Translate this page into:
The association between adiponectin plasma level and rs1501299 ADIPOQ polymorphism with atrial fibrillation
⁎Corresponding author at: Department of Medical Laboratory Sciences, Jordan University of Science and Technology, P.O. Box 3030, Irbid 22110, Jordan. khabour@just.edu.jo (Omar F. Khabour)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Atrial fibrillation (AF) is a common heart disease associated with cardiac dysfunction. Adiponectin is an abundant protein produced by adipocytes that regulates metabolism and inflammation in the body. Studies have reported a link between AF and circulatory adipokines. The objective of the study is to investigate the associations between adiponectin serum level and the ADIPOQ rs1501299 (276G/T) SNP with AF.
Methods
The study included a total of 112 AF cases and 112 matched healthy controls. Serum adiponectin was analyzed using an ELISA assay. Genotyping of rs1501299 was performed using PCR-RFLP technique.
Results
The results showed that the level of adiponectin in the serum was significantly higher (P < 0.01) in AF group versus the controls. When the sample was stratified by sex, the magnitude of increases in adiponectin levels among female AF patients (87.6%) was higher than that of male patients (33.2%). Multivariate regression model showed that gender was related to adiponectin levels among the patient group (P < 0.001). In sex-specific multivariate regression, adiponectin was associated with BMI in female patients (P < 0.05) and with glucose blood level among male patients (P < 0.05). Finally, the study showed no association between ADIPOQ rs1501299 SNP and AF (P > 0.05).
Conclusion
The results confirmed the relationship between blood adiponectin and AF. However, the polymorphism of the ADIPOQ rs1501299 gene may not be associated with AF in the population studied.
Keywords
Atrial fibrillation
Adiponectin
ADIPOQ
Rs1501299
Gender
- AF
-
Atrial fibrillation
- ADIPOQ
-
Adiponectin gene
- CVD
-
Cardiovascular disease
- PCR-RFLP
-
Polymerase chain reaction-Restriction fragment length polymorphism
- SNP
-
Single Nucleotide Polymorphism
Abbreviations
1 Introduction
Atrial fibrillation (AF) is a chronic arrhythmia attributable to a disturbance in the control of the heart that can lead to impaired cardiac function (Lau et al., 2019). AF is mainly associated with age and sex, with males and the elderly being more susceptible to the disease compared to females and younger people (Zhang et al., 2021). The worldwide prevalence of AF has reached 37.5 million cases, and it is constantly increasing (Lippi et al., 2021).
Clinical symptoms of AF include tremors, shortness of breath, tiredness, dizziness, and chest pain (Tousoulis, 2019; Milman and Burns, 2021). Persistent AF can cause atrial fibrosis, abnormal collagen deposition, ion channel disruption, cellular energy imbalance, atrial enlargement, and cell death (Nattel, 2017; Chen et al., 2021). In addition, AF is a risk factor for heart failure, myocardial infarction, and subsequent mortality (Prabhu et al., 2017).
AF is usually caused by structural heart problems (Zimetbaum, 2017). AF can also be associated with alcohol consumption, obesity, and some chronic conditions such as diabetes and sleep apnea. (Csengeri et al., 2021). Previous literature has shown that family history can also influence the risk of developing AF (Lozano-Velasco and Franco, 2020). Therefore, it has been suggested that genetic variations in some genes can cause AF or can modulate a person's risk of developing it (Campbell and Wehrens, 2018).
Adipose tissue is a connective tissue composed predominantly of adipocytes that can act as an endocrine organ by releasing various biologically active substances, known as adipokines (Farkhondeh et al., 2020). Patients with AF have significantly more fatty tissue compared to healthy people (Psychari et al., 2018). Adiponectin is a fatty substance that can induce multiple metabolic effects including glucose homeostasis, appetite, and inflammation (Choi et al., 2020; Maeda et al., 2020). Additionally, circulating adiponectin is elevated in AF (Psychari et al., 2018; Agbaedeng et al., 2022) and an independent risk factor for the development of AF (Guo et al., 2019).
The adiponectin gene (ADIPOQ) is carried on chromosome three (Fang and Judd, 2018). Some specific single-nucleotide polymorphisms (SNPs) have been identified in ADIPOQ gene (Breitfeld et al., 2012). Thus, SNPs in ADIPOQ gene may affect adiponectin gene expression (Dalamaga et al., 2012). Among the most studied polymorphisms is + 276G > T (rs1501299) (Amrita et al., 2021). This polymorphism has been associated with obesity and diabetes mellitus, which are well reported risks for AF (Lu et al., 2014; Elghazy et al., 2022). Thus, the aim of the current study was to investigate the relationship of AF with the rs1501299 SNP. In addition, the level of serum adiponectin was compared between subjects with AF and healthy ones. The rs1501299 SNP is predicted to be associated with AF and presumably adiponectin is altered in AF.
2 Materials and methods
2.1 Subjects
Patients with AF aged 23–73 years from Al-Hussein Medical Complex, Amman, Jordan and age and gender (normal sinus rhythm) controls were recruited to participate in the study. Standard diagnostic criteria for atrial fibrillation based on electrocardiogram/ Holter electrocardiogram data were used (Camm et al., 2010). The exclusion criteria were structural heart disease, heart failure, arrhythmia other than AF, malignancy, autoimmune disease, blood disorders, hepatic failure, renal failure, thyroid disease, and chronic infectious diseases (Psychari et al., 2018; Zhu et al., 2021). Written informed consent was obtained from all participants according to the guidelines of the Institutional Ethics Committee. All study procedures, requirements, benefits and potential risks have been explained to the participants before joining. Clinical data were obtained from patient records. Demographic data were obtained from participants using a standardized form.
2.2 Blood sampling
Venous blood was sampled in EDTA and plain tubes from all subjects. EDTA blood samples were used for DNA extraction and genotyping of the ADIPOQ SNP. Serum was collected from plain tubes by direct centrifugation at 1,500 g for five min. The serum was stored in a deep freeze for biochemical analysis (Al-Azzam et al., 2014).
2.3 Biochemical analysis
Serum Adiponectin concentrations were analyzed using a sandwich ELISA assay kit developed by R&D System (USA). The assay involved the use of a capture antibody and a horse radish peroxidase (HRP) labelled detection antibody. Serum samples were diluted 1:800 with the diluting reagent provided by the kit. Addition of the HRP substrate to the reaction results in the formation of a product that absorbs light at 450 nm. Changes in the absorbance of samples were quantified at 450 nm using a BioTek instrument (Winooski, VT, USA). Values of adiponectin were computed from the standard curve as previously described (Khabour et al., 2018).
2.4 Genotyping of the rs1501299 SNP
DNA was extracted from whole blood using a Promega kit (Madison, USA). The quality and quantity of isolated DNA were checked using the SmartSpec 3000 instrument (Bio-Rad, USA). Genotyping of the rs1501299 SNP was performed using polymerase chain-restriction fragment length polymorphism (PCR-RFLP) technique. The primer sequences were forward: 5′- CCT GGT GAG AAG GGT GAGAA-3′, and reverse: 5′-TCATC CTT GGA AGA CCA ACC-3′. A ready to use master mix was purchased from Promega (USA). A total of 100 ng/mL DNA was used in each PCR reaction. The PCR conditions were 6 min at 94 °C, 34 cycles: 45 s at 94 °C, 50 s at 58 °C, and 50 s at 72 °C, and a final extension of 5 min at 72 °C. The 195 bp amplified fragment was restricted with BsmI at 37 °C as previously described (Garba et al., 2020). The digested products were visualized using 2% agarose. The TT genotype gives a 195 bp fragment. The GG genotype gives 104 bp and 91 bp fragments (Garba et al., 2020).
2.5 Statistical analysis
The SPSS package (version 22, Inc, Chicago, IL) was applied for statistical comparisons. Values were presented as means and percentages, while significance was taken into account at P < 0.05. Adiponectin levels in patients versus control group were analyzed using the Student's t-test. In addition, multivariate regression model was applied to infer predictors of adiponectin levels in AF patients. Factors including gender, age, lipid profile, diabetes, ulcers, and hypertension were used in the regression model as potential predictors. The distribution of rs1501299 SNP between patients and controls was compared using SNPstats website tools (https://www.snpstats.net/start.htm).
3 Results
Table 1 shows the characteristics of patients and controls. The comparison analysis showed no differences (P > 0.05) in age, height, weight, BMI, and gender in patients versus controls. In addition, there is no significant difference in the lipid profile (total cholesterol, triglycerides, HDL, and LDL) and the prevalence of diabetes, hypertension, and gastric ulcers between the two groups (P > 0.05). Sex-specific comparisons showed that weight was higher in males with AF than in control males, whereas the frequency of hypertension was higher among females with AF than control females (Table 1, P < 0.05). *BMI: body mass index, LDL: low density lipoprotein, HDL: high density lipoprotein.
Parameters*
Patients (n = 112)
Controls (n = 112)
p-value
Male patients (n = 54)
Male controls (n = 59)
p-value
Female patients (n = 58)
Female controls (n = 53)
p-value
Age (years)
49.7 ± 14.9
49.4 ± 14.7
0.95
48.3 ± 14.9
49.3 ± 15.4
0.721
51.1 ± 15.0
49.6 ± 14.0
0.583
Height (cm)
167.1 ± 8.7
166.7 ± 9.4
0.358
170.8 ± 7.8
168.2 ± 9.1
0.104
163.6 ± 8.1
165.0 ± 9.7
0.404
Weight (kg)
83.5 ± 15.5
82.1 ± 15.6
0.240
90.3 ± 14.7
81.7 ± 15.5
0.003
77.2 ± 13.4
82.5 ± 15.7
0.062
BMI (kg/cm2)
29.8 ± 5.03
29.7 ± 6.5
0.470
30.9 ± 5.5
29.1 ± 6.2
0.094
28.9 ± 4.8
30.6 ± 6.9
0.123
Total Cholesterol
4.38 ± 1.32
4.48 ± 1.50
0.584
4.24 ± 1.23
4.25 ± 1.43
0.959
4.52 ± 1.39
4.74 + 1.54
0.410
Triglycerides
1.81 ± 0.94
1.64 ± 0.927
0.184
2.02 ± 1.17
1.71 ± 0.99
0.130
1.61 ± 0.63
1.56 ± 0.84
0.743
LDL
2.97 ± 1.08
3.05 ± 1.09
0.577
2.82 ± 0.99
2.91 ± 1.09
0.659
3.12 ± 1.15
3.22 ± 1.09
0.622
HDL
0.79 ± 0.23
0.86 ± 0.35
0.094
0.70 ± 0.17
0.77 ± 0.22
0.068
0.88 ± 0.26
0.96 ± 0.44
0.232
Diabetes: N(%)
20 (17.8)
21 (18.7)
0.863
11 (20.4)
11 (19.0)
0.852
9 (16.7)
9 (17.3)
0.930
Hypertension N(%)
23 (20.5)
20 (17.8)
0.610
9 (16.7)
15 (25.4)
0.256
14 (24.1)
4 (7.5)
0.018
Fig. 1 shows that serum adiponectin was higher (P < 0.001) in AF patients versus controls. When the sample was stratified by sex, ANOVA (F = 22.40, P < 0.001) revealed that adiponectin was higher in patients than controls in males (Fig. 2, 2806 ± 144 ng/mL versus 2096 ± 211 ng/mL respectively, P < 0.05) and females (Fig. 2, 4019 ± 159 ng/mL versus 2142 ± 238 ng/mL, P < 0.001). In addition, adiponectin was higher in female patients than in male patients (P < 0.01). However, no statistical differences were found between adiponectin levels in male and female control subjects (P > 0.05).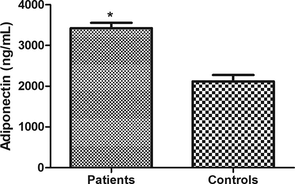
Changes in serum adiponectin in atrial fibrillation. Serum adiponectin was higher in patients than in controls. Values were presented as Mean ± SEM. * indicates P < 0.001.
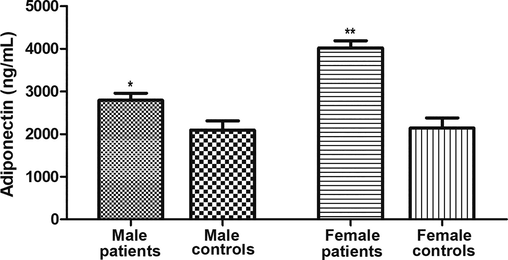
Changes in serum adiponectin in atrial fibrillation across sex. Serum adiponectin were compared between male and female participants. ANOVA indicates significant differences between groups (F = 22.40, P < 0.001). Adiponectin levels were higher in the patient groups than in the control groups in males (P < 0.05) and females (P < 0.001). Adiponectin levels also were higher in female patients than in male patients (p < 0.01). However, no significant difference was found between adiponectin levels in male and female controls (P > 0.05). Values were expressed as Mean ± SEM. * indicates significant difference (P < 0.05). ** indicates significant difference (P < 0.01).
To identify factors that predict adiponectin levels in patients with atrial fibrillation, a multivariate regression analysis was performed. The results showed that gender was strongly associated with adiponectin levels among the AF group (P < 0.001). However, none of the other factors (age, lipid profile, diabetes, ulcers, and hypertension) predicted adiponectin levels in the AF group (Table 2, P > 0.05). When multivariate regression analysis was performed in male and female groups separately (Table 3), adiponectin was associated with BMI in female patients (P < 0.05) and with glucose blood level among male patients (P < 0.05). *BMI: body mass index, LDL: low density lipoprotein, HDL: high density lipoprotein. *BMI: body mass index, LDL: low density lipoprotein, HDL: high density lipoprotein.
Model*
B
Beta
P-value
95% CI
Age
5.014
0.053
0.590
−13.4–23.4
Gender
1180
431
0.000
664.1–1696.8
BMI
1.039
0.004
0.970
−53.85–55.93
Glucose
−2.283
−0.006
0.954
−80.1–75.6
Cholesterol
−24.18
−0.028
0.766
−185.2–136.8
LDL
−2.935
−0.066
0.470
−10.9–5.09
Diabetes
120.2
0.034
0.723
−551.9–792.5
Hypertension
−5.812
−0.002
0.986
−661.3–649.6
Model
B
Beta
P-value
95% CI
Males
Age
0.002
0.056
0.576
−0.005 – 0.008
BMI
−0.013
−0.150
0.126
−0.030 – 0.004
Glucose
−0.042
−0.265
0.016
−0.075 – −0.008
Cholesterol
−0.001
−0.003
0.993
−0.290 – 0.287
LDL
0.034
0.069
0.856
−0.333 – 0.400
HDL
0.096
0.061
0.520
−0.198 – 0.390
Diabetes
0.024
0.019
0.844
−0.219 – 0.267
Hypertension
0.076
0.062
0.534
−0.166 – 0.318
Females
Age
−0.002
−0.057
0.612
−0.009 – 0.005
BMI
0.019
0.237
0.033
0.002 – 0.037
Glucose
−0.030
−0.177
0.114
−0.067 – 0.007
Cholesterol
−0.004
−0.013
0.959
−0.167 – 0.158
LDL
−0.014
−0.033
0.892
−0.224 – 0.195
HDL
0.052
0.030
0.779
−0.315 – 0.419
Diabetes
0.098
0.080
0.454
−0.162 – 0.359
Hypertension
−0.197
−0.145
0.175
−0.484 – 0.090
Table 4 shows the distribution of the rs1501299 SNP between the AF and controls. The distribution of both genotypes and alleles was not statistically different between the AF group and the control group (genotypes: P = 0.387, alleles: P = 0.309). When the sample was stratified according to sex, similar results were obtained (data not shown). Thus, ADIPOQ rs1501299 was not associated with AF in the studied population.
Genotypes
Patients n = 112 (%)
Controls n = 98 (%)
p-value
TT
12 (10.7)
18 (16.1)
0.387
GT
61 (54.5)
62 (55.3)
GG
39 (34.8)
32 (28.6)
Alleles
T
85 (37.9)
98 (43.8)
0.309
G
131 (62.1)
124 (56.2)
4 Discussion
In this investigation, the associations between serum adiponectin level and the ADIPOQ rs1501299 polymorphism with AF were examined. The results showed no differences in the distribution of the ADIPOQ rs1501299 SNP, while serum adiponectin was higher in AF patients than controls.
Adiponectin, a protein hormone, is the most prevalent adipokine in human blood (Cheng et al., 2012 36). It has an insulin-sensitizing, anti-inflammatory, anti-atherosclerotic, and antioxidant effect (Shimano et al., 2008). In addition, literature has also shown that adiponectin may have cardiovascular effects (Nanayakkara et al., 2012). For example, previous studies showed elevated circulatory adiponectin in AF patients (Psychari et al., 2018; Zhu et al., 2021). A meta-analysis reported that elevated circulating adiponectin is an independent risk factor for developing AF (Guo et al., 2019). Increased adiponectin has also been reported in older adults with AF (65 years of age), devoid of clinically overt CVDs (Macheret et al., 2015). Similarly, the present results showed elevated levels of adiponectin in AF. According to previous studies, a high level of adiponectin is important for reducing inflammation and thus removing and preventing the accumulation of dying cells (Macheret et al., 2015). Thus, a high level of adiponectin carries a negative prognosis in late life that may reflect an inadequate or ineffective response to counter regulation of the underlying disease processes (Kizer et al., 2010). An alternative justification for elevated levels of adiponectin is overproduction by muscles. One previous study demonstrated high expression of adiponectin in skeletal muscle with concomitant adiponectin resistance in subjects with heart failure (Van Berendoncks et al., 2010). Another previous study reported elevated plasma adiponectin levels have been associated with persistent AF, and this elevation in adiponectin level may reflect a disconnection between adiponectin and the corresponding receptors in AF resulting in an over-regulation and thus increased secretion of adiponectin (Shimano et al., 2008). Alternatively, the higher level of adiponectin could be due to the accumulation of epicardial adipose tissue and visceral adipose tissue and in patients with AF compared to healthy subjects (Psychari et al., 2018; Sawada et al., 2021). While several studies reported elevated adiponectin in AF, an investigation showed that low circulating adiponectin levels were associated with major cardiovascular events in noncoagulated women with non-valvular AF (Hernández-Romero et al., 2013). In addition, an inverse relationship between adiponectin and the extent of platelet activation and stroke progression has been reported in anticoagulated subjects with AF (Carnevale et al., 2014). Moreover, conditions such as diabetes and obesity are associated with low levels of adiponectin and at the same time are considered major risk factors for AF (Karam et al., 2017). Thus, the relationship between adiponectin and AF appears complex and depends on the etiology and associated conditions. Further studies are needed to determine the exact mechanism responsible for the elevated adiponectin levels observed in AF patients.
The results showed that the magnitude of increases in adiponectin levels among female AF patients (87.6%) was higher than that of male patients (33.2%). In addition, multivariate regression analysis showed that gender is a strong predictor of adiponectin levels in atrial fibrillation. In a previous study, adiponectin was associated with AF in female subjects (Zhu et al., 2021). Similar detection of higher adiponectin has been reported in females with paroxysmal atrial fibrillation compared to male patients (Kim et al., 2018). In a study that examined risk factors for cardiovascular disease, adiponectin was also reported to be about 50% higher in females than in males (Andreasson et al., 2012). The differences between the sexes in the relationship between adiponectin and some conditions were also presented. For example, adiponectin was associated with metabolic risk and diminished glucose tolerance in females but not in males (Eglit et al., 2013). Differences in adiponectin levels can be attributed to sex differences in body physiology. For example, testosterone can suppress adipocyte production of adiponectin in elderly males (Frederiksen et al., 2012). A negative relationship between adiponectin and testosterone was also reported in bulls (Baharun et al., 2021). These findings highlight the importance of including strategies that lower adiponectin levels in AF patients, especially among female patients. The results also showed that adiponectin was associated with BMI in females and with glucose levels among males. While the relationships between BMI and glucose homeostasis and adiponectin levels are well documented (Galler et al., 2007; Tabatabaei-Malazy et al., 2010; Han et al., 2022), the influence of gender on such relationships in AF needs to be investigated.
No association was found between rs1501299 SNP and AF in the current study. Some studies have shown that the genetic factor is responsible for 39–70% of the fluctuation of the serum adiponectin level (Oliveira et al., 2011). The rs1501299 polymorphism is one of the common polymorphisms in the ADIPOQ gene that has been studied (Zhang et al., 2012). The current study is pioneering in examining the relationship between rs1501299 and AF, thus there are no previous studies to compare results with. However, previous studies have shown a contradictory association of the rs1501299 SNP and cardiovascular disease (Amrita et al., 2021). For example, rs1501299 was positively associated with the risk of coronary artery disease in Chinese adults (Gui et al., 2012). In a meta-analysis, rs1501299 was shown to increase the risk of cardiovascular disease (Kanu et al., 2018). Conversely, rs1501299 was associated with a reduced risk of coronary artery disease (Bacci et al., 2004), while the mutant T allele was associated with about 45% reduction in cardiovascular disease risk among diabetics (Qi et al., 2006).
Among the limitations of the current study is the relatively small sample size. Therefore, further investigations are required to verify the association between rs1501299 and AF. In addition, future studies should also explore the role of other polymorphisms in the ADIPOQ gene in the etiology of AF.
5 Conclusion
The serum level of adiponectin but not the ADIPOQ rs1501299 SNP was found to be associated with AF. Adiponectin was higher in females than in males with AF.
Acknowledgements
The authors thank the study subjects for volunteering in the study. Authors thank Jordan University of Science and Technology for funding the present study (grant number: 130-2014).
Statement of financial support
The study was funded by Deanship of Scientific Research at Jordan University of Science and Technology (grant: 130–2014 to OK).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Agbaedeng TA, Zacharia AL, Iroga PE, Rathnasekara VM, Munawar DA, Bursill C, Noubiap JJ. 2022. Associations between adipokines and atrial fibrillation: A systematic review and meta-analysis. Nutrition, metabolism, and cardiovascular diseases : NMCD. https: //10.1016/j.numecd.2022.01.019.
- Al-Azzam SI, Khabour OF, Alzoubi KH, Mukattash TL, Ghanma M, Saleh H. 2014. The role of adiponectin gene variants in glycemic control in patients with Type 2 diabetes. Endocrine research 39:13-17. https: //10.3109/07435800.2013.794427.
- Amrita J, Mahajan M, Bhanwer AJS, Matharoo K. 2021. Association of AdipoQ gene variation (rs1501299) and oxidative stress with cardiovascular disease in North West Indian population of Punjabi women. Journal of medical biochemistry 40:49-59. https: //10.5937/jomb0-24704.
- Andreasson AN, Undén AL, Elofsson S, Brismar K. 2012. Leptin and adiponectin: distribution and associations with cardiovascular risk factors in men and women of the general population. American journal of human biology : the official journal of the Human Biology Council 24:595-601. https: //10.1002/ajhb.22279.
- Bacci S, Menzaghi C, Ercolino T, Ma X, Rauseo A, Salvemini L, Vigna C, Fanelli R, Di Mario U, Doria A, Trischitta V. 2004. The +276 G/T single nucleotide polymorphism of the adiponectin gene is associated with coronary artery disease in type 2 diabetic patients. Diabetes Care 27:2015-2020. https: //27/8/2015 [pii].
- Correlation between age, testosterone and adiponectin concentrations, and sperm abnormalities in Simmental bulls. Veter. World. 2021;14:2124-2130.
- [CrossRef] [Google Scholar]
- Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur. Heart J.. 2010;31:2369-2429.
- [CrossRef] [Google Scholar]
- Genetics of atrial fibrillation: an update. Curr. Opin. Cardiol.. 2018;33:304-310.
- [CrossRef] [Google Scholar]
- Carnevale R, Pastori D, Peruzzi M, De Falco E, Chimenti I, Biondi-Zoccai G, Greco E, Marullo AG, Nocella C, Violi F, Pignatelli P, Calvieri C, Frati G. 2014. Total adiponectin is inversely associated with platelet activation and CHA₂DS₂-VASc score in anticoagulated patients with atrial fibrillation. Mediators of inflammation 2014:908901. https: //10.1155/2014/908901.
- Prevention of pathological atrial remodeling and atrial fibrillation: JACC state-of-the-art review. J. Am. Coll. Cardiol.. 2021;77:2846-2864.
- [CrossRef] [Google Scholar]
- Cheng X, Folco EJ, Shimizu K, Libby P. 2012. Adiponectin induces pro-inflammatory programs in human macrophages and CD4+ T cells. J Biol Chem 287:36896-36904. https: //10.1074/jbc.M112.409516. M112.409516 [pii].
- Multifaceted physiological roles of adiponectin in inflammation and diseases. Int. J. Mol. Sci.. 2020;21:1219.
- [CrossRef] [Google Scholar]
- Alcohol consumption, cardiac biomarkers, and risk of atrial fibrillation and adverse outcomes. Eur. Heart J.. 2021;42:1170-1177.
- [CrossRef] [Google Scholar]
- Dalamaga M, Diakopoulos KN, Mantzoros CS. 2012. The role of adiponectin in cancer: a review of current evidence. Endocr Rev 33:547-594. https: //10.1210/er.2011-1015. er.2011-1015 [pii]
- Eglit T, Lember M, Ringmets I, Rajasalu T. 2013. Gender differences in serum high-molecular-weight adiponectin levels in metabolic syndrome. European journal of endocrinology 168:385-391. https: //10.1530/EJE-12-0688. EJE-12-0688 [pii].
- Biochemical studies of adiponectin gene polymorphism in patients with obesity in Egyptians. Arch. Physiol. Biochem.. 2022;128:43-50.
- [CrossRef] [Google Scholar]
- Adiponectin regulation and function. Compreh. Physiol.. 2018;8:1031-1063.
- [CrossRef] [Google Scholar]
- Farkhondeh T, Llorens S, Pourbagher-Shahri AM, Ashrafizadeh M, Talebi M, Shakibaei M, Samarghandian S. 2020. An Overview of the Role of Adipokines in Cardiometabolic Diseases. Molecules (Basel, Switzerland) 25. https: //10.3390/molecules25215218.
- Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. Eur. J. Endocrinol.. 2012;166:469-476.
- [CrossRef] [Google Scholar]
- Elevated serum levels of adiponectin in children, adolescents and young adults with type 1 diabetes and the impact of age, gender, body mass index and metabolic control: a longitudinal study. Eur. J. Endocrinol.. 2007;157:481-489.
- [CrossRef] [Google Scholar]
- The Association between adiponectin single nucleotide polymorphisms and side effects of isotretinoin in acne patients. Dermatol. Res. Pract.. 2020;2020:3176521.
- [CrossRef] [Google Scholar]
- Gui MH, Li X, Jiang SF, Gao J, Lu DR, Gao X. 2012. Association of the adiponectin gene rs1501299 G>T variant, serum adiponectin levels, and the risk of coronary artery disease in a Chinese population. Diabetes Res Clin Pract 97:499-504. https: //10.1016/j.diabres.2012.05.011. S0168-8227(12)00174-X [pii]
- Adiponectin and the risk of new-onset atrial fibrillation: a meta-analysis of prospective cohort studies. Biosci. Rep.. 2019;39
- [CrossRef] [Google Scholar]
- Role of adiponectin in cardiovascular diseases related to glucose and lipid metabolism disorders. Int. J. Mol. Sci.. 2022;23
- [CrossRef] [Google Scholar]
- The prognostic role of the adiponectin levels in atrial fibrillation. Eur. J. Clin. Invest.. 2013;43:168-173.
- [CrossRef] [Google Scholar]
- Associations between three common single nucleotide polymorphisms (rs266729, rs2241766, and rs1501299) of ADIPOQ and cardiovascular disease: a meta-analysis. Lipids Health Dis.. 2018;17:126.
- [CrossRef] [Google Scholar]
- Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol.. 2017;16:120.
- [CrossRef] [Google Scholar]
- The Relationship of adiponectin level and ADIPOQ gene variants with BMI among young adult women. Dermato-Endocrinol.. 2018;10:e1477902.
- [CrossRef] [Google Scholar]
- Kim TH, Lee JS, Uhm JS, Joung B, Lee MH, Pak HN. 2018. High circulating adiponectin level is associated with poor clinical outcome after catheter ablation for paroxysmal atrial fibrillation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 20:1287-1293. https: //10.1093/europace/eux173
- Kizer JR, Arnold AM, Strotmeyer ES, Ives DG, Cushman M, Ding J, Kritchevsky SB, Chaves PH, Hirsch CH, Newman AB. 2010. Change in circulating adiponectin in advanced old age: determinants and impact on physical function and mortality. The Cardiovascular Health Study All Stars Study. J Gerontol A Biol Sci Med Sci 65:1208-1214. https: //10.1093/gerona/glq122. glq122 [pii]
- New findings in atrial fibrillation mechanisms. Cardiac Electrophysiol. Clin.. 2019;11:563-571.
- [CrossRef] [Google Scholar]
- Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int. J. Stroke : Off. J. Int. Stroke Soc.. 2021;16:217-221.
- [CrossRef] [Google Scholar]
- Lozano-Velasco E, Franco D. 2020. Genetics and Epigenetics of Atrial Fibrillation. 21. https: //10.3390/ijms21165717.
- Association of ADIPOQ polymorphisms with obesity risk: a meta-analysis. Hum. Immunol.. 2014;75:1062-1068.
- [CrossRef] [Google Scholar]
- Macheret F, Bartz TM, Djousse L, Ix JH, Mukamal KJ, Zieman SJ, Siscovick DS, Tracy RP, Heckbert SR, Psaty BM, Kizer JR. 2015. Higher circulating adiponectin levels are associated with increased risk of atrial fibrillation in older adults. Heart 101:1368-1374. https: //10.1136/heartjnl-2014-307015. heartjnl-2014-307015 [pii].
- Adiponectin, a unique adipocyte-derived factor beyond hormones. Atherosclerosis. 2020;292:1-9.
- [CrossRef] [Google Scholar]
- Milman B, Burns BD. 2021. Atrial fibrillation: an approach to diagnosis and management in the emergency department. Emergency medicine practice 23:1-28. https:
- Nanayakkara G, Kariharan T, Wang L, Zhong J, Amin R. 2012. The cardio-protective signaling and mechanisms of adiponectin. Am J Cardiovasc Dis 2:253-266. https:
- Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. JACC Clin. Electrophysiol.. 2017;3:425-435.
- [CrossRef] [Google Scholar]
- Oliveira CS, Giuffrida FM, Crispim F, Saddi-Rosa P, Reis AF. 2011. ADIPOQ and adiponectin: the common ground of hyperglycemia and coronary artery disease? Arq Bras Endocrinol Metabol 55:446-454. https: //S0004-27302011000700003 [pii].
- Atrial fibrillation and heart failure - cause or effect? Heart Lung Circ.. 2017;26:967-974.
- [CrossRef] [Google Scholar]
- Psychari SN, Tsoukalas D, Varvarousis D, Papaspyropoulos A, Gkika E, Kotsakis A, Paraskevaidis IA, Iliodromitis EK. 2018. Opposite relations of epicardial adipose tissue to left atrial size in paroxysmal and permanent atrial fibrillation. SAGE open medicine 6:2050312118799908. https: //10.1177/2050312118799908.
- Qi L, Doria A, Manson JE, Meigs JB, Hunter D, Mantzoros CS, Hu FB. 2006. Adiponectin genetic variability, plasma adiponectin, and cardiovascular risk in patients with type 2 diabetes. Diabetes 55:1512-1516. https: //55/5/1512 [pii].
- Sawada N, Nakanishi K, Daimon M, Hirose K, Yoshida Y, Ishiwata J, Hirokawa M, Koyama K, Nakao T, Morita H, Di Tullio MR, Homma S, Komuro I. 2021. Independent effect of visceral fat on left atrial phasic function in the general population. Nutrition, metabolism, and cardiovascular diseases : NMCD 31:3426-3433. https: //10.1016/j.numecd.2021.08.044.
- Shimano M, Shibata R, Tsuji Y, Kamiya H, Uchikawa T, Harata S, Muto M, Ouchi N, Inden Y, Murohara T. 2008. Circulating adiponectin levels in patients with atrial fibrillation. Circ J 72:1120-1124. https: //JST.JSTAGE/circj/72.1120 [pii].
- Tabatabaei-Malazy O, Hasani-Ranjbar S, Amoli MM, Heshmat R, Sajadi M, Derakhshan R, Amiri P, Namakchian M, Rezazadeh E, Tavakkoly-Bazzaz J, Keshtkar A, Larijani B. 2010. Gender-specific differences in the association of adiponectin gene polymorphisms with body mass index. The review of diabetic studies : RDS 7:241-246. https: //10.1900/rds.2010.7.241.
- Biomarkers in atrial fibrillation; from pathophysiology to diagnosis and treatment. Curr. Med. Chem.. 2019;26:762-764.
- [CrossRef] [Google Scholar]
- Van Berendoncks AM, Garnier A, Beckers P, Hoymans VY, Possemiers N, Fortin D, Martinet W, Van Hoof V, Vrints CJ, Ventura-Clapier R, Conraads VM. 2010. Functional adiponectin resistance at the level of the skeletal muscle in mild to moderate chronic heart failure. Circ Heart Fail 3:185-194. https: //10.1161/CIRCHEARTFAILURE.109.885525. CIRCHEARTFAILURE.109.885525 [pii].
- Zhang H, Mo X, Hao Y, Gu D. 2012. Association between polymorphisms in the adiponectin gene and cardiovascular disease: a meta-analysis. BMC Med Genet 13:40. https: //10.1186/1471-2350-13-40. 1471-2350-13-40 [pii].
- Epidemiology of atrial fibrillation: geographic/ecological risk factors, age, sex, genetics. Cardiac Electrophysiol. Clin.. 2021;13:1-23.
- [CrossRef] [Google Scholar]
- Zhu T, Chen M, Wang M, Wang Z, Wang S, Hu H, Ma K, Jiang H. 2021. Association between adiponectin-to-leptin ratio and heart rate variability in new-onset paroxysmal atrial fibrillation: A retrospective cohort study. Annals of noninvasive electrocardiology : the official journal of the International Society for Holter and Noninvasive Electrocardiology, Inc:e12896. https: //10.1111/anec.12896.
- Zimetbaum P. 2017. Atrial Fibrillation. Annals of internal medicine 166:Itc33-itc48. https: //10.7326/aitc201703070.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102655.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







