A. niger) in groundnut with Trichoderma spp. J. Mycol. Plant Pathol.. 2000;30:221-224.
- [Google Scholar]
- Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Post-harvest Biol. Technol.. 2012;63(1):141-147.
- [Google Scholar]
- Antimicrobial activity of pomegranate peel extracts as affected by cultivar. J. Sci. Food Agric.. 2017;97(3):802-810.
- [Google Scholar]
- Molecular characterization and identification of biocontrol isolates of Trichoderma harzianum from Embu District, Kenya. Trop. Subtrop. Agroecosyst.. 2011;13:81-90.
- [Google Scholar]
- Beneficial effects of pomegranate peel extract and probiotics on pre-adipocyte differentiation. Front. Microbiol.. 2019;10:660.
- [Google Scholar]
- SPSS. 2006. SPSS 15.0 for Windows. SPSS Inc. Chicago, IL
- Identification and nomenclature of the genus Penicillium. St. Mycol.. 2014;78(1):343-371.
- [Google Scholar]
- Study on Postharvest Quality Deterioration and Control Technology of Pomegranate. Chengdu China: Sichuan Agric. Univ.; 2017. Ph.D. Thesis







