The anti-leech potential of the solvent extract of Bornean neem leaves and ultra-high performance liquid chromatography-high-resolution mass spectrometry profiling
⁎Corresponding author. bavmaran@ums.edu.my (Balu Alagar Venmathi Maran)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Aquaculture plays a vital role in the Malaysian economy and food production however, sometimes the development is impeded by parasites. The parasitic leech Zeylanicobdella arugamensis (Hirudinea) infest the sea bass, snappers and groupers. They feed on the blood and cause secondary infection which could cause host death in a short time. In this study, the leaves of the neem plant (Azadirachta indica) (Meliaceae) have been selected as a natural product for the elimination of the parasitic leeches Z. arugamensis and to evaluate the phytochemical composition via ultra-high performance liquid chromatography–high-resolution mass spectrometry system (UHPLC-HRMS). The parasitic leeches were collected from Universiti Malaysia Sabah aquaculture facilities and challenged with various concentration of neem leaves methanol extract. The results revealed significant antileech activity of the methanol extract against Z. arugamensis with total mortality at the concentration of 25, 50 and 100 mg/ml in an average period of 35.81 ± 5.40, 14.18 ± 0.99 and 9.21 ± 1.51 min, respectively. The analysis of UHPLC-HRMS showed the existence of isorhamnetin, myricetin, myricetin 3-O-galactoside, trifolin, and quercetin (flavonoids), 2-(3,4-dihydroxyphenyl) acetamide, apocynin, p-coumaric acid, scopoletin, and, phloretin (phenolics), pulegone, and carvone (terpenoids). Thus, the research showed that methanol extract of neem leaves contained effective bioactive compounds with anti-leech effects. This study will be very helpful to fish farmers in Malaysia and other Southeast Asian countries to control the parasitic leeches in aquaculture using a natural product.
Keywords
Aquaculture
Malaysia
Hybrid groupers
Neem
Antiparasitic activity
Zeylanicobdella arugamensis
Ultra-high-performance liquid chromatography – high-resolution mass spectrometry analysis
Bioactive compounds
1 Introduction
Aquaculture is an important activity in Malaysia and a vital tool for the increment of export revenue, food production and employment (Othman et al., 2017). In 2018 the fisheries department of Sabah reported 159,773.11 tonnes of fishery products from aquaculture in Sabah, Malaysia (Department of Fisheries Sabah, 2020). Aquaculture is an important source of protein for over 30 million population of Malaysia. Unfortunately, the spread of diseases and parasitic infestation is impeding aquaculture developments (Venmathi Maran et al., 2009; Venmathi Maran et al., 2012).
In Malaysia, aquaculture farmers culture various types of marine fishes such as snappers, groupers and sea bass. The highly prevalent species of the groupers are hybrid grouper Epinephelus fuscoguttatus × E. lanceolatus, tiger grouper E. fuscoguttatus, orange-spotted grouper E. coiodes and Malabar grouper E. malabricus. The highly prevalent snappers are the yellow-streak snapper Lutjanus lemniscatus, mangrove snapper L. argentimaculatus, John’s snapper L. johnii and red snapper L. erythropterus (Othman et al., 2017). The culture of groupers and snappers impeded greatly due to the infestation of some parasites (Venmathi Maran et al., 2009) in addition to marine parasitic leech Zeylanicobdella arugamensis de Silva, 1963 (Hirudinea, Piscicolidae) (Cruz-Lacierda et al., 2000; Ravi and Shariman Yahaya, 2017). They attached to the gills, eyes, and fins of the host, feed the blood by sucking and cause lesions and wounds on the body surface which leads to bacterial infection and ultimately result in host mortality within 3–4 days (Cruz-Lacierda et al., 2000). Farmers use formalin, pesticides and other chemicals for the disinfestation of the parasites (Shariff et al., 2000). Such chemicals are highly toxic to fish (Pitten et al., 2000). Thus, it is very important to develop a natural control agent to reduce the consumption of harmful chemicals. Plant-derived extracts can act as a biocontrol agent against leeches due to the presence of different metabolites (Bahmani and Rafieian-Kopaei, 2014).
The neem plant (Azadirachta indica) (Meliaceae) has been reported with insecticidal activity (Schmutterer, 1990), antiparasitic activity (Venmathi Maran et al., 2021) and antivenom activity against black-necked spitting cobra (Naja nigricollis) (Sani et al., 2020). The antihelminthic efficacy of the neem extract has been reported against the gastrointestinal parasite of animals (Jamra et al., 2015). Antibacterial potential of the aqueous extract of neem leaves has been reported against Citrobacter freundii gram-negative bacteria isolated from diseased fingerlings of Oreochromis mossambicus (Tilapia) (Thanigaivel et al., 2015). Malaysian neem plant leaves have been reported with antifungal activity against the fungi Aspergillus niger and Candida albicans (Arumugam et al., 2015). However, no research has been reported on the evaluation of the anti-leech potential of the methanol extract of the plant against Z. arugamensis. The study aimed to provide a natural control agent against the parasitic leech Z. arugamensis by the evaluation of the antiparasitic potential and phytochemical investigation of the methanol extract of the neem leaves.
2 Materials and methods
2.1 Plant collection and extraction
The leaves of the plant were obtained from Sabah, Malaysia. The dried leaves were powdered using a grinder to fine particles. Around 50 g of the leaves powder was dissolved in HPLC grade methanol (250 ml) in a flask and placed on the orbital shaker for 3 days at room temperature. The supernatant was filtered using Whatman No. 41 filter paper. The methanol residues were removed from the extract till it becomes thick via a vacuum rotary evaporator. Just before the sample was dried water (50 ml) was added and continue to run the rotary evaporator to eliminate all the methanol residue. Finally, the moistened samples were kept at −80 0C for 24 h and then lyophilized using a freeze drier.
2.2 Ultra-high performance liquid chromatography
Liquid chromatography (LC) was performed as described by Venmathi Maran et al. (2021) using Dionex UltiMate 3000 UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a Thermo Syncronis C18 column (2.1 mm × 100 mm × 1.7 μm); Thermo Fisher Scientific, Waltham, MA, USA). Briefly, column temperature was maintained at 55 °C and injection volume of 2 µL was applied. Solvent A (water topped with 0.1% HCOOH) and solvent B (acetonitrile topped with 0.1% HCOOH) were used as the mobile phase with a flow rate of 450 µL/min (Shah et al., 2020b; Venmathi Maran et al., 2021) The LC program was initiated at 0.5% of solvent B for 1 min, then from 0.5% to 99.5% solvent B in 15 min and maintained for 4 min. Then, the column was conditioned as initial and stabilized for 2 min before the next injection.
2.2.1 Data acquisition using high-resolution mass spectrometry system
Mass spectrometry (MS) and tandem mass spectrometry (MS/MS) data were acquired on data-dependent acquisition mode using Thermo Scientific Q Exactive HF Orbitrap (Thermo Fisher Scientific, Waltham, MA, USA), which is equipped with heated electrospray ionization (HESI) probe. Both MS and MS/MS data were acquired with the setting as described by Venmathi Maran et al. (2021), and instrument calibration was performed before sample analysis.
2.2.2 Data analysis and compounds identification
Acquired data were processed and analysed using Thermo Scientific Compound Discoverer 3.0 software. Default settings were executed to perform background subtraction, retention time alignment, feature detection, elemental composition analysis, libraries matching, and fragment ion search (FISh) scoring (Venmathi Maran et al., 2021). Identification of compound was primarily based on the matching of MS/MS spectral against mzCloud and mzVault databases. Unmatched signals were reprocessed with ChemSpider database and backed with FISh scoring of above 50.
2.3 Antiparasitic bioassay
The parasitic leeches were collected from the infested hybrid groupers (Epinephelus fuscoguttatus × E. lanceolatus) (Fig. 1) from Universiti Malaysia Sabah. The selected adult parasitic leeches (0.8–1.2 cm long) were categorized into 5 groups (6 leeches per group) negative control (group 1), positive control (group 2, treated with 0.25% formalin solution), extract treatment (groups 3, 4 and 5 administered with 25, 50 and 100 mg/ml of the methanol extract of neem leaves). The selected concentrations were based on optimization. The behaviour of parasitic leeches was noticed keenly during the exposure (Fig. 2). Parasites inactivity and mortality were noted up to 120 mins. At the end of the experiment, the leeches were further observed for 720 mins (after every 60 min interval) (Shah et al., 2020c).

- Infestation of Zeylanicobdella arugamensis on hybrid grouper fish (Epinephelus fuscoguttatus × E. lanceolatus).
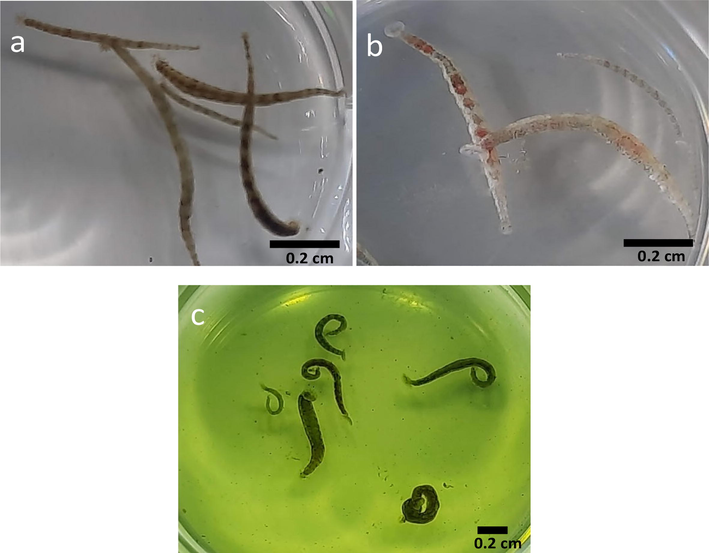
- Control and plant treated leeches. a = control, normal leech, treated with seawater only, b = leech treated with 0.25 % of formalin solution, c = leech treated with methanol extract of neem leaves.
2.4 Physio-chemical parameters
Physio-chemical parameters including temperature, salinity, pH and dissolved oxygen in the solutions of control and extract treated groups were determined via YSI multiparameter.
2.5 Statistical analysis
The data were analyzed using IBM SPSS Statistics 25 Window package (IBM, Armonk, NY, US) and presented as mean ± SD. One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test was performed to determine the significant differences between groups and means were considered as statistically significant if p < 0.05.
3 Results
3.1 Phytochemical profile of neem leaves methanol extract
The name of the metabolites noticed in the methanol extract of neem leaves via UHPLC-HRMS along with the class, retention time and formula are shown in Table 1 and the structures are indicated in Fig. 3. The data showed the presence of flavonoids (isorhamnetin, myricetin, myricetin-3-O-galactoside, trifolin, quercetin), aromatics (1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, 2,4-quinolinediol, indole-3-acrylic acid), phenolics (2-(3,4-dihydroxyphenyl) acetamide, apocynin, phloretin, p-coumaric acid, scopoletin), and terpenoids (carvone, pulegone).
| No. | Metabolites | Class | Retention Time | Formula | m/z | Adduct | Mass Error, ppm |
|---|---|---|---|---|---|---|---|
| [1] | Histidine | Amino acid | 0.69 | C6H9N3O2 | 156.07664 | [M + H]+ | 1.35 |
| [2] | N3,N4-Dimethylarginine | Amino acid | 0.85 | C8H18N4O2 | 203.15031 | [M + H]+ | 0.45 |
| [3] | Arginine | Amino acid | 0.69 | C6H14N4O2 | 175.11884 | [M + H]+ | 0.92 |
| [4] | Valine | Amino acid | 0.75 | C5H11NO2 | 118.08642 | [M + H]+ | 0.26 |
| [5] | Leucine | Amino acid | 0.90 | C6H13NO2 | 132.09036 | [M + H]+ | 1.07 |
| [6] | 1-Aminocyclohexanecarboxylic acid | Amino acid | 1.20 | C7H13NO2 | 144.10190 | [M + H]+ | 0.35 |
| [7] | Proline | Amino acid | 0.94 | C5H9NO2 | 116.07070 | [M + H]+ | 1.04 |
| [8] | Jasmone | Cyclic ketone | 7.04 | C11H16O | 165.12721 | [M + H]+ | 0.97 |
| [9] | 2-Methylcyclohexan-1,3-dione | Cyclic ketone | 1.50 | C7H10O2 | 127.07545 | [M + H]+ | 0.00 |
| [10] | Decanamide | Fatty Acyl | 8.09 | C10H21NO | 172.16940 | [M + H]+ | 0.88 |
| [11] | 4-oxododecanedioic acid | Fatty Acyl | 5.17 | C12H20O5 | 245.13652 | [M + H]+ | 0.66 |
| [12] | 3-Hexenoic acid | Fatty Acyl | 1.87 | C6H10O2 | 115.07545 | [M + H]+ | 1.23 |
| [13] | Isorhamnetin | Flavonoid | 5.54 | C16H12O7 | 317.06509 | [M + H]+ | 1.52 |
| [14] | Myricetin | Flavonoid | 4.91 | C15H10O8 | 319.04434 | [M + H]+ | 1.10 |
| [15] | Myricetin 3-galactoside | Flavonoid | 4.72 | C21H20O13 | 503.07938 | [M + Na]+ | 0.44 |
| [16] | Trifolin | Flavonoid | 5.41 | C21H20O11 | 471.08945 | [M + Na]+ | 1.16 |
| [17] | Quercetin | Flavonoid | 5.03 | C15H10O7 | 303.04953 | [M + H]+ | 1.79 |
| [18] | 6-Methyl-2-pyridinemethanol | Heterocyclic | 1.08 | C7H9NO | 124.07578 | [M + H]+ | 0.73 |
| [19] | Cytidine | Heterocyclic | 0.71 | C9H13N3O5 | 282.04807 | [M + K]+ | 1.07 |
| [20] | Guvacoline | Heterocyclic | 1.07 | C7H11NO2 | 142.08617 | [M + H]+ | 0.14 |
| [21] | Glucosamine | Heterocyclic | 0.61 | C6H13NO5 | 180.08640 | [M + H]+ | 0.78 |
| [22] | Pyroglutamic Acid | Heterocyclic | 0.83 | C5H7NO3 | 130.04984 | [M + H]+ | 0.46 |
| [23] | β,β-Dimethyl-γ-methylene-γ-butyrolactone | Heterocyclic | 1.61 | C7H10O2 | 127.07500 | [M + H]+ | 0.08 |
| [24] | Pipecolic acid | Heterocyclic | 1.05 | C6H11NO2 | 130.08623 | [M + H]+ | 0.85 |
| [25] | 1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid | Heterocyclic aromatic | 1.34 | C10H11NO2 | 178.08582 | [M + H]+ | 0.45 |
| [26] | 2,4-Quinolinediol | Heterocyclic aromatic | 3.24 | C9H7NO2 | 162.05475 | [M + H]+ | 1.37 |
| [27] | Indole-3-acrylic acid | Heterocyclic aromatic | 1.94 | C11H9NO2 | 188.07060 | [M + H]+ | 0.59 |
| [28] | Prolylleucine | Peptide | 1.53 | C11H20N2O3 | 229.15443 | [M + H]+ | 0.53 |
| [29] | 2-(3,4-dihydroxyphenyl)acetamide | Phenolid | 0.93 | C8H9NO3 | 168.06551 | [M + H]+ | 0.54 |
| [30] | Apocynin | Phenolic | 2.93 | C9H10O3 | 167.06998 | [M + H]+ | 0.60 |
| [31] | Phloretin | Phenolic | 5.40 | C15H14O5 | 275.09100 | [M + H]+ | 1.50 |
| [32] | p-Coumaric acid | Phenolic | 3.21 | C9H8O3 | 165.05435 | [M + H]+ | 0.61 |
| [33] | Scopoletin | Phenolic | 4.35 | C10H8O4 | 193.04947 | [M + H]+ | 0.52 |
| [34] | 1-Methyladenine | Purine | 0.83 | C6H7N5 | 150.07732 | [M + H]+ | 0.47 |
| [35] | Guanine | Purine | 0.79 | C5H5N5O | 152.05652 | [M + H]+ | 1.26 |
| [36] | 2′-O-Methyladenosine | Purine | 1.61 | C11H15N5O4 | 282.11963 | [M + H]+ | 1.35 |
| [37] | Adenine | Purine | 0.80 | C5H5N5 | 136.06166 | [M + H]+ | 0.67 |
| [38] | Carvone | Terpenoid | 5.04 | C10H14O | 151.11153 | [M + H]+ | 0.87 |
| [39] | Pulegone | Terpenoid | 3.61 | C10H16O | 153.01779 | [M + H]+ | 0.72 |
| [40] | Nicotinamide | Vitamin B3 | 0.89 | C6H6N2O | 123.05534 | [M + H]+ | 0.66 |
| [41] | Nicotinic acid | Vitamin B3 | 0.84 | C6H5NO2 | 124.03929 | [M + H]+ | 0.49 |
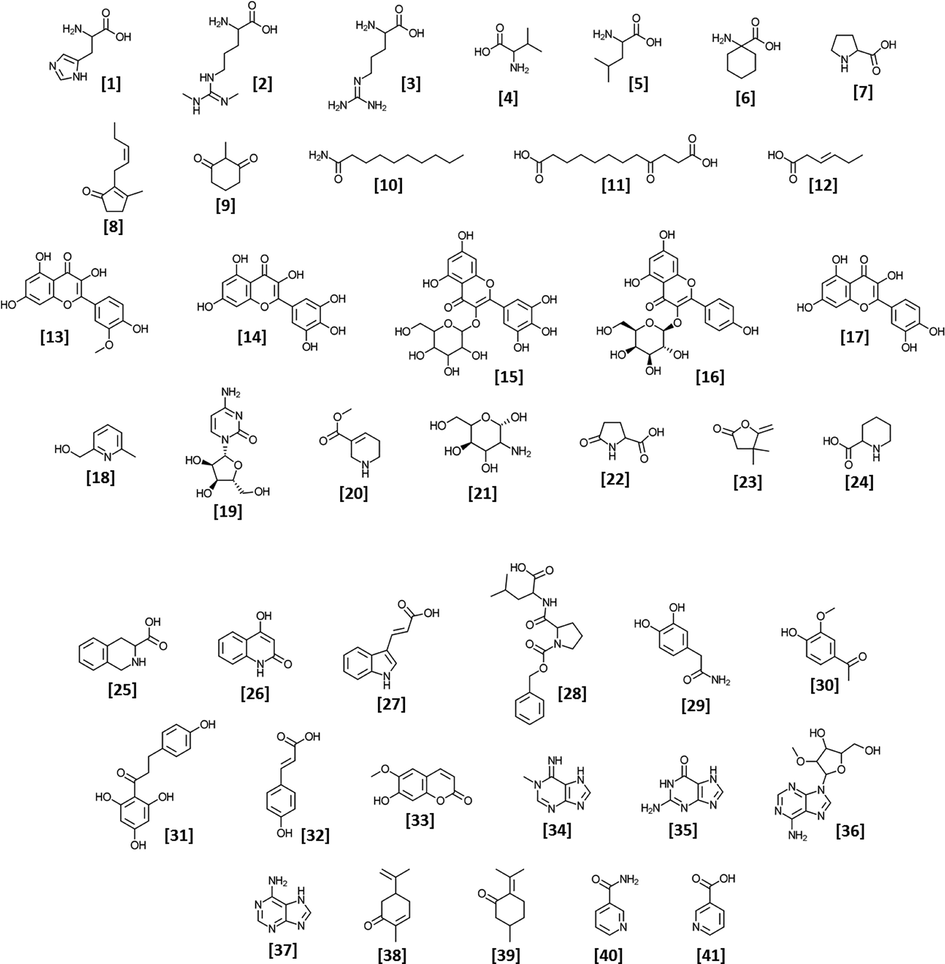
- Structure of the 41 identified metabolites from the methanol extract of neem leaves.
3.2 Antileech activity of neem leaves methanol extract
The administration of neem leaves extract resulted in the killing of leeches at lower (25 mg/ml), medium (50 mg/ml) and higher (100 mg/ml) concentration in an average period of 35.81 ± 5.40, 14.18 ± 0.99 and 9.21 ± 1.51 min. While an average time of 3.77 ± 0.25 min was taken by the formalin solution to kill the leeches. No mortality was noticed in the negative control group treated with seawater. Significance differences were noticed by multiple comparisons among the negative and positive control and extract-treated groups. The differences are highlighted by various symbols (Fig. 4). The mortality percentages of leeches are shown in Table 2. Total mortality (100 %) was noticed in leeches treated with neem leaves extract and formalin solution as compared to normal control group (no mortality).

- Mortality time of parasitic leeches at different concentrations of methanol extract of neem leaves.
| No | Group | Mortality of parasites in percentage (%) |
|---|---|---|
| 1 | Normal control | 0 |
| 2 | Positive control (Formalin 0.25%) (v/v) | 100 |
| 3 | Neem (25 mg/ml) | 100 |
| 4 | Neem (50 mg/ml | 100 |
| 5 | Neem (100 mg/ml) | 100 |
3.3 The behaviour of leeches exposed to neem leaves methanol extract
At the concentration of 25 mg/ml of the extract, the leeches showed active swimming, but the movement reduced after 18 min and at the end of 30 min no movement was noticed. Similarly, active swimming of the leeches was noticed after exposure to 50 and 100 mg/ml of the extracts but after 10 and 2 min, respectively all the leeches become too weak. The leeches exposed to formalin also indicated active movement for 1 min before they stopped moving while the untreated leeches displayed a normal swimming pattern (Fig. 2).
3.4 Physio-chemical parameters
Water quality parameters are indicated in Table 3. Changes in the pH of the plant extract solution were noticed as compared to normal and positive control.
| No | Water parameters | Concentrations | ||||
|---|---|---|---|---|---|---|
| Groups | Normal control | Positive control (formalin 0.25%) (v/v) | Neem leaves (mg/ml) |
|||
| (25) | (50) | (1 0 0) | ||||
| 1 | Temperature (0C) | 24.6 | 25.3 | 24.8 | 24.7 | 25.1 |
| 2 | pH | 7.80 | 7.24 | 4.31 | 4.06 | 5.31 |
| 3 | Salinity (ppt) | 30.0 | 30.9 | 31 | 31.0 | 30.9 |
| 4 | Dissolved oxygen (mg/l) | 7.1 | 6.5 | 7.1 | 6.7 | 6.6 |
4 Discussion
The parasitic leech Z. arugamensis is well distributed throughout the Indian Ocean, affects a wide range of marine fish species (Cruz-Lacierda et al., 2000; Kua et al., 2014; Ravi and Shariman Yahaya, 2017). The leech resulted in the morality of the host in a very short period of time (Kua et al., 2014). Toxic and non-degradable drugs and chemicals are applied for the control of parasitic infestation (Forwood et al., 2013; Pitten et al., 2000). However, the chemicals could be hazardous to living organisms and the environment (Jayaraj et al., 2016; Pitten et al., 2000). Hence, to control the leech infestation, plants with potential bioactive compounds can be applied as a natural remedy (Kayser et al., 2003). Thus, the main reason for this study was to develop a natural and biodegradable control agent against parasitic leech by the application of the plant. Our study indicated that the methanol extract of neem leaves resulted in a total mortality of leeches at a concentration of 25 to 100 mg/ml in an average time period of 35.81 ± 5.40 to 9.21 ± 1.51 min, respectively. Some other plants with antiparasitic properties have also been reported recently. The methanol extract of fern Nephrolepis biserrata (Nephrolepidaceae) (100 mg/ml) resulted in a total mortality of Z. arugamensis in 4.8 min (Shah et al., 2020b), however the chromatographic fraction (2.50 mg/ml) took 1.9 mins (Shah et al., 2021). The methanol extract of neem leaves (100 mg/ml) took more time (9.21 min) for the total mortality of leeches compared to the methanol extract and fraction of N. biserrata. This might be due to the presence of various antiparasitic compounds of different nature in N. biserrata extract and fractions including ivalin, isovelleral, kaempferol 7,4′-dimethyl ether, piscidic acid, chlorogenic acid, phytosphingosine, pyrethrosin, warburganal, isodomedin and pheophorbide a (Shah et al., 2020b; Shah et al., 2021), however the neem leaves extract indicated completely different antiparasitic compounds (Table 1), discussed below in details. Similarly, the methanol extract of shrubby Dillenia suffruticosa (Dilleniaceae) (100 mg/ml) resulted in a total mortality of the parasitic leech in 14 min. (Shah et al., 2020c) and the chromatographic fractions (20 mg/ml) took 31 min. (Shah et al., 2020a). The methanol extract of the neem leaves indicated high antiparasitic efficacy in less time compared to the methanol extract of D. suffruticosa. All plants are available naturally in Sabah without any cost. Baikal Skullcap Scutellaria baicalensis (Lamiaceae) was tested against the marine parasitic leech Piscicola geometra (Hirudinea) infesting Cobia fish (Rachycentron canadum) (Rizky et al., 2018). The leeches were exposed to different serial dilutions (ranging from 1/50x to 1/1000x) of the extract for 96 h and mortality (100%) was recorded in an average time period of 8 h, 40 h, 48 h, 72 h, and 96 h (Rizky et al., 2018). The various serial dilutions (ranging from 1/50x to 1/1000x) of the extract of Noni Morinda citrifolia (Rubiaceae) was applied against Piscicola geometra for 96 h and 80% of leech mortality was reported (Rizky et al., 2018). The methanol extract of the ginger Zingiber officinale (Zingiberaceae) at a concentration of 32 × 104 ppm killed the freshwater leech Limnatis nilotica in 24 min (Forouzan et al., 2012). The above-mentioned reported plants evidently proved that the medicinal plants have anti-leech potential. In addition, compared to other plants the methanol extract of neem plant has shown an effective anti-leech activity in less than 10 min at higher concentration (see: Fig. 4) except N. biserrata.
The UHPLC-HRMS analysis of the methanol extract indicated the presence of compounds such as quercetin, carvone, phloretin, trifolin, jasmone and nicotinamide. The anti-leech activity of these compounds have not yet been reported against Z. arugamensis but they showed a various degree of potential effects against mammalian and plant parasites (Abdel-Rahman et al., 2013; Frank et al., 2002; Juan et al., 2013; Li et al., 2005; Mamani-matsuda et al., 2004; Mead and McNair, 2006; Sereno et al., 2005; Wuyts et al., 2006). In an animal study, a significant reduction in lengths of the gastrointestinal nematodes Haemonchus contortus has been reported in carvone treated sheep (Katiki et al., 2019). The application of carvone does not limit to mammalian parasites but plants as well. Aside from being effective in suppressing potato sprout, carvone has also been reported with antifungal properties against several plant parasitic fungi such as Fusarium, Phoma, and Helminthosporium (Hartmans et al., 1995; Oosterhaven et al., 1995). On the other hand, nicotinamide is also a well-documented antiparasitic compound against Plasmodium falciparum (Prusty et al., 2008), Trypanosoma cruzi (Soares et al., 2012), and Leishmania sp. (Sereno et al., 2005). Nicotinamide significantly deregulated the growth of T. cruzi by changing the conformation of sirtuin TcSir2, a NAD-dependent deacetylase which involved in the parasite cell cycle (Soares et al., 2012). Thus, we believe that the above-mentioned compounds in the methanol extract of neem leaves might contribute to the mortality of the parasitic leech. In the current study, the Bornean neem plant was easily accessible from nature without any cost. As mentioned above the crude extract of the plant possessed a mixture of vital antiparasitic chemical components. The cost of this naturally obtained chemical components mixture is very less as compared to the synthetically prepared chemical components.
5 Conclusion
Our data indicated that the methanol extract of neem leaves showed a significant anti-leech activity against the parasitic leech Z. arugamensis with a total mortality at various concentrations ranging from 25 to 100 mg/ml in average time period from 35.81 ± 5.40 to 9.21 ± 1.51 min, respectively, compared to untreated control. The ultra-high performance liquid chromatography–high–resolution mass spectrometry system revealed the presence of different bioactive compounds such as flavonoids, aromatics, phenolics, and terpenoids. Thus, it is indicated that the methanol extract of neem leaves act as an effective anti-leech agent against Z. arugamensis of cultured groupers due to the presence of vital bioactive compounds. Besides, our current sample indicated 100% mortality of Z. arugamensis in less time as compared to our previous sample D. suffruticosa. This research will be highly beneficial to fish farmers in Sabah, Malaysia to control the leeches in natural manners in aquaculture. However, further isolation, purification and characterization of the pure bioactive compounds with antiparasitic potential is required.
Acknowledgements
This research work was supported by the Fundamental Research Grant Scheme, the Ministry of Education Malaysia, Grant/Award Number: FRG0486-2018, FRGS/1/2018/STG03/UMS/02/2, PRF No.0011-2019 and UMS Grant SDN0073-2019 to BAVM. We thank the UMS fish hatchery staff and our lab members for helping us to collect the samples. We also thank the reviewers for providing critical comments to improve the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Nematicidal activity of terpenoids. J. Environ. Sci. Heal. - Part B Pestic. Food Contam. Agric. Wastes. 2013;48(1):16-22.
- [CrossRef] [Google Scholar]
- Antifungal effect of Malaysian neem leaf extract on selected fungal species causing otomycosis in in-vitro culture medium. Sci: Malaysian J. Med. Heal; 2015. p. :11.
- Medicinal plants and secondary metabolites for leech control. Asian Pacific J. Trop. Dis.. 2014;4(4):315-316.
- [CrossRef] [Google Scholar]
- Marine leech (Zeylanicobdella arugamensis) infestation in cultured orange-spotted grouper, Epinephelus coioides. Aquaculture. 2000;185(3-4):191-196.
- [CrossRef] [Google Scholar]
- Department of Fisheries Sabah, 2020. Anggaran pengeluaran ikan kasar perikanan akuakultur Sabah, 2018. (In Malay)
- Anti-parasitic activites of Zingiber officinale methanolic extract on Limnatis nilotica. Glob. Vet.. 2012;9:144-148.
- [CrossRef] [Google Scholar]
- Efficacy of bath and orally administered praziquantel and fenbendazole against Lepidotrema bidyana Murray, a monogenean parasite of silver perch, Bidyanus bidyanus (Mitchell) J. Fish Dis.. 2013;36(11):939-947.
- [CrossRef] [Google Scholar]
- Feeding deterrent effect of carvone, a compound from caraway seeds, on the slug Arion lusitanicus. Ann. Appl. Biol.. 2002;141(2):93-100.
- [CrossRef] [Google Scholar]
- The use of carvone in agriculture: sprout suppression of potatoes and antifungal activity against potato tuber and other plant diseases. Ind. Crops Prod.. 1995;4(1):3-13.
- [CrossRef] [Google Scholar]
- Antihelminthic efficacy of crude neem (Azadirachta indica) leaf powder against bovine strongylosis. J. Parasit. Dis.. 2015;39:786-788.
- [CrossRef] [Google Scholar]
- Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol.. 2016;9:90-100.
- [CrossRef] [Google Scholar]
- Nicotinamide inhibits the lysosomal cathepsin b-like protease and kills African trypanosomes. J. Biol. Chem.. 2013;288(15):10548-10557.
- [CrossRef] [Google Scholar]
- Evaluation of encapsulated anethole and carvone in lambs artificially- and naturally-infected with Haemonchus contortus. Exp. Parasitol.. 2019;197:36-42.
- [CrossRef] [Google Scholar]
- Kayser, O., Kiderlen, A.F., Croft, S.L., 2003. Natural products as antiparasitic drugs. Parasitol. Res. https://doi.org/10.1007/s00436-002-0768-3
- Effect of salinity and temperature on marine leech, Zeylanicobdella arugamensis (De Silva) under laboratory conditions. J. Fish Dis.. 2014;37(3):201-207.
- [CrossRef] [Google Scholar]
- Antifungal activity of camptothecin, trifolin, and hyperoside isolated from Camptotheca acuminata. J. Agricul. Food Chem.. 2005;53(1):32-37.
- [CrossRef] [Google Scholar]
- Quercetin induces apoptosis of Trypanosoma brucei gambiense and decreases the proinflammatory response of human macrophages. Antimicrob. Agents Chemother.. 2004;48:924-929.
- [CrossRef] [Google Scholar]
- Antiparasitic activity of flavonoids and isoflavones against Cryptosporidium parvum and Encephalitozoon intestinalis. FEMS Microbiol. Lett.. 2006;259:153-157.
- [CrossRef] [Google Scholar]
- S-carvone as a natural potato sprout inhibiting, fungistatic and bacteristatic compound. Ind. Crops Prod.. 1995;4(1):23-31.
- [CrossRef] [Google Scholar]
- Othman, M.F., Hashim, M., Eim, Y.M., Azmai, M.N.A., Iksan, N., Chong, H.G., Merican, Z., 2017. Transforming the aquaculture industry in Malaysia, in: Asian-Pacific Aquaculture 2017. World Aquaculture. The Magazine of the World Aquaculture Society. pp. 16–23.
- Formaldehyde neurotoxicity in animal experiments. Pathol. Res. Pract.. 2000;196(3):193-198.
- [CrossRef] [Google Scholar]
- Nicotinamide inhibits Plasmodium falciparum Sir2 activity in vitro and parasite growth. FEMS Microbiol. Lett.. 2008;282:266-272.
- [CrossRef] [Google Scholar]
- Zeylanicobdella arugamensis, the marine leech from cultured crimson snapper (Lutjanus erythropterus), Jerejak Island, Penang. Malaysia. Asian Pac. J. Trop. Biomed.. 2017;7(5):473-477.
- [CrossRef] [Google Scholar]
- Anti-leech activity of Scutellaria baicalensis and Morinda citrifolia extracts against Piscicola geometra. IOP Conf. Ser. Earth Environ. Sci.. 2018;137:012031.
- [CrossRef] [Google Scholar]
- Schmutterer, H., 1990. Properties and potential of natural pesticides from the Neem tree. Annu. Rev. Entomol. 35:, 271–97.
- Lethality of Naja nigricollis Reinhardt venom and antivenom activity of Azadirachta indica A. Juss. leaf extracts on albino rats. GSC Biol. Pharm. Sci.. 2020;12(2):080-092.
- [Google Scholar]
- In vitro antileishmanial activity of nicotinamide. Antimicrob. Agents Chemother.. 2005;49(2):808-812.
- [CrossRef] [Google Scholar]
- Shah, M.D., Tani, K., Venmathi Maran, B.A., Yong, Y.S., Fui Fui, C., Shaleh, S.R.M., Vairappan, C.S., 2020a. High-resolution chemical profiling and antiparasitic potential of the tropical shrub Dillenia suffruticosa. Fish. Sci. https://doi.org/10.1007/s12562-020-01456-8
- Antiparasitic potential of Nephrolepis biserrata methanol extract against the parasitic leech Zeylanicobdella arugamensis (Hirudinea) and LC-QTOF analysis. Sci. Rep.. 2020;10:1-10.
- [CrossRef] [Google Scholar]
- Antiparasitic activity of the medicinal plant Dillenia suffruticosa against the marine leech Zeylanicobdella arugamensis (Hirudinea) and its phytochemical composition. Aquac. Res.. 2020;51(1):215-221.
- [CrossRef] [Google Scholar]
- Antiparasitic Potential of Chromatographic Fractions of Nephrolepis biserrata and Liquid Chromatography-Quadrupole Time-of-Flight-Mass Spectrometry Analysis. Molecules. 2021;26:499.
- [CrossRef] [Google Scholar]
- Shariff, M., Nagaraj, G., F.H.C, C., Y.G., W., 2000. The use of chemicals in aquaculture in Malaysia and Singapore, in: J.R. Arthur, C.R. Lavilla-Pitogo, in: Use of Chemicals in Aquaculture in Asia : Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia, 20-22 May 1996, Tigbauan, Iloilo, Philippines. pp. 127–140.
- Anti-Trypanosoma cruzi activity of nicotinamide. Acta Trop.. 2012;122(2):224-229.
- [CrossRef] [Google Scholar]
- In vivo and in vitro antimicrobial activity of Azadirachta indica (Lin) against Citrobacter freundii isolated from naturally infected Tilapia (Oreochromis mossambicus) Aquaculture. 2015;437:252-255.
- [CrossRef] [Google Scholar]
- Efficacy of the aqueous extract of Azadirachta indica against the marine parasitic leech and its phytochemical profiling. Molecules. 2021;26:1908.
- [CrossRef] [Google Scholar]
- Records of Caligus (Crustacea: Copepoda: Caligidae) from marine fish cultured in floating cages in Malaysia with a redescription of the male of Caligus longipedis Bassett-Smith, 1898. Zool. Stud.. 2009;48:797-807.
- [Google Scholar]
- Caligus sclerotinosus (Copepoda: Caligidae), a serious pest of cultured red seabream Pagrus major (Sparidae) in Korea. Vet. Parasitol.. 2012;188:355-361.
- [Google Scholar]
- Effects of plant phenylpropanoid pathway products and selected terpenoids and alkaloids on the behaviour of the plant-parasitic nematodes Radopholus similis, Pratylenchus penetrans and Meloidogyne incognita. Nematology. 2006;8(1):89-101.
- [CrossRef] [Google Scholar]







