Translate this page into:
The effect of tomato on weight, body mass index, blood pressure and inflammatory factors: A systematic review and dose-response meta-analysis of randomized controlled trials
⁎Corresponding author. 13931348206wyx@sina.com (Yuxiao Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Tomatoes contain active phytochemicals, including phytoene, phytofluene, beta-caroten, flavonoids, carotenoids, and lycopene but theirs effects on Weight, Body Mass Index, Blood Pressure and Inflammatory Factors is controversial. The aim of this systematic review and meta-analyze is reported the effects of Tomato consumption on Weight, Body Mass Index, Blood Pressure and Inflammatory Factors such as CRP and IL-6 in Randomized Controlled Trials. Literature search was conducted by a systematic search in PubMed/MEDLINE, Scopus, and Web of sciences (WOB) up to August 2019. There was no language or release time data restrictions. Two researcher conducted data extraction, independently. Dersimonian-laird random effect models were conducted to combining result of included studies. PRISMA guidelines (Preferred Items for Reporting of Systematic Reviews and Meta-Analyses) used for this meta-analysis. Nine studies met inclusion criteria for this meta-analysis. Combined results show not significant change in weight of participants (WMD: −0.03 kg, 95%CI: −0.59, 0.53, Pheterogeneity = 0.98) and BMI by tomato intervention (WMD: 0.07 kg/m2, 95%CI: −0.14, 0.29, Pheterogeneity = 0.99). Furthermore, blood pressure change was not significant too but pooled results show an significant reduction in IL-6 levels following intervention by tomato compared to control group (WMD: −0.12 pg/ml, 95% CI: −0.23, −0.02, Pheterogeneity = 0.001). Although, the pooled results from effect of tomato on CRP were not significant (WMD: 0.98 mg/l, 95%CI: −0.23, 219, Pheterogeneity = 0.09) but time-response analysis showed significant time dependent relation between tomato intervention and CRP levels. The results demonstrated a significant reduction in IL-6 by tomato consumption. Tomato reduce CRP levels in short period too.
Keywords
Tomato
Weight
Blood pressure
IL-6
CRP
- BMI
-
Body Mass Index
- BP
-
blood pressure
- WMD
-
weighted mean differences
- CRP
-
C reactive protein
Abbreviations
1 Introduction
Behavioural risk factors, including a plethora of dietary risks, including low intakes of fruit and vegetables, are asserted to possess the greatest potential to promote disease and impair human health, globally (Forouzanfar et al., 2015; Gorji et al., 2019; Rahmani et al., 2019). There exists a large epidemiological evidence-base highlighting that cardiovascular health, including blood pressure, is strongly influenced by a healthy diet; where, in particular, fruits and vegetables are considered an important tenet of a cardio-protective diet (Mozaffarian et al., 2011). One of the most preponderant healthy diets followed, globally, is the Mediterranean diet, and one of its main constituents is tomato which is the most abundant source of lycopene within the diet (Ursini and Sevanian, 2002). Lycopene may be defined as a carotenoid that contains a long-conjugated chain of double bonds, organised linearly; moreover, the antioxidative of lycopene is attributed to its’ structural organisation (Cheng et al., 2017).
Numerous empirical investigations have demonstrated that lycopene possesses biologic activity that can are anti-cancer, -osteoporotic and -atherogenic, respectively, and may possess metabolic disease ameliorative potential (Bowen and Borthakur, 2004; Graner et al., 2006; Ursini and Sevanian, 2002). Metabolic abnormalities, including insulin resistance, obesity or excess adiposity, high blood pressure, and altered blood lipids are shown to increase the risk of developing cardiovascular diseases and type 2 diabetes mellitus (Burton-Freeman et al., 2010). Furthermore, it has been shown that, in healthy humans given tomato juice, levels of cholesterol and low-density lipoprotein are significantly decreased (Lundman et al., 2007; Lundman et al., 1997). In addition, statistically significant decreases in inflammatory bio-markers, and concomitant improvements in levels of antioxidants have been reported (Edirisinghe et al., 2008; Lundman et al., 2007; Sies et al., 2005)
To date, there have been some systematic reviews and meta-analyses investigating the effect of lycopene on indices of oxidative stress (Chen et al., 2013), markers of subclinical inflammation (Chen et al., 2013) and cardiovascular risk biomarkers (Cheng et al., 2017), with all asserting positive outcomes. However, it was reported by Sesso et al. that a reduced risk for cardiovascular disease development was associated with tomato consumption, to a greater extent than lycopene supplementation, indicating that the dietary source, tomatoes, vs. its’ constituent components, such as lycopene, could be more efficacious in attenuating disease risk. However, there is no summative, critical appraisal currently available (Sesso et al., 2003). Thus, we sought to investigate the effect of tomato on weight, body mass index, blood pressure and inflammatory factors, by conducting a systematic review and dose-response meta-analysis of randomized controlled trials.
2 Methods
A systematic literature search was performed in PubMed, Web of sciences and SCOPUS database by Medical subject headings (MeSH) and non-MeSH terms up to August 2019 without each language and time restrictions (Supplemental Table 1). Furthermore, secondary reference checks were performed of reference lists of accepted articles and reviews. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline used for this meta-analysis (Liberati et al., 2009).
2.1 Eligibility criteria
The studies were included if they met the following criteria:studies that had “parallel or crossover RCT” design and used “tomato as intervention” with “sufficient information for outcome in both experimental and the placebo group”. The animals, non-RCTs studies, and studies that were not placebo group excluded from study. The PICOS in this study was: P) public population, I) tomato, C) placebo group, O) Weight, Body Mass Index, Blood Pressure and Inflammatory Factors, S) RCTs.
2.2 Data extraction and quality assessment
Included studies were screened by two researchers, independently. The primary scanning was based on title and abstract and second screening was based on full text of relevant papers. All controversy among study selection was discussed and resolved by the senior researcher.
Study quality was assessed according to the Cochrane criteria (Higgins and Green, 2011) according to following domain: allocation concealment, adequate sequence generation, blinding, number of dropouts (the level of imperfect outcome data), selective outcome reporting and sources of bias other the mentioned ones.
2.3 Data synthesis and statistical analysis
Stata software version 14 used for all statistical analyses in this study. Mean change and standard deviation (SD) of outcomes were used for calculating pooled weighted mean difference (WMD). Following formula was used for calculating the SD difference SD change: SD2 baseline + SD2 final – (2 R* SD baseline + SD final) (Borenstein et al., 2009). DerSimonian and Laird random effect method was used to combining weighted mean difference. I2 index and Q test assessed to evaluation of heterogeneity across included studies (Higgins et al., 2003). The funnel plot, Egger’s and Begg’s test was used to evaluation publication. The nonlinear potential effects of tomato based on length of intervention were examined by polynomial modeling. P < 0.05 was considered as statistically significant.
3 Results
In comprehensive systematic search in PubMed, Scopus, and web of sciences, after removing duplicated studies, 5495 paper were identified to screening. From them 5443 papers removed in title and abstract screen step and 43 papers were examined in full text evaluation step that from them nine papers included in this meta-analysis (Fig. 1) (Cuevas-Ramos et al., 2013; Ghavipour et al., 2013; Maruyama et al., 2001; Michalickova et al., 2019; Osinska et al., 2017; Pourahmadi et al., 2015; Thies et al., 2012; Valderas-Martinez et al., 2016; Yang et al., 2019).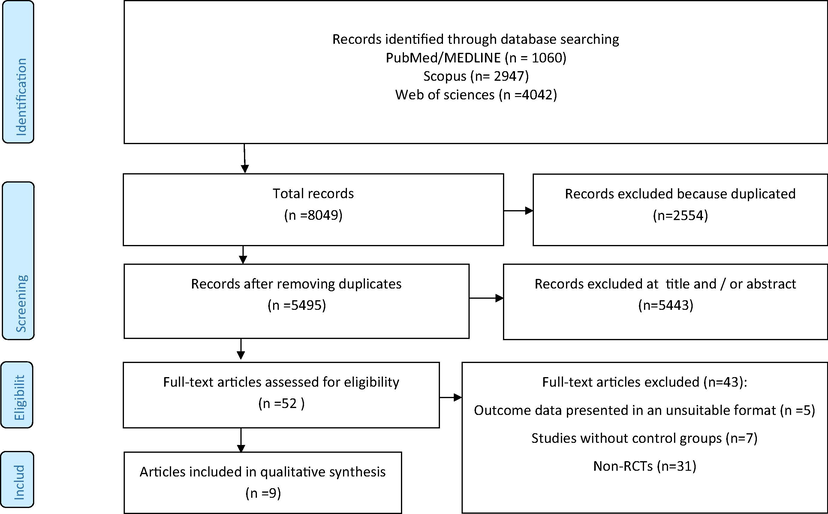
Flow chart of included studies.
3.1 Study characteristics
The characteristics of included studies presented in Table 1. They were conducted in Taiwan (Yang et al., 2019), Serbia (Michalickova et al., 2019), Spain (Valderas-Martinez et al., 2016), Iran (Ghavipour et al., 2013; Pourahmadi et al., 2015), Mexico (Cuevas-Ramos et al., 2013), Scotland (Thies et al., 2012), Poland (Osinska et al., 2017), and Japan (Maruyama et al., 2001). Studies conducted from 2001 to 2019 and three studies were on female (Ghavipour et al., 2013; Maruyama et al., 2001; Pourahmadi et al., 2015) and others were on both genders. Average length of interventions was 5 weeks from 3 to 12. The risk of bias of included studies were assessed by Cochrane collaboration’s tool and most of them had good quality (Fig. 2).
Author
Location
year
Age
Dose
Length of intervention
Gender
(year)
(mg/day)
(week)
(M = Male, F = Female, B = Both)
Yang
Taiwan
2019
post-menopausal under 70
250 g 2 h before sleep/day
8
F
Michalickova
Serbia, Czech republic
2019
45–60
“200 g of tomato fruit juice
4
B
Valdrs-Martinz
Spain
2016
28 ± 11
enriched with ethanolic extract of whole tomato“
4
B
Pourahmadi
Iran
2015
20–30
“7.0 g of Row T (RT group)/kg (BW), 3.5 g of T Sauce (TS group)/kg BW
3
F
Gavipour
Iran
2013
20–40
or 3.5 g of T Sause with Olive Oil (TSOO group)/kg BW“
3
F
Cuevs-Ramos
Mexico
2013
18–65
330 ml tomato juice (60 mg lycopene) 165*2/d
4
B
Thies
Scotland
2012
40–65
330 ml tomato juice (37 mg lycopene) 110*3/day
12
B
Maruyama
Japan
2001
21.3
300 g uncooked tomato
4
F
Osiniska
Poland
2017
28–74
(one capsule per day, each containing 10 mg lycopene, 0.8 mg b-carotene, 0.1 mg c-tocopherol, and 1.3 mg a-tocopherol
4
B
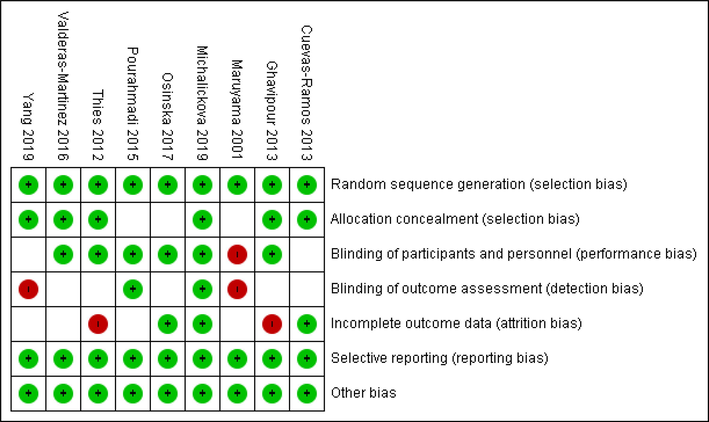
Cochrane risk of bias assessment.
3.2 Meta-analysis results
Three of included studies (Maruyama et al., 2001; Pourahmadi et al., 2015; Yang et al., 2019) with four arms containing 170 participants reported body weight as outcome measures. Combined results with random effect model did not show any significant change in weight of participants (WMD: −0.03 kg, 95% CI: −0.59, 0.53, Pheterogeneity = 0.98) (Fig. 3), Furthermore, BMI change was not significant by tomato intervention (WMD: 0.07 kg/m2, 95% CI: −0.14, 0.29, Pheterogeneity = 0.99).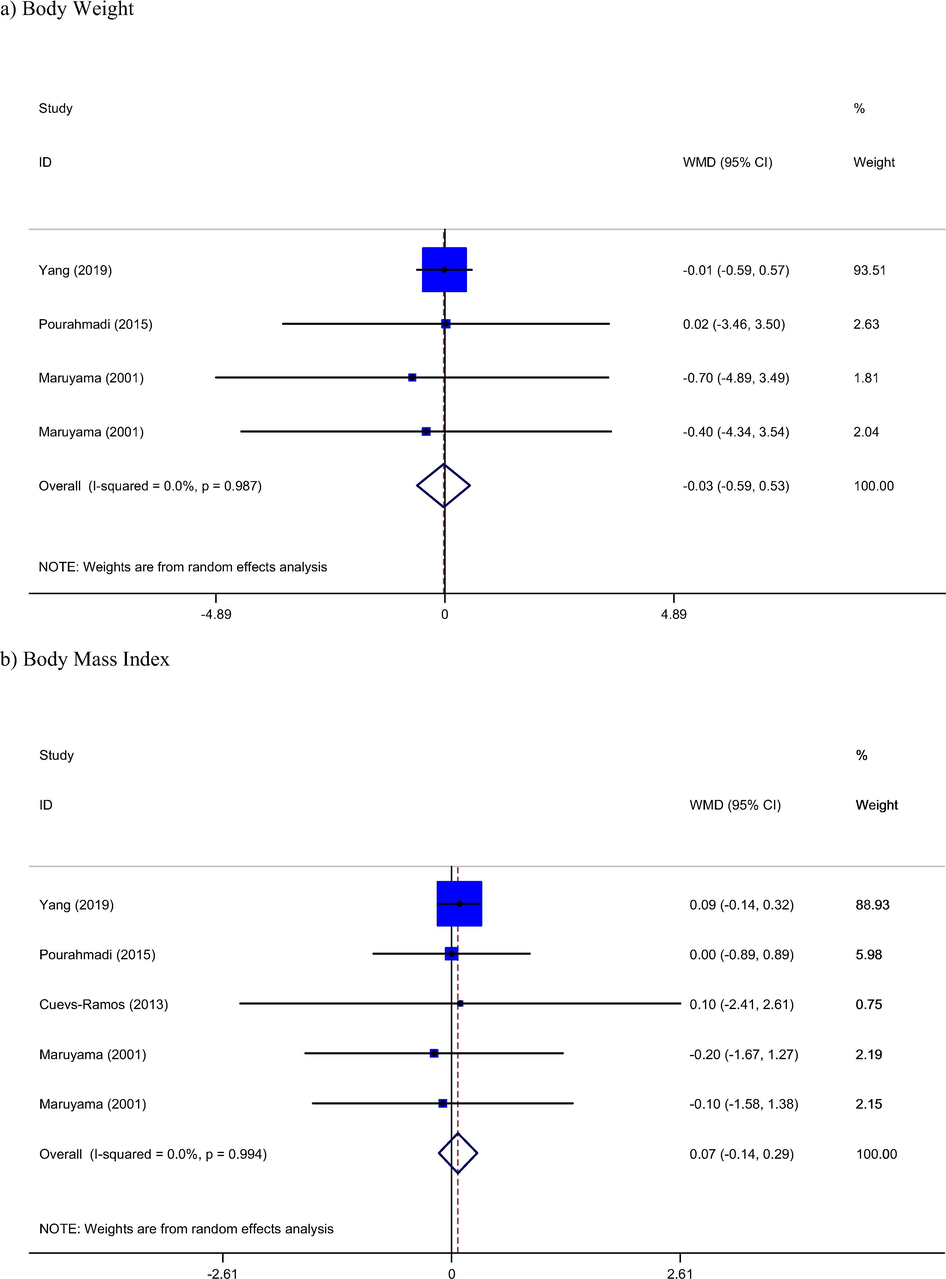
Meta-analysis of effect of Tomato consumption on.
Meta-analysis of effect of Tomato consumption on.
Meta-analysis of effect of Tomato consumption on.
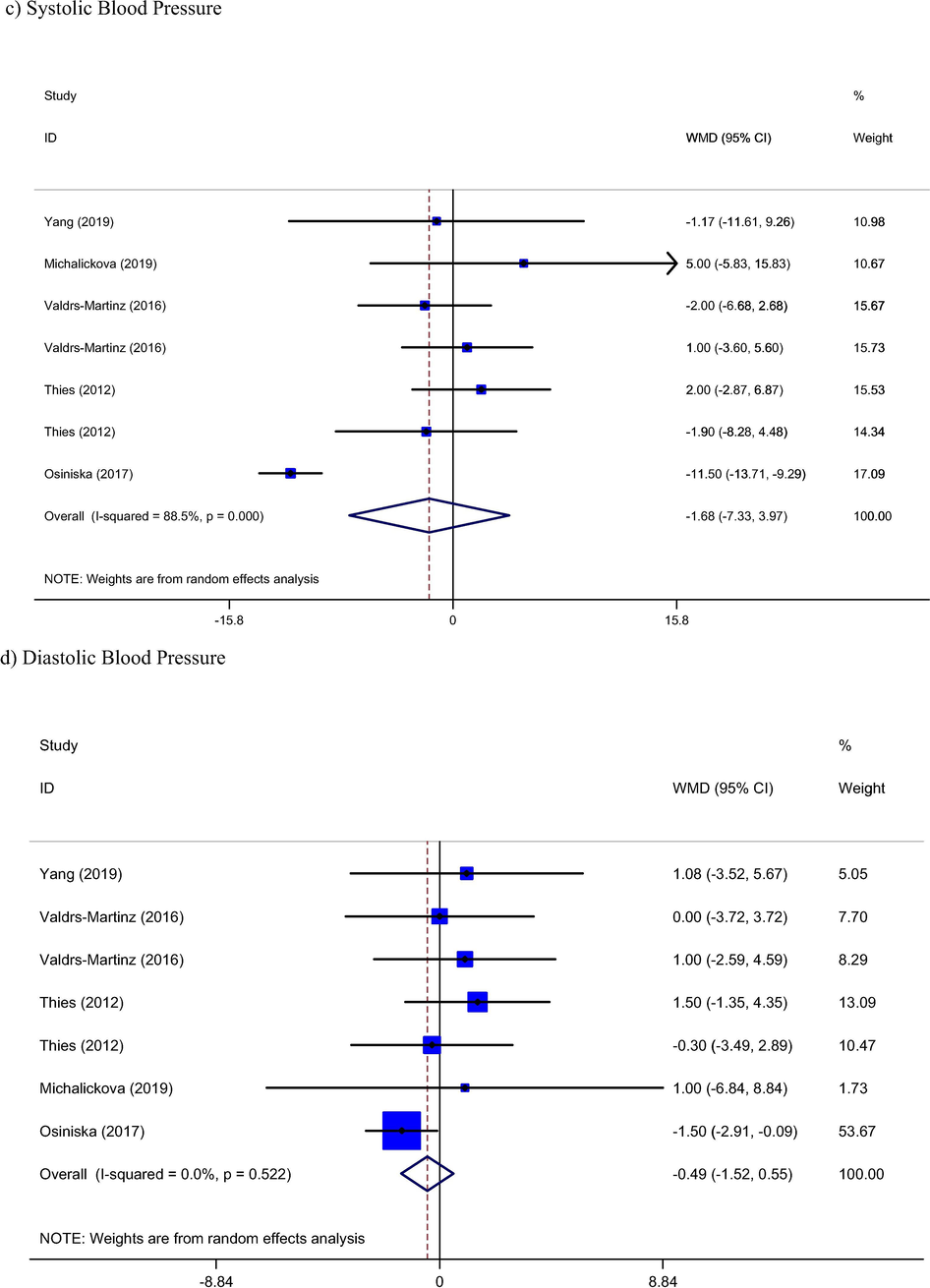
Five studies (Michalickova et al., 2019; Osinska et al., 2017; Thies et al., 2012; Valderas-Martinez et al., 2016; Yang et al., 2019) with seven arms containing 588 participants (294 in control and 294 in intervention groups) reported blood pressure as their outcomes. Pooled results showed not any significant change in SBP and DBP levels by Tomato consumption (WMD: −1.68 mmHg, 95% CI: −7.33, 3.97, Pheterogeneity = 0.001) and (WMD: −0.49 mmHg, 95% CI: −1.52, 0.55, Pheterogeneity = 0.52), respectively.
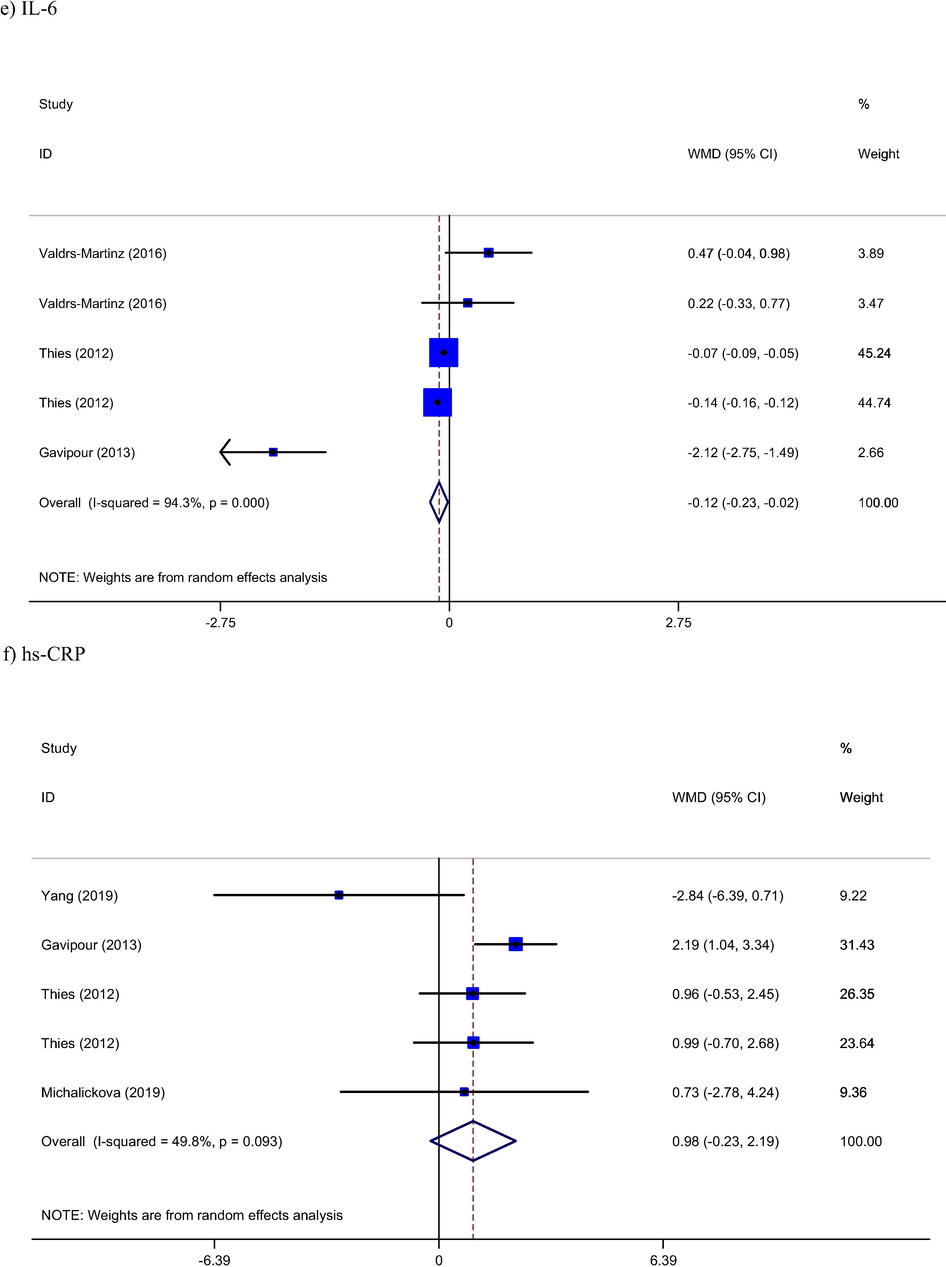
Combined results of included studies (Ghavipour et al., 2013; Thies et al., 2012; Valderas-Martinez et al., 2016) show an significant reduction in IL-6 levels following intervention by tomato compared to control group (WMD: −0.12 pg/ml, 95% CI: −0.23, −0.02, Pheterogeneity = 0.001). Although, the pooled results from effect of tomato on CRP (Ghavipour et al., 2013; Michalickova et al., 2019; Thies et al., 2012; Yang et al., 2019) was not significant (WMD: 0.98 mg/l, 95% CI: −0.23, 219, Pheterogeneity = 0.09) but time-response analysis showed significant time dependent relation between tomato intervention and CRP levels (Fig. 4). According to this relation tomato reduce levels of CRP in short period after intervention (coef = −0.63 and p < 0.01).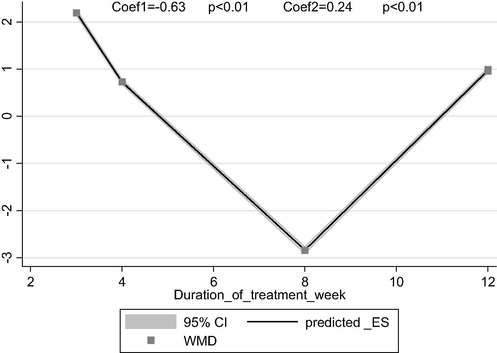
Time-response analysis of effect of Tomato consumption on hs-CRP.
3.3 Risk of bias
Supplemental Fig. 1 showed Funnel plot of publication bias. There is not any asymmetry among included studies. The Egger’s and begg’s tests were (p = 0.23, p = 0.05) for Weight, (p = 0.11, p = 0.62) for BMI, (p = 0.06, p = 0.81) for SBP, (p = 0.05, p = 0.65) for DBP, (p = 0.62, p = 0.14) for IL-6 and (p = 0.09, p = 0.73) for CRP, respectively.
4 Discussion
Tomato is known to possess bio-active phytochemicals, including carotenoids, flavonoids, beta-carotene, and lycopene, which have all have been shown to elicit improvements in weight, body mass index, blood pressure and inflammatory factors. However, no systematic compilation of relevant data has been conducted; thus, the aim of this systematically review and meta-analyze the effects of Tomato consumption on weight, BMI, blood pressure and inflammatory factors, such as CRP and IL-6, as manifest in Randomized Controlled Trials. In accord with the aforementioned aim, combined results show no significant change in weight or BMI following tomato intervention. Furthermore, blood pressure changes were not significant, however, pooled results demonstrated a significant reduction in IL-6 levels, following tomato interventions compared to control group. Notwithstanding, the pooled results did not yield any significant effect on CRP, but time-response analysis showed a significant time dependent relation between tomato intervention and CRP levels.
To date, there have been some systematic reviews and meta-analyses investigating the impact of lycopene supplementation on indices of oxidative stress (Chen et al., 2013), markers of subclinical inflammation (Chen et al., 2013) and cardiovascular risk biomarkers (Cheng et al., 2017), where all studies have reported positive outcomes. However, it was reported by Sesso et al. that a larger decrement in cardiovascular disease risk was associated with tomato, rather than lycopene, intake, indicating that the dietary source possesses greater efficacy in the reduction and management of disease risk (Sesso et al., 2003). Adipose tissue inflammation is common in obesity, and may be mitigated by reducing body fat content (Gregor and Hotamisligil, 2011), however, adipose monocyte chemoattractant protein-1 secretion can stimulate infiltration of macrophages, yielding an inflammatory response (Harman-Boehm et al., 2007). Lycopene has previously been reported to mediate adipokine expression and secretion (Gouranton et al., 2011; Luvizotto et al., 2013), concordantly, dietary-based interventions have highlighted that tomato juice consumption can alter adipokines, towards a more anti-inflammatory profile. Furthermore, such responses can occur independent of an anti-obesity effect, i.e. weight loss, as in this meta-analysis. Moreover, chronic inflammatory responses in overweight participants are shown to be reduced following tomato juice supplementation (Ghavipour et al., 2013). Lycopene anti-inflammatory activity in endothelial cells is believed to be attributable to the inhibition of NF-kB signalling (Armoza et al., 2013). Data from the present study, where significant reductions in IL-6 were evident, suggests that consuming foods abundant with antioxidative properties, including tomato-based products, can positively impact inflammation and oxidative-stress.
4.1 Putative mechanism
It has been suggested that apo-10′-lycopenoic acid supplementation, can counteract a high-fat diet mediated hepatic steatosis (Chung et al., 2012). Such effects may be manifest through SIRT1 activation, which is considered an important regulator in lipogenesis suppression and lipid-catabolism stimulation (Lomb et al., 2010). Lycopene has been shown to decrease binding of fatty-acids of protein 7 levels through increases in hepatic microRNA-21, and in-turn, can yield increases in its; degradation, thus benefiting hepatic steatosis (Ahn et al., 2012). The mechanisms by which tomato juice can reduce cholesterol levels is linked to HMG-CoA reductase inhibition (Navarro-González et al., 2014) and increases in LDL receptor binding activity (Palozza et al., 2012). Numerous empirical data suggests that tomato juice consumption can reduce oxidative stress (Agarwal and Rao, 1998; Bernal et al., 2013; Silaste et al., 2007). Whilst Li et al also found that thiobarbituric levels were lowered substantially, and significantly, following tomato supplementation (Li et al., 2015). However, comparable findings were not present when numerous studies were compiled in the present analysis. Related to the principal, significant, finding of the study; IL-6 and TNF-a are inherently involved in numerous cell processes, including differentiation, proliferation, apoptosis, and inflammation (Biasillo et al., 2010; Libby, 2006). Although the use of naturally-occurring antioxidants as anti-inflammatory supplements has been examined (Gupta et al., 2010; Reuter et al., 2010); the underlying mechanisms are unclear, and thus highlights a strong avenue for further research.
4.2 Strengths and limitations
The available literature, prior to this study, was equivocal, and required a comprehensive meta-analytical perspective. We highlighted that there is enough evidence available to suggest that tomato supplementation can elicit significant, beneficial effects on IL-6, and short-term changes in CRP levels. An additional strength of the study is the sample of participants attained, comprising a range of demographics, ethnic diversity and ages. Notwithstanding, the present study has limitations. Indeed, the analyses were not limited to include participants of only one typology or age; and whilst this enabled numerous studies to be eligible for inclusion in the subsequent analyses, there may be age, gender and disease-specific factors impacting on the efficacy of tomato consumption. In addition, specific differences in tomato type and genus was not assessed, and thus, we must recommend that such differences be further investigated. Some trials included in the present study were relatively small in sample size, which can lead to spurious reporting of larger effect sizes in intervention arms vs. studies with relatively bigger samples (Sterne and Egger, 2001). Finally, an additional limitation of the current investigation was the dearth of suitable studies, evidencing that more high-quality RCT’s are necessary.
5 Conclusion
The results of the current study demonstrated a significant reduction in IL-6 following tomato consumption; whilst short term reductions in CRP levels were also evident. Given that elevated inflammatory markers are associated with a plethora of non-communicable diseases, tomato consumption may represent a viable suggestion. Notwithstanding, however, a larger number of RCT’s are required to confirm the veracity of our results.
Funding
No fund.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Tomato lycopene and low density lipoprotein oxidation: a human dietary intervention study. Lipids. 1998;33(10):981-984.
- [Google Scholar]
- Lycopene inhibits hepatic steatosis via micro RNA-21-induced downregulation of fatty acid-binding protein 7 in mice fed a high-fat diet. Mol. Nutr. Food Res.. 2012;56(11):1665-1674.
- [Google Scholar]
- Tomato extract and the carotenoids lycopene and lutein improve endothelial function and attenuate inflammatory NF-κB signaling in endothelial cells. J. Hypertens.. 2013;31(3):521-529.
- [Google Scholar]
- Lipid biomarkers and metabolic effects of lycopene from tomato juice on liver of rats with induced hepatic steatosis. J. Nutr. Biochem.. 2013;24(11):1870-1881.
- [Google Scholar]
- Inflammatory biomarkers and coronary heart disease: from bench to bedside and back. Intern. Emerg. Med.. 2010;5(3):225-233.
- [Google Scholar]
- Effect sizes for continuous data. In: The Handbook of Research Synthesis and Meta-analysis. 2009. p. :221-235.
- [Google Scholar]
- Postprandial lipid oxidation and cardiovascular disease risk. Curr. Atherosclerosis Rep.. 2004;6(6):477-484.
- [Google Scholar]
- Strawberry modulates LDL oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. J. Am. Coll. Nutr.. 2010;29(1):46-54.
- [Google Scholar]
- Effect of lycopene supplementation on oxidative stress: an exploratory systematic review and meta-analysis of randomized controlled trials. J. Med. Food. 2013;16(5):361-374.
- [Google Scholar]
- Tomato and lycopene supplementation and cardiovascular risk factors: a systematic review and meta-analysis. Atherosclerosis. 2017;257:100-108.
- [Google Scholar]
- Apo-10'-lycopenoic acid, a lycopene metabolite, increases sirtuin 1 mRNA and protein levels and decreases hepatic fat accumulation in ob/ob mice. J. Nutr.. 2012;142(3):405-410.
- [Google Scholar]
- Effect of tomato consumption on high-density lipoprotein cholesterol level: a randomized, single-blinded, controlled clinical trial. Diabetes Metab. Syndr. Obes.. 2013;6:263-273.
- [CrossRef] [Google Scholar]
- Strawberry extract caused endothelium-dependent relaxation through the activation of PI3 kinase/Akt. J. Agric. Food. Chem.. 2008;56(20):9383-9390.
- [Google Scholar]
- GBD 2013 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(10010):2287-2323.
- [Google Scholar]
- Tomato juice consumption reduces systemic inflammation in overweight and obese females. Br. J. Nutr.. 2013;109(11):2031-2035.
- [CrossRef] [Google Scholar]
- The effect of green-coffee extract supplementation on obesity: a systematic review and dose-response meta-analysis of randomized controlled trials. Phytomedicine 2019153018
- [Google Scholar]
- Lycopene inhibits proinflammatory cytokine and chemokine expression in adipose tissue. J. Nutr. Biochem.. 2011;22(7):642-648.
- [Google Scholar]
- Impact of postprandial lipaemia on low-density lipoprotein (LDL) size and oxidized LDL in patients with coronary artery disease. Eur. J. Clin. Invest.. 2006;36(11):764-770.
- [Google Scholar]
- Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev.. 2010;29(3):405-434.
- [Google Scholar]
- Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J. Clin. Endocrinol. Metab.. 2007;92(6):2240-2247.
- [Google Scholar]
- Cochrane Handbook for Systematic Reviews of Interventions (Vol. 4). John Wiley & Sons; 2011.
- Tomato juice supplementation in young women reduces inflammatory adipokine levels independently of body fat reduction. Nutrition. 2015;31(5):691-696.
- [Google Scholar]
- Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr.. 2006;83(2):456S-460S.
- [Google Scholar]
- The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med.. 2009;6(7):e1000100
- [CrossRef] [Google Scholar]
- Sirtuins regulate key aspects of lipid metabolism. Biochim. Biophys. Acta, Proteins Proteomics. 2010;1804(8):1652-1657.
- [Google Scholar]
- A high-fat meal is accompanied by increased plasma interleukin-6 concentrations. Nutr. Metab. Cardiovas. Dis.. 2007;17(3):195-202.
- [Google Scholar]
- Transient triglyceridemia decreases vascular reactivity in young, healthy men without risk factors for coronary heart disease. Circulation. 1997;96(10):3266-3268.
- [Google Scholar]
- Lycopene supplementation modulates plasma concentrations and epididymal adipose tissue mRNA of leptin, resistin and IL-6 in diet-induced obese rats. Br. J. Nutr.. 2013;110(10):1803-1809.
- [Google Scholar]
- Effects of tomato juice consumption on plasma and lipoprotein carotenoid concentrations and the susceptibility of low density lipoprotein to oxidative modification. J. Nutr. Sci. Vitaminol. (Tokyo). 2001;47(3):213-221.
- [Google Scholar]
- Comparison of polyphenol-enriched tomato juice and standard tomato juice for cardiovascular benefits in subjects with stage 1 hypertension: a randomized controlled study. Plant Foods Hum. Nutr. 2019
- [CrossRef] [Google Scholar]
- Components of a cardioprotective diet: new insights. Circulation. 2011;123(24):2870-2891.
- [Google Scholar]
- The inhibitory effects of bioactive compounds of tomato juice binding to hepatic HMGCR: in vivo study and molecular modelling. PLoS ONE. 2014;9(1):e83968
- [Google Scholar]
- The influence of adding tomato extract and acetylsalicylic acid to hypotensive therapy on the daily blood pressure profiles of patients with arterial hypertension and high cardiovascular risk. Kardiochir Torakochirurgia Pol. 2017;14(4):245-252.
- [CrossRef] [Google Scholar]
- Effect of lycopene and tomato products on cholesterol metabolism. Ann. Nutr. Metab.. 2012;61(2):126-134.
- [Google Scholar]
- The effect of tomato juice consumption on antioxidant status in overweight and obese females. Women Health. 2015;55(7):795-804.
- [CrossRef] [Google Scholar]
- The influence of fasting and energy restricting diets on IGF-1 levels in humans: a systematic review and meta-analysis. Ageing Res. Rev. 2019100910
- [Google Scholar]
- Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biol. Med.. 2010;49(11):1603-1616.
- [Google Scholar]
- Dietary lycopene, tomato-based food products and cardiovascular disease in women. J. Nutr.. 2003;133(7):2336-2341.
- [Google Scholar]
- Nutritional, dietary and postprandial oxidative stress. J. Nutr.. 2005;135(5):969-972.
- [Google Scholar]
- Tomato juice decreases LDL cholesterol levels and increases LDL resistance to oxidation. Br. J. Nutr.. 2007;98(6):1251-1258.
- [Google Scholar]
- Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J. Clin. Epidemiol.. 2001;54(10):1046-1055.
- [Google Scholar]
- Effect of a tomato-rich diet on markers of cardiovascular disease risk in moderately overweight, disease-free, middle-aged adults: a randomized controlled trial. Am. J. Clin. Nutr.. 2012;95(5):1013-1022.
- [CrossRef] [Google Scholar]
- Tomato sauce enriched with olive oil exerts greater effects on cardiovascular disease risk factors than raw tomato and tomato sauce: a randomized trial. Nutrients. 2016;8(3):170.
- [CrossRef] [Google Scholar]
- Dietary supplement of tomato can accelerate urinary aMT6s level and improve sleep quality in obese postmenopausal women. Clin. Nutr. 2019
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2019.12.020.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







