Translate this page into:
The effect of aloin in blood glucose and antioxidants in male albino rats with Streptozoticin-induced diabetic
⁎Corresponding author. nsaoiean@qu.edu.sa (Noorah Saleh Al-Sowayan) knaaj1@yahoo.com (Noorah Saleh Al-Sowayan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The anti-diabetic effect of aloin was examined in vivo in the current study, similar to the study, which for four weeks examined how aloin affected the blood sugar, insulin, and antioxidant levels in both healthy and diabetic rats generated by streptozotocin. Adult male albino rats were experimentally given a single intraperitoneal injection of 60 mg/kg of streptozotocin to induced diabetes. The in vitro experiment utilized blood samples, and adult male albino rats' kidneys, pancreas and liver were separated. In comparison to control diabetic rats, aloin isolates (30 mg / kg) body weight for 30 days considerably decreased serum glucose and effectively boosted serum insulin levels. Liver and kidney congeners of malondialdehyde (MDA) were substantially decreased while, Catalase (CAT), superoxide dismutase (SOD) and Congeners Glutathione (GSH) were significantly boosted as contrasted to diabetic rats after aloin therapy. Moreover, a histological assessment of the pancreas was performed.
These results show the promise of aloin at a dose of 30 mg/kg body weight for 30 days as an antidiabetic and antioxidant agent.
Keywords
Diabetes mellitus (DM)
Aloe vera L.
Liliaceae
Aloin
Glutathione (GSH)
Malondialdehyde (MDA)
Catalase (CAT)
Superoxide Dismutase (SOD)
1 Introduction
Diabetes mellitus (DM), a group of metabolic disorders with several different etiologies that are characterized by elevated serum glucose levels or persistent hyperglycemia and abnormalities of insulin hormone secretion, insulin hormone resistance, or both, is one of the biggest threats to human health in the world today (Khan, 2021). Diabetes-related metabolic disorders increase the formation of mitochondrial superoxide in the heart muscle and endothelial cells of either small and large blood arteries (Kelkar, Aditya, Jai, Mehta, & Amoaku, 2018).
Consequently, oxidative stress is a crucial factor in the emergence of microvascular and cardiovascular problems associated with diabetes mellitus. (Tabatabaei S. R., Ghaderi, Tapehebur, Farbood, & Rashno, 2017; Sha, Hu, & Bu, 2020). Studies also show available treatments for controlling diabetes and its complications have various side effects with limited benefits; therefore, the desire for herbal products with anti-diabetic properties and fewer side effects that are more cost-effective furthermore is increasing (Association, 2018). Aloin (Aloe vera L., Liliaceae family), extracted from the outer bark of the aloe vera plant, has diverse applications in medicinal products and cosmetics, while research on animals has shown that aloin can be toxic, there is little indication of this in humans. If consumed excessively, especially over a lengthy period, aloin can be toxic, which signifies that it began having adverse effects on the body that may even be life-threatening. (Zhang, Liu, Liu, Zhao, & Tian, 2016; Association, 2018). Recent approaches suggest that the treatment of diabetes should not only focus on insulin secretion but also antioxidant protection of the β-cell in the pancreas, this may facilitate the repair of β-cells undergoing damage by oxidative stress secondary to hyperglycemia (Tangvarasittichai, 2015; Xu, Li, & Li, 2017) the objective of the study was to investigate how aloin affected blood sugar, insulin, and antioxidants to healthy rats, as well as rats with streptozotocin-induced diabetes, for four weeks.
2 Material and methods
2.1 Chemicals
Streptozotocin was supplied by Alfa Aesar USA and Aloin was supplied by Sigma Aldrich Co USA., Catalase (CAT), Superoxide dismutase activity (SOD), Kit Malondialdehyde (MDA) and Glutathione (GSH), and were also purchased from the NWLSSTM.
2.2 Experimental animals
The College of Pharmacy at King Saud University provided 26 male Wistar rats for this investigation, each weighing 200–250 g. The rats were kept in polyacrylate cages with constant conditions and typical light/dark cycles. Animals were left for one month before experimentation to adapt to laboratory conditions. Throughout the experiment's acclimation phase and duration, a commercial balanced diet (General Organization for Grain Silos and Flour Mills, Riyadh) and water were provided ad libitum.
2.3 Induction of DM and study design
Rats were given intraperitoneal injections of streptozotocin after being starved for the previous night (Alfa Aesar, USA) (60 mg/kg). Throughout the first twenty-four hours following the administration of streptozotocin, mice treated with STZ were given a 5 % glucose solution rather than water to prevent hypoglycemia shock-related mortality. Fasting blood glucose was measured seventy-two hours later utilizing a One Touch glucometer (Icare TD-4279); diabetic rats were defined as those with fasting blood glucose levels>250 mg/dL. (Mestry, Dhodi, Kumbhar, & Juvekar, 2017; Elekofehinti, Onunkun, & Olaleye, 2020).
Six rats each were given to the four groups,
Group 1: was normal control rats given an aqueous solution,
Group 2: The diabetic control rats were given an aqueous solution daily for 30 days, Group 3: The normal control rats were given an aqueous solution daily for 30 days, and Group 4: The diabetic rats were administered Aloin (30 mg/kg) in an aqueous solution daily for 30 days.
2.4 Sampling
In the first, second, third, and fourth weeks, a blood glucose meter (Icare TD-4279) was utilized to check the blood sugar level in the tail vein.
By the completion of the experiment phase (day 30th), The retro-orbital plexus was used to collect blood samples, and serum samples subsequently separated using centrifugation. (Thermo Scientific, USA) and used immediately for Insulin hormone analyses (qing Su, diWang, & yanChi, 2017).
Post-sacrificed by anesthesia, the Liver, kidneys, and pancreas were harvested and rinsed in ice-cold saline, and calculation of liver, and kidney oxidative stress concentrations (MAD, GSH, SOD, CAT) (Sun, Wang, & Lu, 2017).
2.4.1 Determination of glucose
In this experience, we estimate blood glucose based on the use of the enzyme’s method (Glucose oxidase) by the blood glucose meter (Icare TD-4279) (Rahmati, Keshvari, Mirnasouri, & Chehelcheraghi, 2021).
2.4.2 Measurement of serum insulin concentration
Commercial quantitative enzyme-linked immunosorbent assay [(insulin ELISA kit) (DRG International, USA) (Jeffrey, Flier, Kahn, & Roth, 1979) was employed in a microplate reader to evaluate the optical density at an absorbance of 450 nm in order to evaluate the serum insulin levels of STZ-induced diabetic rats and also normal rats (Alborno, 2016).
2.5 Oxidative stress indicators
2.5.1 Sample preparation
Animals were anaesthetized with chloroform and slaughtered at the conclusion of the procedure right after blood was collected. Livers and kidney tissues were quickly collected, collected organs were cleaned from fatty tissues and washed with Phosphate-buffered Heparin 0.16 mg/mL is introduced to saline (PBS), pH 7.4, to eliminate any red blood cells and clots. The samples were then homogenized in cold phosphate buffer using an ultrasonic homogenizer., the homogenate was centrifuged at 4000 rpm for 10 min at 4 °C in a refrigerated centrifuge and the supernatant was collected and kept at − 20 °C and used as a sample for antioxidant assays (Al-musa & Al-hashem, 2013).
2.5.2 Determination of tissue samples
malondialdehyde (MDA), glutathione (GSH)
superoxide dismutase (SOD) and catalase (CAT)
Tissue samples MDA was estimated by the method of Janero (1990). GSH was estimated by the method of Paglia, Valentine (1967). Depending on the enzyme's capacity to prevent autoxidation using the pyrogallol methodology of Martin, Dailey, and Sugarman (1987), SOD activity was determined. Catalase was estimated by the method of Beers and Sizer (1952).
Kit Malondialdehyde (MDA), Glutathione (GSH), Superoxide dismutase activity (SOD), and Catalase (CAT) was used by the NWLSSTM.
2.6 Hematoxylin and eosin staining
Procedure: The pancreas tissues were dried in ethanol at increasing concentrations (70–100 %) before being embedded in paraffin and fixed in a 10 % neutral buffered formalin solution. Using a microtome, tissues were divided into sections that were 4 µm thick, mounted on glass slides, and afterwards dried slides. Following that, routine hematoxylin and eosin staining was performed on all samples. Thereafter, sections were examined at Olympus Optical in Tokyo, Japan, using a light microscope. All groups' slides were examined and captured on camera. (Alkhudhayri, Osman, Alshammari, Al Maiman, & Yahya, 2021; Sarfraz, Khaliq, Hafizur, Raza, & Ullah, 2021).
2.7 Statistical analysis
Data were expressed as the mean ± SEM. statistically analyzed using the SPSS program (version 23) and With the use of ANOVA and the Tukey multiple comparisons test, the various groups were compared. Significance was acceptable at P < 0.05.
* Significant variance unlike the standard control.
a significant variance unlike the standard control.
3 Result
3.1 Effect of 30 days daily dose administration of Aloin on serum glucose and insulin levels.
Figs. 1 and 2 depict the levels of serum-glucose as well as insulin in healthy and diabetic rats caused by streptozotocin, respectively. When contrasted to the matching control group, the serum glucose level was significantly elevated during diabetes, and the serum insulin level was significantly reduced. The current study demonstrates a significantly gradual decline in the serum glucose level. In the second week in the diabetic and Aloin group compared to the diabetic control group and was significantly lower versus normal control groups in the 4th week, and the values of insulin levels and serum glucose tended to be close to normal in the aloin group.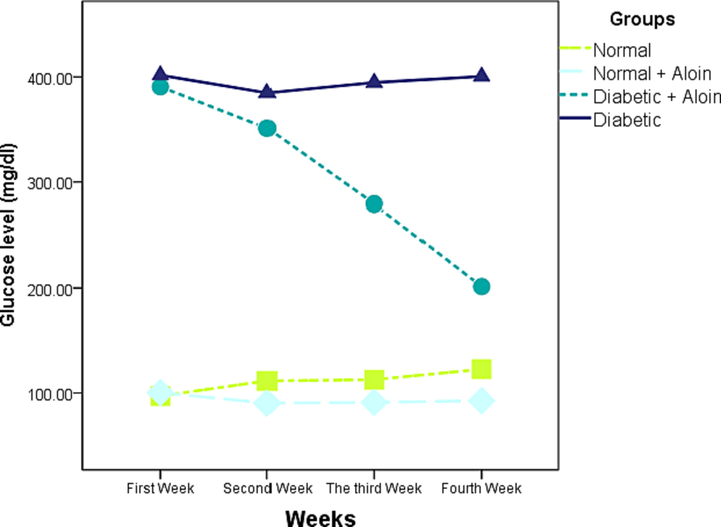
Effect of Aloin on glucose level * Significant different unlike the standard control a significant different unlike the standard control ns P < 0.05.
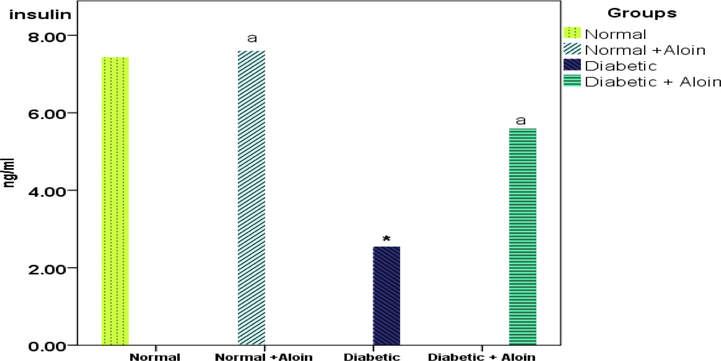
Effect of Aloin on insulin level * Significantly variance unlike the standard control a significantly variance unlike the diabetic control ns P < 0.05.
3.2 Effect of 30 days daily dose administration of aloin on liver and kidney tissue malondialdehyde (MDA) levels
MDA level was significantly increased in the diabetic control group compared with the normal controls group, in the liver and kidney tissue re- respectively,(Fig. 3). With, MDA levels were decreased in the Diabetic + Aloin group contrast with the Diabetic control group, However, no significant (P > 0.05) difference in malondialdehyde level in the normal + Aloin group contrast to the normal control group.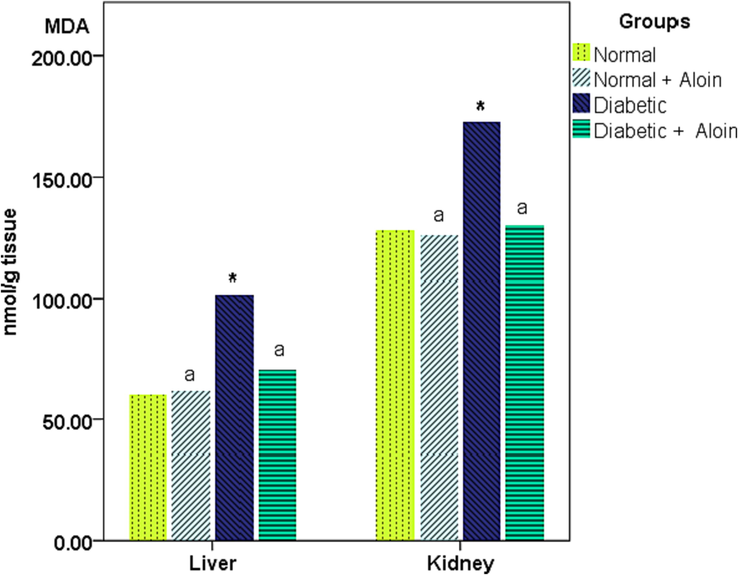
Effect of Aloin on MDA * Significant variance unlike the standard control a significant variance unlike the diabetic control ns P < 0.05.
3.3 Effect of 30 days daily dose administration of aloin on liver and kidney tissue Glutathione (GSH) levels
Liver and kidney tissue Glutathione activity were considerably lower in the Diabetic control group contrasted with the normal control group, respectively. GSH activity was significantly increased in the Diabetic + Aloin group contrast with the Diabetic control group. However no significant (P > 0.05) difference in GSH level in the normal + Aloin contrast to the usual control group (Fig. 4).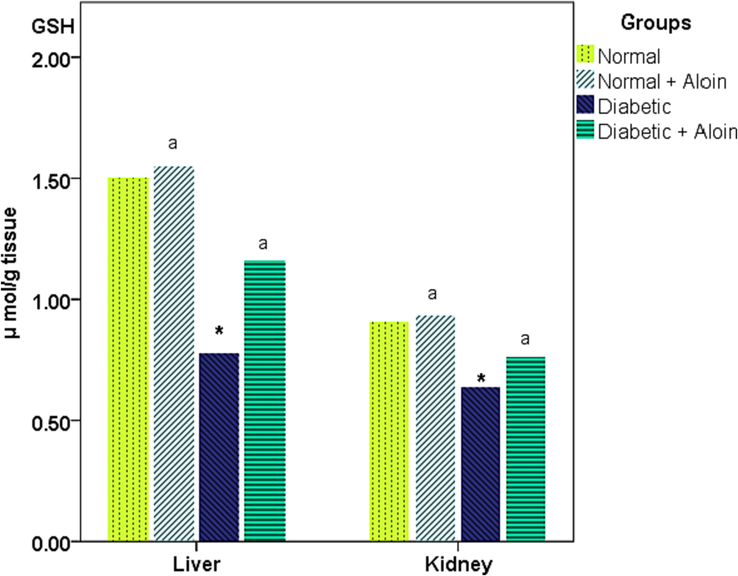
Effect of Aloin on GSH * Significant variance unlike the standard control a significant variance unlike the diabetic control ns P < 0.05.
3.4 Effect of 30 days daily dose administration of aloin on liver and kidney tissue Superoxide dismutase (SOD) levels
Liver and kidney tissue Superoxide dismutase activity was considerably increased in the Diabetic + Aloin group contrasted with the Diabetic control group, respectively. SOD activity was significantly lower in the Diabetic control group contrast with the normal control group. However no significant (P > 0.05) difference in SOD level in the normal + Aloin group contrast to the usual control group (Fig. 5).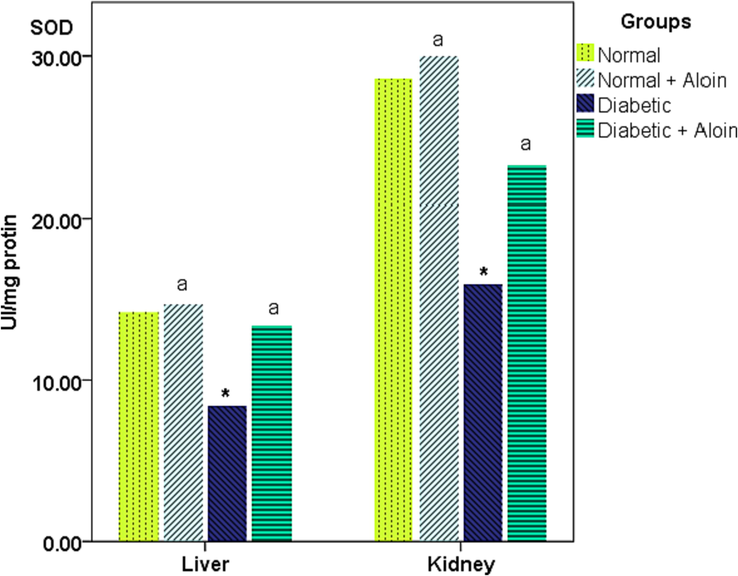
Effect of Aloin on SOD * Significantly variance from the normal control. a significantly variance from the diabetic control ns P < 0.05.
3.5 Effect of 30 days daily dose administration of aloin on liver and kidney tissue Catalase (CAT) levels
Liver and kidney tissue Catalase level was significantly lower in the Diabetic control group compared with the normal control group, respectively. CAT level was significantly increased in the Diabetic + Aloin group contrast with the Diabetic control group. However, no significant (P > 0.05) difference in CAT level in the normal + Aloin group contrast to the usual control group (Fig. 6).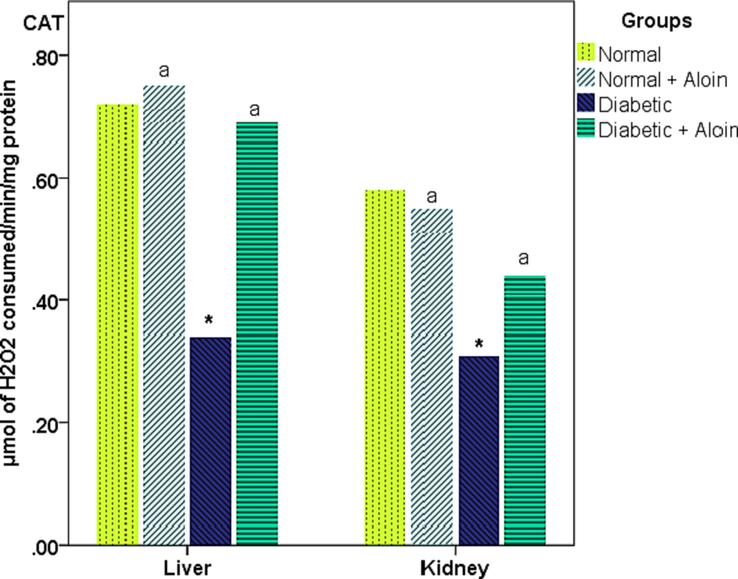
Effect of Aloin on CAT * Significantly variance unlike the standard control. a significantly variance unlike the diabetic control ns P < 0.05.
3.6 Histological results
Both groups are showing normally sized circular islets of Langerhans intact α-cells (peripheral small cells with dark nuclei (small arrow) and β-cells (central larger with light nuclei (arrowhead).
There was a severe reduction in the size of the islets of the Langerhans, with obvious cytoplasmic denegation and a reduction in the number of both α and βcell (short arrow). Note the increase in inflammatory cell infiltration (long arrow). In C and D: there was a considerably increase in the size of the islets of the Langer-hans (long arrow) with an increase in the number of both α (short arrow) and β-cell (Arrowhead).
4 Discussion
The current investigation's findings overwhelmingly point to aloin's potential as an anti-diabetic. When compared to diabetic controls, aloin-treated rats' in-vivo blood glucose and insulin levels were higher depicted in Fig. 1 and Fig. 2. (Abo-Youssef & Messiha 2013; Wexler, 2014). In conjunction, earlier writers stated that aloin had such an anti-diabetic effect in laboratory animals. (Patel & Patel, 2013).
There are many theories as to why aloin has an anti-diabetic effect.
The first theory is that Aloin's capacity to boost insulin sensitivity and encourage insulin production from the remaining β-cells may be the cause of these effects. (abatabaei, Ghaderi, Tapehebur, & Farbood, 2017).
The strong antioxidant properties of aloin extract provide the second justification. It has long been understood that aloin has antioxidant potential by reducing the production of free radicals and improving cellular thiol status. (Lee, Jeong, Chang Baek, Kwang Ku, & Sup Bae, 2019; Cichocka, Wietchy, Nabrdalik, & Gumprecht, 2016), Additionally, it is said to increase the activity of the enzyme glutathione-S-transferase. (Baruah, Bordoloi, & Baruah, 2016).
The quantitative determination of their metabolic end-product malondialdehyde (MDA), which is quantified in nmol/g utilizing the thio-barbituric acid test, is a straightforward and sensitive approach for estimating lipid peroxide concentration (TBA-test). Malondialdehyde is a very hazardous consequence of lipid oxidation that is produced in part by free radicals. Its concentration is dramatically elevated in diabetes mellitus, according to numerous research. MDA combines with proteins and phospholipids in both irreversible and reversible ways, and it can have serious consequences (ManalKamal, MonaSalem, NaglaaKholousi, & KhadegaAshmawy, 2009). MDA levels in the kidney and liver of all groups were measured. When comparing the control group of diabetics to the ordinary control group, we found a substantial rise in malondialdehyde (MDA). These results are consistent with those achieved by (Gina, Aulani, & Mahdi, 2016).
Our observation significantly confirmed aloin's ability to act as an antioxidant, since it was discovered to reduce excessive levels of malondialdehyde in tissue samples and to strengthen kidney and liver tissue. Glutathione, Superoxide dismutase, and Catalase levels (Gina, Aulani, & Mahdi, 2016). Recent strategies emphasize the significance of oxidative stress in pancreatic β-cells damage (see Figs. 7 and 8).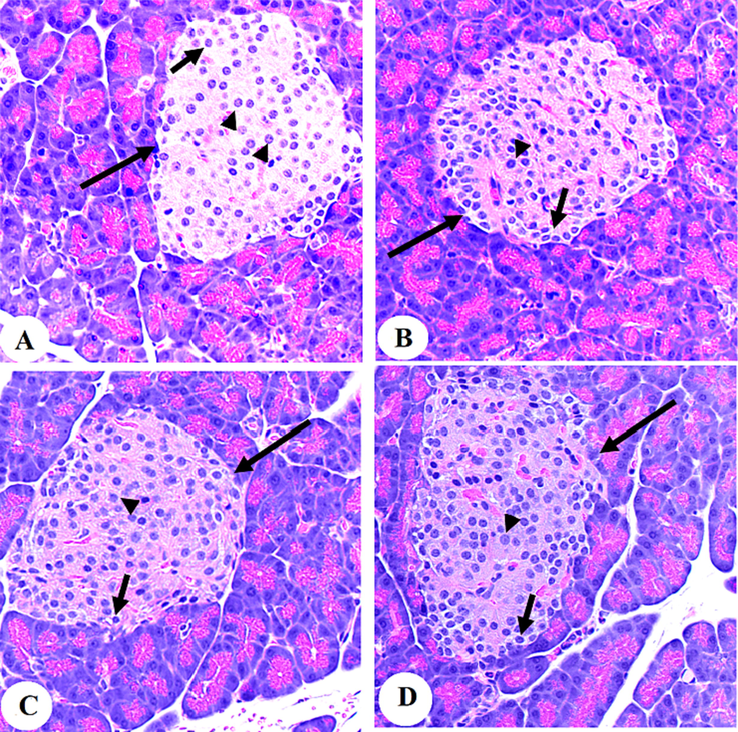
Photomicrographs of the pancreases from the control (A&B) and control + Aloin (30 mg/kg)-treated rats (C&D).
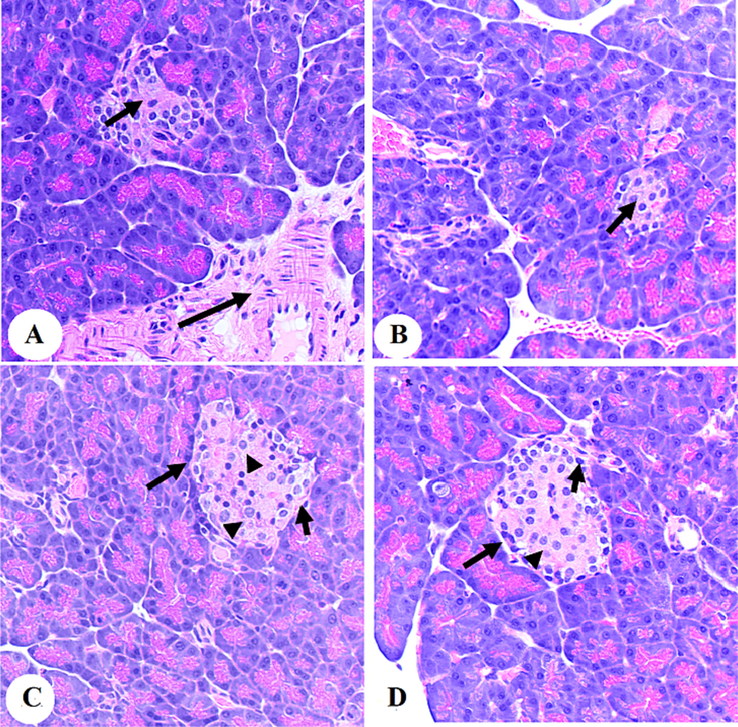
Photomicrographs of the pancreases from the diabetic rats (A and B) and diabetic + Aloin (30 mg/kg)-treated rats. In C and D:
(Bajaj & Khan, 2012; Sumi, et al., 2019; ManalKamal, MonaSalem, NaglaaKholousi, & KhadegaAshmawy, 2009). The aetiology of diabetes mellitus involves oxidative stress, however antioxidants including aloin could have a real antidiabetic impact due to their antioxidant activity (Aldayel, Grace, & Lila, 2020; Ariani, Gofur, Listyorini, & Susanto, 2015).
5 Conclusion
Antioxidant GSH, SOD, and CAT levels in the kidney and liver tissues of cured rats were significantly greater, whereas MDA levels were substantially lower after the program comparison with control rats consequently these data suggest that aloin may have promise as diabetes preventative and/or therapeutic agent, moreover, toxicological and clinical experiments are required to evaluate this plant extract and its possible applications in the medical and pharmaceutical fields.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- abatabaei, S. R., Ghaderi, S., Tapehebur, M. B., & Farbood, Y. 2017. Aloe vera gel improves behavioral deficits and oxidative status in streptozotocin-induced diabetic rats. Biomedicine & Pharmacotherapy, 96, 279-290. doi:https://doi.org/10.1016/j.biopha.2017.09.146.
- Beneficial effects of Aloe vera in treatment of diabetes: Comparative in vivo and in vitro studies. Bulletin of Faculty of Pharmacy, Cairo University. 2013;51(1):7-11. Retrieved from
- [CrossRef] [Google Scholar]
- Alborno, N. T. 2016. erum vaspin level in type 2 diabetic patients from gaza strip. doi:http://hdl.handle.net/20.500.12358/21587.
- LC-MS characterization of bioactive metabolites from two Yemeni Aloe spp. with antioxidant and antidiabetic properties. Arabian Journal of Chemistry. 2020;13:5040-5049. Retrieved from
- [CrossRef] [Google Scholar]
- Alkhudhayri, D. A., Osman, M. A., Alshammari, G. M., Al Maiman, S. A., & Yahya, M. A. 2021. Moringa peregrina leaf extracts produce anti-obesity, hypoglycemic, anti-hyperlipidemic, and hepatoprotective effects on high-fat diet fed rats. doi:https://doi.org/10.1016/j.sjbs.2021.02.078.
- Al-musa, h., & Al-hashem, f. 2013. hypoglycemic hepato renal and antioxidant potential effects of chamomile recutita flowers ethanolic extract in streptozotocin-diabetic rats. American journal of pharmacology and toxicology, 9(1), 1-12. doi:http://thescipub.com/pdf/ajptsp.2014.1.12.pdf.
- Ariani, N. L., Gofur, A., Listyorini, D., & Susanto, H. 2015, Sep. EFFECT OF Aloe vera GEL ON SUPEROXIDE DISMUTASE (SOD) LEVEL IN STREPTOZOTOCIN (STZ)-INDUCED DIABETIC WISTAR Rattus novergicus LIVER. Retrieved from researchgate.net: DOI:10.18502/kls.v2i1.116.
- Association, A. D. 2018, Mar 22. Economic Costs of Diabetes in the U.S. in 2017. Retrieved from diabetesjournals.org ; 41(5):917–928: https://doi.org/10.2337/dci18-0007.
- Antioxidants and diabetes. Indian J Endocrinol Metab. 2012;16(2):s267-s271.
- [CrossRef] [Google Scholar]
- Aloe vera: A multipurpose industrial crop. Industrial Crops and Products. 2016;94:951-963.
- [CrossRef] [Google Scholar]
- spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195(1):133-140.
- [Google Scholar]
- Cichocka, E., Wietchy, A., Nabrdalik, K., & Gumprecht, J. 2016. Insulin therapy - new directions of research. Retrieved from pubmed.ncbi.nlm.nih.gov; 67(3):314-24: DOI: 10.5603/EP.2016.0044.
- Elekofehinti, O. O., Onunkun, A. T., & Olaleye, T. M. 2020. Cymbopogon citratus (DC.) Stapf mitigates ER-stress induced by streptozotocin in rats via down-regulation of GRP78 and up-regulation of Nrf2 signaling. 262. doi:https://doi.org/10.1016/j.jep.2020.113130Get rights and content.
- Gina, L. P., Aulani, A., & Mahdi, C. 2016, April. MDA and Histologic Profile of Pancreatic Diabetic-Rats Model Administered With Extract of Glycine max (L.) Merr. Retrieved from researchgate.net: DOI:10.21776/ub.jpacr.2016.005.01.226.
- Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biology and Medicine. 1990;9(6):515-540.
- [CrossRef] [Google Scholar]
- Receptors, Antireceptor Antibodies and Mechanisms of Insulin Resistance. The New England Journal of Medicine. 1979
- [CrossRef] [Google Scholar]
- ManalKamal, MonaSalem, NaglaaKholousi, & KhadegaAshmawy. (2009, December). Evaluation of trace elements and Malondialdehyde levels in type II diabetes mellitus. Retrieved from sciencedirect.com; Volume 3, Issue 4, pp 214-218: https://doi.org/10.1016/j.dsx.2009.07.007.
- Kelkar, Aditya, Jai, Mehta, & Amoaku. 2018, Oct. Cataract surgery in diabetes mellitus. Retrieved from journals.lww.com; 66(10):p 1401-1410: DOI: 10.4103/ijo.IJO_1158_17.
- Khan, I. A. 2021, Aug 27. Do second generation sequencing techniques identify documented genetic markers for neonatal diabetes mellitus? Retrieved from cell.com; VOLUME 7, ISSUE 9: DOI:https://doi.org/10.1016/j.heliyon.2021.e07903.
- Lee, W., Jeong, G.-S., Chang Baek, M., Kwang Ku, S., & Sup Bae, J. 2019. Renal protective effects of aloin in a mouse model of sepsis. Food and Chemical Toxicology, 132. doi:https://doi.org/10.1016/j.fct.2019.110651.
- MartinJr, J. P., Dailey, M., & Sugarman, E. 1987. Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. 255(2), 329-336. doi:https://doi.org/10.1016/0003-9861(87)90400-0.
- Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. Journal of Traditional and Complementary Medicine. 2017;7:273-280. Retrieved from
- [CrossRef] [Google Scholar]
- Paglia , D., & Valentine, W. 1967. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. doi:https://pubmed.ncbi.nlm.nih.gov/6066618/.
- Patel, K., & Patel, D. K. 2013. Medicinal importance, pharmacological activities, and analytical aspects of aloin: A concise report. Journal of Acute Disease, 2, 262-269. Retrieved from https://doi.org/10.1016/S2221-6189(13)60141-9.
- qing Su, L., diWang, Y., & yanChi, H. 2017. Effect of curcumin on glucose and lipid metabolism, FFAs and TNF-α in serum of type 2 diabetes mellitus rat models. Saudi Journal of Biological Sciences, 24(8), 1776-1780. doi:https://doi.org/10.1016/j.sjbs.2017.11.011.
- Exercise and Urtica dioica extract ameliorate hippocampal insulin signaling, oxidative stress, neuroinflammation, and cognitive function in STZ-induced diabetic rats. Biomedicine & Pharmacotherapy. 2021;139
- [CrossRef] [Google Scholar]
- Effect of black pepper, turmeric and ajwa date on the endocrine pancreas of the experimentally induced diabetes in wister albino rats: A histological and immunohistochemical study. Endocrine and Metabolic Science. 2021;4
- [CrossRef] [Google Scholar]
- Mitochondrial dysfunction and pancreatic islet β-cell failure (Review) Exp ther med. 2020;20(6)
- [CrossRef] [Google Scholar]
- Phenolic Content Analysis of Aloe vera Gel and Evaluation of the Effect of Aloe Gel Supplementation on Oxidative Stress and Fibrosis in Isoprenaline-Administered Cardiac Damage in Rats. Prev Nutr Food Sci. 2019;24(3):254-264.
- [CrossRef] [Google Scholar]
- Lead Exposure Induces Weight Gain in Adult Rats, Accompanied by DNA Hypermethylation. plos one. 2017;12(7)
- [CrossRef] [Google Scholar]
- Aloe vera gel improves behavioral deficits and oxidative status in streptozotocin-induced diabetic rats. Biomedicine & Pharmacotherapy. 2017;96:279-290. Retrieved from
- [CrossRef] [Google Scholar]
- Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. Worid journal of diabetes. 2015;6(3):456-480.
- [CrossRef] [Google Scholar]
- Wexler, P. (2014, Mar 11). Encyclopedia of Toxicology. Retrieved from elsevier.com: https://www.elsevier.com/books/encyclopedia-of-toxicology/wexler/978-0-12-386454-3?original=true.
- Xu, D.-P., Li, Y., & Li, H.-B. (2017). Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. int J MOI Sci, 18(1). doi: 10.3390/ijms18010096.
- Efficacy of Aloe Vera Supplementation on Prediabetes and Early Non-Treated Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled. Trials.. 2016;8(7):388. Retrieved from
- [CrossRef] [Google Scholar]
Further Reeading
- Hirano, T. 2018, July 12. Pathophysiology of Diabetic Dyslipidemia. Retrieved from jstage.jst.go.jp: https://doi.org/10.5551/jat.RV17023.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102589.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







