Translate this page into:
Taphonomic signatures on the pearl oyster Pinctada from Arabian Gulf, Saudi Arabia
⁎Corresponding author.
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A total of 886 valves of the pearl oyster Pinctada were collected from 12 sites in Al-Uqair beach along the Saudi Arabian Gulf coast in January 2021 in order to document their taphonomic signatures. Thirteen ichnospecies of 5 ichnogenera were identified and illustrated. These traces were produced by clionid sponges (Entobia cretacea, E. ovula, E. geometrica, E. laquea, E. cateniformis, Entobia isp.), durophagous drillers (Oichnus paraboloides, O. ovalis, O. simplex, and Oichnus isp.), traces of vermetid gastropods (Renichnus isp.) polychaete annelids (Caulostrepsis isp.) and barnacle attachment scars (Anellusichnus circularis). The Pinctada shells act as hard substrate for colonization by serpulid worm, Spirorbis sp., bryozoans, barnacles, and other bivalves. Ichnogenus Oichnus was the most abundant (53.73%), followed by Entobia (44.58%), Anellusichnus (0.51%), Caulostrepsis (0.34%), and Renichnus (0.84%). The thin-shelled and smooth skeletons of Pinctada were preferable for the abundant durophagous drillers (Oichnus traces) and clionid sponges (Entobia traces) during the lifetime of the pinctadas, in contrast to endolithic bivalves (Gastrochaenolites borings) which need thicker seashells for the settlement. Occurrence of different encrusters and bioeroders on the internal surfaces of many pinctadas confirmed the postmortem origin of the signatures. Disarticulation, fragmentation, and abrasion among the collected pinctadas might be attributed to their mode of life as epifaunal byssate, filter-feeder bivalves in the shallow littoral and sublittoral zones of the continental shelf under strong currents conditions.
Keywords
Bioerosion
Encrustation
Pinctada
Arabian Gulf
Saudi Arabia
1 Introduction
The genus Pinctada is a bivalve that belongs to family Pteriidae, it is the pearl oyster of the Arabian Gulf and represents an important source of pearls before the advent of culture methods in Japan (Cunha et al., 2011). It is distributed through the Indo-Pacific and Caribbean regions and successfully spread throughout the Mediterranean Sea and the Adriatic Sea (Lodeiros et al., 2002; Aideed et al., 2014). Pinctada is represented in the Indo-Pacific region by many species (e.g., P. margaritifera, P. radiate, P. nigra, P. maxima, and P. fucata). Temperature, depth, salinity, substrate type, silt and mud supply, currents, and pollution are the most important environmental factors affecting the distribution of pearl oysters (Gervis and Sims, 1992). Shells of Pinctada in the Indo-Pacific region range from 50 to 95 mm in length, and are subequivalve, subquadrate, subcircular to squarish in shape. These shells have long, straight hinge and straight to concave posterior margin. Muscle scars are more or less regular ellipse with a broad, poorly demarcated, dorsal tail. Sculpture lamellose with radial rows of broad, appressed scales, with radial rows of sharp appressed spines (Crossland, 1957). Ecologically, Pinctada is an epifaunal suspended feeder, and fouling bivalve. In the subtidal zone, it lives attached by byssus either to rocks or to the root systems of marine seagrasses. Pinctada radiata is also attached to the surface of other macroinvertebrate species and fixed to artificial substrata. In the Mediterranean Sea, density of individuals varied between 0 and 62.67 individuals/m2. It prefers to be attached to vertical solid substrata (natural or artificial) within marine habitats with relatively high hydrodynamic conditions (Tlig-Zouari et al., 2009). P. margaritifera usually lives under big rocks as well as inhibiting crevices and corners of the hard bottoms and among the coral habitats away from aggregations of the sea urchins. It is commonly absent in coasts with sandy – muddy bottoms which are devoid of hard rocky formulations (Aideed et al., 2014).
The Arabian Gulf coastline has been subjected to intensive environmental studies (e.g. El-Sorogy and Youssef, 2015; Alharbi et al., 2017a, b, 2019; El-Sorogy et al., 2016, 2018, 2019; Al-Kahtany et al., 2015, 2018; Al-Hashim et al., 2021, 2022). These studies evaluated the sources of heavy metals in coastal sediments, seawaters, and marine skeletons. However, published articles concerned with taphonomic signatures in the Arabian Gulf are very scarce (El-Gendy et al., 2015; El-Sorogy et al., 2018, 2020). These articles dealt primarily with bioerosion and encrustation processes on skeletons of several taxonomical groups, such as bivalves, gastropods, and corals. The objectives of the present work are to: a) identify the bioeroders taxonomy which affected Pinctada shells collected from the Al-Uqair beach, Eastern Saudi Arabia, b) document the encrusters using Pinctada shells as hard substrate, and c) interpret the environmental parameters and the ecological significance of the identified ichnoassemblages.
2 Material and methods
Al-Uqair beach is located between longitudes 50°00′–50°20′ E and latitudes 25°37′ 25°58′–N (Fig. 1). Al-Uqair beach shores consist of three sediment types, namely, sandy, muddy or gravel-filled, and skeleton-dominated. Sandy shores have fine to very coarse sand grains. The sandy beach consists of clastic sediments with varying proportions of skeletal fragments Gravel-filled or muddy shores consist of silt- and clay-sized materials and are rich in pebble-sized gravels. Skeleton-dominated shores consist of large and small seashells of gastropods, bivalves, and foraminifers. The skeletal-dominated beach consists of piles of bivalve and gastropod shells Moreover, these shells were transported by tidal currents, Lastly, seagrass is frequently present on all of the shores, especially in the sandy and skeleton-dominated shores. In this study, a total of 886 Pinctada valves were collected from 12 sites along Al-Uqair beach in January 2021 (Fig. 1). Field samples have been documented and photographed using digital camera. Pinctada samples were collected in plastic boxes in order to protect them from crushing. The large distance between sites 3 and 4 represents a protected area, which explains the paucity of sampling in this area. The bioeroded and encrusted specimens were washed, examined and identified using a binocular microscope and differential distributions on the skeletal surfaces were evaluated.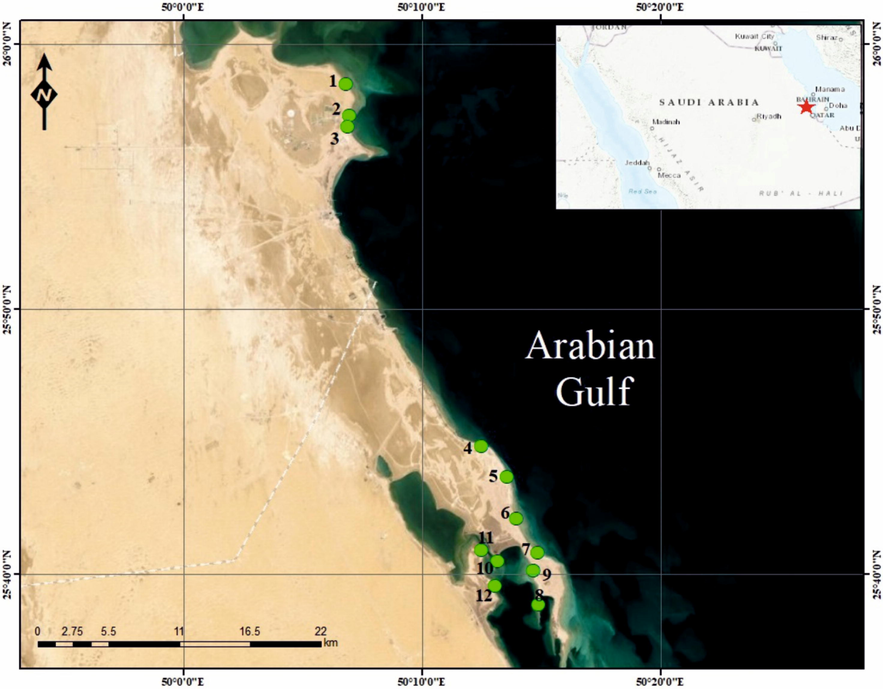
Location map of the study area and sample stations (modified after Al-Hashim et al., 2021).
3 Systematic ichnology
Thirteen ichnospecies belonging to 5 ichnogenera have been identified and illustrated from 371 pinctada specimens (Fig. 2). These traces were produced by clionid sponges, durophagous drillers, polychaete annelids, endolithic bivalves, vermetid gastropods and barnacle attachment scars. Table 1 presents the abundance of the recorded ichnospecies and encrusters in the studied sites.
Ichnofamily Oichnidae Wisshak, Knaust, and Bertling, 2019.
Ichnogenus Oichnus Bromley, 1981.
Oichnus paraboloides Bromley, 1981.
Fig. 3A-E, I, 4C

- Abundance of pinctadas in the study area. A. low abundance, site 1; B. high abundance, site 3.

- (A) Oichnus paraboloides (a), E. ovula (b), E. geometrica (c), Entobia isp.(d), and E. cretacea (e) on a right valve of Pinctada margaritifera, site 1; (B) O. paraboloides (black arrows) on a right valve of P. margaritifera, site 1; (C) O. paraboloides (a), Entobia isp.(b), with serpulid worm tubes (Se) on a right valve of P. margaritifera, site 6; (D,E) O. paraboloides on right valves of P. margaritifera, sites 1 and 3 respectively; (F) O.ovalis (a,b) with serpulid worm tubes (Se) on a right valve of P. margaritifera, site 6; (G) O. simplex on the adductor muscle scar of a right valve of P. radiata, site 6; (H) O. simplex on a left valve of P. margaritifera, site 1; (I) O. ovalis (a), O. paraboloides (b) on a right valve of P. margaritifera, site 6; (J) O. simplex with Spirorbis sp.(Sr) on an internal surface of a left valve of P. margaritifera, site 1; (K) O. ovalis (black arrow) on right valves of P. margaritifera, sites 6; (L) O. ovalis (a) and Entobia isp. (b) with Spirorbis sp.(Sr) on right valves of P. radiata, sites 6 and 1, respectively.
| Sample Number | Oichnus parabolides | O. ovalis | O. simplex | Oichnus isp. | Gastrochaenolites dijugus | Entobia cretacea | E. ovula | E. geometrica | Entobia isp. | E. laquea | E. cateniformis | Caulostrepsis isp. | Renichnusisp. | Anellusichnus circularis | Spirorbissp. | Worm tubes | Balanus | Bryozoz | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | 8 | 18 | 10 | 0 | 8 | 3 | 1 | 16 | 0 | 0 | 0 | 0 | 0 | 8 | 26 | 26 | 0 | 159 |
| 2 | 29 | 0 | 8 | 13 | 1 | 1 | 2 | 2 | 5 | 0 | 0 | 0 | 0 | 1 | 2 | 19 | 7 | 0 | 90 |
| 3 | 33 | 6 | 6 | 12 | 0 | 0 | 0 | 2 | 5 | 1 | 0 | 0 | 0 | 0 | 1 | 32 | 12 | 0 | 110 |
| 4 | 3 | 0 | 1 | 3 | 0 | 3 | 4 | 8 | 31 | 3 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 63 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 5 | 3 | 1 | 0 | 11 |
| 6 | 3 | 9 | 7 | 12 | 1 | 4 | 10 | 8 | 59 | 7 | 0 | 1 | 1 | 2 | 2 | 40 | 3 | 1 | 170 |
| 7 | 26 | 6 | 2 | 11 | 1 | 1 | 4 | 32 | 19 | 4 | 5 | 0 | 0 | 0 | 3 | 12 | 0 | 0 | 126 |
| 8 | 4 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 12 |
| 9 | 5 | 1 | 0 | 4 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 16 |
| 10 | 14 | 0 | 0 | 4 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 10 | 6 | 3 | 39 |
| 11 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 3 | 14 |
| 12 | 8 | 0 | 0 | 12 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 3 | 0 | 36 |
| Total | 160 | 31 | 42 | 84 | 3 | 18 | 27 | 54 | 144 | 15 | 5 | 2 | 1 | 3 | 23 | 167 | 60 | 7 | 846 |
Material and occurrence: 160 traces (91 on left valves and 69 on right valves): 35 traces (site 1), 29 (site 2), 33 (site 3), 3 (site 4), 3 (site 6), 26 (site 7), 4 (site 8), 5 (site 9), 14 (site 10), and 8 (site 12).
Description: Parabolic drill holes, perpendicular to the pinctadas surfaces, 1.4–2.8 mm in diameter with outer diameters exceeding the inner ones. Some shells showed incomplete drills. The parabolic drill holes account for 50.47% of the Oichnus traces and 27.12% of the total traces.
Remarks: O. paraboloides is previously recorded on the Paleocene ostracods from Argentina (Villegas-Martin et al., 2019), the modern and fossil Turritella from northern Gulf of California region (Walker, 1998), the Middle Eocene to Middle Miocene White Limestone Group, Jamaica (Blissett and Pickerill, 2004), the bivalve Mya arenaria from New Haven Harbor, USA. (Dietl and Kelley, 2006), Pleistocene – Holocene, Uruguay (Lorenzo and Verde, 2004.), Recent bivalves of the northern Red Sea Coast, Egypt (El-Sorogy, 2015), and the Quaternary bivalves and gastropods of the Arabian Gulf and Red Sea coasts, Saudi Arabia (El-Sorogy et al., 2018, 2020, 2021; Demircan et al., 2021).
Oichnus simplex Bromley, 1981.
Fig. 3G-H, J
Material and occurrence: 42 traces (22 on left valves and 20 on right valves): 18 traces (site 1), 8 (site 2), 6 (site 3), 1 (site 4), 7 (site 6), and 2 (site 7).
Description: Circular to subcircular drill holes, 1.5–2.3 mm in diameter, more or less perpendicular to the shell surfaces. Some drills end as a shallow depression. The circular to subcircular drill holes account for 13.25% of Oichnus traces and 7.12% of the total traces.
Remarks: O. simplex is recorded from the Paleocene ostracods from Argentina (Villegas-Martin et al., 2019), the Cenomanian oysters in France (Breton et al., 2017), the modern and fossil Turritella from the northern Gulf of California region (Walker, 1998), the Pliocene Roussillon Basin, France (Gibert et al., 2007), the Middle Eocene to Middle Miocene White Limestone Group, Jamaica (Blissett and Pickerill, 2004), Recent bivalves of the northern Red Sea Coast, Egypt (El-Sorogy, 2015), and the Quaternary bivalves and gastropods of the Arabia Gulf and Red Sea coasts, Saudi Arabia (El-Sorogy et al., 2018, 2020, 2021; Demircan et al., 2021).
Oichnus ovalis Bromley, 1993.
Fig. 3F, I, K, L, 4A-B, L
Material and occurrence: 31 traces (19 on left valves and 12 on right valves): 8 traces (site 1), 6 (site 3), 9 (site 6), 6 (site 7), 1 (site 8), and 1 (site 9).
Description: Ovoid drill holes. The holes pass right through the substrate as a penetration, tapering from a relatively large external aperture to a minute inner one. The ovoid drill holes make up to 9.78% of Oichnus traces and 5.25% of the total traces.
Remarks: O. ovalis is previously recorded from the Middle Eocene to Middle Miocene of Jamaica, the Eocene to Recent from the Mediterranean region and Paratethys, the Quaternary of Iceland, and the Quaternary of Saudi Arabia (Blissett and Pickerill, 2004; Ruggiero and Bitner, 2008; Pokorny and Stofik, 2017, El-Sorogy et al., 2021).
Oichnus isp.
Fig. 4G, 5I
Material and occurrence: 84 traces (48 on left valves and 36 on right valves): 10 traces (site 1), 13 (site 2), 12 (site 3), 3 (site 4), 12 (site 6), 11 (site 7), 3 (site 8), 4 (site 9), 4 (site 10), and 9 (site 12).
Description: Circular to slightly ovoid drill holes, perpendicular to the shell surfaces, 1.5–2.2 mm in width. Making up to 26.50% of Oichnus traces and 14.24% of the total traces.
Ichnofamily Entobiaidae Wisshak, Knaust and Bertling, 2019.
Ichnogenus Entobia Bronn, 1837
Entobia geometrica Bromley and D’Alessandro, 1984.
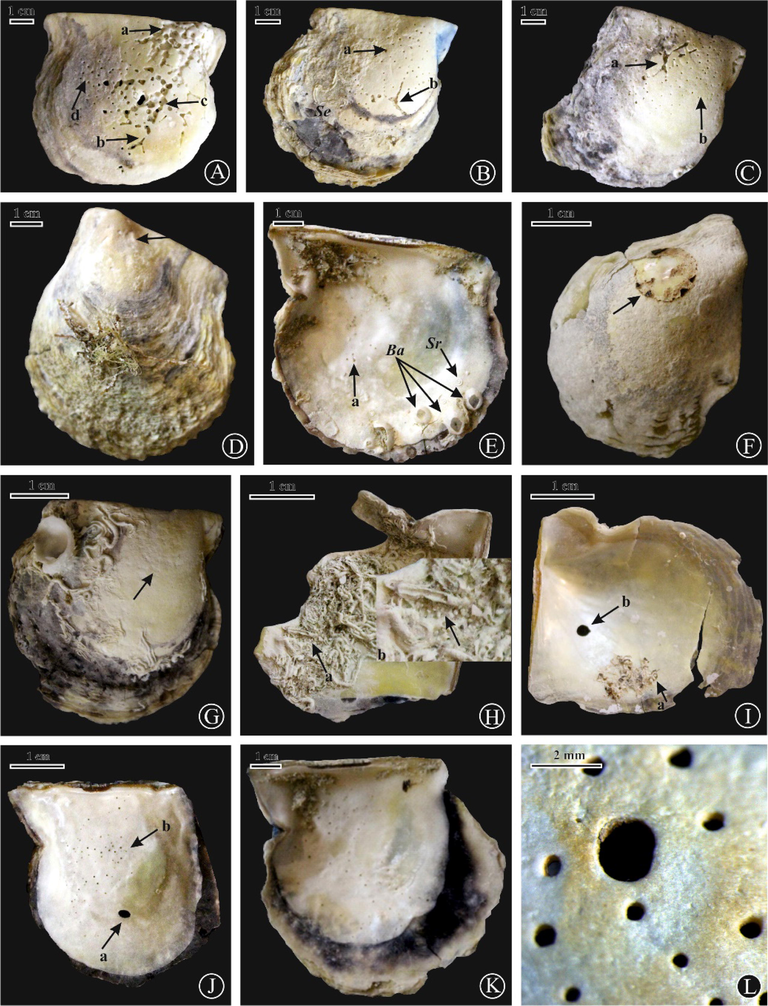
Fig. 3A, 4C-F, H, J, 5A
Material and occurrence: 54 traces (29 on right valves and 25 on left valve): 1 trace (site 1), 2 traces (site 2), 2 traces (site 3), 8 traces (site 4), 8 traces (site 6), 32 traces (site 7), and one trace (site 11).
Description: Networks of chambers with circular apertures, interconnected by irregularly distributed cylindrical galleries. Chambers with 2.3–3 mm in diameter for larger apertures, and 1–2 mm in diameter for the smaller ones. Making up to 20.53% of the recorded Entobia traces (n = 263) and 9.15% of the total traces (n = 590).
Remarks: E. geometrica is previously recorded from the late Eocene from Egypt, the Miocene in southern Spain, the late Miocene from Balearic Islands, the late Miocene of Turkey, the Miocene of NW Algeria, and the Quaternary of the Arabia Gulf and Red Sea coasts, Saudi Arabia. (Rashwan et al., 2019; Santos et al., 2011; Johnson et al., 2010; Demircan, 2012; Naimi et al., 2021; El-Sorogy et al., 2020, 2021; Demircan et al., 2021).
Entobia ovula Bromley and D’Alessandro, 1984.
Fig. 3A, 4H-J, 5A, 6B
Material and occurrence: 27 traces (15 on right valves and 12 on left valve): 3 traces (site 1), 2 traces (site 2), 4 traces (site 4), 10 traces (site 6), 4 traces (site 7), one trace (site 10), 2 traces (site 11), and one trace (site 12).
Description: Borings on the external surface of pinctadas present in four stages. Stage A of narrow and branched tunnels, about 1 mm in diameter. Stage B curved rows with elongate chambers, 1.8–3 mm in diameter. Stage C oval, closely spaced chambers, 2.8–3.3 mm in diameter. Stage D small spherical to ovoid chambers, with an average diameter of about 3.2 mm. Making 10.27% of the Entobia traces and 4.58% of all the studied traces.
Remarks: E. ovula is previously recorded from Upper Cretaceous of Egypt, the Middle Eocene to Middle Miocene of Jamaica, the Early Eocene from India, the Middle Miocene of Egypt, the Miocene of Spain, and the Quaternary of the Arabia Gulf and Red Sea coasts, Saudi Arabia (El-Hedeny, 2007; Blissett and Pickerill, 2004; Gurav and Kulkarni, 2018; El-Hedeny and El-Sabbagh, 2018, Santos et al., 2011; El-Sorogy et al., 2018, 2021; Demircan et al., 2021).
Entobia laquea Bromley and D’Alessandro, 1984.
Fig. 4G, J, K
Material and occurrence: 15 traces (9 on right valves and 6 on left valve): One trace (site 3), 3 traces (site 4), 7 traces (site 6), and 4 traces (site 7).
Description: Traces of oval, elongate to subangular chambers are 1.5–2.5 mm in diameter, making up to 5.70% of the Entobia traces and 2.54% of the total traces.
Remarks: E. laquea is previously recorded from the Middle Miocene of Egypt, and the Quaternary of the Red Sea coast, Saudi Arabia (El-Hedeny and El-Sabbagh, 2018; El-Sorogy et al., 2021).
Entobia cretacea Portlock, 1843
Fig. 3A, 5A-C
Material and occurrence: 18 traces (10 on left valves and 8 on right valve): 8 traces (site 1), one trace (site 2), 3 traces (site 4), 4 traces (site 6), one trace (site 7), and one trace (site 11).
Description: Most commonly in the form of networks of uniform multiple oval chambers, accounting for 6.86% of the Entobia traces and 3.05% of the total traces.
Remarks: E. cretacea is previously recorded from the Late Cretaceous (chalk) of England (Donovan and Fearnhead, 2015), the Late Cretaceous oysters of Egypt (El-Hedeny and El-Sabbagh, 2007), the Cenomanian oysters from France (Breton et al., 2017), and the Quaternary bivalves and gastropods of the Red Sea coasts, Saudi Arabia (El-Sorogy et al., 2021; Demircan et al., 2021).
Entobia cateniformis (Bromley and D’Alessandro, 1984)
Fig. 5H, I
Material and occurrence: 5 traces (3 on left valves and 2 on right valves from site 6).
Description: Chambers in long cylinders with T or L shaped at intersections. Apertures small, with well-developed apertural canals (Gurav and Kulkarni, 2018). Making 1.90% of the Entobia traces and 0.85% of the total ones.
Remarks: E. cateniformis is recorded from the Middle Eocene to Middle Miocene White Limestone Group, Jamaica (Blissett and Pickerill, 2004) and the Early Eocene Kachchh Basin, India (Gurav and Kulkarni, 2018).
Entobia isp.
Fig. 3A, C, L, 4C, D, G, I, K, L, 5A-C, E, K, L, 6F, J
Material and occurrence: 144 traces (76 on left valves and 68 on right valve): 16 traces (site 1), 5 traces (site 2), 5 traces (site 3), 31 traces (site 4), 2 traces (site 5), 59 traces (site 6), 19 traces (site 7), one trace (site 8), 3 traces (site 9), and 3 traces (site 11).
Description: Traces represented by networks of linear chambers, with circular apertures, 0.3–1.4 mm in diameter, make up to 54.75% of the recorded Entobia traces and 24.41% of the total traces.
Ichnofamily Osteichnidae Hopner and Bertling, 2017.
Ichnogenus Caulostrepsis Clarke, 1908.
Caulostrepsis isp.
Fig. 6F, G
Material and occurrence: 2 traces on right valves (sites 6 and 11).
Description: It is a pouch-shaped boring, and long galleries with a figure-of eight-shaped across-section. Making 0.34% of the total traces.
Remarks: Caulostrepsis is very common polychaete boring in Messinian Rhodolith beds in Algeria (Naimi et al., 2021). Caulostrepsis is produced by polychaetes at a water depth between 7 and 15 m (Wisshak et al., 2005).
Ichnofamily Renichnidae Knaust, 2012
Ichnogenus Renichnus Mayoral, 1987.
Renichnus isp
Material: 5 traces on a left valve of P. margaritifera, site 6.
Description: It is observed a half-moon or kidney-shaped depression. There is a maximum of 2 depressions per specimen. Depressions are separated by walls that are perpendicular to slightly oblique to the surface. Making 0.84% the total traces.
Remarks: Renichnus is the result of etching trace of vermetid gastropods (Mayoral, 1987; Uchman et al., 2017).
Ichnofamily Centrichnidae Wisshak, Knaust, and Bertling, 2019.
Ichnogenus Anellusichnus Santos, Mayoral and Muñiz, 2005.
Anellusichnus circularis
Fig. 5F, G, 6D
Material: 3 traces (two on left and one on right valves (sites 2 and 6).
Description: Anellusichnus circularis is surface traces of circular or subcircular to oval. It is revealed by a color difference in the substrate or, by the presence of a very shallow ring-shaped furrow pathway. Its outer furrow has very faint circular, oval or subpolygonal concentric striations (Santos et al., 2005). Making 0.51% of the total traces.
Remarks: It was identified by Lister (1687) for the first time as attachment scars from Balanus. It is observed from late Miocene to Holocene (Santos et al., 2005).
4 Discussion
The main physical factor for fragmentation of Pinctada shells in the study area is the active currents and tides. Fragmentation occurs as the presence of shell fragments. Approximately 22.45% of the collected specimens were still bivalved or articulated shells. Shell movement on coastal sediment and over each other by wave actions is often blamed for shell abrasion, mainly in the form of loose the outer, thin, horny coat of the periostracum and lack of luster on the inner nacre conchiolin and aragonitic layer (Nielsen, 2004). Skeletons of Pinctada act as lithified substrate for bioeroders of clionid sponge (Entobia traces), rare polychaete annelids (Caulostrepsis isp.), and carnivorous gastropods (Oichnus traces), which produce traces of dwelling and predation based on the fundamental behavioral patterns (Seilacher, 1964). Concerning the abundance of the identified ichnotaxa, it is noticed that 53.73% of the studied pinctadas were bioeroded by carnivorous gastropods, 44.58% by clionid sponge, and 1.69% by endolithic bivalves, polychaete annelids and barnacles.
Structurally, the pearl oyster shell consists of three parallel layers (Poirot, 1980). The outer, thin, horny coat of the periostracum, the middle prismatic layer of polygonal prisms of calcite, which lie perpendicular to the surface, while the inner nacre consists of layers of conchiolin, interspersed with thin sheets of aragonite. The nacre has high tensile strength and plasticity compared with other mollusc shells, making it highly resistant to crushing forces and therefore providing good defense against a number of predators (Currey, 1977). Traces of predatory gastropods on the Pinctada surfaces indicate production during the lifetime of these Pinctadas and likely have caused their death (Bromley, 1981). Moreover, presence of more than one Oichnus drill holes on some shells (Fig. 3B, C, F, I), is presumably the result of further attempts by the predatory gastropod to kill its prey (see Kowalewski et al., 2000; Hauser et al., 2008). The ended drills of Oichnus within the pinctadas substrate as a shallow to deep depression or short, subcylindrical pit may be attributed to the strength of the inner nacre making it highly resistant to drill by the naticid and muricid gastropods (Currey, 1977; Klompmaker et al., 2015). The thin-shelled and smooth skeletons of Pinctada were easier to drill by the abundant durophagous drillers (Oichnus traces) and clionid sponges (Entobia traces) during their lifetime. The traces of Entobia range from few scattered borings to entirely bioeroded surfaces (Fig. 4D, G, H, L). The external surfaces were intensively bioeroded than the internal ones, indicating bioerosion during their lifetime.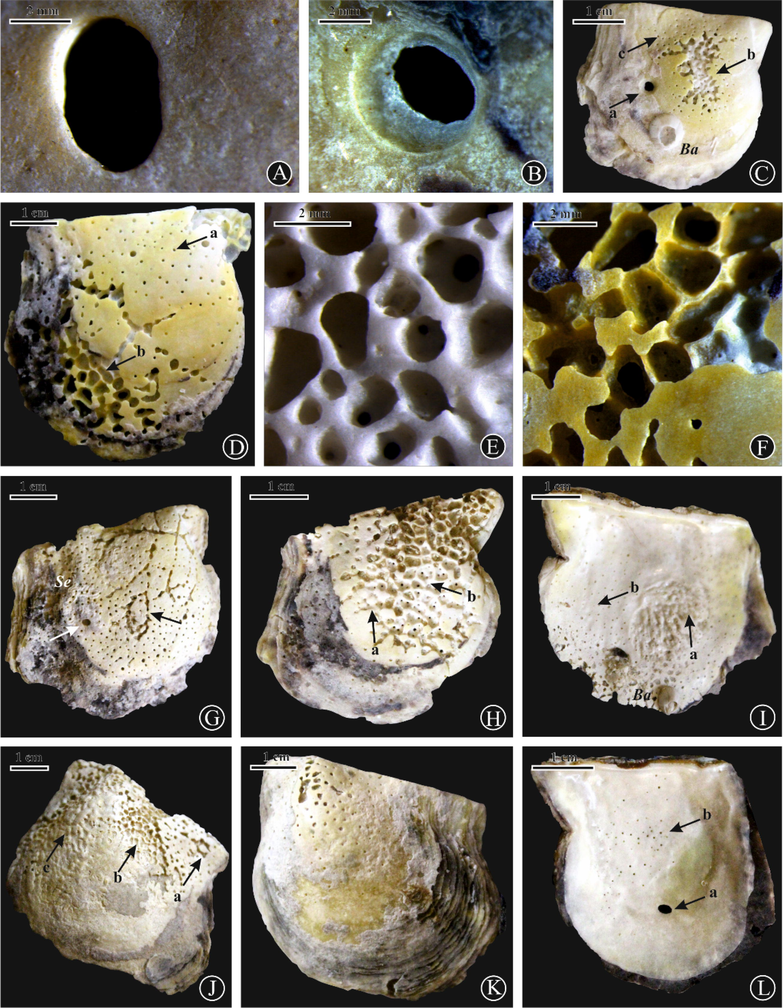
(A, B) Oichnus ovalis on left valves of P. radiata, sites 9 and 2, respectively; (C) O. paraboloides (a), Entobia geometrica (b), Entobia isp. (c) and Balanus (Ba) on a left valve of P. margaritifera, site 1; (D) Entobia isp.(a) and E. geometrica (b) on a left valve of P. margaritifera, site 7; (E, F) E. geometrica on internal and external surfaces of right valves of P. margaritifera, site 7; (G) Oichnus isp. (white arrow), E. laquea (black arrow), Entobia isp., with serpulid worm tubes on a right valve of P. margaritifera, site 6; (H) E. ovula (a) and E. geometrica (b) on a right valve of P. margaritifera, site 7; (I) E. ovula (a), Entobia isp.(b), and encrusted internal surface of a right valve by in part living balanids, site 6; (J) E. laquea (a), E. geometrica (b) and E. ovula (c) on a left valve of P. margaritifera, site 6; (K) E. laquea with Entobia isp. on a left valve, site 6; (L) Entobia isp., and O. ovalis on a right valve of P. radiata, site 6.
(A) E. ovula (a), Entobia cretacea (b), E. geometrica (c), and Entobia isp. (d) on a right valve of P. margaritifera, site 2; (B) Entobia isp. (a), Entobia cretacea (b) serpulid worm tubes, on a right valve of P. margaritifera, site 6; (C) Entobia cretacea (a), Entobia isp. (b) with serpulid worm tubes on a right valve of P. margaritifera, site 6; (D) Renichnus isp. (black arrow) on a left valve of P. margaritifera, site 6; (E) Entobia isp. (a), Balanus (Ba) with Spirorbis sp. (Sr) on a right valve of P. margaritifera, site 1; (F) Anellusichnus circularis, site 2; (G) Anellusichnus circularis with serpulid worm tubes and fixed Chama on the external surface of a right valve of P. margaritifera, site 6; (H) E. cateniformis on a right valve of P. margaritifera, site 7; (I) E. cateniformis (a) and Oichnus isp.(b) on a left valve of P. margaritifera, site 7; (J) Oichnus ovalis (a) and Entobia isp. (b) on an internal surface of right valve of P. radiata, site 7; (K) Entobia isp. on an internal surface of right valve of P. margaritifera, site 6; (L) Entobia isp. on an internal surface of right valve of P. radiata, site 6.
Temperature, depth, salinity, substrate type, mud and silt load, currents, and pollution are the environmental factors affecting distribution and abundance of pinctadas (Gervis and Sims, 1992). The temperature determines the rate of deposition of nacre on shells. Pinctadas prefer shallow water and, therefore, their growth rate is decreased in deeper water, probably due to lower temperatures and reduction of phytoplankton. Similar to other organisms inhabiting the intertidal zone, pinctadas prefer seawaters of normal salinity, but most can tolerate a wide range of salinities. Moreover, strong currents are required to bring in food and oxygen, to remove wastes and to distribute the planulae. In general, all the above mentioned environmental factors are appropriated at the study area but the difference in abundance of bioeroders among the studied sites (e.g. low in sites 4, 5, 8–10, 12, and high in sites 1–3, 6, 7) may be attributed to the abundance of pinctadas shells in each site, which is dependents consequently on the topography of the coast and the wind direction.
The skeletons of Pinctada act as hard substrates for colonization by encrusting invertebrates, including serpulid worms, Spirorbis sp., bryozoans, and barnacles. The serpulid worm tubes are the most common encrusters on Pinctada specimens (167 encrusters, 91 on left valves and 76 on right ones from all studied sites). Serpulids are represented by their tubes, circular to sub-circular in cross-section. They mostly grew as solitary individuals or dense coverings on the internal and external surfaces of Pinctada (Fig. 6A, E, G, I, J). Acorn barnacles were the second abundant (60 encrusters, 36 on left valves and 24 on right ones, from all sites except 4, 7, 8, and 11). Barnacles occur as solitary or as aggregates encrusting the external surface of Pinctada and some living ones encrust the internal surfaces (Fig. 4C, 6A, E, H). Spirorbis sp. is a small white sinistral coiled polychaete that lives attached to pinctadas (23 encrusters on 13 left and 10 right valves). The tubes have peripheral flanges for attachment to the substrate (Fig. 3J, 6C, I, K). The bryozoans are the least abundant, with 7 encrusters of Holloporella, Membranipora, Hippopodina, Celleporaria, and Watersipora spp. They encrusted the internal smooth surface and the external surface of left and right valves of Pinctada (Fig. 6K, L). Bryozoan colonies are sheet-like, a few millimeters to centimeters in size, on encrusted surfaces. The presence of different encrusters on the internal surfaces of many pinctadas has been confirmed a postmortem colonization, while those on the external surfaces indicated a colonization process mostly took place during the lifetime of the pinctadas.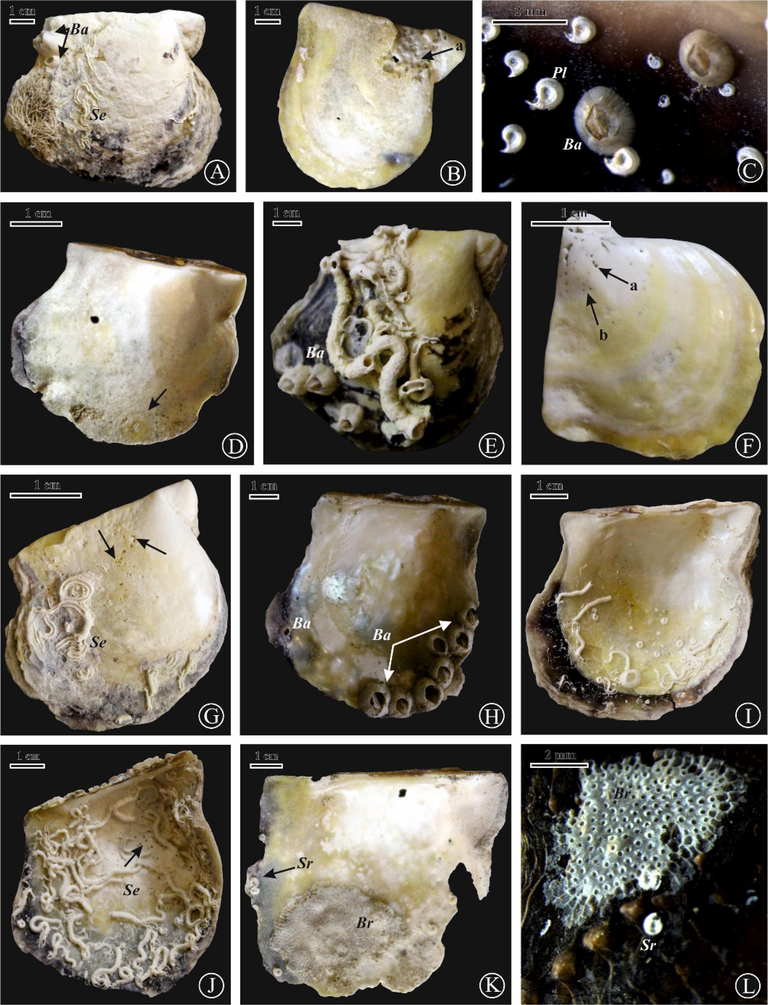
(A) Sponge spicules, Balanid (Ba) with serpulid worm tubes (Se) on a right valve of P. margaritifera, site 6; (B) E. ovula (a) on a right valve of P. radiata, site 2; (C) Balanus (Ba) with Spirorbis sp.(Sr) on an internal surface of a left valve of P. margaritifera, site 1; (D) Anellusichnus circularis (black arrow) on a left valve of P. margaritifera, site 6. (E) Worm tubes with balanids (Ba) internal surface of a right valve of P. margaritifera, site 12; (F) Caulostrepsis isp. (a) and Entobia isp. (b), site11; (G) Caulostrepsis isp. (black arrows) with serpulid worm tubes (Se), site 6; (H) Internal surface of a left valve encrusted by balanids (Ba), site 12; (I) Spirorbis sp.(Sr), serpulid worm tubes (Se) with Balanus (Ba) on an internal surface of a left valve, site 12; (J) Entobia isp. (black arrow), Spirorbis sp. (Sr), with serpulid worm tubes on an internal surface of a left valve, site 7; (K) Bryozoa (Br) and Spirorbis sp. (Sr) on internal surface of a fragmented left valve of P. margaritifera, site 10; (L) Bryozoa and Spirorbis sp. (Sr) on internal surfaces of left valves of P. margaritifera, sites 11, respectively.
5 Conclusions
-
Thirteen ichnospecies have been identified and illustrated on the pearl oyster Pinctada from the Al-Uqair coastline, eastern Saudi Arabia. These ichnospecies were produced by clionid sponges, polychaete annelids, durophagous, acrothoracican barnacle, and barnacle attachment scars. Moreover, the Pinctada shells acted as hard substrate for colonization by serpulid worm, Spirorbis sp., bryozoans, barnacles, and other bivalves.
-
The identified ichnospecies belong to 6 ichnogenera. Ichnogenus Oichnus was most abundant (53.73%, 4 ichnospecies), followed by Entobia (44.58%, 6 ichnospecies), Anellusichnus (0.51%, one ichnospecies), Caulostrepsis (0.34%, one ichnospecies), and Renichnus (0.84%, one ichnospecies). The thin-shelled and smooth skeletons of Pinctada were easier to drill by the abundant durophagous drillers and clionid sponges during their lifetime, in contrast to endolithic bivalves which need thicker seashells for the settlement.
-
Drill holes of Oichnus on the Pinctada surfaces indicated production during the lifetime of the pearl oyster. The shallow depressions or pits of Oichnus within the pinctadas substrate may be attributed to the high tensile strength and plasticity of the inner nacre. Traces of Entobia ranged from few scattered borings to entirely bioeroded surfaces. The external surfaces were intensively bioeroded than the internal ones, indicating bioerosion during their lifetime.
-
Disarticulation, fragmentation, and abrasion in the investigated pinctadas might be due to their mode of life as epifaunal byssate, in shallow strong currents. Moreover, the difference in abundance of bioeroders among the studied sites may be attributed to the abundance of pinctadas shells along the coastline, which depends on the topography of the coast and the wind direction.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project no (IFKSUOR3-455-1).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Existence, growth and reproduction of pearl oyster Pinctada margaritifera in Hadhramout coast/Gulf of Aden. Egypt. J. Aquat. Res.. 2014;40:473-481.
- [Google Scholar]
- Spatial distribution and metal contamination in the coastal sediments of Al-Khafji area, Arabian Gulf, Saudi Arabia. Environ. Monit. Assess.. 2017;189:634.
- [Google Scholar]
- Metal pollution in Al-Khobar seawater, Arabian Gulf, Saudi Arabia. Mar. Pollut. Bull.. 2017;119:407-415.
- [Google Scholar]
- Assessment of seawater pollution of the Al-Khafji coastal area, Arabian Gulf, Saudi Arabia. J. Afr. Earth Sc.. 2019;129:458-468.
- [Google Scholar]
- Contamination and ecological risk of heavy metals in Al-Uqair coastal sediments, Saudi Arabia. Mar. Pollut. Bull.. 2021;171:112748
- [Google Scholar]
- Environmental Assessment of Surface Seawater in Al-Uqair Coastline. Eastern Saudi Arabia. Water. 2022;14:3423.
- [Google Scholar]
- Geochemical and foraminiferal analyses of the bottom sediments of Dammam coast, Arabian Gulf, Saudi Arabia. Arab. J. Geosci.. 2015;8:11121-11133.
- [Google Scholar]
- Heavy metals in mangrove sediments of the central Arabian Gulf shoreline, Saudi Arabia. Arab. J. Geosci.. 2018;11:155.
- [Google Scholar]
- Blissett, D.J., Pickerill, R.K., 2004. Observations on bioerosional structures from the White Limestone Group of Jamaica. In: Donovan, S.K. (Ed.), The Mid-Cainozoic White Limestone Group of Jamaica. Cainozoic Research 3, 167–187.
- Parasitic gastropod bioerosion trace fossil on Cenomanian oysters from Le Mans, France and its ichnologic and taphonomic context. Acta Palaeontol. Pol.. 2017;62(1):45-57.
- [Google Scholar]
- Concepts in ichnotaxonomy illustrated by small round holes in shells. Acta Geologica Hispanica. 1981;16:55-64.
- [Google Scholar]
- Predation habits of octopus past and present and a new ichnospecies, Oichnusovalis. Bull. Geol. Soc. Den.. 1993;40:167-173.
- [Google Scholar]
- The ichnogenus Entobia from the Miocene, Pliocene and Pleistocene of southern Italy. Riv. Ital. Paleontol. Stratigr.. 1984;90:227-296.
- [Google Scholar]
- The beginnings of dependent life. New York State Museum Bulletin. 1908;121:146-196.
- [Google Scholar]
- The cultivation of the mother of pearl oyster in the Red Sea. Aust. J. Mar. Freshw. Res.. 1957;8:111-130.
- [Google Scholar]
- Evolutionary Patterns in Pearl Oysters of the Genus Pinctada (Bivalvia: Pteriidae) Mar. Biotechnol.. 2011;13:181-192.
- [Google Scholar]
- Currey, J.D., 1977. Mechanical properties of mother of pearl in tension. In: Proceedings of the Royal Society of London, 196B, 443-463.
- Determination of a Late Miocene rocky palaeoshore by bioerosion trace fossils from the Bozcaada Island, Çanakkale, Turkey. C.R. Palevol. 2012;11:331-344.
- [Google Scholar]
- Bioerosional structures from the Late Pleistocene coral reef, Red Sea coast, northwest Saudi Arabia. Turk. J. Earth Sci.. 2021;30:22-37.
- [Google Scholar]
- Can naticid gastropod predators be identified by the holes they drill? Ichnos. 2006;13(3):103-108.
- [Google Scholar]
- Exceptional fidelity of preservation in a reworked fossil, Chalk drift, South London, England. Geol. J.. 2015;50:104-106.
- [Google Scholar]
- Taphonomic Signatures on Some Intertidal Molluscan Shells from Tarut Bay (Arabian Gulf, Saudi Arabia) Pak. J. Zool.. 2015;47:125-132.
- [Google Scholar]
- Ichnology of the Upper Cretaceous (Cenomanian–Campanian) sequence of western Sinai, Egypt. Egyptian Journal of Paleontology. 2007;7:269-288.
- [Google Scholar]
- Entobia ichnofacies from the Middle Miocene carbonate succession of the northern Western Desert of Egypt. Ann. Soc. Geol. Pol.. 2018;88:1-19.
- [Google Scholar]
- Taphonomic processes of some intertidal gastropod and bivalve shells from northern Red Sea coast, Egypt. Pak. J. Zool.. 2015;47(5):1287-1296.
- [Google Scholar]
- Distribution of intertidal molluscs along Tarut Island coast, Arabian Gulf, Saudi Arabia. Pak. J. Zool.. 2016;48(3):611-623.
- [Google Scholar]
- Bioerosion structures in high-salinity marine environments: A case study from the Al–Khafji coastline, Saudi Arabia. Estuar. Coast. Shelf Sci.. 2018;204:264-272.
- [Google Scholar]
- Molluscan assemblage as pollution indicators in Al-Khobar coastal plain, Arabian Gulf, Saudi Arabia. J. Afr. Earth Sc.. 2019;158:103564
- [Google Scholar]
- Gastrochaenolites ichnofacies from intertidal seashells, Al-Khobar coastline, Saudi Arabia. J. Afr. Earth Sc.. 2020;171:103943
- [Google Scholar]
- Taphonomic signatures on modern molluscs and corals from Red Sea coast, southern Saudi Arabia. Palaeoworld. 2021;31:365-381.
- [Google Scholar]
- Assessment of heavy metal contamination in intertidal gastropod and bivalve shells from central Arabian Gulf coastline, Saudi Arabia. J. Afr. Earth Sc.. 2015;111:41-53.
- [Google Scholar]
- Integrated assessment of the Tarut Island coast, Arabian Gulf, Saudi Arabia. Environ. Earth Sci.. 2016;75:1336.
- [Google Scholar]
- The biology and culture of pearl oysters (Bivalvia: Pteriidae) ICLARM Stud. Rev.. 1992;21:49.
- [Google Scholar]
- Gibert, J.M., de, Domènech, R., Martinell, J., 2007. Bioerosion in shell beds from the Pliocene Roussillon Basin, France: Implications for the (macro) bioerosion ichnofacies model. Acta Palaeontologica Polonica, 52, 783–798.
- Natural casts of Early Eocene Entobia from the Kachchh Basin, India. Ichnos. 2018;25(4):261-268.
- [Google Scholar]
- Taphonomic signatures on modern Caribbean bivalve shells as indicators of environmental conditions (Belize, central America) PALAIOS. 2008;23:586-600.
- [Google Scholar]
- Ichnofacies and microbial build-ups on Late Miocene rocky shores from Menorca (Balearic Islands), Spain. Facies. 2010;57(2):255-265.
- [Google Scholar]
- The fossil record of drilling predation on barnacles. Palaeogeogr. Palaeoclimatol. Palaeoecol.. 2015;426:95-111.
- [Google Scholar]
- Drill holes in shells of Permian benthic invertebrates. J. Paleo.. 2000;74(3):532-543.
- [Google Scholar]
- Growth and survival of the pearl oyster Pinctada imbricata (Roding 1758) in suspended and bottom culture in the Golfo de Cariaco, Venezuela. Aquac. Int.. 2002;10:327-338.
- [Google Scholar]
- Estructuras de bioerosión en moluscos marinos de la Formación Villa Soriano (Pleistoceno Tardio- Holoceno) de Uruguay. Revista Brasileira de Paleontologia. 2004;7(3):319-328.
- [Google Scholar]
- Accion bioerosiva de Mollusca (Gastropoda, Bivalvia) en el Plioceno Inferior de la Cuenca del Bajo Gauadalquivir. Revista Brasileira de Paleontologia. 1987;2:49-58.
- [Google Scholar]
- Polychaete macrobioerosion in the Messinian (Late Miocene) Rhodolith beds of the western Mediterranean. Neues Jahrb. Geol. Palaontol. Abh.. 2021;301:307-315.
- [Google Scholar]
- Bioerosion in Ostrea lamellosa shells from the Messinian of the Tafna basin (NW Algeria) Carnets de Géologie. 2021;21:127-135.
- [Google Scholar]
- Taphonomy in the light of intrinsic shell properties and life habits: Marine bivalves from the Eemian of northern Russia. Paläontol. Z.. 2004;78(1):53-72.
- [Google Scholar]
- Elements de gemmologie. Paris: Institut National de Gemmologie; 1980.
- Evidence of bioerosive structures in Quaternary glaciomarine sediments from southwestern Iceland. Ichnos. 2017;24(3):204-221.
- [Google Scholar]
- Sclerobiont assemblages on the late Eocene bivalve Carolia placunoides: Composition, distribution and their paleoecological significance. Proceedings of the Geologists’ Association. 2019;130:612-623.
- [Google Scholar]
- Bioerosion on brachiopod shells — a Cenozoic perspective. Earth and Environmental Science, Transactions of the Royal Society of Edinburgh Earth Sciences. 2008;98:369-378.
- [Google Scholar]
- Bioerosion scars of acorn barnacles from the southwestern Iberian Peninsula, upper Neogene. Riv. Ital. Paleontol. Stratigr.. 2005;111:181-189.
- [Google Scholar]
- Bioerosive structures from Miocene marine mobile-substrate communities in southern Spain, and description of a new sponge boring. Palaeontology. 2011;54(3):535-545.
- [Google Scholar]
- Distribution, habitat and population densities of the invasive species Pinctada radiata (Molluca: Bivalvia) along the Northern and Eastern coasts of Tunisia. Cah. Biol. Mar.. 2009;50:131-142.
- [Google Scholar]
- Macroborings, their tracemakers and nestlers in clasts of a fan delta: the Savignone Conglomerate (Lower Oligocene), Northern Apennines, Italy. Neues Jahrb. Geol. Palaontol. Abh.. 2017;283(1):35-51. in English
- [Google Scholar]
- A small yet occasional meal: Predatory drill holes in Paleocene ostracods from Argentina and methods to infer predation intensity. Palaeontology 2019:1-26.
- [Google Scholar]
- Endobionts on modern and fossil Turritella from the northern Gulf of California region. Ichnos. 1998;6(1–2):99-115.
- [Google Scholar]
- Bioerosion alonga bathymetric gradient in a cold temperate setting (Kosterfjord, SW Sweden): an experimental study. Facies. 2005;51:93-117.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102870.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







