Translate this page into:
The beneficial efficacy of liposomal resveratrol against doxorubicin-induced hepatotoxicity in rats: Role of TGF-β1 and SIRT1
⁎Corresponding author. aelhusaini@ksu.edu.sa (Ahlam M. Alhusaini)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Doxorubicin (DOXR) belongs to the antineoplastic anthracycline antibiotic and despite its prevalent use for various types of cancer, it is associated with side effects that occur even after treatment cessation, particularly on the heart, blood, liver and kidney. This study aimed to study the efficiency of carvedilol (CAR), resveratrol (RES) or liposomal RES (LIPO-RES) and their combination to treat DOXR-induced hepatotoxicity and to investigate other driving mechanisms that are involved.
Methods
Rats were injected twice weekly with DOXR for five weeks to induce liver injury. A week before DOXR injection commenced, rats were pretreated singly or simultaneously with CAR, RES or LIPO-RES for six weeks.
Results
The significant elevation in serum alanine aminotransferase (ALT) and the changed the hepatic tissue structure following DOXR administration indicating hepatic injury. Additionally, DOXR injection caused upregulation of the hepatic malondialdehyde (MDA), inflammatory cytokines, and transforming growth factor-β1 (TGF-β1) levels. The endogenous glutathione (GSH) and sirtuin1 (SIRT1) expressions in the liver were downregulated following DOXR administration. CAR, RES or LIPO-RES as their alternative combinations attenuated the liver injury by controlling oxidative stress, inflammation, and fibrosis. These beneficial effects were apparent upon using combined CAR and LIPO-RES, as proved by restoring the balance of MDA and GSH levels, decreasing hepatic cytokines levels of IL-6 and TNF-α, upregulating SIRT1 and downregulating TGF-β1 levels.
Conclusion
The current study indicated that the use of CAR, RES or LIPO-RES alone or in combination could prevent DOXR-induced hepatic damage in rats by inhibiting oxidative stress, inflammation, and fibrosis.
Keywords
Hepatotoxicity
TGF-β1
SIRT1
Carvedilol
Doxorubicin
Resveratrol
1 Introduction
Doxorubicin (DOXR) represents one of the commonly used chemotherapeutic agents in many types of malignancy that mainly inhibit DNA synthesis. Apart from its high antitumor activity, DOXR has been linked to many adverse effects, especially cardiotoxicity and hepatotoxicity. The hepatotoxicity that is associated with DOXR occurs in the form of hepatocyte degeneration and hyperplasia of the bile duct together with focal necrosis (Shivakumar et al., 2012). The major mechanism thought to be implicated in DOXR-induced hepatotoxicity is the overproduction of reactive oxygen species (ROS) as a consequence of its hepatic metabolism, which leads to oxidative stress. The induction of ROS production is linked to p53 activation and results in elevation of Bax and Fas expressions which in turn induces the expression of caspase-3 and eventually elicits apoptotic signaling (Nagai et al., 2016). In addition, DOXR is associated with the initiation of inflammatory response in the liver through oxidative stress by upregulation of the mRNA expression of pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α) and connective tissue growth factor (CTGF) which can be involved in transforming growth factor-β1 (TGF-β1) downstream actions (Cengiz, 2021). DOXR causes activation of nuclear factor-kappa B (NF-κB) signaling through the inhibition of sirtuin 1 (SIRT1) and this results in inflammatory reaction (Song, 2019). Moreover, DOXR disrupts the mitochondrial function through ROS production as it enters the mitochondrial matrix with a high affinity to its inner membrane (Guven et al., 2018). Mitochondrial dysfunction is attributed either to the reduction of inorganic phosphate and thereby decreasing adenosine triphosphate (ATP) level, or inhibition of mitochondrial DNA replication (Guven et al., 2018).
Based on the molecular mechanism of DOXR-hepatotoxicity, the use of antioxidant and anti-inflammatory agents can effectively improve the hepatic complications elicited by this chemotherapeutic agent. Resveratrol (RES) is a natural polyphenolic compound that possesses antioxidant, anti-inflammatory and anti-aging properties present largely in some edible plants like grapes (Giovinazzo et al., 2012). Similarly, carvedilol (CAR) is an agent that belongs to third generation β-blocker which inhibits β-1, β-2 and α-1 adrenoceptors and has an antioxidant effect that may exert additional beneficial action to protect against DOXR-induced hepatotoxicity (Kumar et al., 2000). In cardiomyocytes, CAR inhibits lipid peroxidation and protects them from deleterious effects of oxidative stress (Noguchi et al., 2000). Likewise, it inhibits activated neutrophils from releasing superoxide anion (Yue et al., 1992). Moreover, CAR was shown to protected cultured endothelial cells against cell injury caused by ROS (Yue et al., 1995).
As mentioned earlier, DOXR therapy is associated with harmful effects on the liver, and unfortunately, there are limited treatment options to overcome this limitation. Therefore, there is an urgent need for the emergence of new and advanced strategies for controlling DOXR’s hepatic side effects. Liposomal form of RES is considered as one of these promising protective approaches. This form of RES can increase drug bioavailability, decrease immune reactivity, and target organs with the specific drug. This study aims to evaluate the beneficial activities of RES, CAR or liposomal analog of RES against the DOXR-induced liver injury in rats, focusing on their antioxidative, anti-inflammatory, and antifibrotic actions.
2 Materials and Methods
2.1 Chemicals
RES and CAR were obtained from Sigma (St. Louis, USA). DOXR (Ebewe Pharma Co, Unterach Am Attersee, Austria) was kindly provided from a local pharmacy in Riyadh (Saudi Arabia), and the liposomal Trans RES® formulation (RES encapsulated in liposomes with particle size = 200 nm) was obtained from Lipolife® (Drakes Lane, UK). Primary antibodies for TGF-β1 (ab92486), SIRT1 (ab7343) and GAPDH (ab9483) were obtained from Abcam® (Cambridge, USA). The secondary antibodies conjugated to horseradish peroxidase (HRP) (sc-2357 and sc-2354) were purchased from Santa Cruz Biotechnology (Dallas, USA).
2.2 Animal and experimental design
Male Wistar albino rats of an average weight of 165 ± 10 g were obtained from the Animals Care Centre at the College of Pharmacy, King Saud University, Riyadh, Saudi, KSA. Prior to the experiment, the rats were allowed to adapt to the laboratory conditions (adjusted temperature at 23 ⁰C with alternation of 12/12 h light/dark cycle) for at least one week with free access to food and water ad libitum. All experiments were conducted according to the guidelines and regulations of the Research Ethics Committee at King Saud University (Ref. No: KSU-SE-18–31).
A total of forty-two rats were distributed into seven groups, with six rats each as follows:
Group I (Control): received 1% carboxymethylcellulose (CMC) orally which was used as the vehicle of the drugs for 6 weeks. Physiological saline was given intraperitoneally (i.p.) to this group, starting from week 2 to week 6 twice every week.
Group II (DOXR): received 1% CMC orally for 6 weeks and the DOXR treatment was given twice every week (2 mg/kg, i.p.) starting from week 2 to week 6 to give rise to a total cumulative dose of 20 mg/kg (Tatlidede, 2009).
Group III (CAR): treated with CAR (30 mg/kg) orally for 6 weeks and DOXR treatment was given from the second week as referred in group II (Arozal, 2010).
Group IV (RES): treated with RES (20 mg/kg) orally for 6 weeks and DOXR treatment was given from the second week as referred in group II (Chehl et al., 2009).
Group V (CAR/RES): received a combination of CAR (30 mg/kg) and RES (20 mg/kg) orally for 6 weeks and DOXR treatment was given from the second week as referred in group II.
Group VI (LIPO-RES): treated with LIPO-RES (20 mg/kg) orally for 6 weeks and DOXR treatment was given from the second week as referred in group II.
Group VII (CAR/LIPO-RES): received a combination of CAR (30 mg/kg) and LIPO-RES (20 mg/kg) orally for 6 weeks and DOXR treatment was given from the second week as referred in group II.
After completion of treatment courses, all rats were anesthetized, and blood samples were collected and centrifuged to collect serum. Then, the livers were collected from rats and washed. Parts from the livers were fixed in 10% formalin for histopathological examination, whereas the remaining parts were homogenized in chilled phosphate buffered saline (PBS) and proteinase inhibitors and then centrifuged. The supernatants were kept at −80 °C for the subsequent experiments.
2.3 Assay of liver function, cytokines levels and oxidative stress markers
Serum alanine aminotransferase (ALT) was measured employing a kit obtained from Randox (Crumlin, UK). Hepatic tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) were assayed using ELISA kits (MyBioSource, CA, USA) as instructed by the manufacturer. Hepatic level of malondialdehyde (MDA), a well-known indicator of lipid peroxidation, was assayed as described before. Briefly, the homogenate tissue was mixed with TBA, and acetate buffer, and then heated in a boiling water bath for one hour. Once cooling, n-butanol was added to the mixture, which was centrifuged, the absorbance was measured at 532 nm (Shoukry, 2017). Hepatic GSH level was determined according to the method of Ellman; this analysis is based on the reaction of GSH with 5,50-dithio-bis (2-nitrobenzoic acid) and measurement of the absorbance at 412 nm (Ohkawa et al., 1979).
2.4 DNA fragmentation
The level of DNA fragmentation in hepatic cells as an indicator for cellular death was analyzed by detecting the labeled DNA fragments in the samples using a DNA fragmentation kit (Cat no. 6137, Takara Bio, USA).
2.5 Reverse transcription-polymerase chain reaction (RT-PCR)
According to kit instructions, frozen liver tissues were used to extract total RNA by RNeasy Mini Kit (Qiagen, Cat. No. 74104). The reverse transcription step was performed to synthesize the complementary DNA (cDNA) by First Strand cDNA Synthesis Kit (Cat. No. NP100041). Primers for heme oxygenase-1 (HO-1), Ferritin and β-actin genes are listed in Table 1. After that, the primers were mixed with cDNA, Platinum Blue PCR SuperMix (Invitrogen) and nuclease-free water in PCR tubes. The cycling reactions were performed using Biorad T100 thermal cycler. The amplified DNA samples were loaded into agarose gel mixed with ethidium bromide and allowed to run for 30 min at 120 V in Tris-acetate-EDTA buffer (TAE). The gels were visualized with the Gene Genius Bioimaging System (Syngene).
Gene
Primer sequence
HO-1
F: 5′- −3′ AGCATGTTCCCAGGATG
R: 5′- −3′ GCTCAATGTTGAGCACA
Ferritin
F: 5′- −3′ GCC CTG AAG AAC TTT GCC AAA T
R: 5′- −3′ TGC AGG AAG ATT CGT CCA CCT
β-actin
F: 5′- −3′ CTG TCCCTGTATGCCTCT
R: 5′- −3′ ATGTCACGCACGATTTCC
2.6 Western blotting
The expression of TGF-β1 and SIRT1 in the liver samples was estimated by Western blotting, where the hepatic cells were lysed with RIPA buffer. After protein concentration measurement, 40 μg of the total protein was loaded on 12% SDS/PAGE and subjected to electrophoresis to separate the proteins based on their molecular sizes. The proteins were transferred into nitrocellulose membranes and then blocked with 5% skimmed milk. After that, the membranes were incubated with either anti-TGF-β1, anti-SIRT1 or anti-GAPDH for overnight. Then, the membranes were washed several times and probed with secondary antibodies for 2 h. Finally, the membranes were washed and imaged in ImageQuant LAS 4000 (GE Healthcare, USA) using ECL chemiluminescent detection kit (Bio-Rad, USA). The protein bands were scanned and quantified using ImageJ (NIH, USA).
2.7 Histological studies
The formalin fixed liver samples were subjected to dehydration, they had been inlaid on paraffin wax. The livers were cut into 5-μm-thick sections µm, then, stained with hematoxylin and eosin (H&E) after deparaffinization. The sections were dehydrated and examined under a light microscope.
2.8 Statistical analysis
The data were presented as mean ± standard error of the mean (SEM). Significant differences between the study groups were measured by employing one-way ANOVA followed by Tukey’s post-hoc test using GraphPad 7 software (GraphPad, USA) and considering P-value of 0.05 as statistically significant.
3 Results
3.1 DOXR-induced hepatotoxicity, oxidative stress and cell damage were ameliorated by RES, CAR and LIPO-RES
To investigate the possible protective effects of RES, LIPO-RES and/or CAR on DOXR-induced hepatotoxicity, we measured serum liver enzyme ALT, hepatic DNA fragmentation. DOXR elicited a significant elevation of ALT serum level, which was restored to the normal level after treatments (Fig. 1). In addition, to assess the effect of the earlier mentioned treatment on DOXR-induced oxidative stress on the liver, we measured MDA and GSH levels. In DOXR-intoxicated group, there was a significant elevation in MDA level and reduction in GSH level; However, these deleterious changes in the oxidative stress markers were ameliorated after administration of CAR, RES or LIPO-RES profoundly decrease MDA and increase GSH levels (Fig. 2). Also, to evaluate the impact of current treatments on hepatic cellular death, we measured the DNA fragmentation in hepatic tissue samples. As expected, the DOXR-intoxicated group showed a significant DNA fragmentation level compared to the control group (p < 0.001). On the contrary, treatment with CAR, RES or LIPO-RES individually or in combination resulted in a significant reduction of DOXR-induced DNA fragmentation (p < 0.001) (Fig. 3).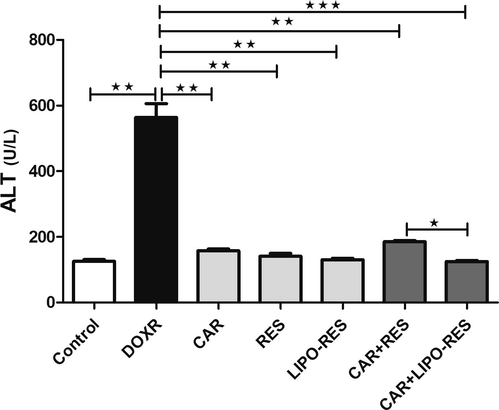
Carvedilol (CAR) and/or resveratrol (RES) and LIPO-RES reduced serum ALT in DOXR-intoxicated rats. Data are expressed as mean ± SEM, (n = 6). *** p < 0.001.
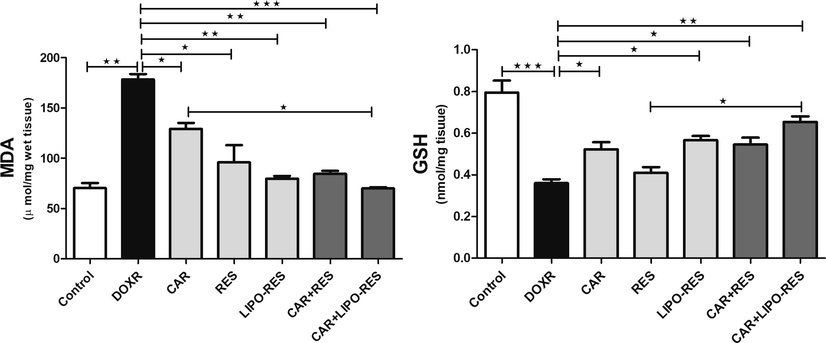
Carvedilol (CAR) and/or resveratrol (RES) and LIPO-RES restored hepatic MDA and GSH levels in DOXR-intoxicated rats. Data are expressed as mean ± SEM, (n = 6). *** p < 0.001, ** p < 0.01, * p < 0.05, ns- nonsignificant.
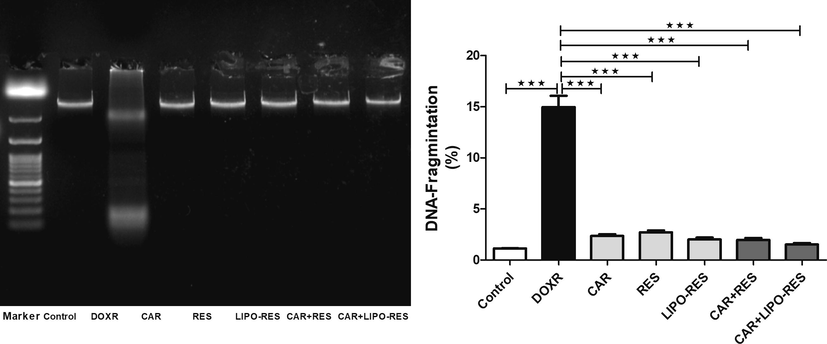
Carvedilol (CAR) and/or resveratrol (RES) and LIPO-RES decreased hepatic DNA fragmentation in DOXR-intoxicated rats. Data are expressed as mean ± SEM, (n = 6). *** p < 0.001.
3.2 CAR, RES and LIPO-RES reduced the levels of inflammatory markers
The levels of TNF-α and IL-6 were significantly elevated in the liver of rats using DOXR; Whereas treatment with CAR, RES or LIPO-RES individually or in combination significantly reduced the hepatic levels of both cytokines (Fig. 4). Among the treatment groups, LIPO-RES containing regimen showed the stronger effect in reducing TNF-α (p < 0.01). However, IL-6 level was significantly reduced in the group that received the combination of CAR and LIPO-RES (p < 0.01).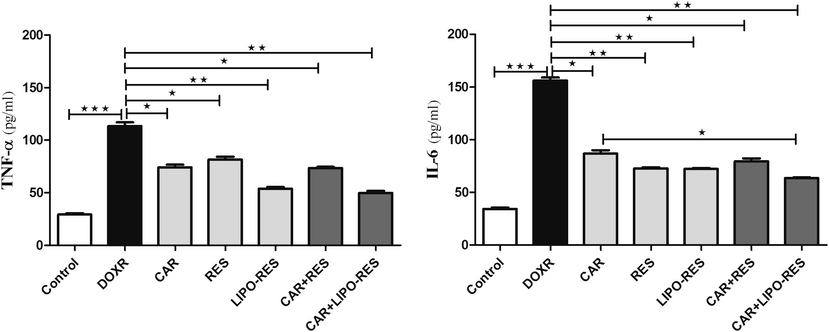
Carvedilol (CAR) and/or resveratrol (RES) and LIPO-RES downregulated the expression of hepatic TNF-α and IL-6 in DOXR-intoxicated rats. Data are expressed as mean ± SEM, (n = 6). *** p < 0.001, ** p < 0.01, * p < 0.05, ns- nonsignificant.
3.3 CAR, RES and LIPO-RES modulated the gene expression of HO-1 and Ferritin
The use of CAR, RES and LIPO-RES treatments increased the gene expression of HO-1 that was reduced with DOXR toxicity. This change was significant with the treatment combinations of CAR + RES and CAR + LIPO-RES (p < 0.05 and p < 0.001, respectively). On the contrary, the expression of ferritin gene was reduced after such combinations (p < 0.05 and p < 0.01, respectively), as depicted in Fig. 5.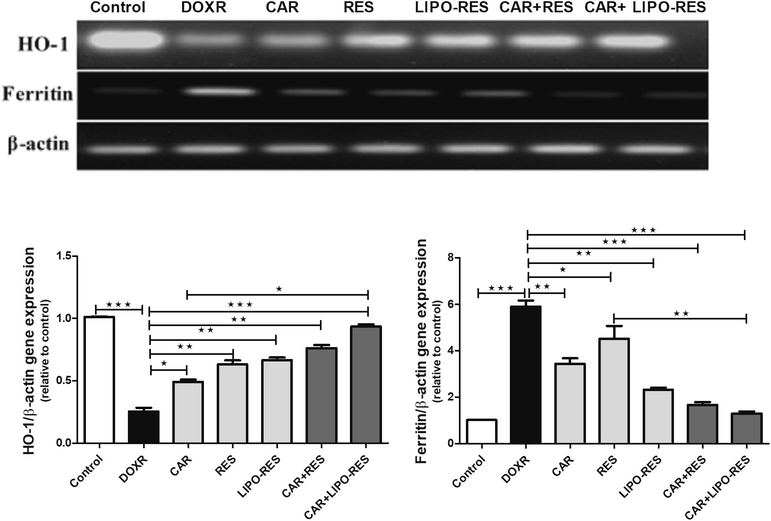
Carvedilol (CAR) and/or resveratrol (RES) and LIPO-RES modulated hepatic HO-1 and ferritin gene expression. Data are expressed as mean ± SEM, (n = 6). *** p < 0.001, ** p < 0.01, * p < 0.05.
3.4 The roles of SIRT1 and TGF-β1 in DOXR-induced hepatotoxicity:
To investigate the involvements of SIRT1 and TGF-β1 in DOXR-induced hepatic abnormalities and whether the use of CAR, RES and LIPO-RES can control the expression of these proteins, Western blotting was employed. Fig. 6 showed that CAR, RES and LIPO-RES treatments significantly increased SIRT1 and decreased TGF-β1 and protein expression.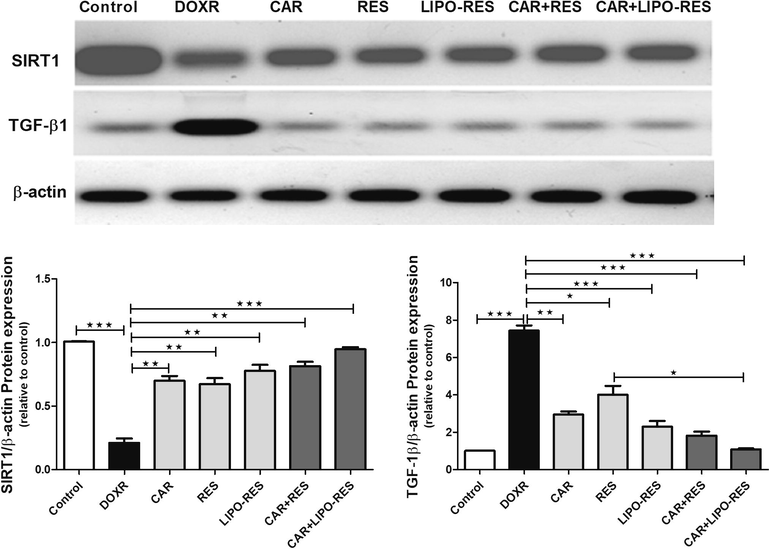
Carvedilol (CAR) and/or resveratrol (RES) and LIPO-RES ameliorated hepatic protein expression of SITR and TGF-β1. Data are expressed as mean ± SEM, (n = 6). *** p < 0.001, ** p < 0.01, * p < 0.05.
3.5 CAR, RES and LIPO-RES ameliorated the hepatic histopathological changes in response to DOXR
Histopathological studies showed that the liver section from the DOXR treated group showed congestion of sinusoids, central vein, and portal tract vein with the appearance of binucleated hepatocytes. Treatment with CAR resulted in normal structure and architecture of hepatic tissue with mild hydropic changes and congested sinusoids. RES treated group showed the same features as CAR and congested central vein, binucleated cells, and aggregates of lymphocytes at portal tract. LIPO-RES group showed almost the same effects as RES group without aggregation of lymphocytes. The combination groups showed hepatic tissue with normal morphology, hepatocytes arranged in thin plates and dilated sinusoids and central vein (Fig. 7).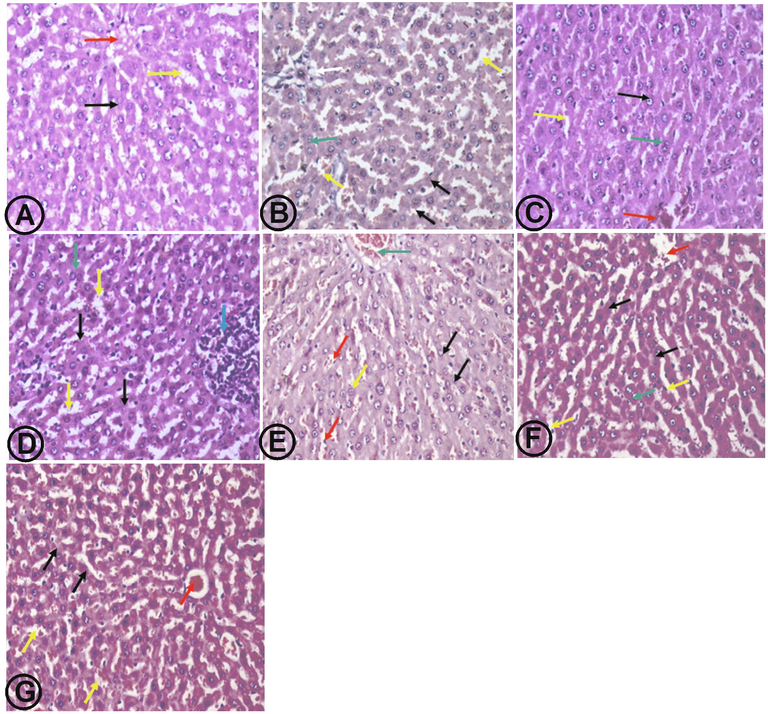
(A) liver section from normal control group showed hepatic tissue with normal architecture, hepatocytes arranged in thin plates (black arrow), with normal sinusoids (yellow arrow) and dilated central vein (red arrow). (B) DOXR group showed preserved lobular hepatic architecture, hepatocytes arranged in thin plates (black arrow), congested sinusoids (yellow arrow), binucleated hepatocytes (green arrow), portal tract vein congestion (white arrow). (C) CAR group showed hepatic tissue with normal structure, hepatocytes arranged in thin plates with mild hydropic changes (black arrow), congested sinusoids (yellow arrow), and dilated central vein (red arrow). (D) RES group showed hepatic tissue with normal structure and architecture, hepatocytes arranged in thin plates with mild hydropic changes (black arrow), dilated sinusoids (yellow arrow), congested central vein (red arrow), binucleated nuclei (green arrow), and aggregates of lymphocytes at portal tract (blue arrow). (E) LIPO-RES group showed hepatic tissue with almost normal structure, hepatocytes arranged in thin plates (black arrow) and dilated central vein (red arrow), binucleated hepatocytes (yellow arrow), and congested portal vein (green arrow). (F) CAR + RES group showed hepatic tissue with normal structure, hepatocytes arranged in thin plates (black arrow) and dilated sinusoids (yellow arrow), dilated central vein (red arrow), and congested portal vein (green arrow). (G) CAR + LIPO-RES group showed hepatic tissue with normal structure, hepatocytes arranged in thin plates (black arrow) and dilated sinusoids (yellow arrow), dilated central vein (red arrow). (H&E x400).
4 Discussion
Hepatotoxicity is one of the clinical consequences associated with DOXR therapy in a significant proportion of patients (about 30%) (Ellman, 1959). The exact underlying molecular mechanisms of DOXR-induced hepatotoxicity are not fully understood, and little is known about effective treatments for such adverse events. So, this study was conducted to examine the effectiveness of combining two well-known antioxidants, namely RES and CAR, in alleviating the DOXR-induced hepatotoxicity in rat model. In the current study, DOXR-induced liver damage was established by increasing ALT activity level, whereas CAR, RES, LIPO-RES, and the combination treatments decreased the activity level of ALT enzyme. The combination of CAR and LIPO-RES treatment exerted a significant reduction in ALT level matched with the combination of CAR and RES treatment, which indicates the beneficial effect of liposomal preparations on the bioavailability of RES (Damodar et al., 2014). ALT enzyme is the most sensitive and the primary indicator for liver function, as its elevation level is associated with liver injury (Song, 2019; Narayanan et al., 2009).
Herein, LIPO-RES alone and the combination of CAR with either RES or LIPO-RES exhibited protective effects against DOXR-induced oxidative stress via restoring the normal levels of MDA and GSH. CAR is a potent antioxidant agent that is possessed due to its carbazole moiety (Ibrahim et al., 2010). Another mechanism is related to the sequestering of ferrous (Fe2+) ion that inhibits lipid peroxidation (Brar, 2001). Moreover, previous studies provided evidence that RES can decrease ROS production as it has free radical scavenging property (Salehi, 2018; Colica et al., 2018), and has the ability to inhibit NADPH oxidase enzyme (Shoukry, 2017). RES showed a protective effect in hepatocytes culture against oxidative stress via increasing the actions of antioxidant enzymes such as catalase, superoxide dismutase and glutathione peroxidase (Passarino et al., 2014).
DOXR‐induced hepatocyte inflammation is considered an important mechanism responsible for its hepatotoxicity through stimulation of pro-inflammatory cytokines release (Yu et al., 2018; Wali, 2020). We found that treatment with LIPO-RES individually or in combination with CAR highly produced reduction in TNF-α and IL-6 concentrations. Our results agree with those reported by Kandil et al., who examined the protective effect of RES against CCl4-induced hepatic damage, and documented that RES pre-treatment significantly reduced pro-inflammatory cytokines such as IL-6 (Kandil et al., 2017). Numerous studies revealed the anti-inflammatory property of RES, as it reduced intercellular adhesion molecule 1 (ICAM-1), inducible nitric oxide synthase (iNOS), TNF-α- and IL-6 as well as NF-κB levels (de Sá Coutinho et al., 2018).
In addition, DOXR toxicity is also related to its ability to down-regulate the nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2), a cellular transcription factor, which exerts antioxidant defense mechanisms against oxidative stress. Nrf-2 induces the transcription process of essential antioxidant enzymes such as glutathione S-transferase, NAD(P)H1 and catalase (Bai and Wang, 2019). Nrf-2 also regulates the gene expression of HO-1. HO-1 is an important antioxidant, anti-inflammatory, and anti-apoptotic agent. Bai et al. reported that DOXR downregulated Nrf-2, and subsequently, the gene expressions of HO-1 (Araujo et al., 2012). Consistent with these observations, our results showed that DOXR reduced HO-1 gene expression.
Further, the importance of iron in DOXR-induced cardiac and liver toxicity is extensively recognized. Ferritin is the major iron storage protein and the enhancement of its production as in case of DOXR treatment, is considered a defensive mechanism to reduce the amount of iron available for ROS synthesis, and subsequently prevent oxidative stress (Gammella et al., 2014). In the present study, DOXR-induced hepatocyte damage was further manifested by the significant elevation in ferritin gene level, while these effects were prevented by the combination of CAR with either RES or LIPO-RES. The effect of RES on HO-1 was documented in previous studies; it suppressed NF-κB and stimulated Nrf2/HO-1 pathways in rats with acute liver injury (Farkhondeh et al., 2020). Moreover, a recent study by Zhang et al. reported that the combination of CAR and carnosic acid significantly increased HO- 1 expression (Zhang et al., 2019).
Our results indicated that the administration of DOXR significantly decreased SIRT1 protein expression, also it increased the DNA damage proved by DNA fragmentation result. While SIRT1 is an NAD+- dependent deacetylase protein, it affects the metabolism of numerous target proteins in several tissues as the heart, liver, muscle, and adipose tissue. SIRT1 plays a critical role in oxidative stress, and it has a protective action against many diseases, including neurologic, cancer and cardiovascular diseases (Ruan, 2015). Several studies have shown that the activation of SIRT1 is considered a cardioprotective strategy in cardiovascular diseases, including DOXR-induced cardiotoxicity (De Angelis, 2015). In the present work, treatment with CAR, RES, LIPO-RES and the combination of CAR with either RES or LIPO-RES increased SIRT1 protein expression as well as decreased DNA fragmentation. Studies have shown that RES protects cardiac cells in rat animal models against myocarditis via up-regulating the expression of SIRT1 that reduced apoptosis mediated by FoxO1 pathway (Chen et al., 2009).
Our data showed that DOXR administration progressed fibrosis by increasing TGF-β1 protein expression inconsistent with a prior study (Abbas and Kabil, 2017). TGF-β1 is a key fibrogenic factor and plays a leading role in the fibrotic process in different tissues, including cardiac, renal, and hepatic tissue (Shi, 2017). DOXR up-regulated TGF- β1/Smad3 pathway in DOXR-induced heart failure rats (Sun, 2020). However, we found that treatment with LIPO-RES alone and the combination of CAR with either RES or LIPO-RES reduced TGF-β1 level. Interestingly, according to our research results, the highest reduction of TGF-β1 expression was achieved by the combination of CAR and LIPO-RES treated group compared with the DOXR group. A recent study by Zhang et al. reported that RES treatment significantly decreased the TGF-β1 by affecting Smad7 protein expression (Zhang, 2018). Furthermore, another study conducted by Serna et al. in a liver cirrhosis model found that CAR administration caused a reduction in TGF-β1 level (Serna-Salas, 2018). Taken together, these results expanded our understanding of DOXR hepatotoxic mechanisms by highlighting the potential role of SIRT1 and TGF-β1 in such toxicity.
CRediT authorship contribution statement
Ahlam M. Alhusaini: Conceptualization, Methodology, Validation, Investigation, Writing – review & editing, Supervision. Abeer M. Alanazi: Conceptualization, Methodology, Validation, Writing – review & editing. Laila M. Fadda: Conceptualization, Investigation, Supervision. Qamraa H. Alqahtani: Writing – review & editing, Data analysis. Wedad S. Sarawi: Investigation, Writing – review & editing, Data analysis. Iman H. Hasan: Validation, Investigation, Writing – review & editing, Data analysis.
Acknowledgement
This research project was supported by a grant from the Research Center of the Female Scientific and Medical Colleges, Deanship of Scientific Research, King Saud University, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Liraglutide ameliorates cardiotoxicity induced by doxorubicin in rats through the Akt/GSK-3β signaling pathway. Naunyn. Schmiedebergs. Arch. Pharmacol.. 2017;390(11):1145-1153.
- [Google Scholar]
- Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol.. 2012;3:119.
- [Google Scholar]
- Protective effect of carvedilol on daunorubicin-induced cardiotoxicity and nephrotoxicity in rats. Toxicology. 2010;274(1–3):18-26.
- [Google Scholar]
- Genistein protects against doxorubicin-induced cardiotoxicity through Nrf-2/HO-1 signaling in mice model. Environ. Toxicol.. 2019;34(5):645-651.
- [Google Scholar]
- Reactive oxygen species from NAD (P) H: quinone oxidoreductase constitutively activate NF-κB in malignant melanoma cells. Am. J. Physiol. Physiol.. 2001;280(3):C659-C676.
- [Google Scholar]
- Use of selenium to ameliorate doxorubicin induced hepatotoxicity by targeting pro-inflammatory cytokines. Biotech. Histochem.. 2021;96(1):67-75.
- [Google Scholar]
- Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. Hpb. 2009;11(5):373-381.
- [Google Scholar]
- Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1–FoxO1 pathway. Biochem. Biophys. Res. Commun.. 2009;378(3):389-393.
- [Google Scholar]
- A systematic review on natural antioxidant properties of resveratrol. Nat. Prod. Commun.. 2018;13(9)
- [Google Scholar]
- An evaluation of hepatotoxicity in breast cancer patients receiving injection Doxorubicin. Ann. Med. Health Sci. Res.. 2014;4(1):74-79.
- [Google Scholar]
- SIRT1 activation rescues doxorubicin-induced loss of functional competence of human cardiac progenitor cells. Int. J. Cardiol.. 2015;189:30-44.
- [Google Scholar]
- Anti-inflammatory effects of resveratrol: mechanistic insights. Int. J. Mol. Sci.. 2018;19(6):1812.
- [Google Scholar]
- The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother.. 2020;127:110234
- [Google Scholar]
- Resveratrol biosynthesis: plant metabolic engineering for nutritional improvement of food. Plant Foods Hum. Nutr.. 2012;67(3):191-199.
- [Google Scholar]
- Mitochondrial dysfunction associated with doxorubicin. In: Mitochondrial diseases. IntechOpen; 2018. p. :323.
- [Google Scholar]
- Modulating effect of carvedilol on doxorubicin-induced cardiomyopathy and hepatic damage. J Am Sci. 2010;6(12):20-32.
- [Google Scholar]
- Resveratrol pretreatment reduces circulating inflammatory interleukins in CCl4-induced hepatotoxicity rats. Bull. Fac. Pharmacy, Cairo Univ.. 2017;55(2):319-323.
- [Google Scholar]
- Carvedilol: a beta blocker with antioxidant property protects against gentamicin-induced nephrotoxicity in rats. Life Sci.. 2000;66(26):2603-2611.
- [Google Scholar]
- Protective effects of taurine on doxorubicin-induced acute hepatotoxicity through suppression of oxidative stress and apoptotic responses. Anticancer Drugs. 2016;27(1):17-23.
- [Google Scholar]
- Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int. J. Cancer. 2009;125(1):1-8.
- [Google Scholar]
- Antioxidant action of the antihypertensive drug, carvedilol, against lipid peroxidation. Biochem. Pharmacol.. 2000;59(9):1069-1076.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2):351-358.
- [Google Scholar]
- Aging and longevity between genetic background and lifestyle intervention. Hindawi 2014
- [Google Scholar]
- SIRT1 suppresses doxorubicin-induced cardiotoxicity by regulating the oxidative stress and p38MAPK pathways. Cell. Physiol. Biochem.. 2015;35(3):1116-1124.
- [Google Scholar]
- Doxazosin and carvedilol treatment improves hepatic regeneration in a hamster model of cirrhosis. Biomed Res. Int.. 2018;vol:2018.
- [Google Scholar]
- Transforming growth factor beta1 from endometriomas promotes fibrosis in surrounding ovarian tissues via Smad2/3 signaling. Biol. Reprod.. 2017;97(6):873-882.
- [Google Scholar]
- A study on the toxic effects of doxorubicin on the histology of certain organs. Toxicol. Int.. 2012;19(3):241.
- [Google Scholar]
- Prophylactic supplementation of resveratrol is more effective than its therapeutic use against doxorubicin induced cardiotoxicity. PLoS ONE. 2017;12(7):e0181535
- [Google Scholar]
- Protective effects of dioscin against doxorubicin-induced hepatotoxicity via regulation of Sirt1/FOXO1/NF-κb signal. Front. Pharmacol.. 2019;10:1030.
- [Google Scholar]
- Qiliqiangxin improves cardiac function and attenuates cardiac remodelling in doxorubicin-induced heart failure rats. Pharm. Biol.. 2020;58(1):417-426.
- [Google Scholar]
- Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radic. Res.. 2009;43(3):195-205.
- [Google Scholar]
- Naringenin regulates doxorubicin-induced liver dysfunction: impact on oxidative stress and inflammation. Plants. 2020;9(4):550.
- [Google Scholar]
- Recent progress in doxorubicin-induced cardiotoxicity and protective potential of natural products. Phytomedicine. 2018;40:125-139.
- [Google Scholar]
- Carvedilol, a new β-adrenoceptor antagonist and vasodilator antihypertensive drug, inhibits superoxide release from human neutrophils. Eur. J. Pharmacol.. 1992;214(2–3):277-280.
- [Google Scholar]
- Carvedilol prevents low-density lipoprotein (LDL)-enhanced monocyte adhesion to endothelial cells by inhibition of LDL oxidation. Eur. J. Pharmacol.. 1995;294(2–3):585-591.
- [Google Scholar]
- Resveratrol inhibits the TGF-β1-induced proliferation of cardiac fibroblasts and collagen secretion by downregulating miR-17 in rat. Biomed Res. Int.. 2018;vol:2018.
- [Google Scholar]
- Carvedilol (CAR) combined with carnosic acid (CAA) attenuates doxorubicin-induced cardiotoxicity by suppressing excessive oxidative stress, inflammation, apoptosis and autophagy. Biomed. Pharmacother.. 2019;109:71-83.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101640.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







