Translate this page into:
Talaromyces purpurogenus from a marine-polluted environment inhibits pro-inflammatory markers in LPS-stimulated RAW 264.7 cells and in carrageenan-induced paw edema
⁎Corresponding author at: Laboratório de Imunomodulação e Protozoologia, Pavilhão Carlos Chagas, Sala 213, Manguinhos, Rio de Janeiro 21040-360, Brazil. calabrese@ioc.fiocruz.br (Kátia da Silva Calabrese)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
Talaromyces purpurogenus is a fungus species widely found in the marine environment. When in a hostile environment, it produces a series of secondary metabolites with important biotechnological potential, such as meroterpenoids. In our previous report, we demonstrated that the culture broth ethyl acetate extract from T. purpurogenus reduced the cellular infiltration of lymphoid organs and circulating levels of TNF-α. To elucidate this anti-inflammatory effect, this work aimed to study T. purpurogenus isolated from a marine-polluted environment regarding the chemical composition of its culture broth and mycelial ethyl acetate extract (CBEAE and MEAE respectively), as well as its action on pro-inflammatory markers in LPS-stimulated RAW 264.7 cells and carrageenan-induced mice paw edema.
Methods
Chemical characterization was performed by HPLC-DAD-UV. Cytotoxicity in vitro and endotoxin quantification in extracts were preformed prior the experiments. In vitro inhibitory effect of T. purpurogenus extracts were evaluated by cytokines and nitrite quantification in cell supernatant of RAW 264.7 cells. The effect of T. purpurogenus in murine acute inflammation model of carrageenan-induced paw edema was verified by thickness kinetic and histology evaluation.
Results
The CBEAE demonstrated a richer chemical composition than MEAE, presenting nine meroterpenoid compounds, while MEAE displayed only two meroterpenoid and one phenolic compound. Cytotoxicity in RAW 264.7 macrophages was not observed at any concentration treatment for both extracts. Endotoxin quantification also demonstrated absence of endotoxins in both extracts. At 500 μg/mL, the CBEAE and MEAE extracts inhibited nitrite (p = 0.0450 and p = 0.0497), TNF-α (p = 0.0076 and p = 0.0001) and IL-1β (p = 0.0042 and p = 0.0031), but only CBEAE inhibited IL-6 levels in LPS-stimulated cells (p = 0.0234). CBEAE, but not MEAE, also inhibited nitrite (p = 0.0353) and IL-1β (p = 0.0386) in non-stimulated cells. In addition, CBEAE at 250 and 500 mg/kg inhibited edema thickness and mast cells (p = 0.0303 and p = 0.0130) in λ-carrageenan-induced mice paw edema.
Conclusions
Talaromyces purpurogenus isolated from marine-polluted environment inhibited pro-inflammatory markers in LPS-stimulated RAW 264.7 cells and in murine model of paw edema. The promising anti-inflammatory activity in vitro and in vivo, especially the culture broth ethyl acetate extract, demonstrates its potential for further studies to elucidate its mechanism of action.
Keywords
Meroterpenoid
Fungus
Cytokine
Inflammation
Nitric oxide
Mast cells
- CBEAE
-
culture broth ethyl acetate extract
- MEAE
-
mycelial ethyl acetate extract
- LPS
-
lipopolysaccharide
- HPLC-DAD-UV
-
High-performance liquid chromatograph coupled to diode-array ultra violet-vis detector
- IL
-
interleukin
- TNF
-
tumor necrosis factor
- PDA
-
Potato Dextrose Agar
- DMSO
-
dimethyl sulfoxide
- DMEM
-
Dulbecco Modified Eagle Medium
- FBS
-
fetal bovine serum
- MTT
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- ELISA
-
Enzyme-Linked Immunosorbent Assay
- NO
-
nitric oxide
- MIP
-
macrophage inflammatory protein
- MCP
-
monocyte chemoattractant protein
- PBS
-
phosphate-buffered saline
Abbreviations
1 Introduction
Inflammation is a biological response to several harmful stimuli that protect the host from infection and tumorigenesis. It occurs by activating humoral and cellular cascades, increasing pro-inflammatory cytokines and leukocytes in circulating blood (Mueller et al., 2010; Sacdalan and Lucero, 2021). An excessive or prolonged inflammatory response results in numerous severe acute or chronic diseases. There is strong evidence that the chronic systemic inflammatory response results in classic features of cancer cachexia, including the preferential loss of lean muscle mass as well as the systemic inflammatory response syndrome, acute lung injury, inflammatory bowel diseases and rheumatoid arthritis (Kraft et al., 2015; Dolan et al., 2017).
There are several biochemical mediators that modulates the inflammatory process, such as cytokines, chemokines, and reactive oxygen or nitrogen species. Tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6 are pro-inflammatory cytokines largely produced in acute phase of inflammation. Nitric oxide is other important pro-inflammatory mediator, and its secretion occurs by various stimuli such as the bacterial cell wall component (lipopolysaccharides, LPS) in RAW 264.7 macrophages (Tilg and Moschen, 2008). The balance of pro-inflammatory marker is crucial for a proper inflammatory response and it is the aim of several search of new compounds with anti-inflammatory activity. Various studies point out that secondary metabolites of plants or fungi exert anti-inflammatory effects by inhibiting pro-inflammatory markers, which can act as chemopreventive substances (Oliveira et al., 2019; Teles et al., 2020).
There is a need for drugs that modulate the immune system, playing a role as anti-inflammatory and immunosuppressant agents directed to pro-inflammatory factors or their receptors by inhibiting or controlling inflammatory cascades. The identification of active substances with pro or anti-inflammatory potential is useful for the development of therapeutic drugs for inflammatory diseases (Mo et al., 2013; El-Shitany et al., 2015).
Studies carried out in the marine environment prove its potential as a rich source of active metabolites with pharmacological potential. Fungi isolated from this environment can produce a multitude of substances. An example is strains of Talaromyces purpurogenus, first identified as Penicillium purpurogenum, that appear with a vast diversity and as a rich source of metabolites of different chemical classes. Described by Stoll (1903–1904) and isolated in a culture contaminant of Aspergillus oryzae, T. purpurogenus presents dark colonies with mycelium varying from pinkish to yellow and yellow red, and over culture time produces its characteristic red pigments on potato agar. Benjamin (1955) introduced the Talaromyces nomenclature, in which 40 species from Penicillium subg. Biverticillium were relocated and combined into Talaromyces genus (Samson et al., 2011; Yilmaz et al., 2012).
Metabolites of T. purpurogenus have wide structural diversity with varied biological and pharmacological properties, such as antimicrobial, anti-tumor and anti-inflammatory activities (Etoh et al., 2013; Shaaban et al., 2016; Wang et al., 2016; Li et al., 2017; Jin et al., 2018; Wang et al., 2020). It was also reported the anti-inflammatory effects of a mutant strain of P. purpurogenum but the activity was above the cytotoxic dose (Etoh et al., 2013). Meroterpenoids of P. purpurogenum MHZ 111, a mutant strain resistant to antibiotics, showed inhibitory effect on NO production by macrophages stimulated with LPS demonstrating its anti-inflammatory potential (Sun et al., 2016; Sun et al., 2017).
In our previous report using T. purpurogenus strain isolated from polluted marine environment, the Jansen lagoon in the state of Maranhão, Brazil, we demonstrated that the culture broth ethyl acetate extract from T. purpurogenus reduced the cellular infiltration of lymphoid organs and circulating levels of TNF-α (Teles et al., 2020). To elucidate this anti-inflammatory effect using proper models, we aimed in this study to evaluate the effect of ethyl acetate extracts from T. purpurogenus culture broth and mycelium, isolated from the marine environment, on inflammatory makers in LPS-stimulated RAW 264.7 cells in vitro, and in vivo in murine model of carrageenan-induced acute paw edema.
2 Materials and Methods
2.1 Culture of T. purpurogenus and obtaining ethyl acetate extracts
Talaromyces purpurogenus MA52 was cultured on Potato Dextrose Agar (PDA) at 28 °C for 7 days until complete growth. After this period, superficial agar circles containing mycelium were subsequently grown in BDA broth for fermentation at 28 °C for 21 days on a rotary shaker (Quimis, São Paulo, Brazil) at 150 rpm. The culture gave rise to the culture broth ethyl acetate extract (CBEAE) already described elsewhere (Teles et al., 2020) and the mycelial ethyl acetate extract (MEAE) in which the mycelial biomass, after total removal of the culture medium by vacuum filtration, was stored in a flask containing 250 mL of 70% ethanol and kept under constant agitation at 115 rpm for 24 h. The obtained extract was filtered and concentrated under reduced pressure until total removal of ethanol. To the remaining aqueous part, ethyl acetate was added in the proportion 1:3, which after separation in a separatory funnel and elimination of the solvent in a rotary evaporator and lyophilization, led to the MEAE used during our research. To perform the biological tests, the CBEAE and MEAE were solubilized in 500 μL dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St Louis, MO, USA) at 100× the final concentration for the in vitro tests, and the final test concentrations was diluted in Dulbecco Modified Eagle Medium (DMEM; Sigma-Aldrich, St Louis, MO, USA). The culture medium showed less than 0.5% DMSO in in vitro tests. For in vivo assay, the dilution extracts were prepared immediately before use by dilution in PBS.
2.2 High-performance liquid chromatograph coupled to diode-array UV–Vis detector (HPLC-DAD-UV)
High-performance liquid chromatograph coupled with a diode-array UV–Vis detector (HPLC-DAD-UV) analysis performed out using a Shimadzu Nexera XR® liquid chromatograph (Shimadzu, Kyoto, Japan) coupled to a UV detector with an SPDM20A diode array. A CBM20A controller, a DGU20A degasser, an LC20AD binary pump, a CTO20A oven, and an SILA20A auto-injector are also components of the system. The chromatographic separation of samples was achieved on a reversed-phase HPLC column (Shimpack CLC-ODS, Thermo, Waltham, MA, United States, 250 mm × 4.6 mm i.d. × 5 μm particle size). The column temperature was maintained at 50 °C, the injection volume was 10.0 μL, and a 1.0 mL/min flow rate was applied using a linear gradient of 0.0% (w/w) solvent B (methanol, HPLC grade, Tedia, Rio de Janeiro, Brazil) in ultrapure water (solvent A). The optimized gradients employed for the extracts consisted of 0–20% B in A over 0–8.5 min, subsequently rising to 100% of B in 68.5 min, and finally staying at this concentration up to 90 min. Compounds were analyzed at 254 nm. Data were acquired and processed with Shimadzu LabSolutions Software Version 5.3 (Shimadzu, Kyoto, Japan).
2.3 Quantification of endotoxins
The endotoxin quantification in CBEAE and MEAE extracts dilutions at 100, 250 and 500 µg/mL in DMEM culture medium (Sigma-Aldrich, St Louis, MO, USA) was performed following the references of the Pierce ™ LAL Chromogenic Endotoxin Quantitation Kit (Thermo Scientific, Carlsbad, CA, USA).
2.4 Cell culture
In vitro tests were carried out with murine macrophages RAW 264.7 cell line (ATCC® TIB-71™) cultured in DMEM supplemented with 10% fetal bovine serum (FBS) (Gibco, Gaithersburg, MD, USA), penicillin (100 U/mL) and streptomycin (100 μg/mL) (Sigma-Aldrich, St Louis, MO, USA) at 37 °C and 5% CO2 in 75 cm2 cell culture flasks.
2.5 Cytotoxicity assay
The cytotoxic assay was performed in 96-well plates. A volume of 100 µL per well of culture cell suspension at 2 × 106 cells/mL were incubated overnight at 37 °C and 5 % CO2. Then, the medium was completely removed, and cells were treated with 100 µL of one of T. purpurogenus extracts at 100, 250 and 500 µg/mL, LPS (Sigma-Aldrich, St Louis, MO, USA) at 10 µg/mL, or dexamethasone (Sigma-Aldrich, St Louis, MO, USA) at 100 µM. Cells treated with DMSO 1% in DMEM and wells only with medium were maintained as controls and blanks, respectively. After 48 h, cell viability was obtained using the colorimetric MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (Sigma-Aldrich, St Louis, MO, USA) assay with modifications. In brief, 10 µL of MTT at 5 mg/mL was added to each well and kept at 37 °C and 5% CO2. After 2 h, the supernatant was completely removed, 100 µL of DMSO was added to solubilize the formazan crystals with the aid of a plate shaker for 15 min. The absorbance was determined in a spectrophotometer EZ Read 400 (Biochrom, Cambridge, UK) at a wavelength of 570 nm. Cytotoxicity was demonstrated as a percentage corresponding to viability calculated as described elsewhere (Teles et al., 2021).
2.6 Treatment of RAW 264.7 macrophages LPS-stimulated with T. purpurogenus
The amount of 500 µL of RAW 264.7 macrophages suspension at 2 × 106 cells/mL were cultured in 24-well plates overnight and after, the medium was completely removed with non-adherent cells. The adherent cells were treated with 1 mL of CBEAE or MEAE (100, 250 and 500 µg/mL), dexamethasone (100 µM) or DMSO 1% (control) for one hour, and then stimulated, or not, with LPS (10 µg/mL). The dilution of compounds used in the treatments were made in DMEM medium. After 48 h, the cell supernatant was collected for nitrite and cytokines quantification.
2.7 Quantification of nitrite and cytokines
Nitrite quantification used the Griess method (Green et al., 1982) with some modifications. In 96-well plates, 50 µL of cell supernatant was mixed to 50 µL of Griess reagent (25 µL of sulfanilamide 1% in 2.5% H3PO4 solution and 25 µL of N-(1-naphthyl) ethylenediamine 0.1% solution) for 10 min protected from light. After, the absorbance was determined with the spectrophotometer EZ Read 400 at a wavelength of 570 nm. Nitrite levels were obtained from a standard curve with known NaNO2 (Sigma-Aldrich, St Louis, MO, USA) concentrations (100 to 3.1 µM) prepared by serial dilution 1:2 (Almeida-Souza et al., 2020). The quantification of cytokine levels TNF-α, IL-1β and IL-6 was performed by Enzyme-Linked Immunosorbent Assay (ELISA) using the kits BD OptEIA™ (BD Bioscience, East Rutherford, NJ, USA) following the manufacturer’s specifications.
2.8 Animals and ethical statement
Female BALB/c mice from six to eight weeks-old from the Institute of Science and Technology in Biomodels (ICTB/FIOCRUZ) were maintained under pathogen-free environments, controlled temperature, and food and water ad libitum. All the protocols with animals followed the National Council for the Control of Animal Experimentation (CONCEA) recommendations and were previously approved by the Ethics Commission for the Use of Animals of the Oswaldo Cruz Institute, license number CEUA/IOC—L053/2016.
2.9 Treatment of mice paw edema induced by λ-carrageenan with T. purpurogenus
Paw edema induced by λ-carrageenan was carried out as described elsewhere (Oliveira et al., 2019). Mice were separated into six groups of five animals. Four groups were pre-treated with 125, 250 or 500 mg/kg of CBEAE by gavage, or with 5 mg/kg of dexamethasone in PBS via intramuscular route. The other two groups were pre-treated with PBS by gavage. After one hour, 25 µL of λ-carrageenan 1% was injected into the left hind footpad. One group was inoculated with PBS and pre-treated with PBS and maintained as control. The footpad inflammation was measured after 1, 2, 3 and 4 h of λ-carrageenan inoculation using a Schnelltaster dial gauge caliper (Kröplin GmbH, Hessen, Germany). The difference in millimeters between the inoculated footpad and the contralateral footpad at the same evaluation time was used to obtain the edema thickness. Four hours after λ-carrageenan inoculation the animals were euthanized with 250 μL intraperitoneal injection of a 1:1 mixture of ketamine (100 mg/mL; Syntec, BRA) and xylazine (20 mg/mL; Syntec, BRA). Footpad fragments were collected for histological processing.
2.10 Histology analysis
For histology evaluation, skin fragments from footpad were fixed in 10% buffered formalin and routinely processed for paraffin embedding. Tissue sections in paraffin were cut at 5 μm thick and stained with Toluidine blue 1% for the quantification of mast cells. The histology slides were analyzed with the aid of a light microscopy by a researcher with expertise, blinded to the experimental groups. Mast cells counting were performed under a light microscope and representative areas in five fields were selected (Oliveira et al., 2019). Images were obtained with Leica DM500 coupled with Leica ICC50 HD using the software Leica Application Suite EZ (Leica Microsystems GmbH, Wetzlar, Germany).
2.11 Statistical analysis
The data was represented as mean ± standard error of mean and plotted in graphs or organized in tables. Statistical analyses were carried out by Kruskal-Wallis followed by Dunn’s multiple comparisons tests (p < 0.05) or by analysis of variance (two-way ANOVA) followed by Bonferroni’s multiple comparisons test (p < 0.05) using GraphPad Prism 7.00 software package (GraphPad Software, San Diego, CA, USA).
3 Results
3.1 T. purpurogenus extracts profile by HPLC-DAD-UV
The extracts profiles from T. purpurogenus were determined by HPLC-DAD-UV analyses. The obtained chromatograms at 254 nm are presented at Fig. 1. CBEAE showed 9 peaks (Fig. 1a), all of them presenting UV λmax in the range of 258–282 nm, suggesting the presence of meroterpenoid compounds while MEAE showed 3 main peaks (Fig. 1b). Their retention times and areas are showed at Table 1. Peaks 2 and 3 were common to both extracts, but peak 9 (Rt = 29.07 min), exclusively found at MEAE, showed characteristic bands different from the other ones, presenting absorption with maximum of 355 nm (band I) and 256 (band II), suggesting the presence of polyphenolic compound (Supplementary material Fig. S1). Rt: retention time; CBEAE: culture broth ethyl acetate extract; MEAE: mycelial ethyl acetate extracts of T. purpurogenus.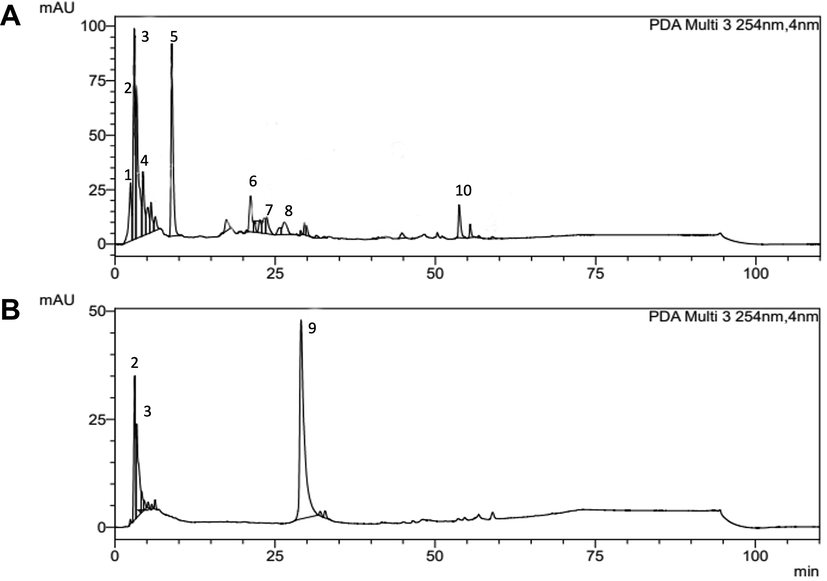
HPLC-DAD-UV chromatograms of Talaromyces purpurogenus extracts at 254 nm: (A) culture broth ethyl acetate extract (CBEAE) showing 9 peaks and (B) mycelial ethyl acetate extracts (MEAE) showing 3 main peaks.
Peak
Rt (min)
Area %
UV (λmax, nm)
CBEAE
MEAE
1
2.46
5.66
–
258
2
3.10
5.88
16.61
275
3
3.37
31.92
18.05
259
4
4.36
8.96
–
264
5
8.87
25.14
–
272
6
20.75
7.24
–
269
7
23.92
5.14
–
276
8
27.06
5.88
–
270
9
29.07
–
65.33
256, 355
10
53.91
4.13
–
278
3.2 T. purpurogenus extracts displayed absence of endotoxin and cytotoxicity
The endotoxin quantification carried out in T. purpurogenus extracts showed endotoxin absence in all the three dilutions used in further cells treatment. Both extracts also not displayed cytotoxicity in cells submitted to the same conditions of treatment and/or stimulation experiments, with all of them presenting not statistically significant difference in the percentage of viable cells when compared to untreated and/or unstimulated cells (Fig. 2).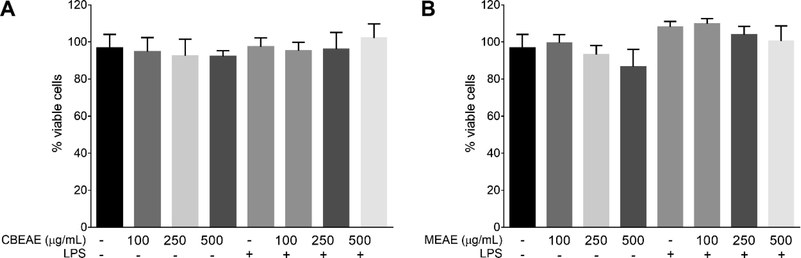
Talaromyces purpurogenus in vitro toxicity in RAW 264.7 macrophages. Viability after 48 h of cells stimulated or non-stimulated with LPS at 10 μg/mL and treated with culture broth ethyl acetate extract (CBEAE) (A) or mycelial ethyl acetate extract (MEAE) (B) of T. purpurogenus. Data represents the mean ± standard error of mean of the experiment carried out at least in quintuplicate.
3.3 T. purpurogenus reduced levels of nitrite and pro-inflammatory cytokines in RAW 264.7 cells
The effect of T. purpurogenus extracts was investigated in RAW 264.7 macrophages stimulated or not with LPS. In cells not stimulated with LPS, CBEAE displayed low levels of nitrite at 250 and 500 μg/mL (p = 0.0388 and p = 0.0353, respectively; Fig. 3a), IL-1β at 500 μg/mL (p = 0.0386; Fig. 3e), and not altered IL-6 levels at any concentration when compared to untreated and non-stimulated cells. In LPS-stimulated cells, both extracts presented low levels of nitrite and cytokines. CBEAE exhibited low nitrite levels at 500 μg/mL (p = 0.0450; Fig. 3a), TNF-α at 250 and 500 μg/mL (p = 0.0132 and p = 0.0076, respectively; Fig. 3c), IL-1β at 250 and 500 μg/mL (p = 0.0013 and p = 0.0042, respectively; Fig. 3e), and IL-6 at 500 μg/mL (p = 0.0234; Fig. 3g). The levels of IL-1β in LPS-stimulated cells treated with CBEAE at 250 and 500 μg/mL (83.33 ± 16.57 and 75.00 ± 11.55 pg/mL, respectively) was similar to that observed in LPS-stimulated cells treated with dexamethasone (85.75 ± 15.32 pg/mL). On the other hand, the TNF-α levels of CBEAE presented a lower mean at 250 and 500 μg/mL (208.3 ± 58.84 and 150.00 ± 46.77 pg/mL, respectively) than that observed in LPS-stimulated cells treated with dexamethasone (249.5 ± 18.44 pg/mL), although its statistically similar. MEAE presented similar reduction pattern than CBEAE in nitrite at 500 μg/mL (p = 0.0497; Fig. 3b), TNF-α at 250 and 500 μg/mL (p = 0.0028 and p = 0.0001, respectively; Fig. 3d), and IL-1β levels at 250 and 500 μg/mL (p = 0.0017 and p = 0.0031, respectively; Fig. 3f), but not in IL-6 in LPS-stimulated cells. Although MEAE induced a reduction concentration-dependent in IL-6 levels of 16.22% and 41.13% in cells treated with 250 and 500 μg/mL respectively, it was not statistically significant when compared to LPS-stimulated and untreated cells (Fig. 3h).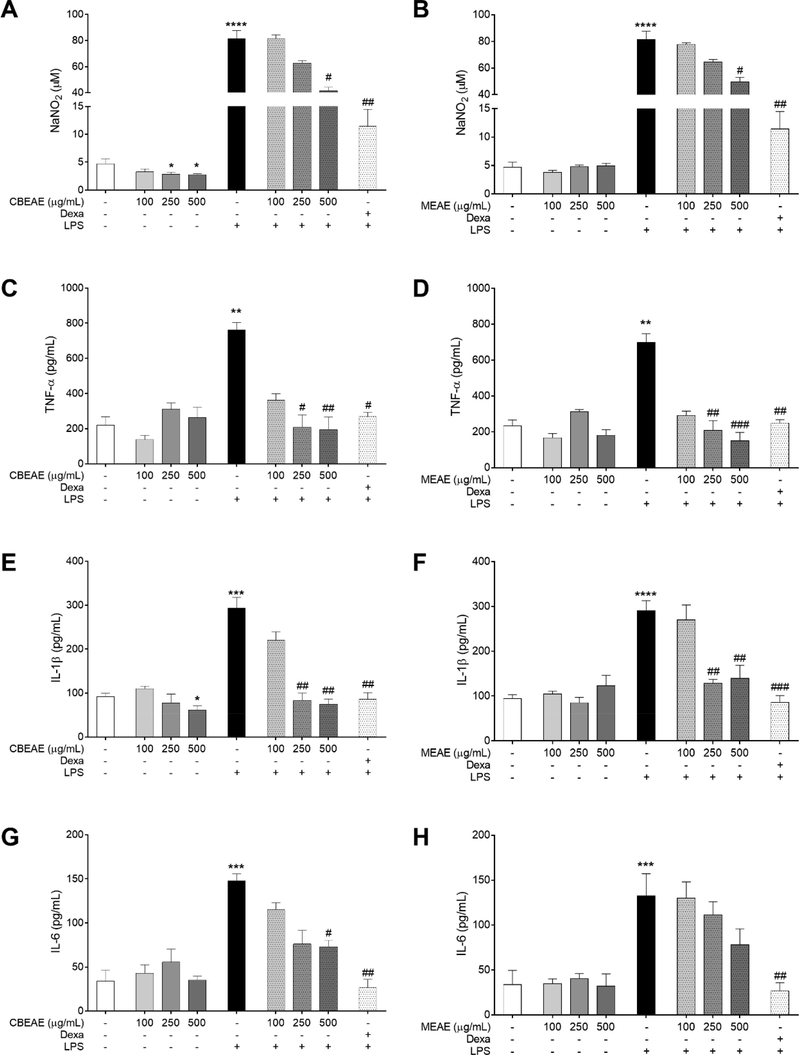
Quantification of pro-inflammatory markers in RAW 264.7 macrophages treated with Talaromyces purpurogenus. Nitrite (A-B) and cytokine levels (C–H) in supernatants of cells culture stimulated or non-stimulated with LPS at 10 μg/mL and treated for 48 h with culture broth ethyl acetate extract (CBEAE) or mycelial ethyl acetate extract (MEAE) of T. purpurogenus, or dexamethasone (Dexa) at 100 μM. Data represent the mean ± standard error of mean of experiment carried out at least in triplicate. **p < 0.01, ***p < 0.001, ****p < 0.0001 when compared with untreated and unstimulated cells and #p < 0.05, ##p < 0.01, ###p < 0.001 when compared with untreated and LPS-stimulated cells by Kruskal-Wallis and Dunn’s multiple comparisons tests.
3.4 T. purpurogenus inhibited λ-carrageenan-induced paw edema
The CBEAE obtained from T. purpurogenus was used to treat mice with λ-carrageenan-induced paw edema. Edema thickness, edema histology and mast cells count of paw edema were used to evaluate the treatment effect (Fig. 4A). Edema thickness showed that CBEAE treatment had a dose-dependent action, with an increase in inhibition effect of edema growth as the treatment doses increase. Thereby, four hours after edema induction, 500 and 250 mg/kg showed the highest inhibition edema, and although 125 mg/kg dose treatment inhibited edema thickness in 24.69%, this inhibition was not statistically significant. The effect of CBEAE treatment at 500 mg/kg was similar to the inhibition induced by dexamethasone treatment at 4 h. CBEAE treatment at 500 mg/kg also showed edema thickness inhibition at three hours after edema induction, demonstrating its potent edema inhibition effect (Fig. 4B and Table 2). The histology analysis of paw edema showed that treatment with 250 and 500 mg/kg presented less inflammatory cells than control group (Fig. 5A). Mast cells quantification exhibited similar result, with 250 and 500 mg/kg displaying less mast cells (12.6 ± 3.04, p = 0.0303; and 8.8 ± 2.53, p = 0.0130 respectively) than group with λ-carrageenan edema treated with PBS (24.6 ± 3.67; Fig. 5B). Data represents the mean ± standard error of mean of experiment performed at least in triplicate. 1No edema was observed in this group at this evaluation time. * p < 0.05, ** p < 0.01, *** p < 0.01 and **** p < 0.0001 when compared to group with edema induced by λ-carrageenan treated with PBS by analysis of variance (two-way ANOVA) and Bonferroni’s multiple comparisons test. +: λ-carrageenan inoculation; –: PBS administration.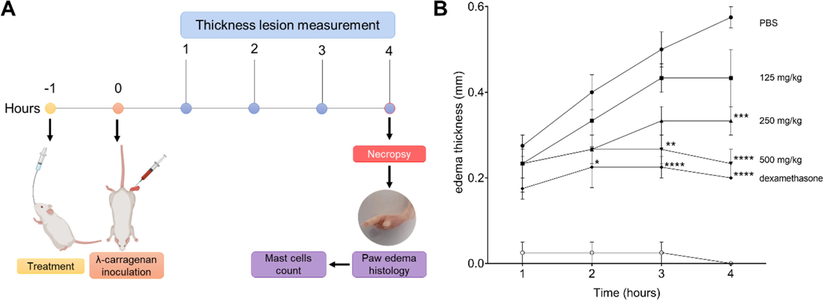
Paw edema thickness of BALB/c mice inoculated with of λ-carrageenan and treated with Talaromyces purpurogenus. (A) Overview of in vivo study design. (B) Lesion kinetic of animals inoculated in footpad with 25 μL of λ-carrageenan 1 % and treated with 100 μL culture broth ethyl acetate extract (CBEAE) of T. purpurogenus at 125, 250 or 500 mg/kg by gavage or with dexamethasone at 5 mg/kg via the intramuscular route. The data represent an experiment carried out in quintuplicate. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, when compared with PBS group by analysis of variance (two-way ANOVA) and Bonferroni’s multiple comparisons tests.
Treatment
λ-Carragenan
Dose
(mg/kg)Edema thickness after treatment (% Edema Inhibition)
1 h
2 h
3 h
4 h
PBS
–
–
0.025 ± 0.050
0.025 ± 0.050
0.025 ± 0.050
– 1
+
–
0.275 ± 0.050
0.400 ± 0.082
0.500 ± 0.082
0.575 ± 0.050
CBEAE
+
125
0.233 ± 0.058
0.333 ± 0.115
0.433 ± 0.058 (13.40)
0.433 ± 0.115 (24.69)
+
250
0.233 ± 0.115
0.267 ± 0.058
0.333 ± 0.058 (33.40)
0.333 ± 0.058 (42.60)***
+
500
0.233 ± 0.058
0.267 ± 0.058
0.267 ± 0.058 (46.60)**
0.233 ± 0.058 (59.47)****
Dexa
+
5
0.175 ± 0.050
0.225 ± 0.096 (43.75)*
0.225 ± 0.050 (55.00)****
0.200 ± 0.000 (65.21)****
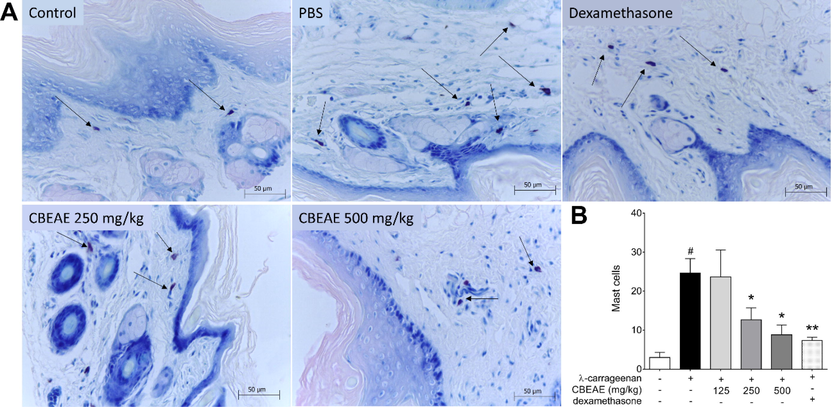
Mast cells of BALB/c mice footpad inoculated with λ-carrageenan 1 % and treated with culture broth ethyl acetate extract (CBEAE) of Talaromyces purpurogenus. (A) Histology of mast cells (arrows) and (B) mast cells counting. Animals were treated with 100 μL of CBEAE at 125, 250 or 500 mg/kg by gavage or with dexamethasone at 5 mg/kg via the intramuscular route. Data represent the mean ± standard error of mean of experiment carried out at least in triplicate. #p < 0.01 compared with the group without stimulation or treatment; *p < 0.05, **p < 0.01 when compared with the λ-carrageenan-stimulated and untreated group after Kruskal-Wallis and Dunn’s multiple comparisons tests. Toluidine blue 1%. 40X magnification.
4 Discussion
The analysis by HPLC-DAD-UV of both T. purpurogenus extracts allowed to point out a significant difference in its chemical composition. The CBEAE was characterized by the predominant presence of 9 meroterpenoidal compounds, while MEAE, in addition to meroterpenoids, also presented one poliphenolic compound. Meroterpenoidal compounds, which are formed by the mixed terpenoid-polyketide biosynthetic pathway, such as macrophorin A, purpurogemutantidine, and purpurogemutantin (Fang et al., 2012; He et al., 2017; Tang et al., 2017), are commonly found at the extracellular extract of this fungus. We previously identified the meroterpenoid compounds purpurogemutantin, purpurogemutantidin macrophorin A, berkeleyacetal C and rubratoxin B in CBEAE (Teles et al., 2020), confirming its rich chemical composition compared to MEAE.
The compounds previously identified in T. purpurogenus have shown interesting anticancer activity. Berkeleyacetal C (Etoh et al., 2013) and rubratoxin B (Wang et al., 2007; Nagashima, 2015) showed anti-inflammatory and anticancer activity in vitro. Pigments obtained from P. purpurogenum, rich in polyphenolic compounds and quinone, showed maximum inhibition of lipid peroxidation (30.15%), hydroxyl radical (74.92%) and NO radical scavenging activity (43.23%) at 20 mg/mL of pigment concentration (Padmapriya and Murugesan, 2016). Another study indicated the presence of flavone derived compound, talaroflavone, and other polyphenolic compound as bioactive metabolite of P. purpurogenum (Shaaban et al., 2016).
Before all the experiments, it was performed the quantification of endotoxins. Their presence it would be an indication of a possible contamination of CBEAE and MEAE. The endotoxin quantification carried out in T. purpurogenus extracts showed endotoxin absence in all the three dilutions used in further cells treatment. Endotoxin contamination, specially LPS, might result in repeated stimuli exposure which can lead to a state of tolerance that reprograms the inflammatory response, resulting in reduced inflammatory cytokine production in vitro and in vivo (Seeley and Ghosh, 2017). Thus, the results obtained in the posterior experiments did not suffer contaminant interference and express the action only of the compounds present in the extracts.
Both extracts also not displayed cytotoxicity in murine macrophages RAW 264.7 cells submitted to the same conditions of treatment and/or stimulation experiments, with all of them presenting not statistically significant difference in the percentage of viable cells when compared to untreated and/or unstimulated cells. The maintenance of the amount of cells in all treatments makes it possible to ensure that changes in the quantification of pro-inflammatory markers occur due to the direct action of the treatment in the cells, rather than to changes in the amount of cells.
The MEAE not changed nitrite or cytokine levels in the treatment concentrations used in non-stimulated cells. These results are important because the cells used in the treatments are macrophages that can trigger an inflammatory response, secreting several pro-inflammatory mediators and chemokines, such as NO, TNF-α, IL-6, IL-1β, MIP-1α and MCP-1, in which activation has been associated with the pathogenesis of acute and chronic inflammatory disorders (Li et al., 2017). The inhibition in non-stimulated cells reassure the lack of contamination, indicating an inhibitory effect of CBEAE even in normal situations.
In our experiments, NO was indirectly measured by nitrite quantification on cell culture supernatant. NO is an inflammatory mediator synthesized mainly by NO synthase enzymes using its isomorphic iNOS (NOS inducible), which can be induced by LPS, TNF-α, IL-1β in macrophages during the inflammatory response. Substances that produce anti-inflammatory activity by various signaling pathways can be found by quantifying NO (Farlik et al., 2010). Our results verified the reduction of nitrite quantification in RAW 264.7 cells treated with both CBEAE and MEAE, showed an inhibitory effect on NO production, as well as on the production of cytokines IL-6 and IL-1β.
The IL-1β when in low concentrations induce a local inflammatory response that ends up provoking a protective immune response (Apte and Voronov, 2002) The reduction of IL-6 by extracts is of fundamental relevance as IL-6 was found in increased levels in cancer patients and biopsies (Kai et al., 2005). The extract acting on the reduction of cytokines is in fact contributing to the inhibition of the development of a neoplasm.
Comparing the chemical composition of CBEAE with MEAE, the results obtained by in vitro experiments can be justified by the richer meroterpenoid composition observed in CBEAE than that in MEAE. Thus, we decided to continue the further experiments only with CBEAE. The use of CBEAE also is an advantage from the point of view of biotechnology, since the fungus can continue to be cultivated producing more metabolites that would be externalized to the culture broth medium. It was performed the in vivo inflammation model of carrageenan-induced mouse paw edema, a model of classic acute inflammation that is susceptible to the anti-inflammatory effects of drugs as well as the extract under study. The model shows us an acceptable response because carrageenan induces several reactions in humans very similar to acute inflammation, such as local telangiectasia, increased vascular permeability, plasma leakage and rapid edema (Dai et al., 2019).
The edema settles down due to vascular phenomena that generate vasodilatation, leading to deregulation of the osmotic balance and exudate effusion. The vasodilatation allows leukocyte extravasation by diapedesis, and cellular migration by chemotaxis to the inflammatory site (Kumar et al., 2011; Almeida-Souza et al., 2016). One of the cells that participate actively in cell migration is mast cells, therefore we quantified mast cells in paw edema of animals treated with CBEAE. Histologically, it was verified that in the highest doses there were fewer inflammatory cells, the same being observed in the quantification of mast cells when compared to the group with λ-carrageenan edema treated with PBS.
Mast cells are located in the mucosa and connective tissues, where they decrease inflammation after the release of pro-inflammatory molecules, such as histamine, proteases, proteoglycans, chymosins, arachidonic acid, growth factors and TNF-α (Popovici et al., 2014). Mast cells have a fundamental protective role in wound healing, thus acting directly on anti-inflammatory activity and defense against pathogens (Stone et al., 2010). The inhibition of mast cells by compounds as flavonoids showed an anti-inflammatory reaction (Weng et al., 2012), and mast cell cellularity in inflamed tissues reflects a good or bad prognosis and efficacy of anti-inflammatory drugs (Popovici et al., 2014). Our results demonstrate that the decrease in its cellularity is an important sign of the anti-inflammatory action of the CBEAE.
5 Conclusion
The two extracts obtained from T. purpurogenus demonstrated the presence of meroterpenoids. The CBEAE revealed to have a richer chemical composition than MEAE, presenting nine meroterpenoid compounds, while MEAE displayed only two meroterpenoid and one phenolic compound. Both extracts inhibited nitrite and IL-1β, but only CBEAE inhibited IL-6 production in LPS-stimulated cells. The in vitro inhibition activity in LPS-stimulated cells may be related to the presence of meroterpenoids observed in CBEAE. CBEAE also inhibited edema thickness and mast cells in λ-carrageenan-induced mice paw edema. The CBEAE from T. purpurogenus isolated from marine-polluted environment demonstrated a meroterpenoid-rich chemical composition, and a promising anti-inflammatory activity observed in acute inflammation in vivo model and in vitro LPS-stimulated cells model. The inhibitory effect on pro-inflammatory marker demonstrated the potential of T. purpurogenus for further studies to elucidate its mechanism of action.
Acknowledgement
This research was funded by the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior do Brazil—CAPES) [grant number Finance Code 001]. The APC was funded by the Oswaldo Cruz Institute (Instituto Oswaldo Cruz—IOC). Fernando Almeida-Souza is a postdoctoral researcher fellow of CAPES [grant number 88887.363006/2019-00]. Dr. Ana Lucia Abreu-Silva is a research productivity fellow of the National Scientific and Technological Development Council (Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq) [grant number 309885/2017-5].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Morinda citrifolia Linn. reduces parasite load and modulates cytokines and extracellular matrix proteins in C57BL/6 mice infected with Leishmania (Leishmania) amazonensis. PLoS Negl. Trop. Dis.. 2016;10(8)
- [CrossRef] [Google Scholar]
- 1,4-Disubstituted-1,2,3-triazole compounds induce ultrastructural alterations in Leishmania amazonensis promastigote: an in vitro antileishmanial and in silico pharmacokinetic study. Int. J. Mol. Sci.. 2020;21(18)
- [CrossRef] [Google Scholar]
- Interleukin-1-a major pleiotropic cytokine in tumor-host interactions. Semin. Cancer Biol.. 2002;12(4):277-290.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effects of different elution fractions of Er-Miao-San on acute inflammation induced by carrageenan in rat paw tissue. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res.. 2019;25:7958-7965.
- [CrossRef] [Google Scholar]
- The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. Hematol.. 2017;116:134-146.
- [CrossRef] [Google Scholar]
- Evaluation of the anti-inflammatory, antioxidant and immunomodulatory effects of the organic extract of the Red Sea marine sponge Xestospongia testudinaria against carrageenan induced rat paw inflammation. PloS One. 2015;10(9):e0138917.
- [Google Scholar]
- Anti-inflammatory effect of berkeleyacetal C through the inhibition of interleukin-1 receptor-associated kinase-4 activity. Eur. J. Pharmacol.. 2013;698:1-3.
- [CrossRef] [Google Scholar]
- Purpurogemutantin and purpurogemutantidin, new drimenyl cyclohexenone derivatives produced by a mutant obtained by diethyl sulfate mutagenesis of a marine-derived Penicillium purpurogenum G59. Mar. Drugs. 2012;10(6)
- [CrossRef] [Google Scholar]
- Nonconventional initiation complex assembly by STAT and NF-kappaB transcription factors regulates nitric oxide synthase expression. Immunity. 2010;33(1)
- [CrossRef] [Google Scholar]
- Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem.. 1982;126(1):131-138.
- [Google Scholar]
- Quinone/hydroquinone meroterpenoids with antitubercular and cytotoxic activities produced by the sponge-derived fungus Gliomastix sp. ZSDS1-F7. Nat. Prod. Res.. 2017;31(5):604-609.
- [CrossRef] [Google Scholar]
- Chemical composition, security and bioactivity of the red pigment from Penicillium purpurogenum Li-3. Chem. Biodivers.. 2018;15(12):e1800300
- [CrossRef] [Google Scholar]
- Involvement of proinflammatory cytokines IL-1beta and IL-6 in progression of human gastric carcinoma. Anticancer Res.. 2005;25(2A)
- [Google Scholar]
- Inflammatory response and extracorporeal circulation. Best. Pract. Res. Clin. Anaesthesiol.. 2015;29(2):113-123.
- [CrossRef] [Google Scholar]
- Pathogen recognition by the innate immune system. Int. Rev. Immunol.. 2011;30(1):16-34.
- [CrossRef] [Google Scholar]
- Berkeleyacetal C, a meroterpenoid isolated from the fungus Penicillium purpurogenum MHZ 111, exerts anti-inflammatory effects via inhibiting NF-κB, ERK1/2 and IRF3 signaling pathways. Eur. J. Pharmacol.. 2017;814:283-293.
- [CrossRef] [Google Scholar]
- Topical anti-inflammatory and analgesic activities of standardized pomegranate rind extract in comparison with its marker compound ellagic acid in vivo. J. Ethnopharmacol.. 2013;148(3):901-908.
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem.. 2010;122(4):987-996.
- [CrossRef] [Google Scholar]
- Rubratoxin-B-induced secretion of chemokine ligands of cysteine-cysteine motif chemokine receptor 5 (CCR5) and its dependence on heat shock protein 90 in HL60 cells. Environ. Toxicol. Pharmacol.. 2015;40(3):997-1000.
- [CrossRef] [Google Scholar]
- Vernonia Polysphaera Baker: anti-inflammatory activity in vivo and inhibitory effect in LPS-stimulated RAW 264.7 cells. PloS One.. 2019;14(12):e0225275.
- [Google Scholar]
- Characterization of methanolic extract of red pigment from Penicillium purpurogenum and its antioxidant activity. J. Pure Appl. Microbiol.. 2016;10(2):6.
- [Google Scholar]
- Mast cells as key players in periodontal disease. Arch. Biol. Sci.. 2014;66(2):801-809.
- [CrossRef] [Google Scholar]
- The association between inflammation and immunosuppression: implications for ICI biomarker development. Onco Targets Ther.. 2021;14:2053-2064.
- [Google Scholar]
- Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud. Mycol.. 2011;70(1)
- [CrossRef] [Google Scholar]
- Molecular mechanisms of innate memory and tolerance to LPS. J. Leukoc. Biol.. 2017;101(1):107-119.
- [CrossRef] [Google Scholar]
- New bioactive metabolites from Penicillium purpurogenum MM. Z. Naturforsch. B. 2016;71(4):287-295.
- [CrossRef] [Google Scholar]
- IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol.. 2010;125(2):S73-S80.
- [CrossRef] [Google Scholar]
- Nitric oxide inhibitory meroterpenoids from the fungus Penicillium purpurogenum MHZ 111. J. Nat. Prod.. 2016;79(5):1415-1422.
- [CrossRef] [Google Scholar]
- Anti-neuroinflammatory constituents from the fungus Penicillium purpurogenum MHZ 111. Nat. Prod. Res.. 2017;31(5):562-567.
- [CrossRef] [Google Scholar]
- Late-stage terpene cyclization by an integral membrane cyclase in the biosynthesis of isoprenoid epoxycyclohexenone natural products. Org. Lett.. 2017;19(19):5376-5379.
- [CrossRef] [Google Scholar]
- Marine-derived Penicillium purpurogenum reduces tumor size and ameliorates inflammation in an Erlich mice model. Mar. Drugs. 2020;18(11):541.
- [CrossRef] [Google Scholar]
- GC-MS Characterization of antibacterial, antioxidant, and antitrypanosomal activity of Syzygium aromaticum essential oil and eugenol. Evid. Based Complement. Alternat. Med.. 2021;2021:1-12.
- [CrossRef] [Google Scholar]
- Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med.. 2008;14:222-231.
- [CrossRef] [Google Scholar]
- A new cyclic dipeptide penicimutide: the activated production of cyclic dipeptides by introduction of neomycin-resistance in the marine-derived fungus Penicillium purpurogenum G59. Arch. Pharm. Res.. 2016;39(6):762-770.
- [CrossRef] [Google Scholar]
- Penicimutanin C, a new alkaloidal compound, isolated from a neomycin-resistant mutant 3-f-31of Penicillium purpurogenum G59. Chem. Biodivers.. 2020;17(7)
- [CrossRef] [Google Scholar]
- Anti-tumor effects of Rubratoxin B on cell toxicity, inhibition of cell proliferation, cytotoxic activity and matrix metalloproteinase-2,9. Toxicol. In Vitro. 2007;21(4):646-650.
- [CrossRef] [Google Scholar]
- Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PloS One. 2012;7(3):e33805.
- [Google Scholar]
- Delimitation and characterisation of Talaromyces purpurogenus and related species. Persoonia. 2012;29
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102021.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







