Translate this page into:
The effect of almonds consumption on blood pressure: A systematic review and dose-response meta-analysis of randomized control trials
⁎Corresponding author. scientist789@sina.com (Fuyu Yin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Almond is rich in antioxidants and phytochemicals such as methylquercetin, protocatechuic acid, catechin, flavonoids, p-hydroxybenzoic acid, resveratrol, vanillic acid, and kaempferol. The aim of the present study was to systematically review and dose-response meta-analyses the effects of almond consumption on systolic and diastolic blood pressure (SBP/DBP), respectively, in Randomized Controlled Trials (RCTs). A systematic search was performed in PubMed/MEDLINE, web of sciences and SCOPUS by 2 researchers, independently to identify randomised controlled trials up to July 2019. There were no time or language restrictions. PRISMA guidelines were followed in conducting this meta-analysis. Fifteen studies with 21 arms, containing 853 participants, reported SBP as an outcome measure. Pooled results showed significant reduction in SBP (WMD: −0.90 mmHg, 95% CI: −1.74, −0.06, Pheterogeneity = 0.94) by almond intervention. There is no significant effect from almond consumption on DBP (WMD: 0.67 mmHg, 95% CI: −1.93, 0.60, Pheterogeneity = 0.001). Meta-regression analysis showed dose of used almond (g/d) as source of heterogeneity between results of DBP. In conclusion results of this meta-analysis showed reduce effect of Almonds on systolic blood pressure.
Keywords
Almond
Systolic blood pressure
Diastolic blood pressure
Meta-analysis
- SBP
-
systolic blood pressure
- DBP
-
diastolic blood pressure
- RCT
-
Randomized Controlled Trials
- WMD
-
weighted mean diffrence
Abbreviations
1 Introduction
Hypertension is one of the important causes of mortality in worldwide (Mills et al., 2016). Its prevalence is increasing dramatically; there was a 10.5% increase of the death rate attributed to high blood pressure in the US from 2005 to 2015 (Benjamin et al., 2018). Worldwide, 1.3 billion hypertensive people have been diagnosed in 2010. According to WHO, the number 1 cause of death in 2016 is the ischemic heart disease and the stroke, a main complication of this silent killer. Eleven percent of children and young adolescents in the US were prone to this non-communicable disease in 2012 according to the American Heart Association (Mills et al., 2016).
Nutrition, in conjunction with pharmacotherapy, plays an important role in either increasing or managing the high blood pressure; high sodium chloride intake increases the incidence and alcohol consumption may cause an acute elevation in blood pressure. In contrast, high intake of potassium, polyunsaturated fatty acids, and protein may help in lowering blood pressure (Savica et al., 2010).
Almonds, a type of tree nut, are rich in vitamins, minerals, mono-saturated and polyunsaturated fatty acid (Jaceldo-Siegl et al., 2004). Recently, almonds have been advocated as a promising part of a healthy diet improving the blood pressure, weight, and the lipid profile (O’Neil et al., 2016). Many studies reported almond's role in reducing serum levels of triglycerides, low-density lipoprotein, and total cholesterol (Griel and Kris-Etherton, 2006; Sabaté et al., 2010). In addition, almonds affect the appetite and the post-ingestive metabolism positively without increasing the body mass index (Tan and Mattes, 2013).
While some studies have shown almond to have a potential reducing effect on SBP and DBP (Choudhury et al., 2014; Dhillon et al., 2016), other studies have not shown significant effects (Chen et al., 2015; de Souza et al., 2018; Foster et al., 2012; Liu et al., 2018). The aim of present dose-response meta-analysis was to determine the effect of almonds consumption on blood pressure in adults.
2 Methods
2.1 Literature search
For conducting the present systematic review and dose-response meta-analysis, the Preferred Reporting Items of Systematic Reviews and Meta-Analysis (PRISMA) statement guideline was followed (23). Two authors (MB and CC) independently conducted a search of the databases MEDLINE/PubMed, Web of sciences, and Scopus up to July 2019. Medical subject headings (MeSH) and non-MeSH (title and/or abstract) terms were used for searching (Supplemental Table 1) and reference lists of included studies were searched manually, too. No date or language or grey literature restrictions were applied. Any disagreements between authors were resolved by way of cooperative triangulation with the senior author. Data from articles were extracted by two authors, independently.
2.2 Study selection
Studies screened based on title and abstract in first step of screening. In second step, we evaluate studies based on our inclusion criteria in full text of them. Studies that met inclusion criteria included in analysis.
2.3 Eligibility criteria
Studies were included if they involved: a) Parallel or cross over randomized clinical trial (RCT) designs, b) consumption of Almond as intervention c) reported blood pressure as outcome measure. d) adults Animal, in-vitro, studies without control group, and review papers were excluded. PICOS for this study was: P – public population, I – intervention with Almond, C – placebo group, O – blood pressure, and S – RCTs.
2.4 Statistical analyses
Effect sizes and 95% confidence intervals were reported for each study.SD change of the mean difference was calculated using the SD2 = [(SD baseline2 + SD final2) − (2 × r × SD baseline × SD final)] formula (Higgins and Green, 2011) if the studies did not report, it. Included studies results combined by random effect model. Meta-regression analysis based on used dose of almond conducted to find source of heterogeneity. Heterogeneity among the studies was assessment using the I-squared (I2) statistics. Funnel plots, Begg’s and Egger’s weighted regression tests were used to assessment publication bias (2); the trim and fill approach was used to adjust for publication bias (Duval and Tweedie, 2000). Sensitivity analysis was additionally conducted to investigate the individual effect of each study on the overall analysis. Cochrane collaboration’s Risk of Bias tool was used to assess included studies (4). STATA software version 12 (STATA Corp, College119 station, Texas) was used in all analyses.
3 Results
In our initial comprehensive systematic search from PubMed, Scopus, and web of sciences, 104 papers were located. Fig. 1 depicts a flow diagram of the search strategy. After removing duplicates, 66 articles were identified for title and/or abstract screening. Screening led to the removal of 39 articles Subsequently, 11 additional articles were removed because they did not meet the inclusion criteria during afull text screening. Finally, 15 articles with 21 arms were included for meta-analysis (Abazarfard et al., 2014; Chen et al., 2015, 2017; Choudhury et al., 2014; de Souza et al., 2018; Dhillon et al., 2016, 2018; Foster et al., 2012; Jamshed et al., 2015; Jenkins et al., 2002; Johnston et al., 2017; Liu et al., 2018; Richmond et al., 2013; Sweazea et al., 2014; Tan and Mattes, 2013; Wien et al., 2010).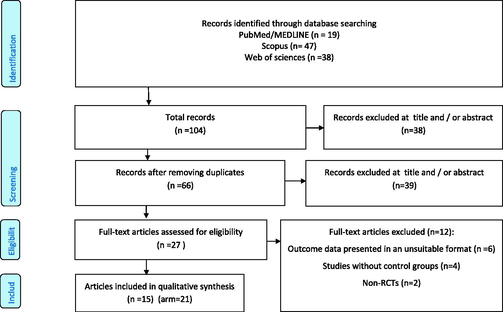
Flow chart of included studies.
3.1 Study characteristics
Characteristics of the eligible studies are detailed in Table 1. Studies were published from 2002 to 2018. Sample sizes ranged from 6 to 137. Seven studies were conducted in the USA (Chen et al., 2015; Dhillon et al., 2016, 2018; Foster et al., 2012; Johnston et al., 2017; Sweazea et al., 2014; Wien et al., 2010), 1 in New Zealand (Richmond et al., 2013), 1 in Canada (Jenkins et al., 2002), 1 in Taipei (Chen et al., 2017), Brazil (de Souza et al., 2018), UK (Choudhury et al., 2014), Korea (Liu et al., 2018), Pakistan (Jamshed et al., 2015), and Australia (Tan and Mattes, 2013). Length of follow up ranged from 3 to 78 weeks. The quality of eligible studies was evaluated using the Cochrane collaboration’s Risk of Bias tool for quality assessment of RCT’s, where the majority studies were assigned low risk (Fig. 2).
First Author
Country
Year
Study Population (n)
Age
Sex (1. male 2. female 3. both)
Dose of Almond
basic participant disease
type of Almond
Duration of treatment (week)
Chen
USA
2015
45
61.8
3
85 g/d
CAD patients
Raw whole almonds
2 × 6 wk intervention phases
Chen
USA-collected in Taipe
2017
33
54.9
3
60 g/d
T2DM
Roasted almonds
12 wk × 2 phases (CO)
Choudhury
UK
2014
60
Middle age 56y; young male 22.1y; young male with risk factors 27.3y; CL gp allocated from the above 3 groups
1
50 g/d
healthy middle-aged (MA); Healthy yound (HY); “young, at risk (YR); Habitual (control)
not specified-2 bags
4wk
Dhillon
USA
2016
50
18–60
3
providing 15% of Energy
Overweight and obese individuals
Dry roasted, lightly salted
12 wks
Foster
USA
2012
123
46.8
3
two 28-g packages of almonds (24) Over the first 5 wk of treatment, participants received whole, raw almonds only.
At week 6, roasted almonds were introduced and, over time, a variety of isocaloric, flavored almonds were used.Overweight and obese individuals
first 5 wk – whole, raw almonds only.At week 6, roasted almonds were introduced over time:over time,a variety of isocaloric, flavored almonds were used.
18 months
Jaapna Dhillon
USA
2018
73
18
3
56.7 g/day
–
dry roasted almonds
12 wks
Jamshed
Pakistan
2015
113
32–86
3
10 g/d PA: Pakinstan almonds AA: american almonds
Included CAD patients with optimal LDL cholesterol and low HDL cholesterol
Pakistan almonds (Talwar (sword shape) & American almonds (Carmel variety, California Shelled. Soak overnight and eat after removing the skin, before breakfast
12 wk
Jenkins
Canada
2002
27
64
3
73.3 g/d
hyperlipidemic subjects
whole raw unblanched almonds
3 phases 1 month each
Johnston
USA
2017
12
45–60
3
2.5 oz/d
sedentary older adults
raw, whole
8 wks (wk 1–5 walking) but group comparisons with almonds vs cookie butter were on wk 6–8
Lui
Rep Korea
2018
85
26
3
56 g/d
Healthy adults
whole
20wks
Richmond
New Zealand
2018
22
62
2
30 g/d
postmenopausal women with type 2 diabetes
whole nature almonds
3wk x2 (CO)
Souza
Brazil
2018
46
20–59
2
20 g
Adult women , overweight or obese
roasted Baru almonds
8 wks
Sweazea
USA
2014
21
55
3
1.5 oz/5–7 days a wk
Health adults with T2D
skins
12 wk
Tan
Australia
2013
137
31
3
43 g/d in 4 conditions
Risk of T2D
Dry roasted, lightly salted
4 wks 5 groups
Wien,
USA
2010
6
53
3
Participants consumed an ADA diet with 20% from almonds and avoided other tree nuts and peanuts (intervention) energy from almonds
Prediabetes
no mentioned
16 wks
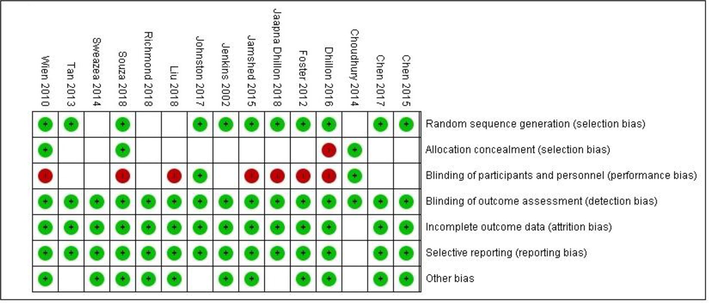
Cochrane risk of bias assessment.
3.2 Meta-analysis results
Sixteen studies, with 21 arms, containing 853 participants, reported SBP as an outcome measure. Pooled results did not show any significant reductive effect from almonds on SBP (WMD: −0.90 mmHg, 95% CI: −1.74, −0.06, I2 = 00%, Pheterogeneity = 0.94) (Fig. 3).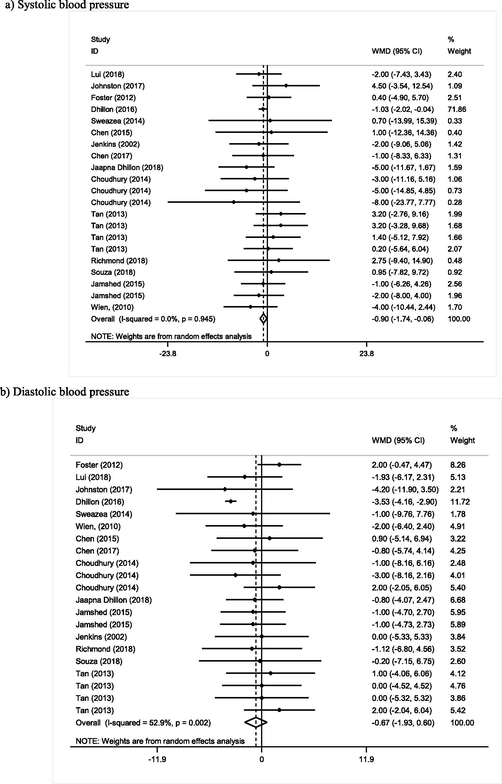
Meta-analysis of effect of Almond consumption on:
Almond consumption did not show any significant effect on DBP (WMD: 0.67 mmHg, 95% CI: −1.93, 0.60, I2 = 52%, Pheterogeneity = 0.001). Meta-regression analysis based on used dose of almond was performed and showed dose of almond as a source of heterogeneity (I2 = 0%). There was inverse relation between dose of almond and blood pressure (Coef = −0.0097) but this relation was not statistically significant (p = 0.72) (Supplemental Fig. 1).
3.3 Publication bias and sensitivity analysis
There is not any asymmetry in the funnel plot of included studies for SBP (Fig. 4), but there was an asymmetry between included studies for DBP. The Egger’s and Begg’s tests for SBP were p = 0.63 and p = 0.80, respectively, and p = 0.01 and p = 0.09 for DBP, respectively. A Trim and fill method was used to adjust for publication bias for DBP; results showed 32 papers and a significant reduction in DBP (WMD: −2.91 mmHg, 95% CI: −4.11, −1.71). The sensitivity analysis showed no significant differences beyond the 95% CI of calculated combined results for each of included studies (Supplemental Fig. 2).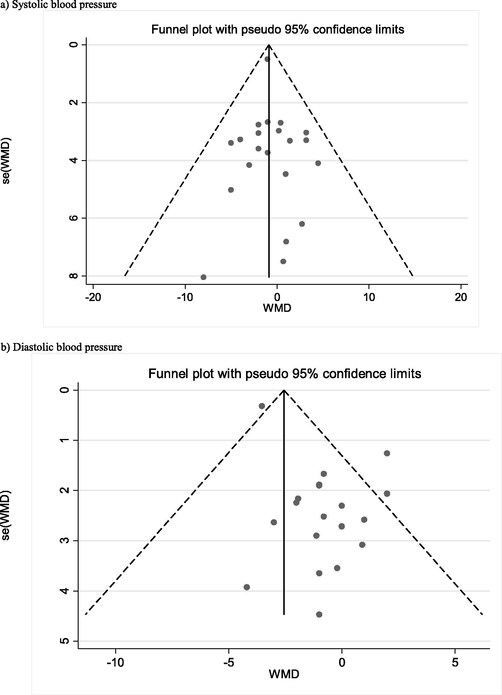
Funnel plot to assess publication bias.
4 Discussion
Whilst consumption of almonds has been shown to elicit improvements in triglycerides, total cholesterol (Sabaté et al., 2010), low density lipoprotein-cholesterol, and various cardiovascular disease risk factors (Griel and Kris-Etherton, 2006; Li et al., 2010); its’ widescale recommendation and uptake remains inconsistent. Observational studies have reported that almond consumption is associated with a reduced risk of developing various non-communicable diseases, including; elevated blood pressure (hypertension), coronary artery disease, and type 2 diabetes (Jiang et al., 2002; Kris-Etherton et al., 2001). However, despite numerous reports suggesting almond consumption may elicit positive effects on vascular health, there still exists some equivocality, particularly when considered concurrently with weight-loss and blood lipid markers (Foster et al., 2012). Moreover, to date, no systematic compilation of relevant data has taken place; thus, we sought to conduct a systematic review and dose-response meta-analysis of the effect of almond consumption on blood pressure. In accord with the aim of this study, we found that, overall, almond consumption had significant reduction in SBP but on DBP. However, following meta-regression analyses, we found that a reduction in DBP by higher dose of Almond.
Lifestyle modifications consisting of regular adherence to physical activity guidelines, reduced consumption of fat, increased consumption of plant-based foods and smoking cessation are recommended to complement pharmacological regimens in the management and prevention of hypertension, and related comorbidities (Booth et al., 2014). However, a recent meta-analysis investigating dietary-based almond interventions asserted that the current evidence base does not support almond consumption specifically for lipid altering effects (Phung et al., 2009), whilst dietary intervention with almonds demonstrably improves biomarkers of insulin sensitivity in prediabetic adults (Wien et al., 2010). Almond consumption has been shown to positively impact markers of inflammation and hemostasis, respectively, independent of dose (Rajaram et al., 2010). Furthermore, almonds represent major dietary sources of α-tocopherol and mono-unsaturated fatty acids and regular consumption evidently diminishes the risk of early mortality attributed to coronary heart disease, myocardial infarction (Hu et al., 1997), and sudden cardiac death (Albert et al., 2002). Moreover, nutrients constituent within almonds are associated with beneficial cardiovascular outcomes, such as antioxidant flavonoids and l-arginine, which is key for the synthesis of the vasodilatory molecule, nitric oxide. The precise nutrient composition of almonds differs dependent on the cultivar (Barreira et al., 2008), and the overall positive effects of almonds may conceivably be attributable to the milieu of nutrients.
Some studies, such as Dhillon et al., have reported that almond consumption results in greater proportional reductions of trunk and total body fat as well as DBP, and thus may help to lower metabolic disease risk in obesity (Dhillon et al., 2016); however, in such examples, participants were concurrently following an energy restriction protocol and were overweight or obese at study commencement. Thus, whilst consuming almonds, alongside calorie-restriction, was more beneficial than calorie restriction alone.
Epidemiological and clinical evidence demonstrates that adherence to a diet enriched in mono-unsaturated fatty acids and tocopherols from nuts, can reduce blood pressure (SBP and DBP) and blood lipid levels (Einarsson et al., 1985). Indeed, regular nut consumption, demarcated by 1–4 times per week, has been reported to diminish the risk of early mortality attributed to cardiovascular disease by, roughly, one-quarter (Dreher et al., 1996). Indeed, mechanistically, muscle relaxation is putatively mediated by the availability of nitric oxide (Hijmering et al., 2002). Almonds are abundant in l-arginine, which is a rate-limiting substrate for the production of endothelial nitric oxide and in the synthesis of vitamin E, and acts as a scavenger of free radicals; in combination, this is asserted to increase bioavailable nitric oxide. Age-dependent vascular stiffness might also be a mitigating factor, where, although the mechanism by which baroreceptor control is mediated via nutrients is not well defined, some empirical evidence suggests that redox imbalances might precipitate high blood pressure and baroreflex responses, whilst tocopherol can modulate vascular responses (El-Mas et al., 2012; Wu et al., 2011). Furthermore, lipid dysfunction may intermediate this effect, where hyperlipidemia in hypertensive and obese patients has been attributed to baroreceptor reflex impairment, resulting in hypertension (SBP and DBP) (Gadegbeku et al., 2002).
5 Strengths and limitations
The primary strength of the present study was that we assessed the impact of almond consumption on blood pressure, which has not previously been reported; and given the potential influence on clinical and holistic practice, this represents an important addition to the literature. The evidence base, prior to this investigation, was far from consensual, and thus necessitated a meta-analytical evaluation, which we have provided. An additional strength of the current meta-analysis is the heterogeneous sample assimilated, with a range of demographics, ethnicities, and ages. Moreover, we were also able to stratify our analyses based upon dosage of supplementation. However, there are some limitations that need to be appreciated within this meta-analysis. Varying diets, ad libitum or prescribed, may elicit different magnitudes of weight change during trials, possibly confounding the results of the meta-analysis; moreover, it should be considered that control group diets designed to mimic the overall energy content of almonds may also represent a confounding variable. Bias associated with attrition may be a concern in the present meta-analysis, however, the compliance of participants to study diets was scarcely reported; consequently, we were unable to assess attrition bias in the included studies and their consequential impact on the overall results of the meta-analysis.
6 Conclusion
The current body of evidence supports the ingestion of almonds for their beneficial effect on blood pressure. However, both the vascular moderating effects and the safety and acceptability of almond consumption should be further investigated in large, randomized, double-blind, placebo-controlled trials of longer duration, across age groups.
Funding
No funding to report.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
References
- The effect of almonds on anthropometric measurements and lipid profile in overweight and obese females in a weight reduction program: a randomized controlled clinical trial. J. Res. Med. Sci.. 2014;19(5):457-464.
- [Google Scholar]
- Nut consumption and decreased risk of sudden cardiac death in the Physicians' Health Study. Arch. Intern. Med.. 2002;162(12):1382-1387.
- [Google Scholar]
- Antioxidant activity and bioactive compounds of ten Portuguese regional and commercial almond cultivars. Food Chem. Toxicol.. 2008;46(6):2230-2235.
- [Google Scholar]
- Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67
- [Google Scholar]
- Effect of sustaining lifestyle modifications (nonsmoking, weight reduction, physical activity, and mediterranean diet) after healing of myocardial infarction, percutaneous intervention, or coronary bypass (from the REasons for Geographic and Racial Differences in Stroke Study) Am. J. Cardiol.. 2014;113(12):1933-1940.
- [Google Scholar]
- Effect of almond consumption on vascular function in patients with coronary artery disease: a randomized, controlled, cross-over trial. Nutr. J.. 2015;14(1)
- [CrossRef] [Google Scholar]
- Almonds ameliorate glycemic control in Chinese patients with better controlled type 2 diabetes: a randomized, crossover, controlled feeding trial. Nutr. Metab. (Lond.). 2017;14:51.
- [Google Scholar]
- An almond-enriched diet increases plasma alpha-tocopherol and improves vascular function but does not affect oxidative stress markers or lipid levels. Free Radic. Res.. 2014;48(5):599-606.
- [Google Scholar]
- A baru almond-enriched diet reduces abdominal adiposity and improves high-density lipoprotein concentrations: a randomized, placebo-controlled trial. Nutrition. 2018;55:154-160.
- [Google Scholar]
- Almond consumption during energy restriction lowers truncal fat and blood pressure in compliant overweight or obese adults. J. Nutr.. 2016;146(12):2513-2519.
- [Google Scholar]
- Glucoregulatory and cardiometabolic profiles of almond vs. cracker snacking for 8 weeks in young adults: a randomized controlled trial. Nutrients. 2018;10(8)
- [Google Scholar]
- The traditional and emerging role of nuts in healthful diets. Nutr. Rev.. 1996;54(8):241-245.
- [Google Scholar]
- Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463.
- [Google Scholar]
- Influence of age on secretion of cholesterol and synthesis of bile acids by the liver. N. Engl. J. Med.. 1985;313(5):277-282.
- [Google Scholar]
- Redox imbalances incite the hypertensive, baroreflex, and autonomic effects of cyclosporine in rats. Eur. J. Pharmacol.. 2012;694(1–3):82-88.
- [Google Scholar]
- A randomized trial of the effects of an almond-enriched, hypocaloric diet in the treatment of obesity. Am. J. Clin. Nutr.. 2012;96(2):249-254.
- [Google Scholar]
- Raising lipids acutely reduces baroreflex sensitivity. Am. J. Hypertens.. 2002;15(6):479-485.
- [Google Scholar]
- Tree nuts and the lipid profile: a review of clinical studies. Br. J. Nutr.. 2006;96(S2):S68-S78.
- [Google Scholar]
- Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2011.
- Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J. Am. Coll. Cardiol.. 2002;39(4):683-688.
- [Google Scholar]
- Dietary fat intake and the risk of coronary heart disease in women. N. Engl. J. Med.. 1997;337(21):1491-1499.
- [Google Scholar]
- Long-term almond supplementation without advice on food replacement induces favourable nutrient modifications to the habitual diets of free-living individuals. Br. J. Nutr.. 2004;92(3):533-540.
- [Google Scholar]
- Dietary almonds increase serum HDL cholesterol in coronary artery disease patients in a randomized controlled trial. J. Nutr.. 2015;145(10):2287-2292.
- [Google Scholar]
- Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation. 2002;106(11):1327-1332.
- [Google Scholar]
- Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA. 2002;288(20):2554-2560.
- [Google Scholar]
- Almond ingestion contributes to improved cardiovascular health in sedentary older adults participating in a walking intervention: a pilot study. J. Funct. Foods. 2017;39:58-62.
- [Google Scholar]
- The effects of nuts on coronary heart disease risk. Nutr. Rev.. 2001;59(4):103-111.
- [Google Scholar]
- Pistachio nuts reduce triglycerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12-week weight loss program. J. Am. Coll. Nutr.. 2010;29(3):198-203.
- [Google Scholar]
- Time and intervention effects of daily almond intake on the changes of lipid profile and body composition among free-living healthy adults. J. Med. Food. 2018;21(4):340-347.
- [Google Scholar]
- Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441-450.
- [Google Scholar]
- Almond consumption is associated with better nutrient intake, nutrient adequacy, and diet quality in adults: national health and nutrition examination survey 2001–2010. Food Nutr. Sci.. 2016;7(07):504.
- [Google Scholar]
- Almonds have a neutral effect on serum lipid profiles: a meta-analysis of randomized trials. J. Am. Diet. Assoc.. 2009;109(5):865-873.
- [Google Scholar]
- Effect of almond-enriched high-monounsaturated fat diet on selected markers of inflammation: a randomised, controlled, crossover study. Br. J. Nutr.. 2010;103(6):907-912.
- [Google Scholar]
- Markers of cardiovascular risk in postmenopausal women with type 2 diabetes are improved by the daily consumption of almonds or sunflower kernels: a feeding study. ISRN Nutr.. 2013;2013:626414
- [Google Scholar]
- Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch. Intern. Med.. 2010;170(9):821-827.
- [Google Scholar]
- Almond supplementation in the absence of dietary advice significantly reduces C-reactive protein in subjects with type 2 diabetes. J. Funct. Foods. 2014;10:252-259.
- [Google Scholar]
- Appetitive, dietary and health effects of almonds consumed with meals or as snacks: a randomized, controlled trial. Eur. J. Clin. Nutr.. 2013;67(11):1205-1214.
- [Google Scholar]
- Almond consumption and cardiovascular risk factors in adults with prediabetes. J. Am. Coll. Nutr.. 2010;29(3):189-197.
- [Google Scholar]
- Ascorbic acid and α-tocopherol supplement starting prenatally enhances the resistance of nucleus tractus solitarius neurons to hypobaric hypoxic challenge. Brain Struct. Funct.. 2011;216(2):105-122.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.01.013.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







