Tannin acyl-hydrolase production by Bacillus subtilis KMS2-2: Purification, characterization, and cytotoxicity studies
⁎Corresponding authors at: School of Agro-Industry, Faculty of Agro-Industry, Chiang Mai University, Mae‐Hia, Chiang Mai 50100, Thailand. biogovindarajan@gmail.com (Rasiravathanahalli Kaveriyappan Govindarajan), chartchai.k@cmu.ac.th (Chartchai Khanongnuch)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Objective
Bacterial mediated tannin acyl-hydrolase production was investigated Bacillus subtilis KMS2-2 in this study. Tannin acyl-hydrolase used as catalysts in the production of glucose and gallic acid. Also, it has potential application in beverage and food processing.
Methods
This bacterial TAH enzyme was purified by a single methods approach consisting of size exclusion chromatography (sephadex G-100) and characterization of purified enzyme using different methods followed.
Result
The molecular mass of purified TAH was determined as (∼43 kDa) on 12% (PAGE) and it was confirmed by (MALDI-TOF/MS). Characterization of purified tannin acyl-hydrolase by (HPLC) confirmed that gallic acid was formed as a by-product during hydrolysis of tannic acid as a substrate and (FT-IR) spectroscopy showed the functional groups such as O–H, C-O and C–C. The purified tannin acyl-hydrolase retained the enzyme activity upto 80% under the conditions at 50 °C and pH 6.0 after 60 min incubation. The TAH comprises a typical secondary structure at pH 6.0 and contains α-(16.8%), β-(45.1%), and Turns (38.4%). Also, thermodynamics parameters and different additives like inhibitors, chelators, and metal ions were studied on the tannin acyl hydrolase. Further, the purified TAH enzyme has no cytotoxicity on the vero cell line as well as rat model study.
Conclusion
From this experiment, the B. subtilis KMS2-2 would provide a possible resource for more efficient tannin-acyl hydrolase production and can be used for various industrial purposes, in particular food, feed, pharmaceutical industry.
Keywords
Bacillus subtilis
Bacterial Tannin acyl-hydrolase (TAH)
Sephadex G-100
SDS-PAGE
In vivo model
1 Introduction
The tannin acyl-hydrolase (TAH) belonging to the superfamily of esterase catalyzes hydrolysis of galloyl ester molecules of tannins and yields the gallic acid as a major source. Tannins are naturally occurring in various plant materials as water-soluble polyphenols with varying molecular weight. Moderate levels of tannins can be beneficial for animal and other ruminants' guts and adverse feeding properties in ruminant insect mid-gut microbes (Govindarajan et al., 2019; Aharwar and Parihar, 2021). The hydrolysis of ester and depside bonds in hydrolysable tannin by releasing as gallic acid and glucose (Govindarajan et al., 2016a, 2016b; Xu et al., 2019). This enzyme is used in different applications such as drugs, food, beer, wine, coffee, aromatized soft drinks and food drinks. The most important hydrolytic products of enzymes are tannic acid and gallic acid as the major substrates that are used in skin-care, artificial foods. And potent antioxidant activity this enzyme can use illustrative dye agents to decrease the haze and sweetness in case of liquid drinking food and commercial beverages juices and also the treatment of contaminated wastewater and degradation of tannery effluent (Tapingkae et al., 2018).
TAH extracted from various sources (animals, plants and fungi,bacteria) has been utilized to a great extent, as a valuable source for different sectors. Potential TAH enzyme producers have been identified from environmental samples such as soil, water and ruminant gut microbes (Thiyonila et al., 2020; Muslim et al., 2017). Various microbes have been identified as good TAH producers, in particular, fungal species such as Penicillum sp, Aspergillus sp, Aspergillus ruber, A. versicolor and Aspergillus tamarii showed good TAH producer whereas, many bacterial strains such as., Bacillus sp. PAB2 and Bacillus licheniformis KBR6, Lactobacillus paraplantaraum, Enterobacter cloacae for their possible significance towards TAH enzyme production (Hidayathulla et al., 2018; Govindarajan et al., 2016a, 2016b). Hence, the microbial TAH enzyme is considered due to its up-stream and downstream activity. An extreme stability observed in enzymes produced by microorganisms when grown under extreme temperature, pH, substrate-level and specific metal ions. TAH enzyme production through various microbes has been performed by different methods such as submerged, solid-state conditions and alternatives such as liquid fermented cultures able to carry out in a different way specifically mentioned (Kumara et al., 2015; Sharma et al., 2017). Therefore, submerged fermentation by cultures has been recommended to control the progress and very easily revitalization of extracellular other bacterial enzymes (Belmares et al., 2004).
Microbes which grow in higher temperature conditions can produce an enzyme capable of temperature resistant (Kanpiengjai et al., 2020). Large-scale fermentation is an advantage because the control mechanism in this process is more comfortable to modulate for advanced aspects (Jana et al., 2013; Lekshmi et al., 2020). Tannin are the most important molecule in the medical fields owing to their anti-tumor, antioxidant properties; it is also a requirement for learning and recognizing the structural and functional TAH associations (Kanpiengjai et al., 2019). The cost of infinite applications, fermentation parameters have to be configured to acquire maximum and environmentally viable yield for gut microbial TAH enzyme (Beniwal et al., 2010). Thus, an attempt was made to study the enzyme production, purification, and characterization of TAH produced by Bacillus subtilis KMS2-2 in this study. In addition, the biocatalytic property of tannin acyl hydrolase was also investigated.
2 Material and methods
2.1 Bacterial strain, & TAH enzyme production
The chemical and reagents used in this experiment were analytical grades. The enzyme production capability of Bacillus subtilis KMS2-2 (GenBank ID: MN065454.1) was investigated and this strain was isolated in the earlier study of Mathivanan et al. (2019). TAH production by B. subtilis KMS2-2 was performed in tannic acid agar media as the method described by Roy et al. (2018), the tannic acid was used as a substrate for TAH production.
Enzyme activity was evaluated according to the methods described by Aguilar et al. (2007). Tannin acyl hydrolase production was calculated by the enzyme units, where one unit of tannin acyl hydrolase activity was defined as the amount of enzyme required for the release of 1 µ/mol of gallic acid per/min under standard assay. The estimation of protein content was done according to the method of Bradford et al. (1976).
2.2 Enzyme purification Gel-filtration chromatography
The enzyme purification was done by dialysis method (bag cut off size approximately 15 kD), as well as chromatography method (Sephadex G-100). For TAH purification, ammonium sulfate was added graudally to collected crude enzyme upto an concentration of 50% in anticipation of precipitation. The precipitated proteins were separated by centrifugation at 10,000 × g & re-suspended in the smallest amount of sodium phosphate buffer (0.2 M, pH 6.0). To remove the residual ammonium sulfate, the dialysis process was performed against the same buffer using dialysis membrane bags 15 kD cut off. Then, the dialyzed TAH was purified by Sephadex G-100 column with an bed size and volume of 1.5x55cm and 90.0 mL respectively. The column was initially equilibrated with sodium phosphate buffer (0.2 M, pH 6.0), and was fraction eluted with the same buffer at a flow rate of 1 mL/min. More than one hundred fractions of 1 mL size were collected infraction (Pharmacia Biotech, USA) using at 280 nm a spectrophotometer (Jana et al., 2013). Then, the TAH enzyme activity in this collected sample was evaluated and the fractions showed high activity was pooled together to concentrate and their purity was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). The step-wise purification process for each sample was conducted at 4 °C.
3 Quantification of Protein, mass spectrometry analysis
Protein concentration was determined as the method of Bradford (1976) and Homogeneity and probable molecular weight of the purified TAH was confirmed by SDS-PAGE (12%) using standard marker. Further, zymogram confirmation was carried out by the methods of Aoki et al. (1979). The digested outcome was confirmed by MALDI-TOF/MS analysis. For analysis, the purified enzyme whole fraction was carryout aliquots contains minimum 5p/mol of a digested sample, together with µL of matrix [10 mg/ml-cyano-4-hydroxycinnamic acid in acetonitrile 50%]. The m/z spectra representing the mono-isotopic masses of the digested specific protein bands were acquired in the MALDI-TOF/MS and analyzed by Protein Pilot software.
3.1 HPLC analysis of the purified enzyme
The investigation of purified TAH was carried out on HPLC withan UV–visible detector. The sample was analysed in a solvent comprised of acetonitrile/acetic acid/water (10:4:80 v/v/v with a reversed-phase Nova-pack C18 cartridge) run speed of 0.6 mL /1min. UV–visible spectrum was detectedin a wavelength of 254 nm. The chromatography prepared with a 600/MS machine organizer, a 717 + auto detector & a 996 photodiode array (PDA) detector was used.
3.2 FT-IR analysis of the purified enzyme
The purified TAH was determined using a IR spectrophotometer, (Shimadzu, Kyoto, Japan). The entire sample was collected in liquid form with KBr and packed together into disks at 600 kg/cm2 to obtain solid samples. The spectrum was collected range in 4000–400 cm−1 with resolutions of 2 cm−1 at 15 spectrum scans.
3.3 Circular dichroism spectroscopy (CD)
Circular dichroism (CD), spectra were recorded using a JASCO J-810CD spectropolarimeter and cuvettes with the path lengths 1 or 0.05 cm, depending on the protein concentration and wavelength region. Wavelength range 190–250 nm using a 0.5 nm band width, 1 s response time, and 250 nm/min scan speed. Protein structures calculation was achieved using CDNN software. The effect of pH (6–8) on the production of TAH was 1 h of incubation at different pH and 1 h of various temperatures (30–70 °C) (Greenfield, 2006).
3.4 Effect of temperature and pH
The effect of temperature on purified TAH activity was assessed to determine the thermal stability. In brief, the TAH enzyme was incubated at various temperatures between 30 and 100 °C and their activity was checked every 15 min intervals for a period of 1 h. The TAH assays were performed in twice.
To know the stability of the TAH at various pH, the purified TAH of B. subtilis KMS2-2 was incubated in the various buffer system. The buffer system used in the study was acetate buffer (pH 3–5), sodium phosphate buffer (pH 6–7), Tris-HCl buffer (pH 8), and glycine-NaOH buffer (pH 9–10).
3.5 Effect of reaction time
For this study, the TAH enzymes and the substrate in tannin content was incubated at various time durations (15 to 90 min). The standard protocol for TAH assay was referred according to Mondal et al. (2001).
3.6 Determination of kinetic studies
A Km and Vmax value of enzyme was determined by the various concentration of tannic acid from 5 mM to 15 mM in 0.2 M sodium phosphate buffer (pH 6.0). Standard condition was identified for each purified enzyme. The experiment data was fitted to the micahlis-menten equation using Sigmaplot 12.0 version.
3.7 Effect of different additives on enzyme activity
Differents inhibitor such as (gallic acid, n-Bromosuccinic acid, methyl gallate, pyrogallol, n-propyl gallate, sodium bi-sulfate and sodium thioglycolate), Chelators (EDTA, SLS) and metal ions (MgCl2, FeSO4, CuSO4, ZnSO4, FeCl3, BaCl2, HgCl2, AgNO3, NaCl and KCl) were dissolved in sodium phosphate buffer in pH 6.0 at incubating time at 50 °C for 60 min. Enzyme standard assay was performed using a buffer and the effect of these additives on TAH activity was examined.
3.8 Cytotoxicity assay
MTT assay was used to examine the biological activity of enzymes. The vero cells obtained from ATCC, and isolated mice peritoneal macrophage were seeded into 96 plates well culture with 1.5 × 105 cell/mL concentration. Cell culture was developed in a concentration of 1000 mg/mL were incubated at 37 °C; 5% CO2 for 24 h using serial dilutions of purified enzyme (0, 0.5, 1, 2, 4, 8, 16, 32, 64 and 128 mg/mL -1) as described in previous report (Khan et al., 2017). Consequently, after 4 h of incubation, 10 μL of MTT reagent (2.5 mg/mL3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added to isopropanol and the OD492 nm in ELISA microplate reader. The viability was calculated in this formula.
3.9 In vivo cytotoxicity studies
In vivo cytotoxicity of TAH was studied as the method described by Jana et al. (2013). In this experiment, three months old male albino Wistar rats (Rattus norvegicuc) was taken and their weight wasas 100 ± 10 g each. The wister rats taken in this study were classified into 3 groups, Group I was used as control (untreated) and Group II and III were treated by the enzyme atdoses of 1000 mg respectively for 6 weeks, at the rate of 10 mL/kg/day. The end of the treatment, the animals were sacrificed to collect the tissue and fixed in 10% (v/v) formalin. Then, the collected tissue section (thickness 4–5 μm) was stained with hematoxylin and eosin and digital format observed by a light microscope.
4 Result and discussion
4.1 Production and purification of enzyme
The production of TAH by B. subtilis KMS2-2 in the liquid culture was collected after 24 h and purified bya specific purification process consisted of (NH4)2SO4 precipitation methods (50%) saturation followed by chromatography (Sephadex G-100). The purification fold was 322.00 and 10.8% yield with a specific activity of 2.58 (µ/mg) Table 1. Further, the TAH was eluted for single peaks through Sephadex G-100 column in this study and this was different from the observation made by Bhardwaj et al. (2003). The TAH has been determined by the SDS-PAGE and the showed a single band, it exhibited zymogram enzyme activity, indicating the clarity of protein (Fig. 1). The molecular mass for purified TAH was found to be ∼ 43 kDa. Therefore, mass spectrometry (MALDI/TOF/MS) were uses to prove the purity of enzyme with direct out of single digest at m/z 1800 (Fig. 2a). Previous, studies revealed that the TAH produced by Lactobaciilus plantarum and Enterobacter cloacae 41 were found to be a monopolymer structure with a weight of 47 & 45 Da, respectively (Iwamoto et al., 2008; Govindarajan et al., 2019).
| Purification step | Total activity (U) | Total protein (mg) | Specific activity (U/mg) | Purification fold | Yield % |
|---|---|---|---|---|---|
| Crude Tannase | 2.58 | 36.5 | 0.070 | 1.00 | 100 |
| (NH₄)₂SO₄, precipitation | 1.90 | 13.9 | 0.13 | 1.85 | 73.64 |
| Sephadex G-100 | 0.28 | 0.0124 | 2.58 | 322.0 | 10.8 |
Total activity = Activity (U) × Total volume (mL).
Total protein = Protein (mg) × Total volume (mL).
Specific activity = Total activity (U)/ Total protein (mg).
Purification fold = Specific activity of the sample/initial specific activity.
Yield = [total activity of the sample/Initial total activity] × 100.
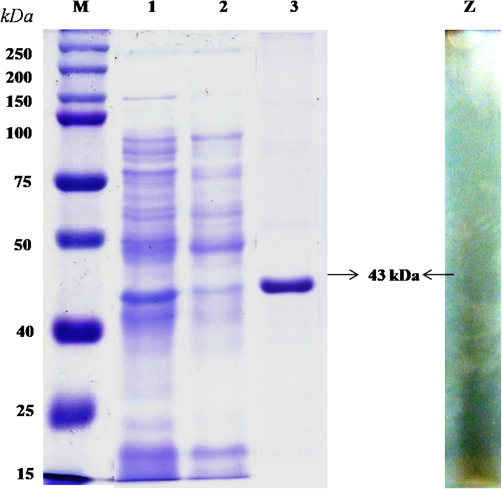
- Molecular mass of purified Bacillus subtilis KMS2-2 tannin acyl-hydrolase determined by SDS-PAGE. M, Marker proteins (kDa) using molecular weight marker (Amersham Biosciences); Lane 1, Crude extract tannin acyl hydrolase; Lane 2, Ammonium sulphate precipitation; Lane 3, Sephadex G-100 from B. subtilis KMS2-2; Lane Z, Zymogram analysis.
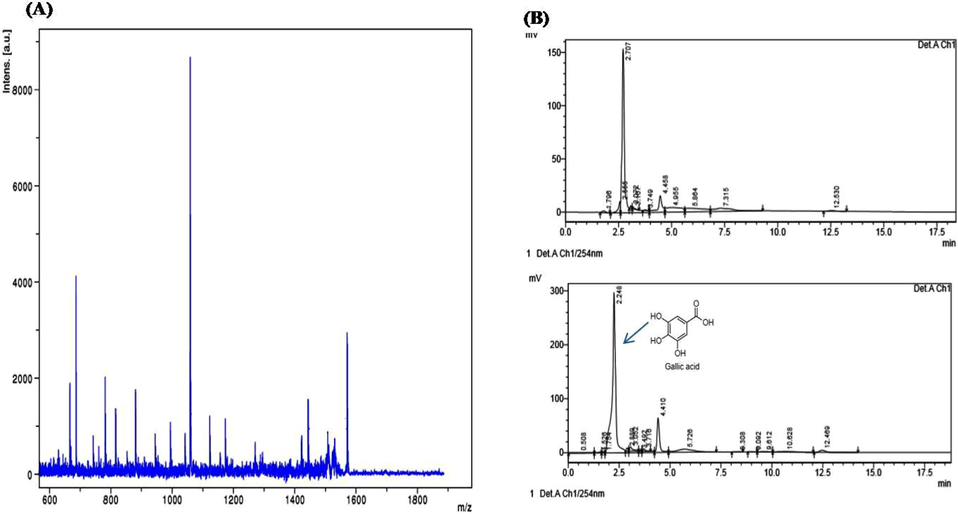
- (a) Purified tannin acyl hydrolase enzyme analyzed by MALDI-TOF-MS/MS. (b) HPLC analysis of purified tannin acyl hydrolase enzymatic reactions. Upper panel: tannic acid; lower panel: B. subtilis KMS2-2 purified tannin acyl hydrolase enzymatic reactions.
4.2 HPLC analysis purified TAH from Bacillus subtilis KMS2-2
The chromatogram of HPLC analysisfor the enzymatic reaction mixtures was performed after incubationin the semi-synthetic under enzymatic conditions. This enzyme can cleave the ester molecules in the tannic acid and formed by-product of gallic acid. The standard tannin content regulation showed major significant peaks with the largest amount of 150 mV at the retention time of 2.707 and 2.5 min (Fig. 2b). Therefore, purified tannin acyl-hydrolase enzyme confirmed major shifted peaks is the rention time of 2.5 min by a profusion of 300 mV, other than observed new peaks at 2.24, 4.410 and 12.5/min in the TAH, which is shown the hydrolyzed into get by-products. However, Results indicated that tannin acyl-hydrolase enzyme from B. subtilis KMS2-2 was able to hydrolyze both ester and other molecules finally to be confirmed as gallic acid. Nelson et al. (1995) observed as an byproduct during tannic acid degradation by a ruminal bacterium and gallotannin degradation by Aspergillus fumigates and by Aspergillus foetidus, Rhizopus oryzae 85.67%, 95% conversion to gallic acid was recorded (Mukherjee and Banerjee, 2004).
4.3 FT-IR analysis
The IR spectral examination of purified TAH showed bands at different peaks as 3436.1, 1628.5, 1359.7 and 776.3 cm−1. The result obtained from the FT-IR analysis of the tannic acid hydrolyzed after the transesterification reaction (Fig. 3), indicated the presence of the OH stretch rates in involving intermolecular of hydrogen bonds pattern and C–C, C-O molecule of the benzene structure bonds. The peaks at 779.3 and 1628.5 cm−1 were due to the C-O stretch with superiority effect with O–H and confirming the presence of gallic acid. These results indicated the suitability of the TAH enzyme obtained from Bacillus subtilis KMS2-2, for the cleave ester band and the by-products gallic acid and glucose. The purified enzyme was crystallized under medium thermal conditions and analyzed. Govindarajan et al. (2019) have been reported absorbance peaks at 3440.91, 2911.37, 2358.72, 1656.81, 1466.68, 1054.60, and 669.07 cm−1. The TAH is degraded using tannic acid as substrate and formed to simple ester molecule in which are the activity mainly occurred in numerous micro-organisms in the ruminant digestive path systems (Sharma and Gupta, 2003).
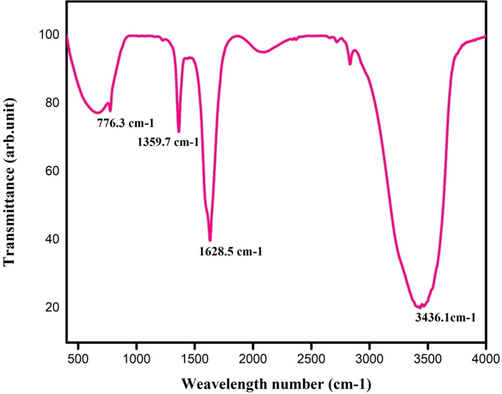
- FT-IR analysis of purified tannin acyl hydrolase enzymatic reactions.
4.4 Circular dichroism of purified TAH from B. subtilis KMS2-2
The structures and composition of TAH of B. subtilis KMS2-2, found to be composed of α-16.8%, β-45.1% and Turns 38.4% at standard pH 6.0. Its has been exposed that β- structure possibly of very important TAH activity. The difference in the TAH structure under pH (6–8) between α, β, and Turns % was examined & schematically in a graphical way as shown in Fig. 4a,b. The result showed that β-confirmation would have an over riding responsibility for enzyme activity. Hence, the composition and secondary structure of enzyme from B.subtilis KMS2-2 is similar to that of Penicillium herquei (Jana et al., 2013; Qiu et al., 2011). In general, TAH is presumably similar both in the bacteria and fungi. The modification of α, β and Turns percent in the TAH structure at different pH and temperature was examined and it was exposed with the intention of α helical structure decreased compared to alkaline pH 7.0 so far acidic/basic pH ranges.Therefore, the all changes in helix conent was not significant at wide pH ranges. Furthermore, β-structure was increased at a lower level to higher (pH 5–6) and higher (pH 7–8). The maximum content was exhibited at a range of isoelectric pH of acidic enzyme activity pH 6.0, which are maybe due to forced precipitation, the difference in the temperature in Fig. 4b. The due to the pH & temperature dependent of destabilization of ester bond and hydrogen, this would be a stabilizing force for the secondary enzyme structure.
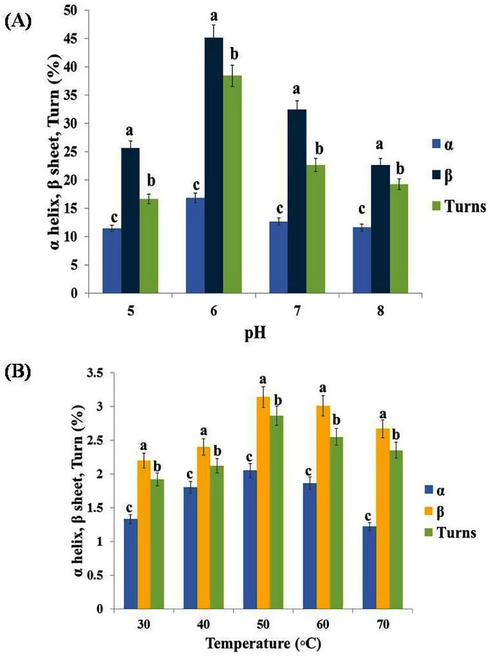
- Circular dichroism (CD) analysis of purified tannin acyl hydrolase from Bacillus subtilis KMS2-2 at different acidic, neutral and basic (pH 5–8) and temperature (30-70◦C) range for the prediction of secondary structureof tannin acyl hydrolase. Error bars indicate standard deviation from triplicate determinations. Data are mean of three independent readings with significance of P < 0.05.
4.5 Effect of temperature and pH for TAH activity
The temperature for greater TAH enzyme activity was studied in the range of 30–100 °C is shown in Fig. 5. This revealed the higher activity of the broader temperature ability of enzyme activity was recorded as 3.43U/mL at 50 °C. This obtained TAH activity is similar to that activity by L. pentosus (Kanpiengjai et al., 2019) and P. crustosum (Chavez-Gonzalez et al., 2012). The effectiveness of this TAH can be solitary of the more significant data, while determining and the cost effective possibility to prevent them from the food industry & various pharmacy industries and to maintain their activity. Therefore, the purified TAH has been subjected to the different pH conditions and has higest activity contained byafew partial ranges with precise performance. Notably, the effect of pH (Fig. 5) mixtures of the TAH reaction on the activity showed that the pre-dominantly minimum at pH 3.0 (0.483U/mL), pH 4.0 (0.687U/mL) respectively. The effective pH 6.0 (3.12U/mL) for TAH activity, potential value in food, pharmaceutical industries wherever the acidic (pH 6.0) are suitable. These findings were also consistent with those reported for TAH enzyme produced by Enterobacter cloacae 41 and Verticillium sp. Bacillus licheniformis KBR 6 and other lactic acid bacteria as compared many reports regarding temperature and pH variability among tannin acyl hydrolase TAH produced by varius bacterial and fungal (Govindarajan et al., 2019; Ueda et al., 2014).
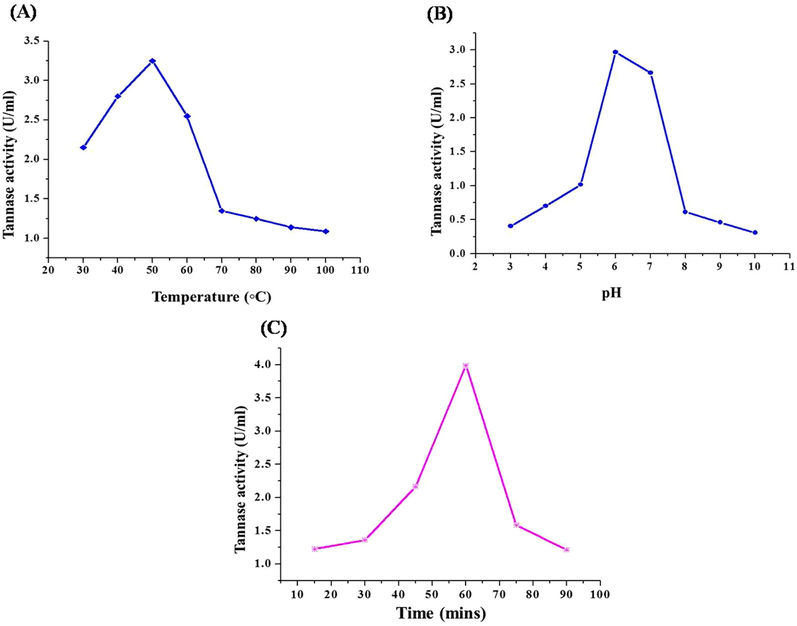
- (a) Effect of temperature on purified tannin acyl hydrolase enzyme. (b) Effect of pH on purified tannin acyl hydrolase enzyme. (c) Effect of incubation period on tannin acyl hydrolase enzyme. Error bars indicate standard deviation from triplicate determinations. Data are mean of three independent readings with significance of P < 0.05.
4.6 Incubation time on purified TAH activity
The outcome showed that the TAH activity were appeared after 15 min of incubation, and increased slowly as the incubation time was increased. The maximum TAH activity was observed at 60 min (3.71U/mL), but the TAH activity decreased substantially after 75 min. Previously, this enzyme is stated to be produce during the important growth phase and consequently, the enzyme activity gradually decreased due to the decline phase of TAH productions and the degradation at the final stages (Suseela and Nandy, 1985).
4.7 Effective of substrate concentration
The TAH was determined from plotting velocity against the concentration of substrate was found to be 10–15 mM and 7.12 U/mL for tannic acid, respectively (Fig. 6). The double reciprocal Line weaver Burk plot was used for measuring the kinetic of data. The observation of this study is not similar to the earlier reports which maybe due to variation in the TAH reaction (Iwamoto et al., 2008), but other similar reports from the same organism showed Km value of 0.7 × 10-4 & 2.0 × 10-4 min a semi-solid condition and submerge fermentation correspondingly (Jimenez et al., 2014).
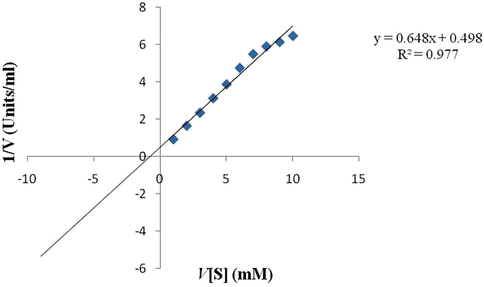
- Lineweare-Burk plot of tannin acyl hydrolase of Bacillus subtilis KMS2-2 data points represents the three replicate with standard deviation ± 5%.
4.8 Effective of different additives on TAH activity
The studies of different additives on TAH activity provided information about structure patterns and the binding nature of the active site. For this study, the inhibitore such as gallic acid, n-bromosuccinicacid, pyrogallol, n-propyl gallate, methylgallate, sodium-bi-sulfate & sodium-thio-glycolate were used to test the TAH activity (Table 2). Methyl gallate was found to be an effective inhibitor of TAH activity, where inhibition of 92.5% defeat in unique enzyme activity, followed by pyrogallol, n-propyl gallate and sodium thio-glycolate shown moderate inhibitions. The inhibitor, β-mercaptoethanol showed more inhibition activity for TAH produced by P.herquei and Aspergillus heteromorphus Jana et al. (2013). Govindarajan et al. (2019) found DMSO & n-bromosuccinimide had efficiently inactivated the TAH activity.
| Inhibitors (1 mM) | 60 min | |
|---|---|---|
| Relative activity | % Inhibition | |
| Control | 100 | – |
| Gallic acid | 76 | 24 |
| n-Bromosuccinic acid | 42 | 58 |
| Methyl gallate | 7.5 | 92.5 |
| Pyrogallol | 34.5 | 65.5 |
| n-Propyl gallate | 30 | 70 |
| Sodium bisulfate | 61 | 39 |
| Sodium thioglycolate | 28 | 72 |
| Surfactants/Chelator | ||
| EDTA (1 mM) | 88 | 12 |
| SDS (0.5%) | 70 | 30 |
| Triton X 100 (0.5%) | 65 | 35 |
| Tween 20 (0.5%) | 30 | 70 |
| Tween 80 (0.5%) | 45 | 55 |
| Metal Ions | 5 mM Concentration | |
| Mg2+ | 110 | – |
| Zn4+ | 92 | 08 |
| Cu4+ | 60 | 40 |
| Fe4+ | 44 | 56 |
| Fe3+ | 69 | 31 |
| Ba2+ | 61 | 39 |
| Ag3+ | 77 | 23 |
| Hg2+ | 52 | 48 |
| Na+ | 94 | 06 |
| K+ | 68 | 32 |
The surfactant/chelator on purified TAH activity was investigated. The sodium dodecyl sulfate (SDS) (0.5%) showed inhibitions effect (30%), Triton x-100 (35%) inhibitions moderate effects Tween 20, Tween 80 had maximum inhibitions activity (70,55%) respectively (Table 2), reported that Furth (1980), this chelator, it can well interelated to resistance against polar and non-polar interaction. Earlier reports Goncalves et al. (2011) enzyme activity in the presence of SDS is an E.nidulans and Aspergillus heteromophus purified TAH activity.
The different metal ions on TAH activity are showed in Table 2 In metal ions, the ions serve as salt and complex ions to preserve TAH conformation or to stabilize the binding of a specific substratum molecule as a co-factor. Amongst, the metal ions such as, Cu4+ (40%), Fe4+ (56%), Fe3+(31%), Ba2+ (39%), Hg2+ (48%) and K+ (32%) showed strong inhibitory actions where as, Zn4+ (08%), Ag3+ (23%) and Na+ (6%) showed moderate inhibitions activity and this can be able to binding to thiol groups and specific strong inhibitions, tryptophan residues or the carboxyl groups attached responsible amino acid in the TAH. Therefore, TAH inhibitions the being there of Hg2+ were investigative of the natural history of the molecule's thiol hydrolase. Many researchers have previously reported on the existence of thiol-hydrolase in TAH enzyme (Jana et al., 2013).
4.9 In vitro and in vivo cytotoxicity assay
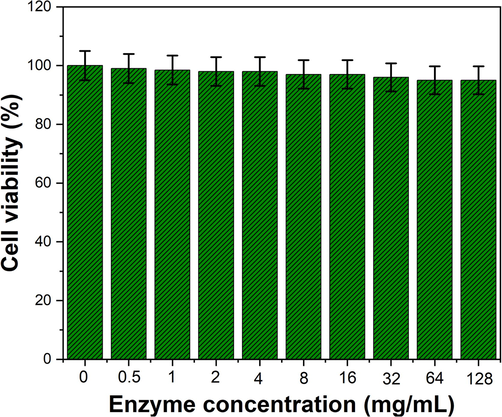
The cytotoxicity assay of TAH of B. subtilis KMS2-2 was determined by test the minimum concentration with observable cytotoxic impact. TAH with a concentration of up to 1000 mg/mL did not show any cytotoxic effects on vero cells, suggesting, it is no toxicity to the cells. For CC50, the TAH enzyme values was observed as 128 mg/mL was chosen as the concentration of TAH in against vero cells assay that was lower than the maximum non-toxic effects (Fig. 7). Till now, no such evidence report is available on in vitro cytotoxic activity of TAH enzyme.
-
Invitro cytotoxicity of purified tannin acyl-hydrolase enzyme was evaluated on Vero cells and absorbance at 492 nm was interpreted as a function of cell viability. The whole experiment repeated twice. Results are expressed as means ± standard deviation.
In vivo cytotoxicity was studied on rats, and no abnormal behavior was found during the experimental time. The histology sections of the rat liver & spleen treated by purified TAH showed the architecture of cellular functions with no pathological cell damages (Abdallah et al., 2009; Chih et al., 2001). The microscopic histological section analysis revealed no abnormal signs and showed the symptom such as liver hepatocyte damages, and spleen hematopoietic cell damages (Fig. 8). In general, the liver is an important organ in toxicological research, as it’s the primary source of detoxification of all drugs/toxins in the animal model. The overall (In vitro /In vivo) toxicological data observed for TAH of KMS2-2 strain provided a potential basis of advancing biotechnological attention, which, considering its GRAS (Generally Regarded as Safe) status, may find it additional exploitation in food, pharamaceutical industry. This is the first report on TAH, produced by B.subtilis KMS2-2 and its safety aspects have been dealt in this study (Govindarajan et al., 2019; Trivedi and Rawal, 2001).
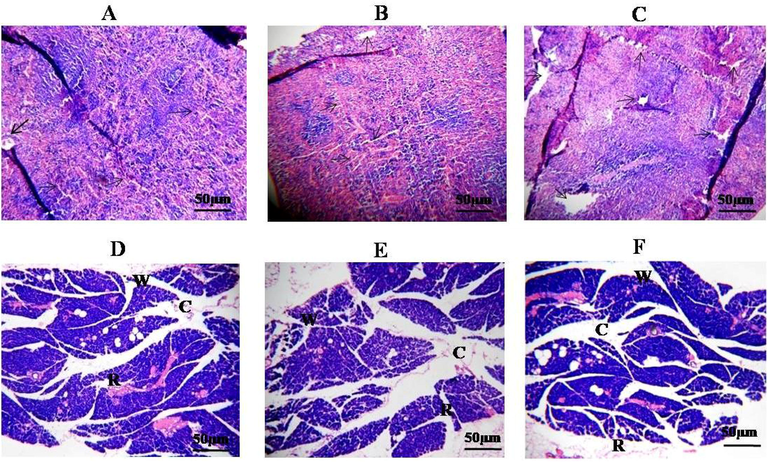
- Hematoxylin and eosine stained section of Liver, Speen at 400× manification. (A, B and C Liver showing normalhistology –arrows depict damages hepatocytes, portal area & dotted arrow indicated koofer cells). D, E and F Spleen rat showing normal histology-arrows. The different text indicated that different portions of the spleen (W, white pulp, R, red pulp, C, central arteriole).
5 Conclusion
The present work explored the purified and advanced the characterization of TAH enzyme produced by Bacillus subtilis KMS2-2 at the molecular level, our finding revealed the TAH of B. subtilis KMS2-2 is appropriate for maximum use in various aspects of the food and pharmaceutical industry as well as molecules for synthesis in the biochemical industry. The bacterial capable of producing TAH can be useful in the treatment of ruminants, healthy vegetation soils. The low molecular weight of the enzyme ∼ 43 kDa consequently assumes that its useful TAH isolated from Ailanthus excels Roxb leaves, other than highly active TAH from the pathogen Fusobacterium sp and novel TAH from endophyticactino actinobacteria (Roy et al., 2018). The main benefits of and purification of TAH and to advance molecular studies from enzyme investigation were to study the stability of the conditions of culture for the production B. subtilis KMS2-2. A conventional step followed by methods with purification or used for enzyme effectively. From this study, B. subtilis KMS2-2 can be a potentialresource for the production of TAH enzyme & can be used in the food and pharmaceutical companies.
CRediT authorship contribution statement
Rasiravathanahalli Kaveriyappan Govindarajan: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing, Visualization. Krishnamurthy Mathivanan: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing, Visualization. Chartchai Khanongnuch: Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing, Visualization. Rajendran Srinivasan: Formal analysis. Kridsada Unban: Investigation, Visualization. Arulanandam Charli Deepak: Writing - review & editing, Visualization. Dunia A. Al Farraj: Writing - review & editing. Khaloud Mohammed Alarjani: Visualization. Fatmah S. Al Qahtany: Visualization.
Acknowledgement
The authors would like to thank the Division of Biotechnology, School of Agro-Industry, Faculty of Agro-Industry, Chiang Mai University, for their facilities. We also acknowledge Science and Technology Park, Chiang Mai University, Mae‐Hia, Thailand.
The authors extend their appreciation to the Researchers supporting project number (RSP-2020/190) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Toxicological assessment of the oleogum resins of Commiphoramolmol and Boswelliapapyrifera in rats. J. Med. Plants Res.. 2009;3:526-532.
- [Google Scholar]
- Microbial tannases: advances and perspectives. Appl. Microbiol. Biotechnol.. 2007;76:47-59.
- [Google Scholar]
- Aharwar, A., Parihar, D.K., 2021. Talaromyces verruculosus tannase immobilization, characterization, and application in tea infusion treatment. Biomass Convers. Biorefin. Doi: 10.1007/s13399-020-01162-6.
- Microbial production of tannase: an enzyme with potential use in food industry. Lebensm. Wiss. Technol.. 2004;37:857-864.
- [Google Scholar]
- Optimization of process parameters for the production of tannase and gallic acid by Enterobacter cloacae MTCC 9125. J. Am. Sci.. 2010;6:389-397.
- [Google Scholar]
- Purification and characterization of tannin acyl-hydrolase from Aspergillus niger MTCC2425. J. Basic Microbiol.. 2003;43:449-461.
- [Google Scholar]
- A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem.. 1976;72:248-254.
- [Google Scholar]
- Biotechnological advances and challenges of tannase: an overview. Food Bioprocess Technol.. 2012;5:445-449.
- [Google Scholar]
- Bullatacin, a potent antitumour annonaceous acetogenin, inhibits proliferation of human hepatocarcinoma cell line 2.2.15 by apoptosis induction. Life Sci.. 2001;69:1321-1331.
- [Google Scholar]
- Removing unbound detergent from hydrophobic proteins. Anal. Biochem.. 1980;109:207-215.
- [Google Scholar]
- Extracellular tannase from Emericellanidulans showing hypertolerance to temperature and organic solvents. J. Mol. Catal. B: Enzymatic.. 2011;71:29-35.
- [Google Scholar]
- Purification, structural characterization and biotechnological potential of tannase enzyme produced by Enterobacter cloacae strain 41. Process Biochem.. 2019;77:37-47.
- [Google Scholar]
- Microbial tannase: current perspectives and biotechnological advances. Biocatal. Agric. Biotechnol.. 2016;6:168-175.
- [Google Scholar]
- Isolation and Characterization of Tannase Producing Bacteria from the gut of Gryllotalpa krishnani. J. Microbiol. Biotech. Food Sci.. 2016;6:813-817.
- [Google Scholar]
- Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc.. 2006;1:2876-2890.
- [Google Scholar]
- Optimization of physicochemical parameters of tannase post-purification and its versatile bioactivity. FEMS Microbiol. Lett.. 2018;365:12.
- [Google Scholar]
- Identification and cloning of a gene encoding tannase (tannin acylhydrolase) from Lactobacillus plantarum ATCC 14917T. Syst. Appl. Microbiol.. 2008;31:269-277.
- [Google Scholar]
- Structural characterization of thermostable, solvent tolerant, cyto safe tannase from Bacillus subtilis PAB2. J. Biochem. Eng.. 2013;77:161-170.
- [Google Scholar]
- Characterization of a bacterial tannase from Streptococcus gallolyticus UCN34 suitable for tannin biodegradation. Appl. Microbiol. Biotechnol.. 2014;98:6329-6337.
- [Google Scholar]
- Expression and Biochemical Characterization of a New Alkaline Tannase from Lactobacillus Pentosus. Protein Expr. Purif.. 2019;157:36-41.
- [Google Scholar]
- Co-production of gallic acid and a novel cell-associated tannase by a pigment-producing yeast, Sporidiobolus ruineniae A45.2. Microb Cell Fact.. 2020;19(95)
- [Google Scholar]
- In vitro evaluation of cytotoxicity, possible alteration of apoptotic regulatory proteins, and antibacterial activity of synthesized copper oxide nanoparticles. Colloids Surf. B. Biointerfaces.. 2017;153:320-326.
- [CrossRef] [Google Scholar]
- Optimization of tannase production by a novel Klebsiella pneumoniae KP715242 using central composite design. Biotechnol. Reports.. 2015;7:128-134.
- [Google Scholar]
- Pomegranate peel is a low-cost substrate for the production of tannase by Bacillus velezensis TA3 under solid state fermentation. J. King Saud Univ. Sci.. 2020;32:1831-1837.
- [Google Scholar]
- Biologically synthesized silver nanoparticles against pathogenic bacteria: synthesis, calcination and characterization studies. Biocatal. Agric. Biotechnol.. 2019;22:101373
- [Google Scholar]
- Synthesis and characterization of nanoparticles conjugated tannase and using it for enhancement of antibacterial activity of tannase produced by Serratia marcescens. Microb. Pathog.. 2017;110:484-493.
- [Google Scholar]
- Colorimetric assay for determination of tannin acyl-hydrolase (E.C. 3.1.1.20) activity. Anal. Biochem.. 2001;295:168-171.
- [Google Scholar]
- Biosynthesis of tannase and gallic acid from tannin rich substrates by Rhizopus oryzae and Aspergillus foetidus. J. Basic Microbiol.. 2004;44:42-48.
- [Google Scholar]
- Isolation and characterization of an anaerobic ruminal bacterium capable of degrading hydrolysable tannins. Appl. Environ. Microbiol.. 1995;61:3293-3298.
- [Google Scholar]
- Properties and secondary structure of Tannase from Penicillium herquei. Biotechnol. Bioprocess Eng.. 2011;16:858-866.
- [Google Scholar]
- Occurrence of a novel tannase (tan BLP) in endophytic Streptomyces sp. AL1L from the leaf of Ailanthus excels Roxb. 3. Biotech.. 2018;8:33.
- [Google Scholar]
- Synthesis of antioxidant propyl gallate using tannase from Aspergillus niger van Teighem in nonaqueous media. Bioorg. Med. Chem. Lett.. 2003;13:395-397.
- [Google Scholar]
- Physical adsorption of lipase onto mesoporous silica. Int. J. Curr. Adv. Res.. 2017;6:3837-3841.
- [Google Scholar]
- Decomposition of tannic acid and gallic acid by Penicillium chrysogenum. Leather Sci.. 1985;32:278-280.
- [Google Scholar]
- Hepatoprotective and antioxidant property of Andrographis paniculata in BHC induced liver damage in mice. Indian J. Exp. Biol.. 2001;39:41-46.
- [Google Scholar]
- Influence of tannase from Serratia marcescens strain IMBL5 on enhancing antioxidant 2 properties of green tea. BCAB-101675. Biocat. Agric. Biotechnol. 2020
- [CrossRef] [Google Scholar]
- Efects of dietary red yeast (Sporidiobolus pararoseus) on production performance and egg quality of laying hens. J. Anim. Physiol. Anim. Nutr.. 2018;102:337-344.
- [Google Scholar]
- Ueda, S., Nomoto, R., Yoshida, K., Osawa, R., 2014. Comparison of three tannases cloned from closely related Lactobacillus species: L. plantarum, L. paraplantarum, and L. pentosus. BMC Microbiol. 14, 87.
- Effects of Tannase and Ultrasound Treatment on the Bioactive Compounds and Antioxidant Activity of Green Tea Extract. Antioxidants. 2019;8:362.
- [Google Scholar]







