Testing the cytotoxicity and genotoxicity of the antimalarial drug mefloquine
*Corresponding author. Address: Zoology Department, Faculty of Science, King Saud University, P.O. Box 22452, Riyadh 11495, Saudi Arabia. Tel./fax: +966 14767296 o_elhabit@yahoo.com (Ola Hassan El-Habit) oelhabot@ksu.edu.sa (Ola Hassan El-Habit)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 29 July 2011
Abstract
Numerous drugs show significant behavioral, developmental, teratogenic and mutagenic effects. This study investigates the cytotoxic and genotoxic effects of two doses (20 and 40 mg/kg body weight) of the antimalarial drug mefloquine (MQ), in male mice. The parameters included the assessment of: mitotic index, cell cycle kinetics, and frequency of chromosomal aberrations in bone marrow cells, translocation frequency in spermatocytes and head and tail abnormalities in sperm. A high dose of mefloquine resulted in a decreased mitotic index, suggesting a dose–response correlation. A notable recovery in mitotic activity was observed during the first and third week post treatment, which approached the mitotic index of control. Chromosomal abnormalities including gaps, breaks, and fragments also increased with the higher dose of mefloquine. Spermatids and secondary spermatocytes were moderately sensitive to the drug; whereas primary spermatocytes were more sensitive, and spermatogonia were highly sensitive to both doses of MQ.
Conclusion
Mefloquine (MQ) at the doses used can be considered as a drug with a potential mutagenic effect. The results obtained from the spermatocyte diakinesis-metaphase 1 test and sperm abnormalities suggest that MQ may contribute to high incidence of birth defects and congenital abnormalities.
Keywords
Mefloquine (MQ)
Cytotoxic
Genotoxic
Spermatocyte diakinesis metaphase-1
1 Introduction
Mefloquine, a 4-quinolinemethanol (MQ), is the only drug proven to be effective against multiple drug-resistant malaria. Experimental studies indicated that mefloquine does not significantly bind to DNA (Davidson et al., 1975). Schupbach (1979) showed no mutagenic activity of mefloquine in three tester strains TA 1535, TA 1537 and TA 1538 of Salmonella typhimurium. Grisolia and Takahashi (1994) showed that the administration of MQ did not lead to any clastogenic activity in the bone marrow of Wistar rats, when given in doses of 150, 300, 600 and 1200 mg/kg, equivalent to the human therapeutic doses. However, in vitro studies showed that mefloquine at doses (100 and 50 μg/ml) significantly altered poly-morphonuclear neutrophils (PMN) while, amodiaquine and chloroquine did not (Labor and Babin-Chevaye, 1988). The genotoxic effect of amodiaquine (AQ), mefloquine (MQ) and halofantrine (HF), investigated in rat liver cells using alkaline Comet assay. AQ, MQ and HF at doses between 0 and 1000 μmol/L significantly increased DNA strand breaks of rat liver cells illustrating dose-dependent relationship in the order of AQ > MQ > HF (Farombi, 2005, 2006). However, the study of clastogenic activity in human lymphocytes has not been done, in vitro or in patients undergoing therapy. Chatterjee et al. (1998), who used similar groups of three antimalarial drugs to examine the genotoxic and mutatoxic potential of MQ revealed that these drugs are weak mutagen, but capable of inducing significant sister chromatid exchange (SCE) and chromosomal aberrations (CA) in the bone marrow cells of mice. Karbwang and White (1990) detected the induction of lymphocytopenia at doses of 30 mg/kg. Genotoxicity tests by Akerele and Obaseiki-Ebor (2002) indicated that MQ was generally not genotoxic, but had the same potential mutagenicity as chloroquine phosphate.
The current study was undertaken to examine the long term treatment on cytotoxic and mutagenic potential of MQ in male mice, administered at 20 and 40 mg doses. The mitotic index of bone marrow cells, chromosomal aberration in metaphase cells and cytogenetic evaluation diakinesis-metaphase 1 in mouse spermatocytes were the focus of the study. Morphological abnormalities of the sperm too, were studied over 3 weeks. Following the last day of the drug treatment, sperms were analyzed at weekly intervals for 6 weeks, to study the effect of the drug on each stage of the spermatogenic cycle, which is completed in about 5 weeks (Okberg, 1956; Wyrobek, 1978).
2 Material and methods
2.1 Experimental animals
Swiss albino mice, strain SWR/J, were used in this study. Adult male mice were obtained from the Central Animal House of King Saud University (Women’s Branch – Malaz, Riyadh, KSA). The animals were housed in well ventilated, hygienic conditions under standardized temperatures. Food and water were provided ad libitum. Male mice were used in order to avoid hormonal interference of the estrous cycle in females. All animals were 10–12 weeks of age, each weigh about 25 g. Animals were randomly selected for experimental control.
2.2 Dosage and treatment
Mefloquine (trade name Mephaquine [Mepha Ltd., Aesch-Basel, Switzerland]) was purchased from a local pharmacy in Riyadh, KSA, and was suspended in Tween 80 (1%) to the desired concentrations (1 mg/0.5 ml, and 0.5 mg/0.5 ml). These doses correspond to human doses of 40 and 20 mg/kg body weight (Preston et al., 1981; Fryauff et al., 2007). The stock solution was freshly prepared before each dose was administered.
For studying cytotoxic and cytogenetic effects of bone marrow (mitotic index, cell cycle kinetics, and chromosomal aberrations in metaphase cells), animals were divided into three large groups of 30 animals each: two treated with a different dose of MQ and the third served as the control. Each large group was divided into six small groups of five animals each.
For spermatocyte diakinesis metaphase-1 test and for studying the morphological abnormalities of sperm head and tail, animals were similarly divided into three groups of 35 male animals each. Two groups were treated with a different dose of MQ and the third group served as control. Each large group was subdivided into seven small groups of five animals each.
2.3 Sampling time
Cells were obtained from bone marrow of both control and treated mice. Mitotic indices, cell cycle kinetics, and analysis of chromosomal aberrations were performed at 24, 48, 72 h and at weekly intervals for 3 weeks following the last dose. For the spermatocyte metaphase-1 test, samples were tested at 24 h and at weekly intervals for 6 weeks following the last dose of drug, which covered a complete spermatogenic cycle. Morphological head and tail abnormalities of the spermatozoa were recorded for 6 weeks at weekly intervals following the last dose.
2.4 Techniques for the study of mitotic index, cell cycle kinetics, and metaphase chromosomes from bone marrow cells
Bone marrow from treated and control mice was flushed out from the two femurs into saline solution. The aspirated cells were divided into two samples; one was processed immediately for the study of mitotic index and cell cycle kinetics (Cho et al., 2011). The second sample is treated with Colchicine for 2 h for the study of metaphase chromosomal aberrations followed by hypotonic treatment with 0.075 M KCl for 20 min at 37 °C. Cells were then fixed in Carnoy’s fixative (3:1 methanol:1 acetic acid). Air dried slide preparations were stained with 5% Giemsa stain (Evans et al., 1964; Adler, 1984). A total diploid number of chromosomes were counted in 100 metaphases and different types of CA were determined and tabulated according to Savage (1975). The effect of MQ on cell cycle kinetics of the bone marrow cells was also examined.
2.5 Analysis of germinal cell tissue, spermatocyte diakinesis-1, and morphological abnormalities of head and tail regions of spermatozoa
To evaluate the effect of MQ on meiotic chromosomes from germinal cells in different stages of spermatogenesis, treated animals and control groups were dissected and testes were removed from the seminiferous tubules. The cytogenetic analysis of diakinesis metaphase-1 spermatocytes involved the detection of reciprocal translocations and other chromosomal aberrations induced in spermatogonia of the treated males (Leonard, 1977).
To examine any morphological abnormalities of sperm, the animals caudae epididymides were removed, dissected and sperm were processed for fixation and stained with 1% eosin for 10–15 min. Preparation of spermatozoa was performed according to Wyrobek (1978).
2.6 Photography
All photography was performed using a photographic research microscope (Olympus Bx41).
2.7 Statistical analysis
Data were evaluated by the Student’s t-test (Byrkit, 1980) and by an analysis of variance (two-way ANOVA) to determine the significance of differences between groups.
3 Results
3.1 Mitotic index (M.I.) and cell cycle kinetics
The M.I. data are shown in Table 1. The results indicate a decreased M.I. with an increase in the dose of MQ, suggesting a dose–response relationship. However, these dose effects were not statistically significant. Percentages of cells in metaphase were not affected by MQ, but the percentages of cells entering mitosis decreased by about 17–29% in the groups treated with 20 mg/kg at all assay times. Regarding the dose 40 mg/kg, there was an even greater decrease in the percentage of cells entering mitosis of about 21–31% at all assay times. Also, there was a notable recovery in mitotic activities at the first and third weeks post treatment, approaching the control index. The cell cycle kinetics data are given in Fig. 1. There was a notable recovery of bone marrow cells toward normal cell cycle kinetics starting 72 h post treatment; this remained stable until 3 weeks post treatment.
| Doses of MQ | No. of dividing cells and % at different assay time | |||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 1 week | 2 weeks | 3 weeks | |
| Control 0 mg/kg | 958.6 ± 134.3 | 996.8 ± 49.2 | 954 ± 95.2 | 950.8 ± 108.7 | 904.6 ± 67.7 | 1004.8 ± 136.9 |
| 47.9% | 49.8% | 47.7% | 47.5% | 45.2% | 50.2% | |
| 20 mg/kg | 779.6 ± 22.5a | 778.4 ± 15.2a | 687.4 ± 69.2a | 805.6 ± 75.5a | 711.2 ± 110.9a | 800.4 ± 50a |
| 39% | 38.9% | 34.4% | 40.3% | 35.6% | 40% | |
| 40 mg/kg | 686.6 ± 29.7a | 683.8 ± 20.8a | 700 ± 29.6a | 720.4 ± 29.6a | 662 ± 63a | 750.4 ± 34.2a |
| 34.3% | 34.2% | 35% | 36% | 33% | 37.5% | |
Five mice per group.
Two thousand cells counted per animal.
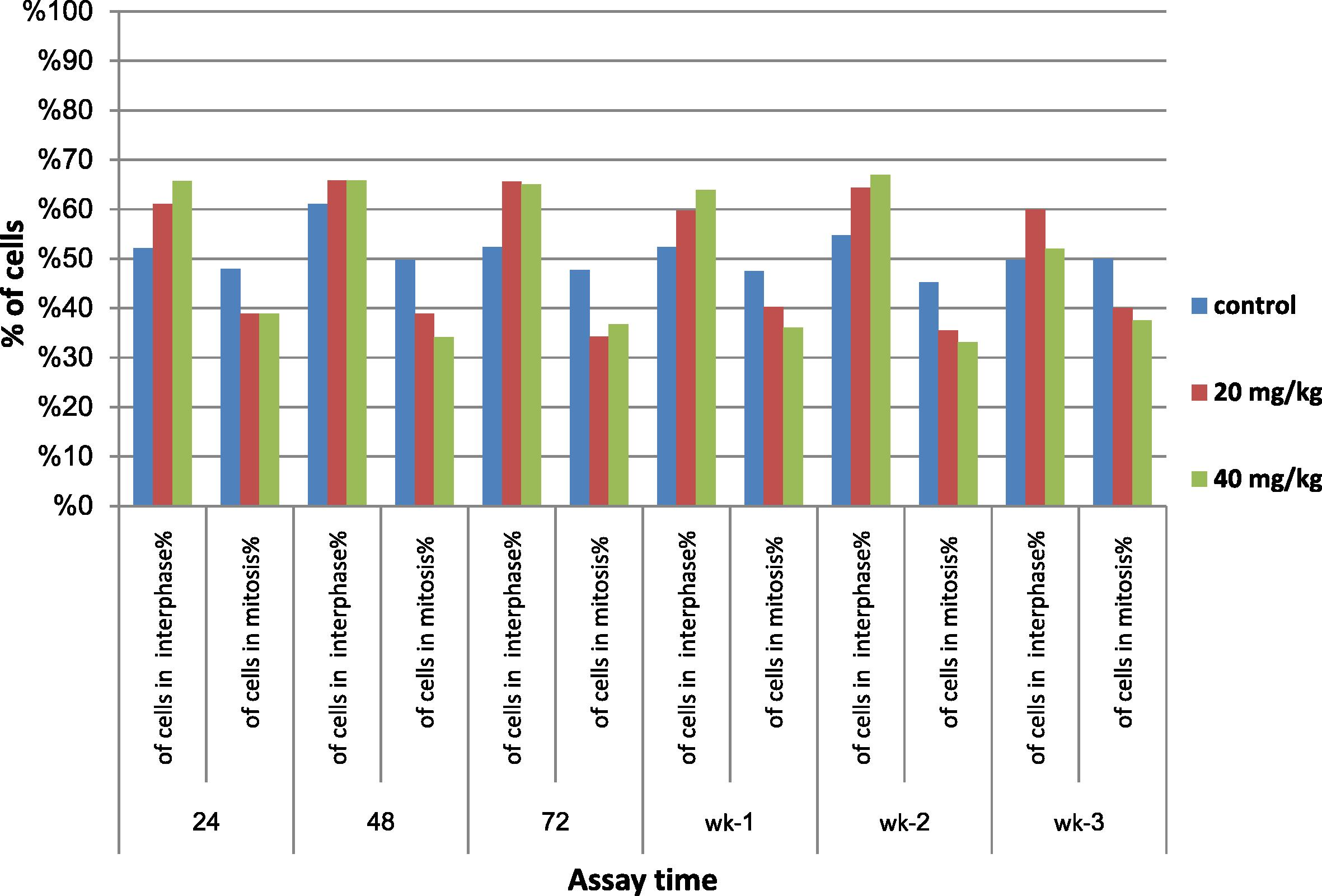
- Effect of mefloquine (MQ) on cell cycle kinetics assayed 24, 48 and 72 h; 1, 2 and 3 weeks.
3.2 Analysis of chromosomal aberrations in bone marrow cells and spermatocyte cells
The effects of the two doses (20 and 40 mg/kg) of MQ on the incidences of different types of chromosomal aberrations induced in bone marrow cells were examined at 24, 48 and 72 h and 1, 2 and 3 weeks post treatment. At 24 h interval post treatment with 20 mg/kg of MQ the percentages of total aberrant cells, chromatid and chromosome gaps, centromeric chromosome break and centromeric fusion showed a significant increase (p < 0.05) (28.4, 18.8, 10.4, 17.6, and 5.2, respectively) and when treated with 40 mg/kg of MQ a significant increase in the percentage of total aberrant cells, chromatid gaps, chromatid breaks, fragment, centromeric chromosome breaks, and centromeric fusion when compared to the control group (19.2, 14, 1.2, 3.2, 16, and 2.8, respectively) (Fig. 2 and Plates 1 and 2). The two-way ANOVA for comparison between groups represented in Table 2 showed significant differences in the percentages of total chromatid gaps and total centromeric chromosome breaks between treated and control groups at six sampling times.
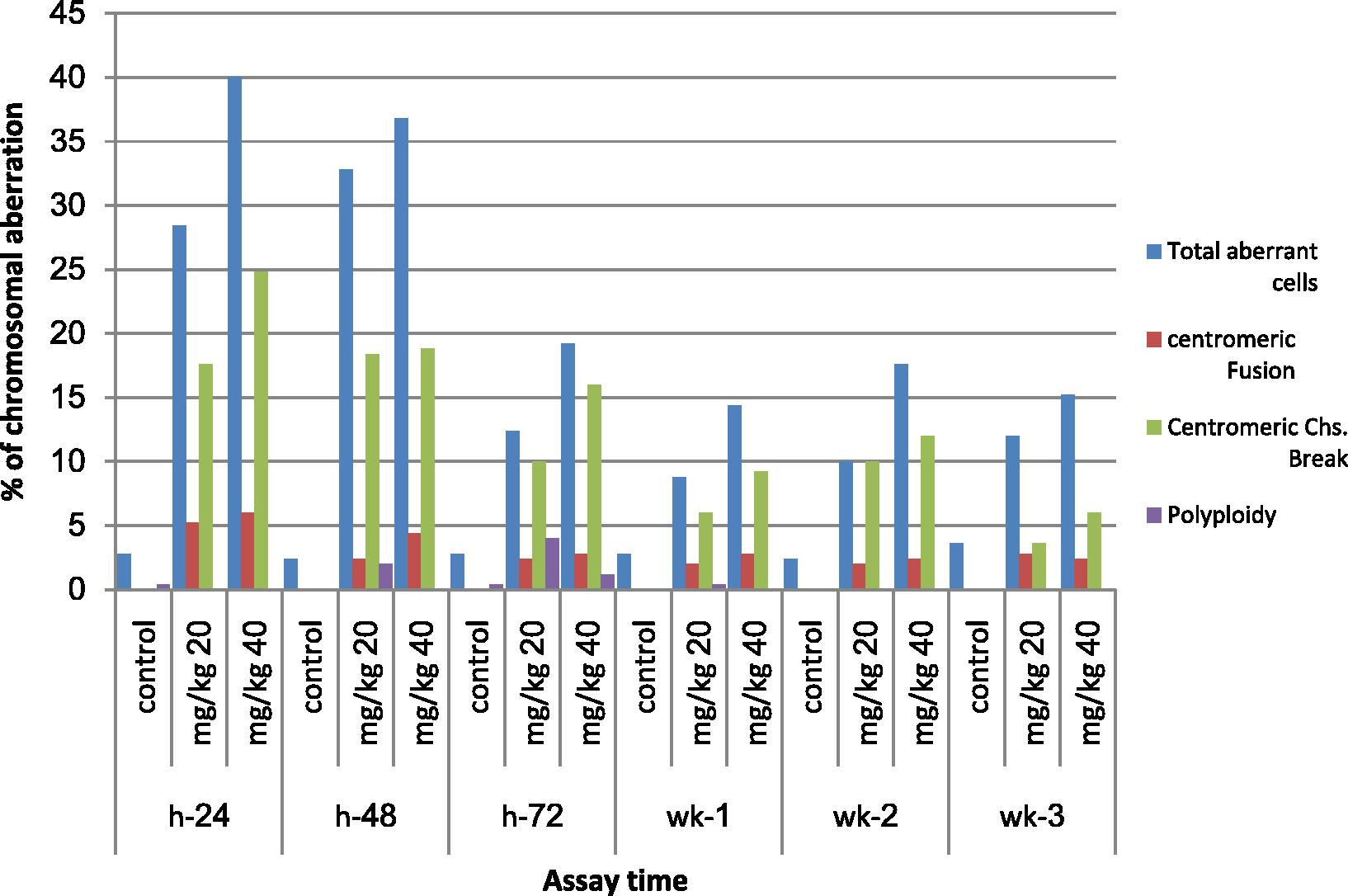
- Effect of mefloquine (MQ) on the incidences of different types of chromosomal aberrations induced in bone marrow cells of mice assayed 24, 48, 72 h; 1, 2 and 3 weeks.
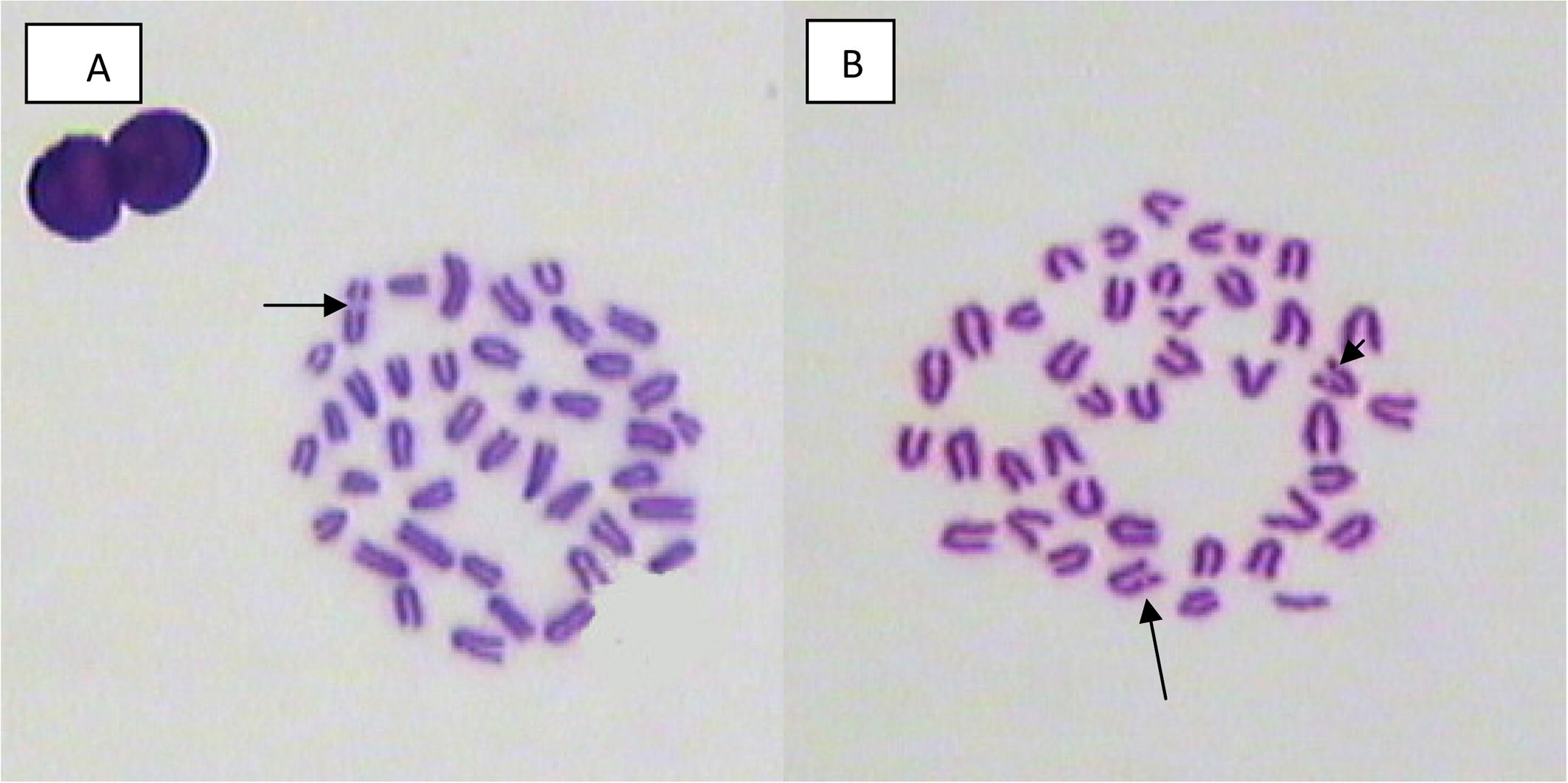
- Bone marrow metaphase spread showing chromosome break from male mouse treated with (A) 20, (B) 40 mg/kg of mefloquine and assessed 2 weeks and 24 h post treatment.
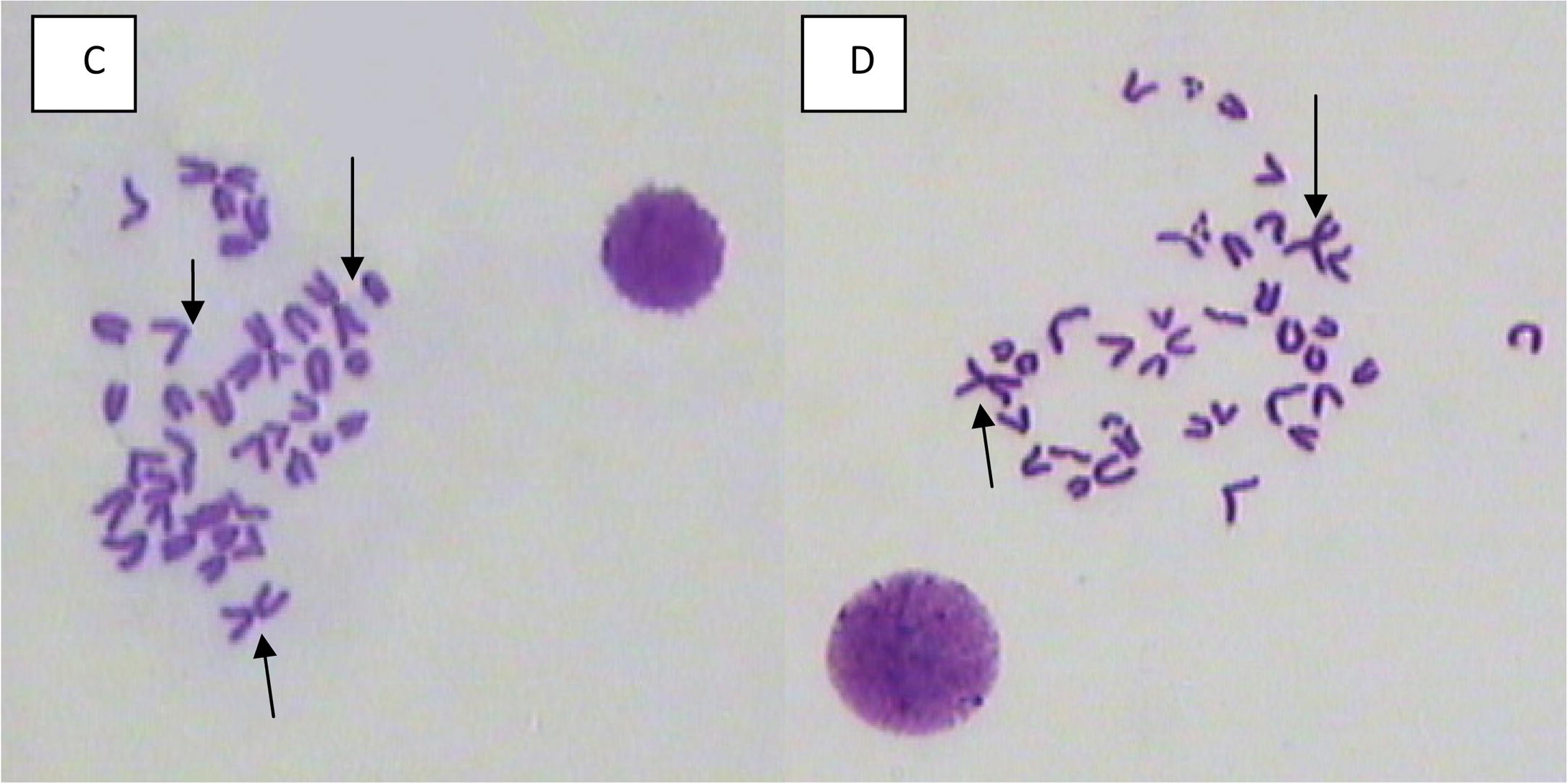
- Bone marrow metaphase spreads showing centromeric fusions, and centromeric breaks from male mouse treated with (C and D) 40 mg/kg of mefloquine assessed 24 and 48 h, respectively, post treatment.
| Comparison between groups | F-test value | |
|---|---|---|
| % of total chromatid gaps | % of total centromeric chromosome break | |
| Control (0 mg/kg) | 2.7 | 0 |
| 20 mg/kg | 10.8a | 10.9a |
| 40 mg/kg | 14.4a | 14.5a |
Five mice were used per dose.
Fifty cells counted per mouse.
Two hundred and fifty cells counted per groups.
Fig. 3 summarizes the results of the spermatocyte diakinesis metaphase-1 assay obtained post treatment with both doses of MQ compared with controls. The induced chromosomal aberration assayed 24 h post treatment was much higher than those assayed from 1 to 5 weeks. The results indicate that primary spermatocytes are the most affected stage of meiosis whereas other stages are equally affected by the MQ treatment analysis of variance (two-way ANOVA) between groups treated with 20, 40 mg/kg of MQ at six sampling times. Significant (p < 0.05) differences in the percentages of total X–Y univalents and non-significant values for total fragments (minutes) are presented in Table 3 and Plate 3.
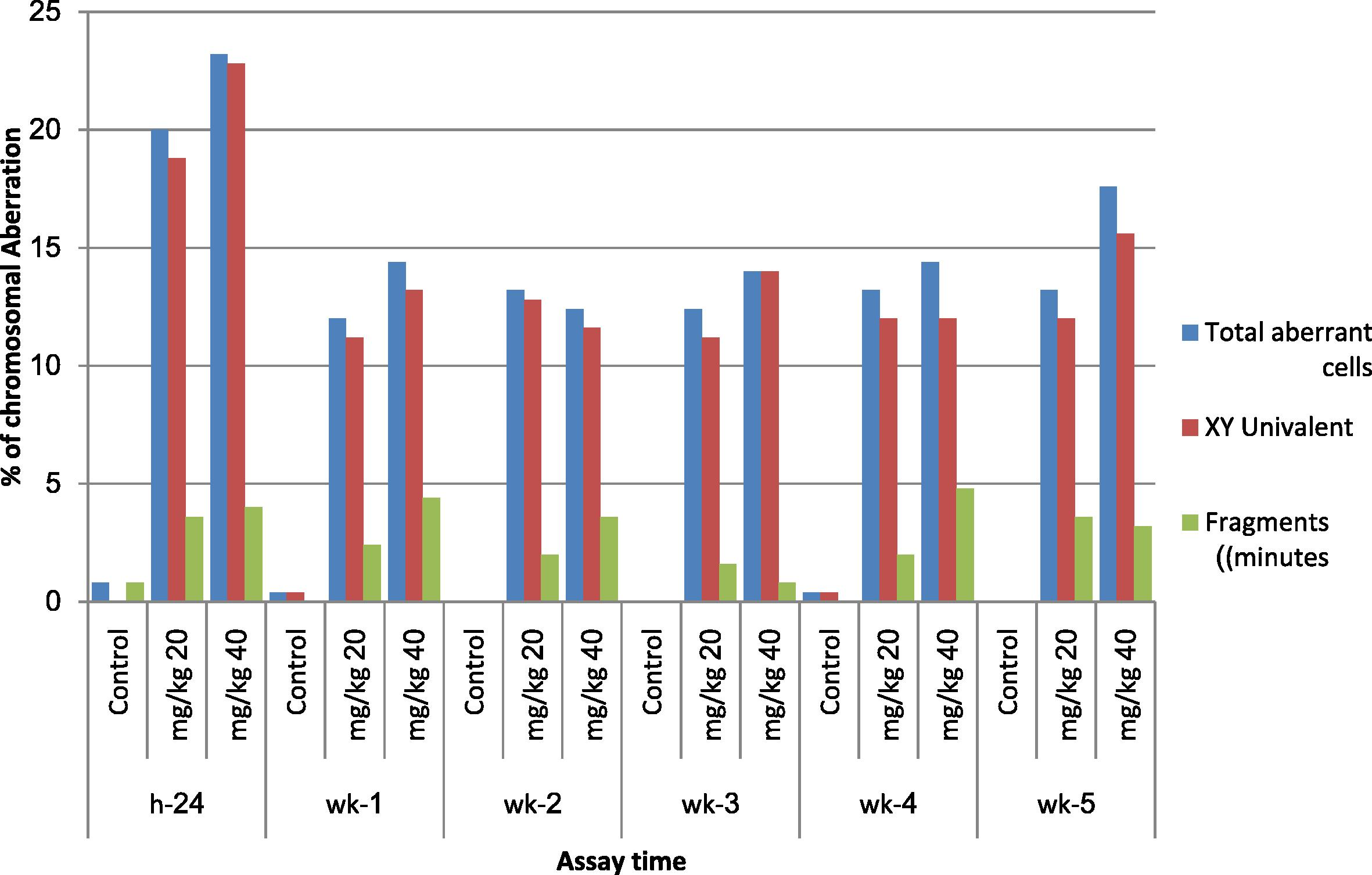
- Effect of mefloquine (MQ) on the incidence of different types of chromosomal aberrations in diakinesis metaphase-1 spermatocytes of mice assayed 24 h; 1, 2, 3, 4 and 5 weeks post treatment.
| Comparison between groups | F-test value | |
|---|---|---|
| % of total X–Y univalent | % of total fragment (min) | |
| Control (0 mg/kg) | 0.13 | 0.13 |
| 20 mg/kg | 13a | 2.53 |
| 40 mg/kg | 14.9a | 3.3 |
Five mice were used per dose.
Fifty cells counted per mouse.
Two hundred and fifty cells counted per groups.
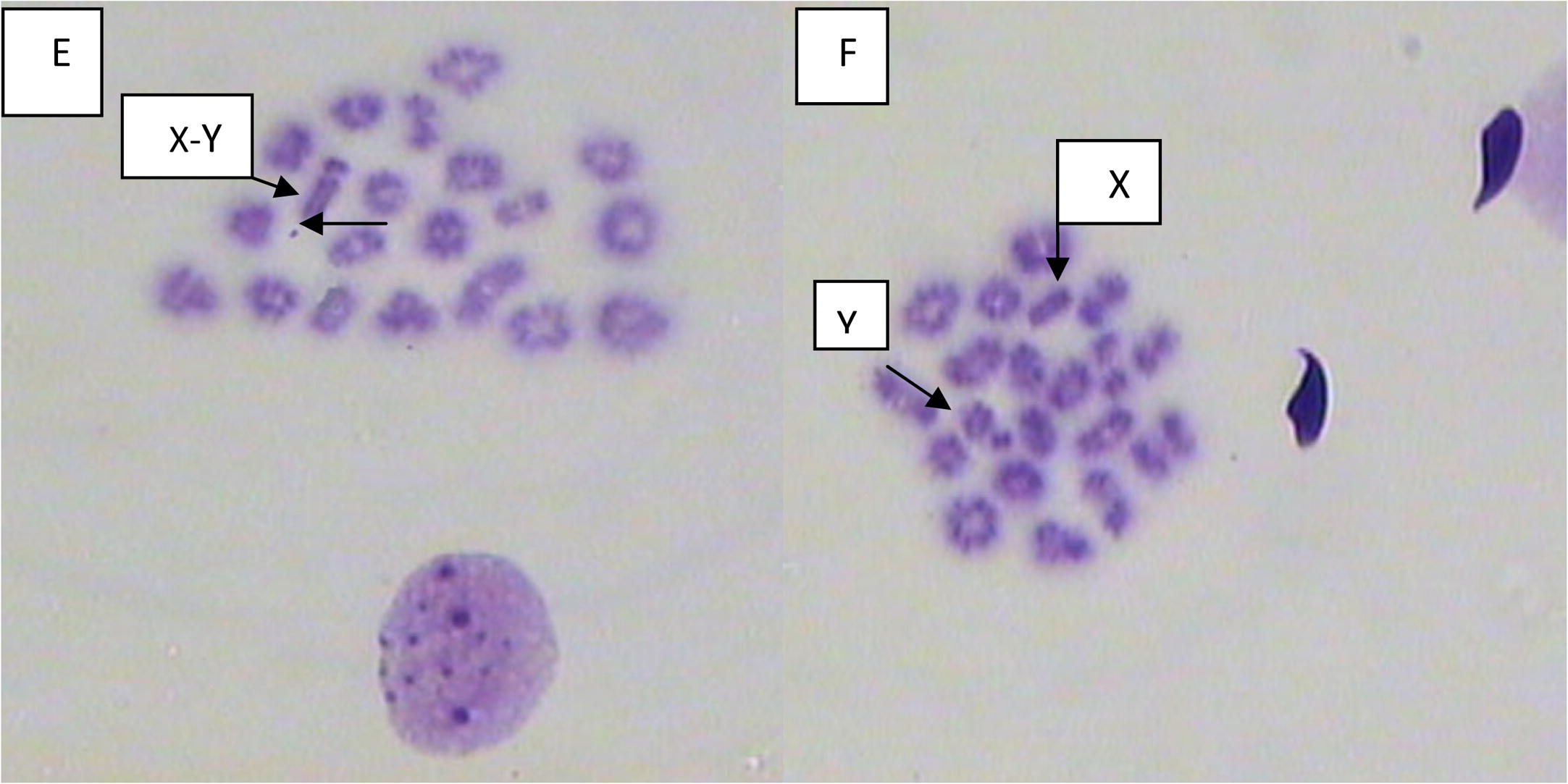
- Spermatocyte diakinesis metaphase-1 spread, showing X–Y univalents and minute, from male mouse treated with (E) 20 and (F) 40 mg/kg of mefloquine and assessed 2 weeks and 4 weeks post treatment, respectively.
3.3 Analysis of sperm head and tail abnormalities of mice treated with mefloquine
The results of sperm head and tail abnormalities in mice treated with MQ for 1, 2, 3, 4, 5, and 6 weeks post treatment showed statistically significant increase with increasing dosage over the whole assay time (Table 4). Higher incidences were observed at the first week post treatment. The increase of the total abnormal head was statistically significant (p < 0.05). The most significant increase was noted in the groups treated with 40 mg/kg and assayed 2, 4, 5 weeks post treatment. The two-way ANOVA, for comparison between groups showed significant differences in the percentages of total abnormal sperm using 20, 40 mg/kg of MQ at 1, 2, 3, 4, 5, and 6 weeks post treatment. These data are presented in Table 5.
| Assay time | % Total abnormal sperm | Abnormal head | % Abnormal tail | |
|---|---|---|---|---|
| % head without hook | % amorphous head | |||
| First week | ||||
| 0 mg/kg | 4.3 | 1.3 | 0.5 | 2.58 |
| 20 mg/kg | 12.9a | 6a | 3.8a | 3.1 |
| 40 mg/kg | 17.2a | 7.6a | 6a | 3.6 |
| Second week | ||||
| 0 mg/kg | 3.2 | 0.9 | 0.7 | 1.7 |
| 20 mg/kg | 11.1a | 5.2a | 2.9a | 2.9a |
| 40 mg/kg | 13.8a | 5.7a | 2.9a | 5.2a |
| Third week | ||||
| 0 mg/kg | 4.4 | 1.1 | 1.3 | 2.1 |
| 20 mg/kg | 8.3a | 3.1a | 1.7 | 3.5a |
| 40 mg/kg | 12.9a | 6a | 3.8a | 3.1 |
| Fourth week | ||||
| 0 mg/kg | 3.7 | 1 | 1.1 | 1.6 |
| 20 mg/kg | 7.3a | 2.6a | 1.6 | 3.1a |
| 40 mg/kg | 9.8a | 3.2a | 2.5a | 4.1a |
| Fifth week | ||||
| 0 mg/kg | 3.8 | 1.2 | 0.5 | 2.1 |
| 20 mg/kg | 6.4a | 2.1a | 1.5a | 2.8 |
| 40 mg/kg | 8.6a | 2.8a | 1.7a | 4.1a |
| Sixth week | ||||
| 0 mg/kg | 3.1 | 0.9 | 0.6 | 1.6 |
| 20 mg/kg | 5 | 1.6a | 1a | 2.4 |
| 40 mg/kg | 6.8a | 2.3a | 1.4a | 3.1a |
Five mice were used per dose.
One thousand sperm counted per mouse.
| Treatment | F-test value | |||
|---|---|---|---|---|
| % of total abnormal sperm | % of total head without hook | % of total amorphous head | % of total abnormal tail | |
| Control (0 mg/kg) | 3.8 | 1.02 | 0.8 | 1.9 |
| 20 mg/kg | 8.5a | 3.4a | 2.1a | 2.96 |
| 40 mg/kg | 11.5a | 4.6a | 3.04a | 3.9a |
Five mice were used per dose.
One thousand sperm counted per mouse.
Five thousand counted per groups.
Two-way ANOVA.
4 Discussion
This study assessed and evaluated the potential cytotoxic and genotoxic effects of two doses of the antimalarial drug MQ in male mice using a number of parameters. The M.I. decreased with an increased dose of antimalarial drug MQ, suggesting a dose–response relationship. However, these dose effects were statistically not significant. A notable recovery was also recorded in mitotic activities during the first and third weeks post treatment. The percentages of cells at metaphase was not affected by MQ, but the percentage of cells entering mitosis decreased by about 17–29% in mice treated with 20 mg/kg MQ at all assay periods. There was an even greater decrease in the percentage of cells entering mitosis (21–31%) in mice treated with 40 mg/kg MQ. This indicates that, when cells suffer genetic damage, the cell cycle kinetics change in such a way that cells are arrested at different cell cycle check points at S phase and M phase (Tobey, 1975; Keiser et al., 2011).
The decrease in M.I. could be explained as a result of cell cycle delay, where cells are arrested in G2, resulting in an accumulation of large number of cells. This cell cycle delay appears to be reversible, as the differences in the percentage of cells in interphase between treated and control groups reduced slowly over time. Data obtained in this study also show a notable recovery of bone marrow cells toward normal cell cycle kinetics starting 72 h post treatment and remaining stable until 3 weeks post treatment, for the two doses of MQ (Zhou and Elledge, 2000; Fryauff et al., 2007).
Analysis of the frequency of chromosomal aberrations indicated a 10- to 14-fold increase in frequency above the spontaneous level in mice treated with both doses of MQ which indicates a potential mutagenic action of the drug. This is also well illustrated by the increase in frequency of aberrant cells analyzed 24 and 48 h post treatment. Frequencies of induced chromosomal aberrations at 72 h post treatment significantly decreased compared to 24 and 48 h post treatment, but were still 4–6 times higher than the spontaneous level. These results clearly show a dose–response relationship at the assayed times. It is also evident that repair mechanisms to eliminate the damaged chromosomes began operating shortly after treatment. Every single type of observed chromosomal aberration behaved similar to the trend represented by total aberrant cells. The high frequency of centrometric chromosome breaks observed illustrates a high sensitivity of heterochromatic DNA regions to MQ or its metabolites (Friedberg et al., 2006). The levels of chromosomal aberrations induced by MQ remained stable 3 days after treatment, and over a period of 3 weeks. Centrometric fusions involving two chromosomes were the only type of structural aberrations that remained at significantly higher levels than controls at almost all assay times. This reflects either stability of the induced chromosome abnormality or persistence of the abnormality in differentiated bone marrow cells.
The data presented in this study are in contrast with the previous results obtained by Grisolia and Takahashi (1994). However, they are in agreement with the findings of Chatterjee et al. (1998), who reported that MQ induced statistically significant increase in sister chromatid exchanges (∼1.5-fold) and chromosomal aberrations (∼2- to 3-fold) in bone marrow cells of mice at the highest dose tested (100 mg/kg intra-peritoneally). It must be emphasized that this study applied different battery test systems, such as spermatocyte diakinesis metaphase-1 and sperm abnormalities that have not been reported in other studies. However, the negative reports of the mutagenicity of MQ may be due to the different methods of administration to the drug (Akerele and Obaseiki-Ebor, 2002). Davidson et al. (1975) and Schupbach (1979) reported that MQ binds weakly to DNA, but is not genotoxic and MQ is a non-mutagenic compound. The results obtained in the present study suggest that spermatids and secondary spermatocytes are moderately sensitive to the action of MQ at the two treated doses (20 and 40 mg/kg bw) in comparison to primary spermatocytes which are more sensitive while the spermatogonia are highly sensitive. The highest percentage of abnormal sperm was recorded in the first week of observation, indicating that spermatogonial stem cells in the seminiferous tubules are also affected by the drug. Whether this damage would be eliminated over a period of time could be judged by an extension of the observation period through 4–5 spermatogenic cycles.
Results of the analysis of diakinesis metaphase-1 spermatocytes showed a significant increase in the total number of aberrant cells, X–Y univalent, and fragments at all assay times. It is obvious that spermatocytes exposed directly to MQ showed the highest incidence in aberrations. Spermatocytes assayed 1–6 weeks post treatment showed a notable decrease in the incidence of aberrations which suggest that the stages of spermatogenesis preceding primary spermatocytes formation are less sensitive to MQ treatment or that significant repair of induced chromosomal damage has taken place. The result also suggests that the high dose of 40 mg/kg was more effective in inducing chromosomal aberrations. The efficiency of the proposed mechanism of repair may be impaired by the quantity of damage, suggesting a limitation of the enzyme repair systems to cope with the induced damage (Zhou and Elledge, 2000; Farombi, 2006; El-Habit and Al-Khamash, 2010).
The results obtained from spermatocyte analysis suggest that the germ cell line is also affected by MQ, similar to the effects of MQ on somatic cells of the bone marrow. The importance of the spermatocyte results is that a high level of abnormalities persisted throughout the process of spermatogenesis, from spermatogonial stages until 6 weeks later, thus providing a high possibility of transmitting these chromosomal aberrations to the gamete cells.
These conclusions draw attention to the potential mutagenicity of MQ to future generations. The teratogenicity of MQ has been previously demonstrated in animals at doses which are higher than the therapeutic doses that are recommended for humans (Zaneveld and Polakoski, 1977; Haward, 1996). In addition, Vanhaumere et al. (1998) reported congenital malformations in children born to mothers who were on MQ therapy at different trimesters during pregnancy. This is an important consideration when prescribing treatment to women of childbearing age. These patients should be warned of the possible risk associated with MQ treatment. Patients on MQ should not conceive for a considerable period of time after cessation of the treatment, to ensure that the germ cell lines are unaffected.
Acknowledgments
The authors thank The Research Center, King Saud University Kingdom of Saudi Arabia, Riyadh for the financial support, and for great support and for offering all their scientific and research facilities.
References
- Cytogenetic tests in mammals. In: Mutagenicity Testing: A Practical Approach. Oxford: IRI Press; 1984. p. :275-306.
- [Google Scholar]
- Studies on the genotoxic and mutagenic potentials of mefloquine. Trop. J. Pharm. Res.. 2002;1(2):91-98.
- [Google Scholar]
- Elements of Statistics (third ed.). New York: D. Van Nostrand Company; 1980. pp. 275–358
- Comparative mutagenic and genotoxic effects of three antimalarial drugs, chloroquine, primaquine and amodiaquine. Mutagenesis (13):619-624.
- [Google Scholar]
- Cho, S., Park, J., Hawang, E.S., 2011. Kinetics of the cell biological changes occurring in the progression of DNA damage-induced senescence., in press.
- Mefloquine, a clinically useful quinolinemethanol anti-malarial which does not significantly bind to DNA. Nature (254):632-634.
- [Google Scholar]
- El-Habit, O.H., Al-Khamash, H.S., 2010. Mutagenic and carcinogenic effect of mefloquine as antimalarial drug. In: The Fourth Saudi Conference, Al-Madinah Al-Munawwarah, Kingdom of Saudi Arabia. POS-Zoo-01, p. 129.
- Drying method for meiotic preparations from mammalian tests. Cytogenetics (3):289-294.
- [Google Scholar]
- Evaluation of genotoxicity of three antimalarial drugs amodaiquine, mefloquine and halofantrine in rat liver cells. DBpia. 2005;97:103.
- [Google Scholar]
- Genotoxicity of chloroquine in rat liver cells: protective role of free radical scavengers. Cell Biol. Toxicol. (22):159-167.
- [Google Scholar]
- DNA Repair and Mutagenesis, Part 3 (second ed.). ASM Press; 2006.
- Mefloquine treatment for complicated Falciparum malaria young children 6–24 months of age in northern Ghana. Am. J. Trop. Med. Hyg.. 2007;76(2):224-231.
- [Google Scholar]
- Assessment of interactions of the antimalarial drugs chloroquine and mefloquine with NaNO2 and HgCl2 in rodents. Mutat. Res. (305):151-156.
- [Google Scholar]
- Efficacy and safety of mefloquine, artesunate, mefloquine–artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin. Infect. Dis.. 2011;50(9):1205-1213.
- [Google Scholar]
- Effects of amodiaquine, chloroquine, and mefloquine on human polymorpholonuclear neutrophil function in vitro. Antimicrob. Agents Chemother. (32):1124-1130.
- [Google Scholar]
- Observations on meiotic chromosomes of the male mouse as a test of the potential mutagenicity of chemicals in mammals. In: Venitt S., Parry J.M., eds. Chemical Mutagens: Principles and Methods of their Detection. Vol vol. 3. New York: Plenum Press; 1977. p. :21-56.
- [Google Scholar]
- Duration of spermatogenesis in the mouse and timing of stages of the cycle of somniferous epithelium. Am. J. Anat. (99):507-516.
- [Google Scholar]
- Mammalian in vivo and in vitro cytogenetic assays: a report of the US EPA’s Gene-Tox Program. Mutat. Res. (87):143-188.
- [Google Scholar]
- Classification and relationship of induced chromosomal structural change. J. Med. Genet.. 1975;12:103-122.
- [Google Scholar]
- Mutagenicity evaluation of the two antimalarial agents chloroquine and mefloquine, using a bacterial fluctuation test. Mutat. Res. (68):41-49.
- [Google Scholar]
- Different drugs arrest cells at a number of distinct stages in G2. Nature (254):245-247.
- [Google Scholar]
- Post-marketing surveillance of prophylactic mefloquine (Lariam) use in pregnancy. Am. J. Trop. Med. Hyg. (58):17-21.
- [Google Scholar]
- The induction of sperm shape abnormalities in mice and humans. In: Hollander A., Serres F.J., eds. Chemical Mutagens Principals and Methods for their Detection. Vol vol. 5. New York: Plenum Press; 1978. (Chapter 11)
- [Google Scholar]
- Collection and physical examination of the ejaculate. In: Hefez E., ed. Techniques of Human Andrology. Amsterdam: Biomedical Press; 1977. p. :47-172.
- [Google Scholar]







