Translate this page into:
Tangeretin alleviates malathion-instigated testicular toxicity via ameliorating biochemical, apoptotic, hormonal & steroidogenic markers
⁎Corresponding author. rabianoorbwn@gmail.com (Rabia Azmat)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Malathion (MLT) is an organophosphate pesticide that instigates severe injuries in the testicular tissues. Tangeretin (TGN) is an important naturally present flavone that shows potential antioxidant and anti-apoptotic activities. This research was planned to ascertain the palliative role of TGN against MLT-instigated testicular toxicity in male Sprague-Dawley (SD) rats. 48 rats were distributed into 4 groups: control, MLT (100 mgkg−1), MLT+TGN (100 + 50 mgkg−1 respectively), and TGN (50 mgkg−1). The results showed that MLT exposure reduced the activities of catalase (CAT), glutathione reductase (GSR), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione (GSH), whereas escalated the levels of reactive oxygen species (ROS) and malondialdehyde (MDA). Moreover, it decreased the levels of luteinizing hormone (LH), plasma testosterone, and follicle-stimulating hormone (FSH). MLT also reduced the expressions of steroidogenic enzymes, including StAR, 3β-HSD, and 17β-HSD. Additionally, MLT exposure increased the expressions of Bax and Caspase-3, while reducing the Bcl-2 expressions. MLT administration also increased the levels of inflammatory markers such as nuclear factor kappa B (NF-κB), tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), interleukin-1 beta (1L-1β) as well as cyclooxygenase-2 (COX-2) activity in testes. Nonetheless, TGN recovered all the MLT induced damages in testes. In conclusion, TGN could ameliorate MLT instigated testicular impairment because of its anti-apoptotic, androgenic and anti-oxidant properties.
Keywords
Malathion
Reproductive dysfunction
Tangeretin
Oxidative stress
Steroidogenesis
Data availability
Data will be made available on request.
1 Introduction
Malathion (MLT) is a commonly used organophosphate pesticide, primarily employed for controlling household pests, ectoparasites, preserving stored grain, and eliminating disease-causing arthropods (Selmi et al., 2018). It accounts for 65 % of all organophosphate pesticides (OP) used in the field. Humans are vulnerable to MLT due to its residues in tap water, food, personal care products containing MLT, and residential areas where MLT is sprayed during pesticide treatment (Coban et al., 2015). The primary cause of MLT toxicity is its metabolite malaoxon, which is 40 times more toxic than MLT. Uzunhisarcikli et al. (2007) stated that MLT can potentially disrupt spermatogenesis that results in higher percentage of sperm abnormality as well as lowered sperm count.
Moreover, MLT impairs the function of sex hormone receptors, thereby exhibiting endocrine-disrupting potential and contributing to the dysfunction of various organ systems, including the reproductive system (Mehri et al., 2016). The ability of MLT to bypass the blood/testis barrier contributes to impairments that disrupts testicular function (Nahid et al., 2016). As spermatogenesis and fertility are critically reliant upon the adequate levels of testosterone (Sharma et al., 2005), the capacity of MLT to decrease their levels could potentially lower the reproductive capability of male mice (Uzun et al., 2009). One possible mechanism by which MLT-prompted abnormalities is production of oxidative stress (OS), which deteriorates the antioxidant defense system (Nahid et al., 2016). These free radicals attack the testicular membranes and induce lipid peroxidation (LP) (Ahmed et al., 2023), which results in abnormal sperm production, as well as lowered sperm count, viability, and motility (Nahid et al., 2016). Furthermore, MLT has the tendency to disturb the sex hormone levels and histomorphometry of the testes.
Tangeretin (TGN) is one of the important members of polymethoxyfavones (PMFs), which is plentiful in peel of citrus fruit (Fu et al., 2017). It acts as a potential bioactive flavonoid with diverse biological potentials i.e., anti-oxidant, anti-inflammatory, antitumor, anti-metastatic, anti-asthmatics, neuroprotective, and anticancer (Ashrafizadeh et al., 2020) effects. It has a pre-eminent benefit over other chemically related flavones as it has sufficient bioavailability (Kurowska et al., 2004). Therefore, this study was planned to appraise the remedial potential of TGN on MLT caused testicular-toxicity in rats.
2 Materials and methods
2.1 Chemicals
MLT TGN were brought from the Merck (US).
2.2 Animals
This research was conducted on 48 male SD rats (Rattus norvegicus, 200–250 g weight, and 6–8 weeks old). They were housed in steal cages in the animal house of University of Agriculture, Faisalabad, under controlled conditions (22-25°C temperature and 12h day/dark light cycles). The animals were provided with unrestricted water and food access with standard pallets. Animals were handled according to the guidelines of European Union of Animal Care and Experimentation (CEE Council 86/609).
2.3 Experimental layout
Firstly, rats were randomly allocated into four groups (n = 12). Control; MLT treated (100 mg/kg by oral gavage); MLT+TGN co-treated (100 mg/kg and 50 mg/kg respectively) and TGN only treated (50 mg/kg orally) groups. MLT and TGN doses were selected according to the previous studies by Al-Attar, 2010 and Arivazhagan and Subramanian, 2015, respectively. All the rats were treated for 56 days as the rats require a duration of about 54–56 days to complete one spermatogenic cycle. On the 56th day of the experiment, rats were euthanized with xylazine (6 mg/kg) and ketamine (60 mg/kg) and decapitated. Heparinized tubes were used to collect blood samples and serum samples were obtained by centrifuging blood at 1000 g for 15 min and stored at − 20 °C for biochemical assays. Testes were removed, one testis was stored in zipper bags for biochemical observation at − 80 °C, testicular tissues were homogenized in Na3PO4 buffer solution at 12,000 rpm for 14–15 min. Finally, various parameters were evaluated by using the supernatant.
2.4 Antioxidant/oxidant assay
The CAT activity was measured using the protocol given by Aebi (1984). Kakkar et al.’s (1984) standard method was used to evaluate SOD activity. GPx activity was evaluated with Lawrence and Burk (1976) method, while GSR activity was estimated using Carlberg and Mannervik’s (1975) method. However, GSH content was assessed by the approach of Sedlak and Lindsay (1968). Hayashi et al.'s (2007) technique was used to determine the ROS concentration, whereas the MDA content was assessed in accordance with the approach of Placer et al. (1966).
2.5 Hormonal assay
The levels of LH, plasma testosterone, and FSH were appraised by using ELISA kits as per company’s instructions (Los Angeles, US). The values of LH and FSH were presented as mlU mL−1 while plasma testosterone concentrations were expressed as ng mL−1.
2.6 RNA extraction and real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Expressions of apoptotic markers and steroidogenic enzymes were estimated by RT-qPCR. The total RNA of the cell was obtained by using TRI-zol reagent. RNA was transcribed to generate cDNA by using a Fast Quant reverse transcription kit (China). The relative expressions of these parameters were assessed by 2−ΔΔCT, with β-actin acting as an internal control (Livak and Schmittgen, 2001). The primer sequences of target gene are depicted in Table 1 as reported by Ijaz et al. (2020).
Gene
Primers 5′ −> 3′
Accession number
3β-HSD
Forward: GCATCCTGAAAAATGGTGGC
NM_001007719
Reverse: GCCACATTGCCTACATACAC
17β-HSD
Forward: CAGCTTCCAAGGCTTTTGTG
NM_054007
Reverse: CAGGTTTCAGCTCCAATCGT
StAR
Forward: AAAAGGCCTTGGGCATACTC
NM_031558
Reverse: CATAGAGTCTGTCCATGGGC
Bax
Forward: GGCCTTTTTGCTACAGGGTT
NM_017059.2
Reverse: AGCTCCATGTTGTTGTCCAG
Bcl-2
Forward: ACAACATCGCTCTGTGGAT
NM_016993.1
Reverse: TCAGAGACAGCCAGGAGAA
Caspase-3
Forward: ATCCATGGAAGCAAGTCGAT
NM_012922.2
Reverse: CCTTTTGCTGTGATCTTCCT
β-actin
Forward: TACAGCTTCACCACCACAGC
NM_031144
Reverse: GGAACCGCTCATTGCCGATA
2.7 Assessment of inflammatory markers
The levels of inflammatory markers, including TNF-α (CSB-E07379r), IL-6 (CSB-E04640r), NF-κB (CSB-E13148r), IL-1β (CSB-E08055r), and COX-2 (CSB-E13399r) activity were evaluated using ELISA kit (Shanghai, China). The quantifications were performed by following the manufacturer’s instructions through ELISA Plate-Reader (BioTek, Winooski-VT, USA).
2.8 Statistical analysis
Data were displayed as Mean ± SE. The significant variations were measured using One-way ANOVA followed by Turkey’s test for group’s comparisons through Minitab. The alpha-level for statistical significance was P<0.05.
3 Results
3.1 Impacts of MLT and TNG on antioxidant/oxidant markers
MLT treatment noticeably (P<0.05) downregulated the activities of antioxidant enzymes, while a notable upsurge was observed in ROS and MDA contents in comparison with control animals. Nonetheless, supplementation of TGN with MLT escalated the activities of antioxidants, while reducing ROS and MDA contents as compared to MLT-exposed animals. Moreover, these parameters in control and TGN only supplemented groups were comparable (Table 2). Values having dissimilar letters are substantially (P<0.05) distinct from other groups.
Groups
CAT (U/mg protein)
SOD (U/mg protein)
GSR (nm NADPH oxidized/min/mg tissue)
GPx (U/mg protein)
GSH (µg/mg)
ROS (U/mg tissue)
MDA (nmol/mg protein)
Control
7.88 ± 0.22 a
5.66 ± 0.11 a
3.50 ± 0.09 a
12.8 ± 0.09 a
347.0 ± 10.1 a
0.86 ± 0.04 a
0.72 ± 0.05 a
MLT
4.53 ± 0.16c
2.36 ± 0.12c
1.52 ± 0.07c
7.67 ± 0.03c
174.9 ± 8.62c
7.18 ± 0.14c
1.91 ± 0.10c
MLT+TGN
7.10 ± 0.06b
4.71 ± 0.13b
2.57 ± 0.07b
10.4 ± 0.10b
311.1 ± 6.45b
2.83 ± 0.08b
1.11 ± 0.05b
TGN
7.86 ± 0.18 a
5.57 ± 0.16 a
3.42 ± 0.09 a
13.4 ± 0.67 a
365.7 ± 8.66 d
1.11 ± 0.05b
0.81 ± 0.06 a
3.2 Impacts of MLT and TNG on hormones
The MLT treatment substantially (P<0.05) decreased the levels of LH, testosterone, and FSH in comparison with the control animals. Nevertheless, TGN supplementation substantially restored these levels in co-treated animals as compared to MLT treated animals. Furthermore, only TGN treated group displayed these levels almost similar to control group (Table 3). Values having dissimilar letters are substantially (P<0.05) distinct from other groups.
Groups
LH
(mlU/mL)FSH
(mlU/mL)Plasma testosterone (ng/mL)
Control
2.57 ± 0.06 a
3.52 ± 0.12 a
4.29 ± 0.11 a
MLT
1.29 ± 0.09c
1.83 ± 0.09c
2.18 ± 0.08c
MLT+TGN
2.07 ± 0.08b
2.92 ± 0.08b
3.91 ± 0.07b
TGN
2.49 ± 0.08 a
3.49 ± 0.12 a
4.38 ± 0.09 a
3.3 Impacts of MLT and TNG on steroidogenic enzymes
MLT administration remarkably (P<0.05) decreased the expressions of StAR, 3β-HSD, and 17β-HSD, expressions as compared to control animals. However, TGN supplementation notably restored these expressions in contrast to MLT treated animals. Furthermore, no substantial variations were noted in the expression of these biomarkers in TGN only supplemented group, in comparison with control group (Fig. 1).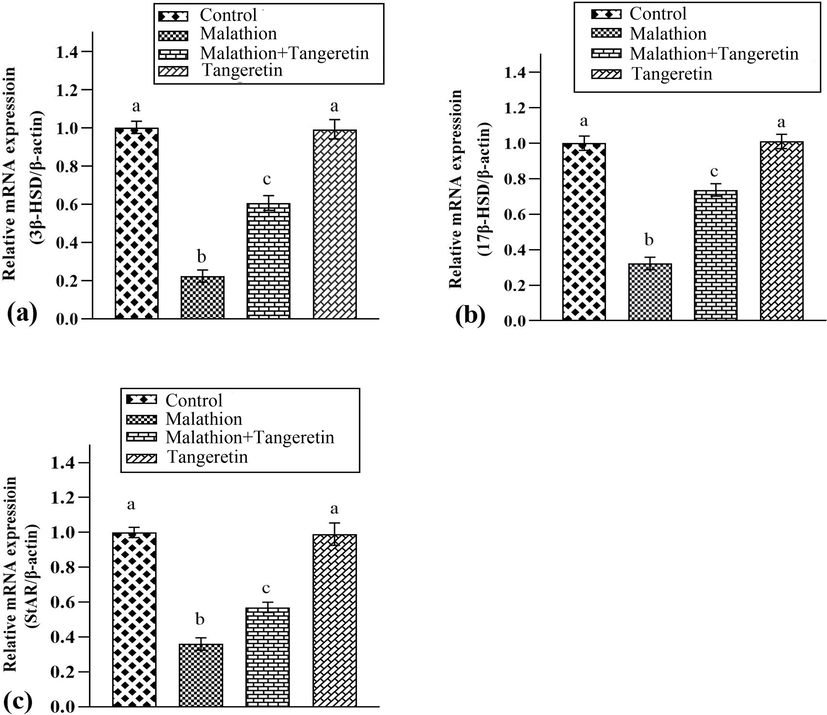
Shows the effects of malathion and tangeretin on the expressions of a) 3β-HSD, b) 17β-HSD and c) StAR. The bars are based on mean ± SEM values. Dissimilar letters on the bars are indicate considerable differences.
3.4 Impacts of MLT and TNG on apoptotic markers
MLT treatment led to remarkable (P<0.05) down-regulation in Bcl-2 expression, on the other hand upregulated Bax and Caspase-3 expressions in comparison to the control animals. Nevertheless, TGN treatment considerably regulated these expressions in MLT+TGN administered rats as compared to MLT administered rats. Moreover, these expressions were comparable in only TGN-treated and control group (Fig. 2).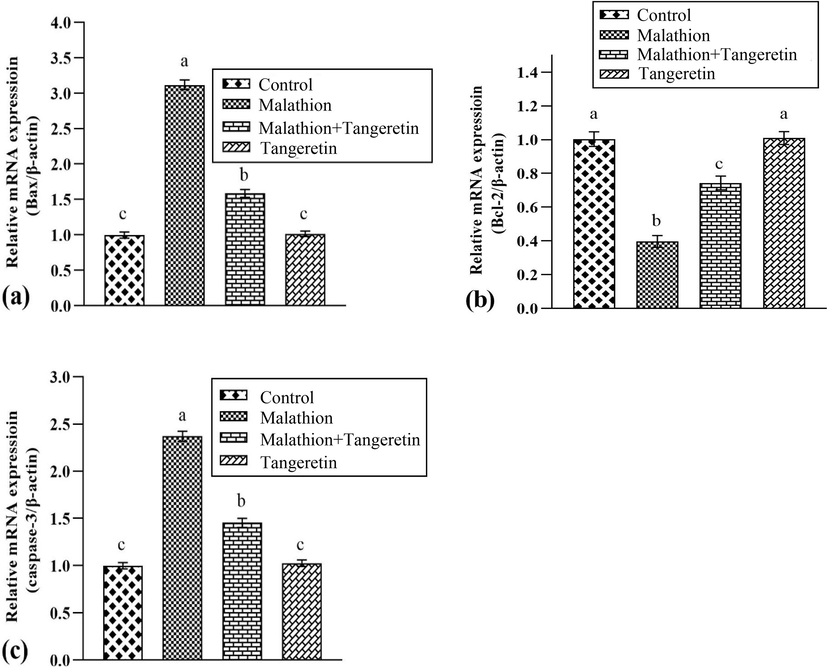
Shows the effects of malathion and tangeretin on the expressions of a) Bax, b) Bcl-2 and c) Caspase-3. The bars are based on mean ± SEM values. Different letters on the bars are indicate significant difference.
3.5 Impacts of MLT and TNG on inflammatory indices
MLT administration noticeably (P<0.05) escalated the levels of TNF-α, NF-κB, IL-1β, IL-6, and COX-2 activity in comparison with the control group. Nevertheless, TNG+MLT supplementation markedly lowered the levels of these inflammatory indices as compared to MLT treated animals. Furthermore, there were no differences in the values of these markers between the control group and the TGN supplemented group (Table 4). Values having dissimilar letters are substantially (P<0.05) distinct from other groups.
Groups
NF-κB
(ngg−1 tissue)
TNF-α
(ngg−1 tissue)
IL-1β
(ngg−1 tissue)
IL-6
(ngg−1 tissue)
COX-2
(ngg−1 tissue)
Control
12.96
c
7.14
0.98c
22.78
c
5.56
c
24.41
c
MLT
65.16
a
38.56
1.48a
72.48
a
56.81
a
92.70
a
MLT+TGN
24.25
b
17.20
1.45b
45.47
b
24.27
b
34.10
b
TGN
12.72
0.79c
7.08
c
22.69
c
5.42
c
24.19
c
4 Discussion
This study aimed to elucidate the adverse effects of MLT on testes of Sprague-Dawley rats and the palliative potential of TGN in ameliorating these damages. The administration of MLT reduced the activities of CAT, SOD, GSR, GPx, and GSH, while increasing ROS and MDA contents. These antioxidants are the first line of defense in the body that protects macromolecules against OS by suppressing production of ROS (Ighodaro and Akinloye, 2018). Nitric oxide, superoxide anion and hydroxyl radicals are known as the key reactive nitrogen and ROS species (Mijatović et al., 2020). OS is a physiological state where excessive ROS generation damages body parts such as cells, tissues and organs (Barbosa et al., 2020). SOD transforms O2– into H2O2 (Ighodaro and Akinloye, 2018), In addition to that, hydrogen peroxide is changed into H2O by CAT and GPx. In these reactions, GSH acts as an electron donor. The content of GSH is maintained by the GSR that restores GSH and helps GPx to perform its function (Ali et al., 2020). Natural flavonoids have potential to improve the activity of antioxidant enzyme (Ijaz et al., 2022). However, TGN supplementation markedly suppressed OS and escalated the activities of CAT, SOD, GPx, GSR, and GSH content, whereas reduced the MDA and ROS contents due to its ROS scavenging and antioxidant activities. Flavonoids' antioxidant properties are ascribed to the presence of several OH– groups and their ability to donate electron, which enables them to counteract ROS and ameliorate OS (Teixeira et al., 2005).
The development and growth of spermatogenic cells depends on the pituitary hormones such as gonadotropins (FSH and LH) and androgens (plasma testosterone), which are produced in response to gonadotropins stimulation (O'Shaughnessy, 2014). MLT exposure notably lowered the levels of gonadotropins and androgens. The generation of ROS stimulates hypothalamic–pituitary–adrenal (HPA) axis that release cortisol (in humans) and corticosterone (in animals). LH secretion from anterior–pituitary is influenced by these hormones (Gao et al., 2002). Reduced LH levels cannot trigger Leydig cells (LCs) to secrete enough androgen (O'Shaughnessy, 2014). Besides, FSH increases sperm growth as well as the release of sperm (Chauhan et al., 2007). However, diminished FSH reduces the flow of androgen binding protein from Sertoli cells (SCs) and subsequently reduces circulating testosterone. However, in the current investigation, the supplementation of TGN potentially restored the above-mentioned hormonal alterations. The outcomes of the study are further endorsed by previous research, which revealed that flavonoids have regulatory effects on hormones i.e. estrogens and androgens (Agarwal and Allamaneni, 2011). Hence, TGN may restore this disturbance in hormonal levels by regulating the HPA axis, which may in turn recover the spermatogenesis.
LCs are the primary site of testosterone generation in mammals and are also responsible for the development and growth of male sex organs (Martin and Touaibia, 2020). 3β-HSD and 17β-HSD control the steroidogenic event and perform vital functions in androgenesis, while StAR has a role as key-protein in the steroidogenic event (Castillo et al., 2015). For testosterone generation, StAR transports cholesterol inside mitochondrial membrane (Das et al., 2012). Both 17β-HSD and 3β-HSD facilitates the testosterone production form cholesterol (Ye et al., 2011). Current analysis revealed that MLT administration considerably suppressed steroidogenic enzymes expression. Above-stated steroidogenic enzymes reduced expressions caused by MLT-administration resulted in a lower testosterone level. Nevertheless, TGN supplementation markedly escalated steroidogenic enzymes expressions. Our results are endorsed by the fact that flavonoids have chemical structure similar to cholesterol that may affect the generation of androgens in LCs (Martin and Touaibia, 2020).
The MLT exposure elevated the expressions of Bax and caspase-3, whereas decreased Bcl-2 expressions. Apoptosis is carried out due to a disturbance in the expressions of apoptotic and anti-apoptotic markers (Sinha et al., 2013). Increased eviction of cyt-C into the cytosol results from a significant alteration in the permeability of the mitochondrial membrane caused by a decrease in Bcl-2 and an increase in Bax (Gu et al., 2017). Bax and Bcl-2 are members of the Bcl-2 family. Bcl-2 boosts the survival of cells, whereas Bax encourages apoptosis (Ghasemi et al., 2018). The relative imbalance in the expression of these markers causes cell death (apoptosis) (Gu et al., 2017). Nonetheless, the supplementation of TGN mitigated these testicular damages by escalating the expressions of Bcl-2, while decreasing caspase-3 and Bax expressions. These outcomes are compatible with the outcomes of Liu et al. (2019), who revealed that TGN supplementation ameliorated the streptozotocin-induced apoptosis in β-cells.
MLT treatment elevated inflammatory indices levels i.e., NF-κB, TNF-α, IL-1β, IL-6, and COX-2. NF-κB activation affects the expression of inflammatory indices, indicating acute inflammation along with other ailments linked to high ROS levels. Inflammation is caused by NF-kB activation, which promotes the production of inflammatory cytokines (Kandemir et al., 2020). COX-2, an additional biomarker, which also leads to inflammation (Kim et al., 2019). However, TNG supplementation decreased levels of biomarker above-mentioned, which is possibly attributable to its anti-inflammatory efficacy.
5 Conclusion
The outcomes of the current study showed that TGN administration efficiently alleviated MLT-provoked testicular damages by improving biochemical profile, reproductive hormones and reducing oxidative stress, inflammation, and apoptosis. Therefore, it can be deduced that TGN might be used as a pharmacological candidate for the treatment of testicular damage.
CRediT authorship contribution statement
Shama Mustafa: Writing – original draft, Methodology, Investigation, Conceptualization. Rabia Azmat: Writing – review & editing, Writing – original draft, Methodology, Investigation. Moazama Batool: Validation, Software, Formal analysis, Data curation. Mohammad Z. Ahmed: Writing – review & editing, Resources, Funding acquisition. Mian Nadeem Riaz: Writing – review & editing, Software, Formal analysis, Data curation.
Acknowledgement
Authors are thankful to the Researchers Supporting Project number (RSPD2024R728), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ameliorative Effects of Rhamnetin against Polystyrene Microplastics-induced Nephrotoxicity in Rats. Pak. Vet. J.. 2023;43:623-627.
- [CrossRef] [Google Scholar]
- Physiological and histopathological investigations on the effects of α-lipoic acid in rats exposed to malathion. Biomed. Res. Int.. 2010;2010:8.
- [CrossRef] [Google Scholar]
- Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem.. 2020;44:13145.
- [CrossRef] [Google Scholar]
- Tangeretin, a citrus flavonoid attenuates oxidative stress and protects hepatocellular architecture in rats with 7, 12-dimethylbenz (a) anthracene induced experimental mammary carcinoma. J. Funct. Foods.. 2015;15:339-353.
- [CrossRef] [Google Scholar]
- Ashrafizadeh, M., Ahmadi, Z., Mohammadinejad, R., Afshar, E.G., 2020. Tangeretin: a mechanistic review of its pharmacological and therapeutic effects. J. Basic. Clin Physiol. Pharmacol. 31 (2020) (ahead-of-print), DOI: doi: 10.1515/jbcpp-2019-0191.
- Barbosa, M.L., de Meneses, A.A.P.M., de Aguiar, R.P.S., e Sousa, J.M.D.C., Cavalcante, A.A.D.C.M., Maluf, S.W., 2020. Oxidative stress, antioxidant defense and depressive disorders: A systematic review of biochemical and molecular markers. Neurol. Psychol. Brain Res. 36, 65–72. doi: 10.1016/j.npbr.2020.02.006.
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250:5475-5480.
- [CrossRef] [Google Scholar]
- The role of mitochondrial fusion and StAR phosphorylation in the regulation of StAR activity and steroidogenesis. Mol. Cell Endocrinol.. 2015;408:73-79.
- [CrossRef] [Google Scholar]
- Suppression of fertility in male albino rats following the administration of 50% ethanolic extract of Aegle marmelos. Contraception. 2007;76:474-481.
- [CrossRef] [Google Scholar]
- Boron attenuates malathion-induced oxidative stress and acetylcholinesterase inhibition in rats. Drug Chem. Toxicol.. 2015;38:391-399.
- [CrossRef] [Google Scholar]
- Taurine protects rat testes against doxorubicin–induced oxidative stress as well as p53, Fas and caspase 12–mediated apoptosis. Amino Acids. 2012;42:1839-1855.
- [CrossRef] [Google Scholar]
- Evaluation of bioactive flavonoids and antioxidant activity in Pericarpium Citri Reticulatae (Citrus reticulata ‘Chachi’) during storage. Food Chem.. 2017;230:649-656.
- [CrossRef] [Google Scholar]
- Glucocorticoid induces apoptosis in rat leydig cells. Endocrinology. 2002;143:130-138.
- [CrossRef] [Google Scholar]
- Ghasemi, A., Khanzadeh, T., Heydarabad, M.Z., Khorrami, A., Esfahlan, A.J., Ghavipanjeh, S., Belverdi, M.G., Fikouhi, S.D., Darbin, A., Najafpour, M., Azimi, A., 2018. Evaluation of BAX and BCL-2 gene expression and apoptosis induction in acute lymphoblastic leukemia cell line CCRF-CEM after high-dose prednisolone treatment. Asian Pac. J. Cancer Prev. 19, 2319. 10.22034/APJCP.2018.19.8.2319.
- Inhibition of chemotherapy-induced apoptosis of testicular cells by squid ink polysaccharide. Exp. Ther. Med.. 2017;14:5889-5895.
- [CrossRef] [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol. Environ. Mutagen.. 2007;613:55-61.
- [CrossRef] [Google Scholar]
- First line defence antioxidants–superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med.. 2018;54:287-293.
- [CrossRef] [Google Scholar]
- Hepatoprotective potential of Genkwanin against aflatoxin B1-induced biochemical, inflammatory and histopathological toxicity in rats. Pak. Vet. J.. 2022;42:499-504.
- [CrossRef] [Google Scholar]
- Nobiletin ameliorates nonylphenol-induced testicular damage by improving biochemical, steroidogenic, hormonal, spermatogenic, apoptotic and histological profile. Hum. Exp. Toxicol.. 2020;40:403-416.
- [CrossRef] [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Protective effects of morin against acrylamide-induced hepatotoxicity and nephrotoxicity: A multi-biomarker approach. Food Chem. Toxicol.. 2020;138:111190
- [CrossRef] [Google Scholar]
- Anti-inflammatory actions of folate-functionalized bioactive ion-releasing nanoparticles imply drug-free nanotherapy of inflamed tissues. Biomaterials. 2019;207:23-38.
- [CrossRef] [Google Scholar]
- Modulation of HepG2 cell net apolipoprotein B secretion by the citrus polymethoxyflavone, tangeretin. Lipids. 2004;39:143-151.
- [CrossRef] [Google Scholar]
- Glutathione peroxidase activity in selenium–deficient rat liver. Biochem. Biophys. Res. Commun.. 1976;71:952-958.
- [CrossRef] [Google Scholar]
- Tangeretin inhibits streptozotocin-induced cell apoptosis via regulating NFκB pathway in INS-1 cells. J. Cell. Biochem.. 2019;120:3286-3293.
- [CrossRef] [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2–DDCT method. Methods. 2001;25:402-408.
- [CrossRef] [Google Scholar]
- Improvement of testicular steroidogenesis using flavonoids and isoflavonoids for prevention of late-onset male hypogonadism. Antioxidants.. 2020;9:237.
- [CrossRef] [Google Scholar]
- Hepatoprotective effect of the root extract of green tea against malathion–induced oxidative stress in rats. J. Herbmed. Pharmacol.. 2016;5:116-119.
- [Google Scholar]
- The double-faced role of nitric oxide and reactive oxygen species in solid tumors. Antioxidants.. 2020;9:374.
- [CrossRef] [Google Scholar]
- Protective role of green tea on malathion–induced testicular oxidative damage in rats. Asian Pac. J. Reprod.. 2016;5:42-45.
- [CrossRef] [Google Scholar]
- Hormonal control of germ cell development and spermatogenesis. Semin. Cell Dev. Biol.. 2014;29:55-65.
- [CrossRef] [Google Scholar]
- Estimation of products of lipid peroxidation (as malondialdehyde) in biochemical systems. Anal. Biochem.. 1966;16:359-364.
- [CrossRef] [Google Scholar]
- Estimation of total, protein bound and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem.. 1968;25:192-205.
- [CrossRef] [Google Scholar]
- Malathion, an organophosphate insecticide, provokes metabolic, histopathologic and molecular disorders in liver and kidney in prepubertal male mice. Toxicol. Rep.. 2018;5:189-195.
- [CrossRef] [Google Scholar]
- Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol.. 2013;87:1157-1180.
- [CrossRef] [Google Scholar]
- Structure–property studies on the antioxidant activity of flavonoids present in diet. Free Radic. Biol. Med.. 2005;39:1099-1108.
- [CrossRef] [Google Scholar]
- Malathion-induced testicular toxicity in male rats and the protective effect of vitamins C and E. FoodChem Toxicol.. 2009;47:1903-1908.
- [CrossRef] [Google Scholar]
- Acute, subacute and subchronic administration of methyl parathion-induced testicular damage in male rats and protective role of vitamins C and E. Pestic. Biochem. Physiol.. 2007;87:115-122.
- [CrossRef] [Google Scholar]
- Inhibitors of testosterone biosynthetic and metabolic activation enzymes. Molecules. 2011;16:9983-10001.
- [CrossRef] [Google Scholar]







