Translate this page into:
Tantalum doped TiO2 nanoparticles induced cytotoxicity and DNA damage through ROS generation in human neuroblastoma cells
⁎Corresponding author at: Department of Zoology, College of Science, King Saud University BOX 2455, Riyadh 11451, Saudi Arabia. salarifi@ksu.edu.sa (Saud Alarifi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The chemoradiotherapy technique is widely applied in the treatment of cancerous disease. Nowadays, nanoparticles are used in drug delivery and it is essential to confirm the nontoxicity of nano-carriers to improve radiotherapy. So nanoparticles have a great research interest to develop chemo radiotherapies. In this experiment, we aimed to examine the toxic nature of TiO2-Ta nanoparticles on human neuroblastoma (SH-SY5Y) cells. The cytotoxic nature of TiO2-Ta NPs was examined by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. We have evaluated the IC50 24 h value of TiO2-Ta nanoparticles as 68 µg/ml by MTT assay. The cytotoxicity of TiO2-Ta nanoparticles was enhanced as the exposure concentration of NPs was increased. Produced oxidative stress such as GSH and LPO were determined and GSH was reduced and LPO was increased as the concentration of TiO2-Ta NPs increased. The Comet test was performed to detect the genotoxic potential of TiO2-Ta nanoparticles. We have noted 34% of DNA damage in SH-SY5Y cells at 300 µg/ml NPs for 24 h. The current study suggests that TiO2-Ta NPs induced major ROS-related cytotoxicity and genotoxicity in SH-SY5Y cells. More detailed studies would be needed to confirm the safety issues related to increased application of TiO2-Ta NPs in chemoradiotherapy. Thus this work presents a new type of mesoporous nano-carrier particularly useful for the delivery of safe and effective chemoradiotherapy.
Keywords
TiO2-Ta nanoparticles
Apoptosis
Oxidative stress
SH-SY5Y cells
1 Introduction
Rich sources of rare earth metals are found in Asian countries. Tantalum metal is found in the Ghurayyah mine, Saudi Arabia. Tantalum is a rare transition metal that is very resistant to corrosion and has major applications in electronic equipment. Nowadays various types of nanomaterials are manufactured using different nanotechnological techniques. Tantalum metal nanomaterials are applied as base materials for dental care and dental implant. The human neuroblastoma (SH-SY5Y) cells are the most frequent and degenerative type of neuronal cell line and it used as the model cell for neurodegenerative disorders (Slanzi et al., 2020). Here we investigate the toxicity of Ta2O5 NPs on SH-SY5Y cells. Bu et al. (2014) reported that compound should be considered as a model agent if it provides both pre-exposure and exposure imaging, permitting the discovery of the location of the tumors before and during the treatment to minimize the damage of the adjacent healthy tissues and improve diagnostic accuracy and therapeutic effectiveness. Presently, rare earth metals are applied in animal husbandry, agriculture, health industry, and (Blinova et al., 2020). Due to the extensive application of rare earth metals in various areas, it gets into the environment, animals, and trophic levels, the human body. Chen et al. (2015) had reported that chronic bioaccumulation of heavy metals or rare earth metals in the human body caused teratogenic and reproductive toxicity. Ta2O5NPs with a strong X-ray lessening, represent an ideal candidate for the assembly of multifunctional imaging agents with which to combine CT and fluorescence imaging. Rare earth metals decreased superoxide dismutase, glutathione, and glutathione peroxidase and increased malondialdehyde levels and apoptotic activity during spermatogenesis. Reactive oxygen species (ROS) generated by mitochondria participate in stress signaling in normal cells but also contribute to the initiation of nuclear or mitochondrial DNA mutations that promote neoplastic transformation (Sabharwal and Schumacker, 2014). Generation of excess ROS make dysfunction of mitochondria (Sabharwal and Schumacker, 2014; Schumacker et al., 2014). Production of ROS and apoptotic effect are the underlying mechanisms of toxicity of NPs. To my knowledge, few studies confirmed the harmful effects of TiO2-Ta on human cell lines. In this study, we determined the role of sensitivity of ROS in the toxicity of TiO2-Ta on SH-SY5Y cells.
2 Materials and methods
2.1 Chemical and reagents
TiO2-Ta nanoparticles (Stock#: US1001TTa, 10–30 nm, titania-doped tantalum film with a cation ratio = 0.53 as-deposited) were purchased from US Research Inc. Houston TX USA. MTT, H2-DCFH-DA), dimethyl sulfoxide (DMSO), were bought from Sigma-Aldrich (St. Louis, Missouri, United States). Dulbecco's Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco, USA.
2.2 Characterization of TiO2-Ta NPs
Physical characterization of TiO2-Ta NPs was done transmission electron microscope (TEM) (JEOL Inc., Tokyo, Japan) operated at 200 kV and average hydrodynamic size and zeta potential of TiO2-Ta NPs in DDW was observed by dynamic light scattering (DLS) (Nano-Zeta Sizer-HT, Malvern, UK) as reported by Alarifi et al. (2015). We have used 80 μg/ ml TiO2-Ta NPs suspension for DLS examination.
2.3 Cell culture and TiO2-Ta NPs exposure
Human neuroblastoma (SH-SY5Y) cells were bought from American Type Culture Collection (ATCC), USA. SH-SY5Y cells were sub-cultured in DMEM with 10% FBS and 10,000 U/ml antibiotics at 5% CO2 incubator at 37 °C.
SH-SY5Y cells were sub-cultured for 24 h before to treatment of TiO2-Ta NPs. The stock solution of TiO2-Ta NPs was made in DDW at the rate of 1 mg NPs /ml DDW and diluted according to the experimental dosage (0–80 µg/ml).
2.4 Cell viability test
After exposure of TiO2-Ta NPs, the cell viability of SH-SY5Y cells was determined by MTT assay by Alarifi et al. (2017) methods.
2.5 Intracellular reactive oxygen species (ROS) generation
After exposure of TiO2-Ta NPs, the generation of ROS in SH-SY5Y cells was measured as described by Alzahrani et al. (2019) methods.
2.6 Oxidative stress
SH-SY5Y cells were exposed to with TiO2-Ta NPs for 24 hr. The it was scrapped by scrapper and lysed by ultra-sonicator (Q700 Qsonica Sonicator Newtown, CT 06470). The cell lysate was used for to investigate oxidative stress enzymes such reduced glutathione (GSH), and lipid peroxidation (LPO) according to the instruction of kits (Cayman Chemical Kit).
2.7 Comet assay
Single strands breakage in SH-SY5Y cells were measured using Comet assay as reported by Ali et al. (2008).
2.8 Western blotting
For Western blotting, 20 mg of protein was applied to the lanes of 4% to 12% Bis-Tris Gels (Life Technologies), then blotted onto Immobilon-P membranes (Millipore, Bedford, MA, USA), and incubated with the relevant primary antibody. Appropriate species-specific conjugated secondary antibodies were commercially obtained (GE Healthcare, Tokyo, Japan). Proteins were detected using the ECL prime Kit or the ECL Kit (GE Healthcare) with an Image Quant LAS 4000 system (GE Healthcare). All protein expression levels were normalized to the levels of β-actin protein expression in each band.
2.9 Statistical analysis
The data were analyzed by SPSS 26.0 software (IBM) and expressed as mean ± standard error (SE). Statically differences between the control group to exposed groups were determined by a one-way ANOVA test with the least significant difference test. *p < 0.05,
** p < 0.01 vs. control were considered statistically significant and highly significant, respectively.
3 Results
3.1 Properties of TiO2-Ta NPs
TiO2-Ta NPs was prepared by doping titania and tantala film with a cation ratio = 0.53 and size have examined TEM operated at 110 V. TiO2-Ta NPs were non-uniform sheet-like structure in shape and showing aggregation pattern as determined by TEM. Fig. 1 (A, B) showed the microphotographs of TiO2-Ta NPs nanoparticle by TEM. After the suspension of TiO2-Ta NPs in Mille Q water and DMEM, their size was determined using a zeta sizer, and size and zeta potential were 180 ± 2.0 nm and ∼9.4 ± 4.6 mV and 138 ± 6.0 nm and ∼10.7 ± 3.3 mV.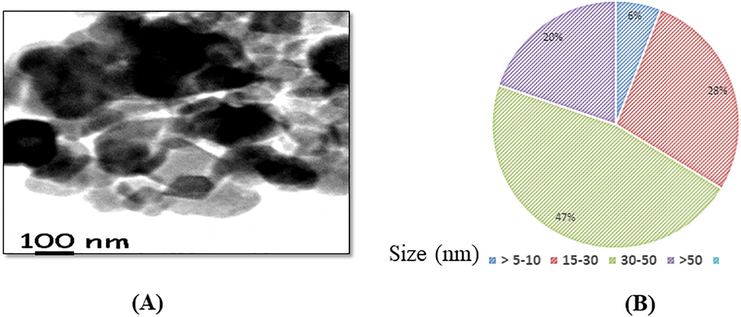
(A) Photomicrograph of T302-Ter nanoparticles by using TEMP (B) Analysis of size distribution of 1102. Ta nanoparticles by TEM.
3.2 Cell viability
The cell viability of SH-SY5Y cells after exposure to TiO2-Ta NPs (0, 5, 10, 20,40, 60 and 80 µg/ml) for 24 hr. Data of cytotoxicity were presented in Fig. 2. The cell viability due to TiO2-Ta NPs (0, 5, 10, 20,40, 60 and 80 µg/ml) was observed 100%, 97.80%, 90%, 83%, 88%, 64% and 38 % for 24 h in SH-SY5Y cells (Fig. 2). TiO2-Ta NPs induced cytotoxicity in cells in a concentration-dependent manner. The highest toxicity of TiO2-Ta NPs was seen in cells at 80 µg/ml (Fig. 2).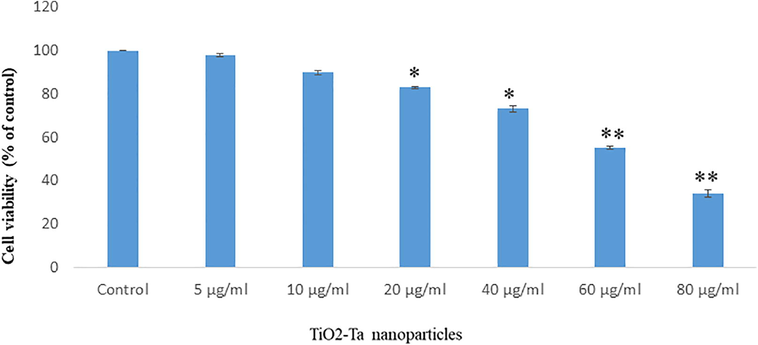
Reduction in viability of SH-SY5Y cells after exposure to TiO2-Ter nanoparticles for 24 h Each value represents the mean 1-.SE of three experassents n = 3. *p < 0 05,*p < 0 01 VS control.
3.3 Oxidative stress
The production of intracellular reactive oxygen species (ROS) was measured by DFDFA fluoro-chrome and DCFDA was oxidized into DCF fluorescence. DCF fluorescence was emit green fluorescence. The green fluorescence intensity was observed more in at 80 µg/ml NPs in cells (Fig. 3 A). DCF fluorescence (green color) intensity was irregularly increasing and decreasing (Fig. 3A, B).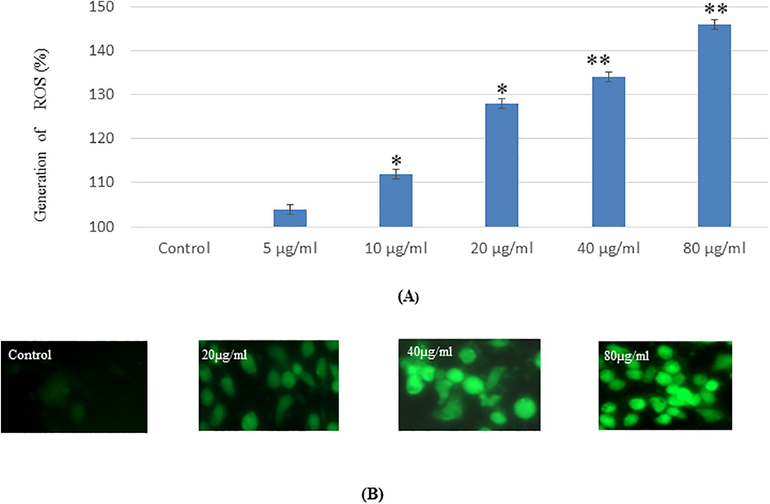
(A). Percent generation of intracellular ROS in SH-SY5′fcells due to Ti02-Ta NPs for 24 h exposure_ as evaluated by DCFDA fluorescence stain assay (13). DCF-fluorescence intensity as marker of ROS generation in cells, Each value represents the mean = SE of three experiments n= *p < 0.05, *p < 0.01 vs. control.
GSH and LPO were determined and statistically analyzed with control cells. Glutathione and lipid peroxide was measured and glutathione level was declined and lipid peroxide was increasing as exposure concentration of NPs was increased (Fig. 4A, B).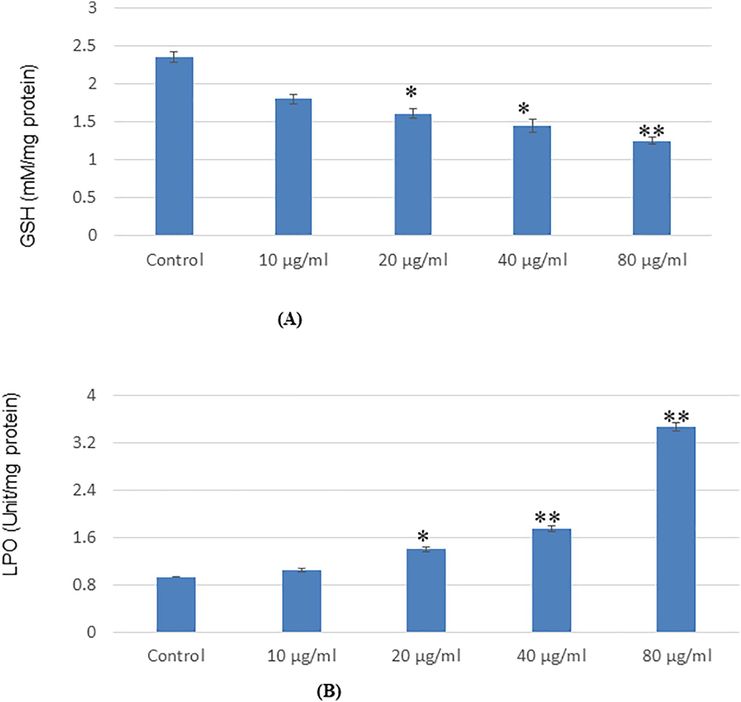
Oxidative stress bromarkers in SH-SYSY cells after exposure to Ti02-Ta nanopartieles for 24 13 (A). Levels of GSH 03) LPO in cells. Each value represents the mean = SE of three experiments. *p < 0 05 and *p < 0.01 vs control.
3.4 Genotoxicity
The declination of single-strand breakage in SH-SY5Y cells due to NPs was measured by comet techniques (Fig. 5). During electrophoresis, the damaged DNA migrates away from the nucleus toward the anode. The quantity of migrated DNA is a measure of the extent of DNA damage in the form of % tail DNA and olive tail moment. DNA damage has occurred in a dose-dependent manner in cells. The highest DNA fragmentation was seen at 80 µg/ml NPs in cells (Fig. 5 A, B). Compared with the control group, DNA damage of NPs was increased significantly (p < 0.05, p < 0.01) in SH-SY5Y cells (Fig. 5 A-B).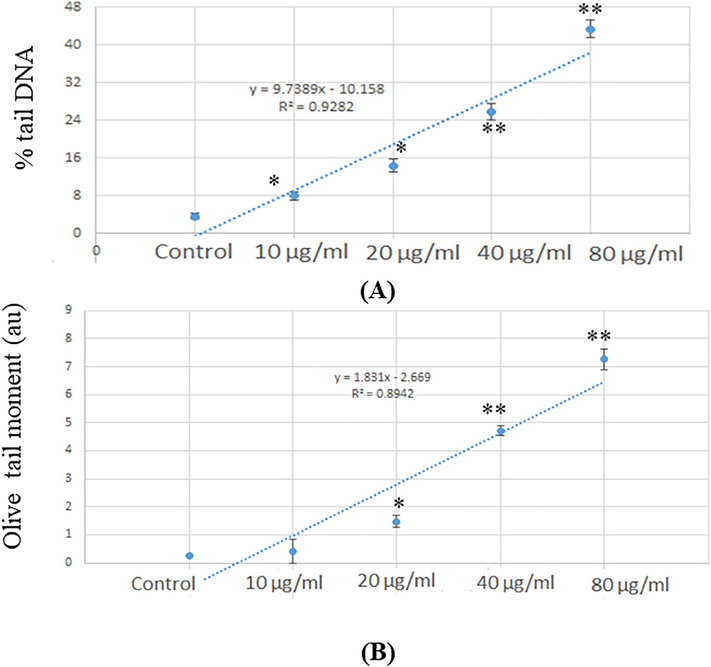
(A) Percent of tail DNA damage in SII-SV5V cells for 24 hr after exposure of NPs 03) Olive tail moment as in SH-SY5Y cells for 24 la after exposure of Tr02-Ter:tiPs Each value represents the mean --L-SE of three experiments *p < 0.05 and *p < 0.01 vs. control.
3.5 Immunoblotting
To confirm the apoptosis due to the effects of NPs in SH-SY5Y cells. We have determined the expression of the apoptotic protein in cells after exposure to NPs. In cells exposed to NPs, there is dose-dependent downregulation of Bax (Fig. 6A, B). Also, NPs exposure increases the upregulation of bcl2 according to the dose-dependent manner (Fig. 6A, B).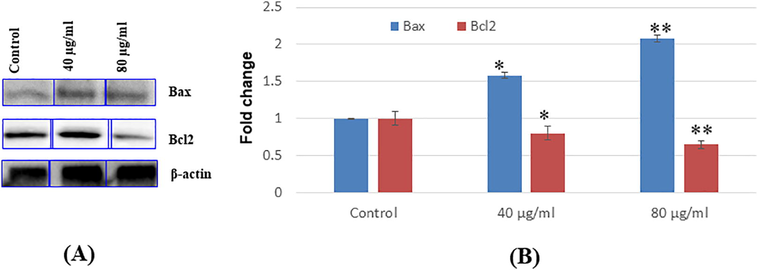
Immuno blotting of proteins involved in apoptosis (A) Bax, and 2c12. expression levels cells (B) Relative quantification of protein expression levels. 0-actin was used as internal control to normalize the data, Data represent mean ± SEM of three experiments *p < 0 05 and **p < 0 01 vs. control.
4 Discussion
Nanomaterials, including tantalum and TiO2 nanoparticles, have become an essential part of human daily life. So, researchers keen to investigate the underlying mechanism of toxicity of NPs (Alarifi et al., 2017; Musial et al., 2020). In the present experiment that when SH-SY5Y cells were exposed with TiO2-Ta NPs for 24 h, we observed significant cytotoxicity and apoptotic effects on neuroblastoma cells. In addition, a significant decrease in glutathione and an increase in LPO were observed. Moreover, a significant increase in DNA fragmentation, and generation of intracellular ROS, was investigated. Consistent with our results, other studies reported that exposure to TiO2 nanoparticles resulted in toxicity. In this experiment, we have observed the toxic potential of NPs on cells. TiO2-Ta NPs was characterized using TEM and Zeta-sizers to confirm the shape and size of nanoparticles. The mean size of TiO2-Ta NPs was found 45 ± 1.5 nm by TEM with a rectangular structure. We have analyzed the production of ROS in SH-SY5Y cells due to TiO2-Ta NPs and production of ROS was increased according concentration. So we assume that ROS has played a specific role in cytotoxicity and apoptosis in SH-SY5Y cells due to TiO2-Ta NPs exposure. Bortner and Cidlowski (2014) has reported that ions trigger apoptosis in cancerous cells. TiO2-Ta NPs has induced cytotoxicity in SH-SY5Y cells at all doses but the highest toxicity exerted in cells at 80 µg/ml. Our results corroborated with finding of finding of Sambale et al. (2015) for toxicity of silver nanoparticles in mammalian cell lines and Zhang et al. (2020) for cytotoxicity and inflammatory to Tantalum Nanoparticles in THP-1-Derived Macrophages. CeO2 and TiO2 nanoparticles induced phytotoxicity and genotoxicity in Hordeum vulgare L. (Mattiello et al., 2015). In this experiment, we have investigated the generation of intracellular reactive oxygen species, and its generation was increased in a dose-dependent manner and subsequently, production of ROS was higher in the cell at higher concentration. The effect of ROS may due to mitochondrial-mediated pathway as we found in our experiment. Lu et al. (2020) reported that in the extrinsic pathway of apoptosis, still not approved ROS as an activator due to NPs. Intrinsic pathways occurred in apoptosis due to nanoparticles (Mkandawire et al., 2015). NPs produced free radicals which degenerate the cells through the generation of ROS. We have confirmed the apoptotic response of TiO2-Ta NPs using the immunoblotting technique. Apoptotic and anti-apoptotic proteins were induced according to the concentration of NPs. Based on the above finding, we confirmed that TiO2-Ta NPs induced toxicity due to concentration upon cells. TiO2-Ta NPs showed cytotoxicity, apoptosis, most likely due to its size effect and induction of ROS and oxidative stress. We have observed less significant differences for single-stranded DNA breaks between the control and lower dose of NPs. Our findings may indicate that TiO2-Ta NPs in SH-SY5Y cells contributes to oxidative stress and DNA damage. However, the increase in DNA breaks in SH-SY5Y cells with TiO2-Ta NPs may also reveal a slow DNA repair capacity. Based on our finding we observed SH-SY5Y cells are more sensitive to TiO2-Ta NPs and induced toxicity. In the future, we will investigate the toxicity of TiO2-Ta NPs in vivo model.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RG- 1441-180).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Oxidative Stress-Induced DNA Damage by Manganese Dioxide Nanoparticles in Human Neuronal Cells. Biomed Res Int.. 2017;2017:1-10.
- [Google Scholar]
- Nanoalumina induces apoptosis by impairing antioxidant enzyme systems in human hepato carcinoma cells. Int. J. Nanomed.. 2015;10(1):3751-3760.
- [Google Scholar]
- Genotoxicity assessment of acute exposure of chlorpyrifos to freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Chemosphere. 2008;71(10):1823-1831.
- [Google Scholar]
- Alzahrani, F., Khadijah, M., Ali, D., Alarifi, S., 2019. Apoptotic and DNA-damaging effects of yttria- stabilized zirconia nanoparticles on human skin epithelial cells. Int. J. Nanomed. 14, 7003-7016.
- Blinova, I., Muna, M., Heinlaan, M., Lukjanova, A., Kahru, A., 2020. Potential Hazard of Lanthanides and Lanthanide-Based Nanoparticles to Aquatic Ecosystems: Data Gaps, Challenges and Future Research Needs Derived from Bibliometric Analysis. Nanomaterials
- Fluorescent imaging of cancerous tissues for targeted surgery. Adv Drug Deliv Rev.. 2014;76:21-38.
- [Google Scholar]
- Ion channels and apoptosis in cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci.. 2014;369(1638):20130104.
- [CrossRef] [Google Scholar]
- Rare earths exposure and male infertility: the injury mechanism study of rare earths on male mice and human sperm. Environ. Sci. Pollut. Res.. 2015;22(3):2076-2086.
- [Google Scholar]
- Zhang L, El-Mustapha H, Hannes B, Ralf B, Shyam P, Julian M. R, Xian L, Max J, Christof B, Dieter C. W, Koroush K, Frank A. S. Investigation of Cytotoxicity, Oxidative Stress, and Inflammatory Responses of Tantalum Nanoparticles in THP-1-Derived Macrophages. Mediators of Inflammation, vol. 2020, Article ID 3824593, 14 pages, 2020
- Evidence of Phytotoxicity and Genotoxicity in Hordeum vulgare L. Exposed to CeO2 and TiO2 Nanoparticles Front. Plant Sci. 2015
- [CrossRef] [Google Scholar]
- Mkandawire, M.M. et al. Induction of apoptosis in human cancer cells by targeting mitochondria with gold nanoparticles. Nanoscale 7, 10634-10640.
- Titanium Dioxide Nanoparticles in Food and Personal Care Products—What Do We Know about Their Safety? Nanomaterials. 2020;10:1110.
- [CrossRef] [Google Scholar]
- Investigations of the toxic effect of silver nanoparticles on mammalian cell lines. Journal of Nanomaterials. 2015;2015:1-9.
- [Google Scholar]
- Mitochondria in lung biology and pathology: more than just a powerhouse. Am J Physiol Lung Cell Mol Physiol. 2014;306(11):L962-L974.
- [Google Scholar]
- Simran S. Sabharwal, Paul T. Schumacker, 2014. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 14(11), 709–721.
- Cytotoxic lanthanum oxide nanoparticles sensitize glioblastoma cells to radiation therapy and temozolomide: an in vitro rationale for translational studies. Sci Rep.. 2020;10:18156.
- [Google Scholar]







