Translate this page into:
Synthesis, structural characterization of silver(I)-NHC complexes and their antimicrobial, antioxidant and antitumor activities
⁎Corresponding author. lmansour@ksu.edu.sa (Lamjed Mansour)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
To prepare a novel series of silver (I) complexes, the interaction of benzimidazolium salts having their two nitrogen atoms substituted by bulky groups with Ag2O in DMF has been carried out. Their structures were characterized by elemental analyses, 1H NMR, 13C NMR and IR spectroscopy techniques. Further, the antibacterial properties of both the salts and their silver(I)-NHC complexes were tested against positive and negative bacteria using the agar dilution procedure. The results show that silver complexes are effective against Salmonella Typhimurium, Listeria monocytogenes, and Micrococcus luteus with moderate to high activity, and their minimum inhibitory concentrations ranging from 0.0024 to 1.25 mg/ml. Moreover, the antioxidant activity determination of these compounds were studied with the DPPH, and compared with (gallic acid “GA“and butylatedhydroxytoluene “BHT “). They exhibited significant antioxidant activities. In addition, the of benzimidazoles salts 2a-j and silver-NHC complexes 3a-j were screened for their antitumor activity. The highest antitumor activity was observed for 3e and 3d Complexes and they exhibited IC50 values 6.85 μg/mL against MCF-7 and 10.75 μg/mL against T47D, respectively.
Keywords
N-heterocyclic carbene
Benzimidazolium salts
Silver(I)-NHC complexes
Antimicrobial
Antioxidant activities and antitumor activities
1 Introduction
In organometallic chemistry, N-heterocyclic carbene (NHC) ligands have generated a great interest (ArduengoIII and Harlow, 1991). They can form stable metal complexes with strong metal-carbon bonds. Silver complexes are of considerable importance among the NHC-metal complex. The reaction of AgO Tf and a free carbene led to the first NHC-Ag(I) complex isolated in 1993 by ArduengoIII et al. (1993). The most common method reported fot synthesis has been the deprotonation with a silver base such as Ag2O, Ag2CO3 and AgOAc (Xia et al., 2017; Patil et al., 2010a,b; Gok et al., 2013). NHC-Ag(I) complexes have been widely used as sources of different metal complexes via transmetallation (Nakamura et al., 2016; Baquero et al.,2013; Deng et al., 2013; Hameury et al., 2014; Iwasaki et al., 2016; Monticelli et al., 2016; Wan et al., 2016; Chardon et al., 2017). These compounds have antimicrobial and anticancer properties (Shahini et al., 2017a,b; Haque et al., 2015; Karatas et al., 2016; Iqbal et al., 2015; Anchez et al., 2016). Also, they have been studied as luminescent materials (Syu et al.,2017; Adhikary et al., 2012; Seth et al., 2013; Lin et al., 2013). There have been limited number of reports related to the use of NHC-Ag(I) complexes in catalysis (Yoshida et al., 2014; Fu et al., 2011; Kilincarslan et al., 2016; Li et al., 2013; Samantaray et al., 2006; Balcan et al.,2013; Fujii et al.,2009; Tasci et al., 2017; Avinash et al., 2008; Datani et al., 2012). The biological interest of benzimidazole derivatives structure comes from their similarity with naturally occurring nucleotides (Cavallo et al.,2005). For this reason, we synthesized a series of new Ag(I) complexes. The study was conducted with two primary objectives. The first objective was to synthetize a series of new Ag(I) complexes and the second objective was to evaluate the antimicrobial, antioxidant and antitumor activities of these compounds. All new benzimidazoles and silver complexes were characterized by elemental analysis including 13C NMR, 1H NMR and FT-IR spectroscopy
2 Experimental
2.1 Preparation of benzimidazolium salts
Reaction of 1-isobutyl-benzimidazole (1 mmol) (1) with various alkyl chloride(1,1mmol) in dimethylformamide (DMF; 5 mL) at 80 °C for 24 h afford benzimidazole salts 2a-j. A white crystalline solid was obtained after adding Diethyl ether (15 mL), which was subsequently filtered off. After washing with diethyl ether (3*10 mL) the solid was dried under vacuum, and the crude product was recrystallized from Dichloromethane/diethyl ether (1:3 ratio).
2.2 1-(isobutyl)-3-(benzyl) benzimidazolium chloride, 2a
Yield 89%, Mp: 128.3 °C, ν(CN) = 1650.9 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 1.06 (d, 6H, CH3 (a,b), J = 4 Hz), 2.44 (Hep, 1H, H2′, J = 8 Hz), 4.44 (d, 2H, H1′), 5.95 (s, 2H, H1′’), 7.30–7.71 (m, 9H, H4, 5, 6, 7, 3″, 4″, 5″, 6″, 7″), 12.07 (s, 1H, H2). 13C NMR (CDCl3, 100 MHz) δ (ppm) 19.8 (Ca,b), 28.8 (C2′), 51.3 (C1″), 54.4 (C1′), 113.1 (C4), 113.8 (C7), 127.0 (C6), 127.1 (C5), 128.3 (C3″;7″), 129.1 (C5′'), 129.3 (C4′';6′'), 131.1 (C9), 131.7 (C8), 132.9 (C2″), 144.0 (C2). Elemental analysis % calcd. (found) for C18H21ClN2:C, 71.866% (71.9); H, 7.036% (7.1); N, 9.312 (9.2).
2.3 1-(Isobutyl)-3-(benzyl)-5.6-dimethylbenzimidazolium chloride, 2b
Yield 95%, Mp: 238.9 °C, ν(CN) = 1566.9 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 1.04 (d, 6H, CH3(a,b), J = 8 Hz), 2.38 (s, 3H, CH3(c)), 2.42 (s, 3H, CH3(d)), 2.44 (Hep, 1H, H2′, J = 8 Hz), 4.36 (d, 2H, H1′, J = 8 Hz), 5.86 (s, 2H, H1′’), 7.30–7.49 (m, 7H, H4, 7, 3″, 4″, 5″, 6″, 7″), 11.81 (s, 1H, H2). 13C NMR (CDCl3, 100 MHz) δ (ppm) 19.8 (Cc,d), 20.7 (Ca,b), 28.7 (C2′), 51.0 (C1″), 54.2 (C1′), 112.8 (C4), 113.3 (C7), 128.1 (C3″;7″), 129.0 (C5′'), 129.3 (C4″;C6″), 129.6 (C8), 130.2 (C9), 133.2 (C5;6), 137.3 (C2″), 142.8 (C2). Elemental analysis % calcd. (found) for C20H25ClN2: C, 73.040 (73.1); H, 7.662% (7.7); N, 8.518% (8.6).
2.4 1-(Isobutyl)-3-(2.3.5.6-tetramethylbenzyl) benzimidazolium chloride, 2c
Yield 89%, Mp: 176.3 °C, ν(CN) = 1660.1 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 1.02 (d, 6H, CH3 (a,b), J = 8 Hz), 2.25 (s, 12H, CH3(c,d,e,f)), 2.38 (Hep, 1H, H2′, J = 8 Hz), 4.49 (d, 2H, H1′, J = 8 Hz), 5.97 (s, 2H, H1′’), 7.07–7.69 (m, 5H, H4, 5, 6, 7, 5″), 11.44 (s, 1H, H2). 13C NMR (CDCl3, 100 MHz) δ (ppm) 16.12 (Cc,f), 19.7 (Cd,e), 20.5 (Ca,b), 28.8 (C2′), 48.0 (C1″), 54.2 (C1‘), 113.0 (C4), 113.8 (C7), 126.8 (C6), 127.0 (C5), 127.9 (C5“), 131.3 (C8), 131.8 (C9), 133.5 (C3”,7”), 134.0 (C4“,6”), 135.0 (C2”), 143.9 (C2).Elemental analysis % calcd. (found) for C22H29ClN2:C, 74.030% (74.1); H, 8.189% (8.2); N, 7.848%(7.9).
2.5 1-(Isobutyl)-3-(2.3.5.6 tetramethylbenzyl)-5.6-dimethylbenzimidazolium chloride, 2d
Yield 87%, Mp: 113.1 °C, ν(CN) = 1558 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm0.99 (d, 6H, CH3 (a,b), J = 4 Hz), 2.30 (m, 18H, CH3(c,d,e,f,g,h)), 2.38 (Hep, 1H, H2′, J = 8 Hz), 4.43 (d, 2H, H1′, J = 8 Hz), 5.86 (s, 2H, H1′’), 6.94–7.36 (m, 3H, H4, 7, 5″), 11.08 (s, 1H, H2). 13C NMR (CDCl3, 100 MHz) δ (ppm)16.1 (Ce,h), 18.4 (Cc,d), 19.7 (Ca,b,f,g), 28.8 (C2′), 47.6 (C1″), 54.1 (C1′), 112.7 (C4), 113.5 (C7), 128.0 (C5“), 129.9 (C8), 130.3 (C9), 133.4 (C3”), 133.0 (C7”), 134.9 (C5;6), 136.9 (C4”), 136.9 (C6”),142.5 (C2”), 142.6 (C2). Elemental analysis % calcd. (found) for C24H33ClN2: C, 74.875% (74.9); H, 8.640% (8.7); N, 7.276% (7.2).
2.6 1-(Isobutyl)-3-(2.3.4.5.6-pentamethylbenzyl) benzimidazolium chloride, 2e
Yield 92%, Mp: 198.2 °C, ν(CN) = 1546 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 1.05 (d, 6H, CH3 (a,b), J = 4 Hz), 2.24 (s, 6H, CH3(c,g)), 2.28 (s, 6H, CH3(d,f)), 2.28 (s, 3H, CH3(e)), 2.38 (Hep, 1H, H2′), 4.51 (d, 2H, H1′), 5.94 (s, 2H, H1′’), 7.22–7.70 (m, 4H, H4, 5, 6, 7), 11.29 (s, 1H, H2). 13C NMR (CDCl3, 100 MHz) δ (ppm) 16.9 (Cc,g), 17.1 (Cd,f), 17.3 (Ce), 19.7 (Ca,b), 28.8 (C2′), 48.5(C1″), 54.2 (C1‘), 113.0 (C4), 113.9 (C7), 125.1 (C5“), 126.8 (C5), 127.00(C6), 131.3 (C4”), 131.8 (C6”), 133.5 (C3”;7”), 133.8 (C8;9), 137.2 (C2”), 143.7 (C2). Elemental analysis % calcd. (found) for C23H31ClN2: C, 74.468% % (74.5); H, 8.423% (8.5); N, 7.552% (7.4).
2.7 1-(Isobutyl)-3-(2.3.4.5.6-pentamethylbenzyl)-5.6-dimethylbenzimidazolium chloride, 2f
Yield 93%, Mp: 219.9 °C, ν(CN) = 1550 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 0.98 (d, 6H, CH3(a,b), J = 8 Hz), 2.14 (Hep, 1H, H2′, J = 8 Hz), 2.25 (s, 6H, CH3(c,d)), 2.30 (t, 12H, CH3(e,f,h,i)), 2.41 (s, 3H, CH3(g)), 2.37 (Hep, 1H, H2′), 4.46 (d, 2H, H1′), 5.80 (s, 2H, H1′’), 7.05 (s, 1H, H7), 7.38 (s, 1H, H4), 10.48 (s, 1H, H2). 13C NMR (CDCl3, 100 MHz) δ (ppm) 16.9 (Cf,h), 17.1 (Ce,i), 17.3 (Cg), 19.7 (Ca,b), 20.6 (Cc,d), 28.7 (C2′), 47.9 (C1″), 54.1 (C1‘), 112.7 (C4), 113.4 (C7), 125.2 (C5′'), 129.9 (C8), 130.4 (C9), 133.8 (C3″;7″), 133.5 (C4″;6″), 136.8 (C5), 136.9 (C6), 137.1 (C2″), 142.3 (C2). Elemental analysis % calcd. (found) for C25H35ClN2: C, 75.253% (75.3); H, 8.841% (8.9); N, 7.021% (7.1).
2.8 1-(Isobutyl)-3-(4- methylbenzyl) benzimidazolium chloride 2g
Yield 87%, Mp: 176.1 °C, ν(CN) = 1550 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 1.05 (d, 6H, CH3(a,b)), 2.31 (s, 3H, CH3(c)), 2.43 (Hep, 1H, H2′), 4.44 (d, 2H, H1′), 5.88 (s, 2H, H1′’), 7.15–7.71 (m, 8H, H4, 5, 6, 7, 3″, 4″, 6″,7″), 12.05 (s, 1H, H2).). 13C NMR (CDCl3, 100 MHz) δ (ppm) 19.8 (Ca,b), 21.1 (Cc), 28.9 (C2′), 51.2 (C1″), 54.3 (C1‘), 113.1 (C4), 113.9 (C7), 127.0 (C5;6), 128.3 (C3″;7″), 129.9 (C5″), 129.9 (C4″;6″), 131.0 (C8), 131.7 (C9), 139.1 (C2″), 143.9 (C2). Elemental analysis % calcd. (found) for C19H23ClN2: C, 72.480% (72.5); H, 7.363% (7.4); N, 8.897% (8.9).
2.9 1-(Isobutyl)-3-(2.4.6-trimethylbenzyl) −5.6-dimethylbenzimidazolium chloride 2h
Yield 89%, Mp: 249.7 °C, ν(CN) = 1550 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 1.01 (d, 6H, CH3(a,b)), 2.29 (d, 6H, CH3(c,d)), 2.32 (s, 6H, CH3(e,g)), 2.40 (s, 3H, CH3(f)), 2.44 (Hep, 1H, H2′), 4.40 (d, 2H, H1′), 5.84 (s, 2H, H1′’), 6.9–7.38 (m, 4H, H4, 7, 4″, 6′’), 11.34 (s, 1H, H2). 13C NMR (CDCl3, 100 MHz) δ (ppm) 19.7 (Ca,b), 20.2 (Cc,d), 20.6 (Ce), 20.8 (Cg), 21.0 (Cf), 28.7 (C2′), 47.1 (C1″), 54.1 (C1‘), 112.6 (C4), 113.5 (C7), 125.4 (C4″;6″), 130.0 (C3″; 5″;7″), 137.02 (C8;9), 137.89 (C5;6), 139.52 (C2″), 142.91 (C2). Elemental analysis % calcd. (found) for C23H31ClN2: C, 74.468% (74.5); H, 8.423% (8.5); N, 7.552% (7.6).
2.10 1-(Isobutyl)-3-(naphthyl)-5.6-dimethylbenzimidazolium chloride 2i
Yield 98%, Mp: 178.3 °C, ν(CN) = 1550 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 1.04 (d, 6H, CH3(a,b)), 2.33 (s, 3H, CH3(d)), 2.39 (s, 3H, CH3(c)), 2.44 (Hep, 1H, H2′), 4.38 (d, 2H, H1′), 6.04 (s, 2H, H1′’), 7.28–7.96 (m, 9H, H4, 7, 3″, 5″, 6″, 7″, 8″, 10″, 11″), 11.52 (s, 1H, H2). 13C NMR (CDCl3, 100 MHz) δ (ppm) (ppm)19.8 (Ca,b), 20.6 (Cc,d), 28.8 (C2′), 51.2(C1″), 54.3 (C1‘), 112.8 (C4), 113.4 (C7), 125.0 (C7″), 126.7 (C3″), 126.8 (C10″), 127.6 (C5″), 127.7 (C8″), 128.1 (C6″), 129.4 (C11″), 130.4 (C8;9), 133.2 (C5;6), 137.4 (C2″), 141.9 (C2). Elemental analysis % calcd. (found) for C24H27 BrN2: C, 68.083% (68.1); H, 6.428% (6.5); N, 6.616% (6.7).
2.11 1-(Isobutyl)-3-(anthracen-9-ylmethyl)-5.6-dimethylbenzimidazolium chloride 2j
Yield 97%, Mp: 269,7 °C, ν(CN) = 1666 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 1.00 (d, 6H, CH3 (a,b)), 1.91 (s, 3H, CH3(d)), 2.23 (s, 3H, CH3(c)), 2.34 (Hep, 1H, H2′), 4.32 (d, 2H, H1′), 6.90 (s, 2H, H1′’), 6.65 (s, 1H, H9″), 7.23–8.58 (m, 10H, H4, 7, 4″, 5″, 6″, 7″, 11″, 12″, 13″, 14″), 11.95 (s, 1H, H2). 13C NMR (CDCl3, 100 MHz) δ (ppm) 19.7 (Ca,b), 20.4 (Cc), 20.4 (Cd), 28.6 (C2′), 45.2 (C1″), 54.2 (C1′), 112.3 (C4), 114.2 (C7), 122.1 (C9″), 123.1 (C5″, 6″, 12″, 13″), 125.4 (C4″, 14″), 128.0 (C7″, 11″), 129.7 (C4″, 15″), 129.8 (C8), 130.1 (C9), 130.4 (C8″), 130.9 (C10″), 131.2 (C2″), 136.65 (C5), 136.72 (C6), 143.04 (C2). Elemental analysis % calcd. (found) for C28H29ClN2: C, 78.392% (78.4); H, 6.814% (6.9); N, 6.530% (6.6).
2.12 General procedure for the preparation of silver(I)-NHC complexes
A solution of benzimidazolium salt (1.0 mmol) (2a-j), Ag2O (1.0 mmol) in dichloromethane (15 mL) was stirred for 24 h at room temperature in dark condition. The reaction mixture was filtered through celite and the solvent removed under reduced pressure. The crude product was recrystallized from dichloromethane/diethyl ether (1:3).
2.13 Chloro[1-isobutyl-3-(benzyl) benzimidazole-2-ylidene] silver(I) (3a)
Yield 75%, Mp: 188 °C, ν(CN) = 1466 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 1.02 (d, 6H, CH3(a,b), J = 8 Hz), 2.38 (Hep, 1H, H2′, J = 8 Hz), 4.425 (d, 2H, H1′, J = 8 Hz), 5.63 (s, 2H, H1′’), 7.25–7.38 (m, 9H, H4, 5, 6, 7, 3″, 4″, 5″, 6″, 7″). 13C NMR (CDCl3, 100 MHz) δ (ppm) 20.3 (Ca,b), 29.3 (C2′), 53.4 (C1″), 56.8 (C1‘), 111.7 (C4), 112.2 (C7), 124.2 (C5), 124.2 (C6), 127.0 (C3″;7″), 127.1 (C8), 127.1(C9), 128.5(C5′'), 129.1 (C4′';6′'), 134.8 (C2″), C2: Ag-Ccarbene: not observed. Elemental analysis % calcd. (found) for C18H20AgClN2: C, 53.029%% (53.1); H, 4.945% (4.8); N, 6.871% (6.9).
2.14 Chloro[1-isobutyl-3-(benzyl)-5.6-dimethylbenzimidazole-2-ylidene] silver(I) (3b)
Yield 78%, Mp: 208.2 °C, ν(CN) = 1400 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 1.00 (d, 6H, CH3(a,b), J = 4 Hz), 2.31 (s, 3H, CH3(c)), 2.37 (s, 3H, CH3(d)), 2.37 (Hep, 1H, H2′, J = 8 Hz), 4.18 (d, 2H, H1′, J = 8 Hz), 5.56 (s, 2H, H1′’), 7.08–7.32 (m, 7H, H4, 7, 3″, 4″, 5″, 6″, 7″). 13C NMR (CDCl3, 100 MHz) δ (ppm) 20.3 (Cc,d), 20.4 (Ca,b), 29.2 (C2′), 53.1 (C1″), 56.7 (C1′), 111.2 (C4), 112.8 (C7), 126.8 (C3″;7″), 128.3 (5“), 129.0 (C4”; 6”), 132.2 (C8), 132.7 (C9), 133.6 (C5), 133.7 (C6), 135.1 (C2”), C2: Ag-Ccarbene: not observed. Elemental analysis % calcd. (found) for C20H24AgClN2: C, 53.029%% (53.1); H, 4.945% (4.8); N, 6.871% (6.9).
2.15 Chloro[1-isobutyl-3-(2.3.5.6-tetramethylbenzyl)benzimidazole-2-ylidene]silver 3c
Yield 85%, Mp: 227.3 °C, ν(CN) = 1450 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 0.94 (d, 6H, CH3(a,b), J = 8 Hz), 2.15 (s, 6H, CH3(d,e)), 2.29 (s, 6H, CH3(c,f)), 2.33 (Hep, 1H, H2′, J = 8 Hz), 4.17 (d, 2H, H1′, J = 8 Hz), 5.49 (s, 2H, H1′’), 7.14–7.49 (m, 5H, H4, 5, 6, 7, 5″). 13C NMR (CDCl3, 100 MHz) δ (ppm)16.1(Cc,f), 20.2 (Cd,e), 20.7 (Ca,b), 29.3 (C2′), 47.6 (C1″), 57.3 (C1′), 111.4 (C4), 111.5 (C7), 124.0 (C5), 124.2(C6), 129.6 (C5′'), 133.1 (C3″;7″), 133.5 (C4′';6″), 133.1 (C2″), 135.3 (C8;9), 189.3 (C2). Elemental analysis % calcd. (found) for C22H28AgClN2: C, 56.973% (56.8); H, 6.085% (6.1); N, 6.040% (6.1).
2.16 Chloro[1-isobutyl-3-(2.3.5.6-tetramethylbenzyl)-5.6-dimethylbenzimidazole-2-ylidene]silver 3d
Yield 82%, Mp: 231.4 °C, ν(CN) = 1466 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 0.92 (d, 6H, CH3(a,b), J = 8 Hz), 2.13 (s, 6H, CH3(f,g)), 2.29 (s, 6H, CH3(e,h)), 2.30 (Hep, 1H, H2′, J = 8 Hz), 2.4 (s, 6H, CH3(c,d)), 4.09 (d, 2H, H1′, J = 8 Hz), 5.40 (s, 2H, H1′’), 7.14 (s, 1H, H5″), 7.21 (d, 2H, H4,7, J = 4 Hz). 13C NMR (CDCl3, 100 MHz) δ (ppm)16.1 (Ce,h), 20.2 (Cc,d), 20.7 (Ca,b,f,g), 29.2 (C2′), 47.1 (C1″), 57.3 (C1′), 111.5 (C4), 111.8 (C7), 129.9 (C5′'), 133.0 (C8;9), 133.3 (C3″;4″), 133.4 (C5;6), 133.5 (C4″;6″), 135.5 (C2″), C2: Ag-Ccarbene: not observed. Elemental analysis % calcd. (found) for C24H32AgClN2: C, 58.607% (58.7); H, 6.558% (6.6); N, 5.696% (5.7).
2.17 Chloro[1-isobutyl-3-(2.3.4.5.6-pentamethylbenzyl)benzimidazole-2-ylidene]silver 3e
Yield 86%, Mp: 275.3 °C, ν(CN) = 1458 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 0.93 (d, 6H, CH3(a,b), J = 4 Hz), 2.19 (s, 6H, CH3(d,f)), 2.28 (s, 6H, CH3(c,g)), 2.30 (Hep, 1H, H2′, J = 8 Hz), 2.33 (s, 3H, CH3(e)), 4.15 (d, 2H, H1′, J = 8 Hz), 5.48 (s, 2H, H1′’), 7.27–7.48 (m, 4H, H4, 5, 6, 7). 13C NMR (CDCl3, 100 MHz) δ (ppm)17.1 (Cc,d,f,g), 17.3 (Ce), 20.2 (Ca,b), 29.3 (C2′), 47.8 (C1″), 57.3 (C1′), 111.4 (C4), 111.6 (C7), 123.9 (C5), 124.1 (C6), 126.5 (C5′'), 132.9 (C4″;6″), 133.2 (C8;9;3″;7″), 137.2(C2″), 189.0 (C2). Elemental analysis % calcd. (found) for C23H30 AgClN2: C, 57.814% (57.9); H, 6.328% (6.4); N, 5.863% (5.9).
2.18 Chloro[1-isobutyl-3-(2.3.5.6-tetramethylbenzyl)-5.6-dimethylbenzimidazole-2-ylidene]silver 3f
Yield 89%, Mp: 208.3 °C, ν(CN) = 1591 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 0.91 (d, 6H, CH3(a,b), J = 8 Hz), 2.18 (s, 6H, CH3(f,h)), 2.24 (Hep, 1H, H2′, J = 8 Hz), 2.28 (s, 6H, CH3(e,i)), 2.33 (s, 3H, CH3(g)), 2.41(s, 6H, CH3(c,d)), 4.08 (d, 2H, H1′, J = 8 Hz), 5.39 (s, 2H, H1′’), 7.20 (s, 1H, H4), 7.27 (s, 1H, H7). 13C NMR (CDCl3, 100 MHz) δ (ppm) 17.0 (Cf,h), 17.1 (Ce,i), 17.3 (Cg), 20.4 (Ca,b), 20.4 (Cc), 20.5 (Cd), 29.1 (C2′), 47.3 (C1″), 57.3 (C1′), 111.5 (C4), 111.8 (C7), 126.7 (C5′'), 132.9 (C8;9), 133.2(C3″), 133.5 (C7″), 134.2 (C5;6;4″;6″), 137.1 (C2″), 185.2 (C2). Elemental analysis % calcd. (found) for C25H34 AgClN2: C, 59.356% (59.4); H, 6.774% (6.8); N, 5.538% (5.6).
2.19 Chloro[1-isobutyl-3-(4--methylbenzyl) benzimidazole-2-ylidene]silver 3g
Yield 79%, Mp: 202.3 °C, ν(CN) = 1468 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 1.01 (d, 6H, CH3(a,b), J = 8 Hz), 2.31 (s, 3H, CH3(c)), 2.83 (Hep, 1H, H2′, J = 8 Hz), 4.24 (d, 2H, H1′, J = 8 Hz), 5.57 (s, 2H, H1′’), 7.11–7.48 (m, 8H, H4, 5, 6, 7, 3″, 4″, 6″,7″). 13C NMR (CDCl3, 100 MHz) δ (ppm) 20.3 (Ca,b), 21.1 (Cc), 29.3 (C2′), 53.3 (C1″), 56.8 (C1′), 112.2 (C4), 111.6 (C7), 124.1 (C5), 124.2 (C6), 127.1 (C3″;7″), 129.7 (C4″;6″), 131.8 (C5″), 133.6 (C8), 134.2 (C9), 138.4 (C2″), C2: Ag-Ccarbene: not observed. Elemental analysis % calcd. (found) for C19H22AgClN2: C, 54.114% (54.2); H, 5.258% (5.3); N, 6.643% (6.7).
2.20 Chloro[1-isobutyl-3-(2.4.6-trimethylbenzyl)-5.6-dimethylbenzimidazole-2-ylidene]silver 3h
Yield 86%, Mp: 273.4 °C, ν(CN) = 1458 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 0.94 (d, 6H, CH3(a,b), J = 4 Hz), 2.23 (s, 6H, CH3(c,d)), 2.28 (Hep, 1H, H2′, J = 8 Hz), 2.34 (s, 3H, CH3(e)), 2.35 (s, 3H, CH3(f)), 2.38 (s, 3H, CH3(g)), 4.11 (d, 2H, H1′, J = 8 Hz), 5.40 (s, 2H, H1′’), 6.97 (s, 2H, H 4″, 6″), 7.18 (s, 2H, H 4, 7). 13C NMR (CDCl3, 100 MHz) δ (ppm) 20.2 (C(c,d)), 20.3 (C (a,b)), 20.4 (Ce), 20.5 (Cg), 21.1 (C f), 29.2 (C2′), 47.4 (C1″), 57.1 (C1′), 111.7 (C4), 111.8 (C7), 126.8 (C4″;6″), 130.2 (C3″;5″;7″), 133.3 (C8;9), 137.4 (C5;6), 139.4 (C2″), 187.9 (C2).Elemental analysis % calcd. (found) for C23H30AgClN2: C, 57.814% (57.9); H, 6.328% (6.4); N, 5.863% (5.9).
2.21 Chloro[1-isobutyl-3-(3-(naphthyl)-5.6-dimethylbenzimidazole-2-ylidene] silver 3i
Yield 85%, Mp: 284.6 °C, ν(CN) = 1400 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 0.98 (d, 6H, CH3(a,b)), J = 8 Hz), 2,26 (s, 3H, CH3(d)), 2.35 (s, 3H, CH3(c)), 2.36 (Hep, 1H, H2′, J = 8 Hz), 4.19 (d, 2H, H1′), J = 8 Hz), 5.71 (s, 2H, H1′’), 7.10–7.76 (m, 9H, H4, 7, 3″, 5″, 6″, 7″, 8″, 10″, 11″). 13C NMR (CDCl3, 100 MHz) δ (ppm) 20.3 (C (a,b)), 20.3 (C (c,d)), 29.2 (C2′), 53.2 (C1″), 56.6 (C1′), 111.8 (C4), 112.2 (C7), 124.4 (C7″), 125.9(C3″), 126.3 (C10″), 126.5(C5″), 127.7 (C8″), 127.9 (C6″), 129.0 (C11″), 132.3 (C8), 132.8 (C9), 133.0 (C5), 133.1 (C6), 133.6 (C2″), 168.6 (C2). Elemental analysis % calcd. (found) for C24H26AgClN2: C, 59.337% (59.4); H, 5.395% (5.4); N, 5.766% (5.8).
2.22 Chloro[1-isobutyl-3-(anthracen-9-ylmethyl) dimethylbenzimidazole-2-ylidene]silver 3j
Yield 86%, Mp: 214.2, ν(CN) = 1468 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 0.90 (d, 6H, CH3(a,b), J = 4 Hz), 2.12 (s, 3H, CH3(d)), 2.22 (Hep, 1H, H2′, J = 8 Hz), 2.31 (s, 3H, CH3(c)), 4.08 (d, 2H, H1′, J = 4 Hz), 6.38 (s, 2H, H1′’), 7.87–8.22 (m, 11H, H4, 7, 4″, 5″, 6″, 7″, 9″, 11″, 12″, 13″, 14″). 13C NMR (CDCl3, 100 MHz) δ (ppm) 20.2(C (a,b)), 20.3 (C (c)), 20.4 (C (d)), 29.1 (C2′), 46.2 (C1″), 57.0 (C1′), 111.7 (C4), 112.3 (C7), 122.9 (C9″), 125.2 (C5″; 6″; 12″; 13″), 127.5 (C4″; 7″; 11″; 14″), 129.9 (C3″, 8″, 10″, 15″), 131.1 (C8;9), 131.4 (C5;6), 132.7 (C2″), C2: Ag-Ccarbene: not observed. Elemental analysis % calcd. (found) for C28H28AgClN2: C, 62.759% (62.8); H, 5.267% (5.3); N, 5.228% (5.3).
Antibacterial activities were prepared according the reported procedures (boubakri et al., 2018)
2.23 Cytotoxicity assay
Cytotoxicity of benzimidazoles salts 2a-j and silver–NHC complexes 3a-j was assessed using the reported procedures (Song et al., 2010).
3 Results and discussion
3.1 Preparation of benzimidazolium salts
benzimidazoles salts (2a-j) were prepared via the two step N-alkylation process as depicted in Scheme 1. Compound 1 was obtained by N-alkylation of benzimidazole with isobutyl bromide (1-Bromo-2-methylpropane) in the presence of KOH in DMSO at 80 °C for 8 h. The benzimidazolium salts (2a-j) were prepared by reacting N-(isobutyl)-benzimidazole (1) with various aryl chloride in DMF at 80 °C for 24 h (Scheme 1). The reaction has been monitored following thin layer chromatography, and after this time the formation of salts (2a-j), has been observed for every target compound. The benzimidazolium salts (2a-j) were air- and moisture-stable both in the solid state and in solution.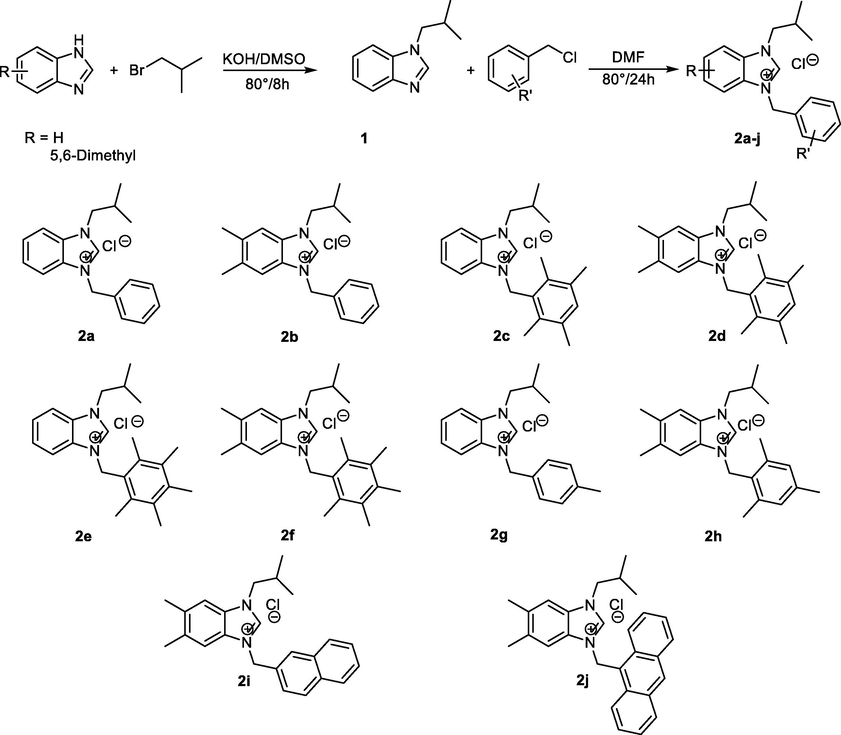
Synthesis of the benzimidazoles salts (2a-j).
The FTIR spectroscopy, 1H- and 13C{1H} NMR spectroscopy, and elemental analysis data of the title compounds confirm the proposed structures.
NMR spectra of all the compounds were analyzed in d-CDCl3. In the 1H NMR spectra, acidic protons (NCHN) for benzimidazolium salts (2a-j) were seen at 12.07, 11.81, 11.44, 11.08, 11.29, 10.48, 12.05, 11.34, 11.52 and 11.95 ppm, respectively, as a characteristic sharp singlet. In the 13C{1H} NMR spectra of benzimidazoles salts (2a-j), the NCHN carbon were detected as typical singlets at 144.5, 142.8, 144.02, 142.66, 143.78, 142.39, 143.96, 142.91, 141.91 and 143.04 ppm, respectively. These values are consistent with related literature. (Hu et al.,2004; Doǧan et al., 2001; Ozdemir et al., 2010; Iqbal et al., 2013).
In the IR spectra, the ν(C⚌N) bands for salts (2a-j) were observed at 1650, 1566, 1660, 1558, 1546, 1550, 1550, 1550, 1550 and 1666 cm−1 respectively.
Aromatic protons of benzimidazolium salts (2a-j) were detected in the range of 6.94–8.64 ppm. –CH– protons of isopropyl group on all benzimidazolium salts where seen as septets in the range of 2.34–2.44 ppm. Methylic protons of isopropyl group on benzimidazolium salts (2a-j) were resonated between 0.98 and 1.06 as doublets however methyl protons of benzimidazolium salts (2a-j) signaled at 2.24–2.44 ppm as singlets. In the 1H NMR spectra of (2a-j) H1′ protons appeared at 4.49 ppm while H1′' protons were detected as typical singulets between at 5.90 ppm (Fig. 1).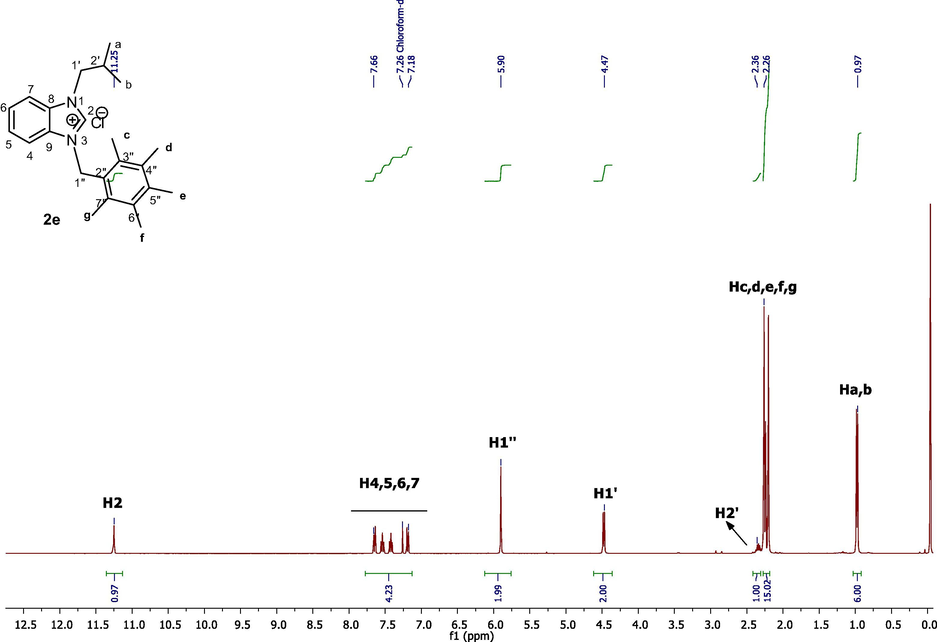
1H NMR spectra of benzimidazole salt 2e in CDCl3.
The 13C NMR spectra showed aromatic carbons of benzimidazolium salts (2a-j) in the range of 112.35–143.97 ppm. NCHN carbons on salts (2a-j) were observed at 144.5, 142.8, 144.02, 142.66, 143.78, 142.39, 143.96, 142.91, 141.91 and 143.04 ppm, respectively. Terminal carbons(a,b) of the isopropyl group of all benzimidazolium salts (2a-j) gave peaks at 19.86, 20.70, 20.57, 19.72, 19.79, 19.71, 19.86, 19.74, 19.87 and 19.73 respectively while –CH carbons of the isobutyl group were observed at 28.89, 28.77, 28.87, 28.87, 28.88, 28.79, 28.90, 28.77, 28.84, 28.69 respectively (Fig. 2).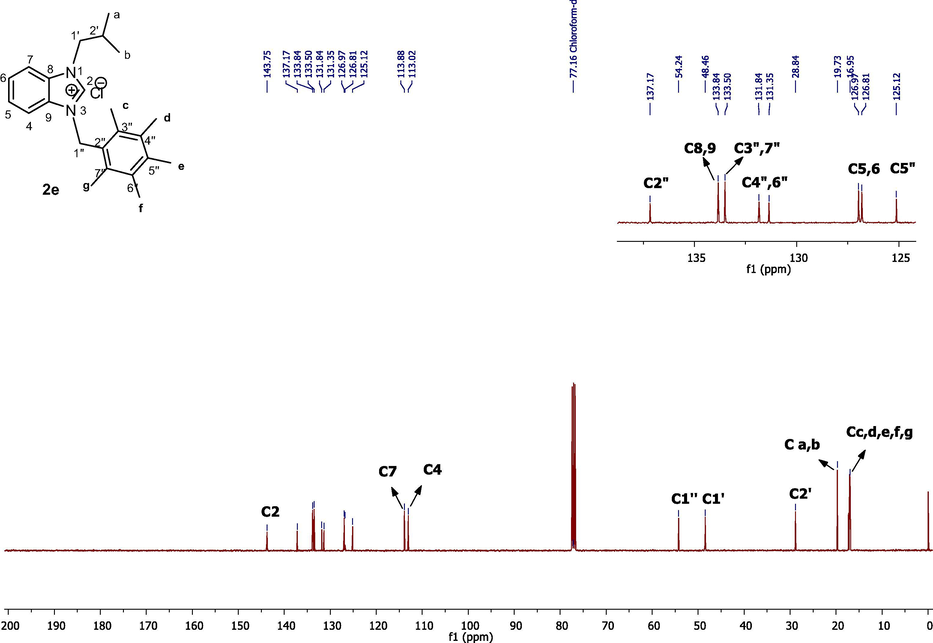
13C NMR spectra of benzimidazole salt 2e in CDCl3.
The general procedure for the preparation of silver(I)-NHC complexes (2a-j) was performed according to the procedure of Organ (O’Brien et al., 2006). Benzimidazolium salts (2a-j) were incorporated into the silver(I)-NHC complexes (3a-j) by reaction with Ag2O in dichloromethane for 24 h resulted in the silver-NHC complex as a white solid. Monitoring of the reaction by 1H NMR spectroscopy in CDCl3 showed that benzimidazolium salts were completely transformed into silver complexes with moderate yields (75–85%) under argon atmosphere. Ag(I) complexes were synthesized in the absence of light and all products were stored in the dark Scheme 2.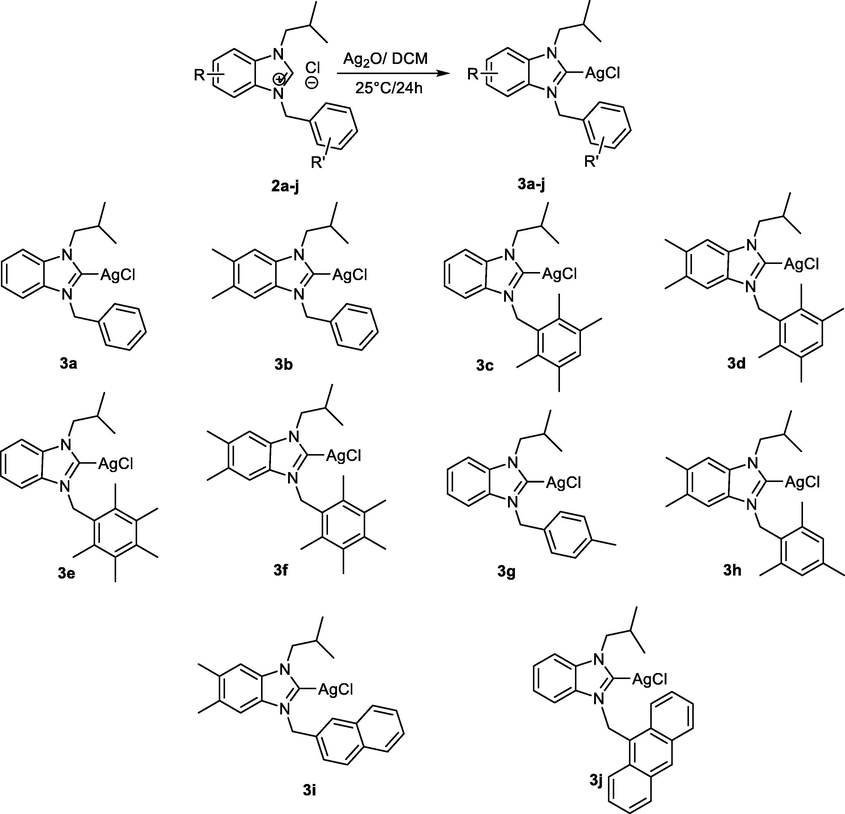
Synthesis of silver(I) complexes 3a-j.
The silver complexes (3a-j) have a good solubility in polar solvents and are stable in the air and toward the moisture.
In the 1H NMR spectra the acidic imino proton of benzimidazolium salts (NCHN) were not observed between δ10.48–12.07 ppm. Fig. 3 Similarly, in the 13C NMR spectra, imino carbon of benzimidazolium salts (NCHN) were not observed between δ 141–144 ppm.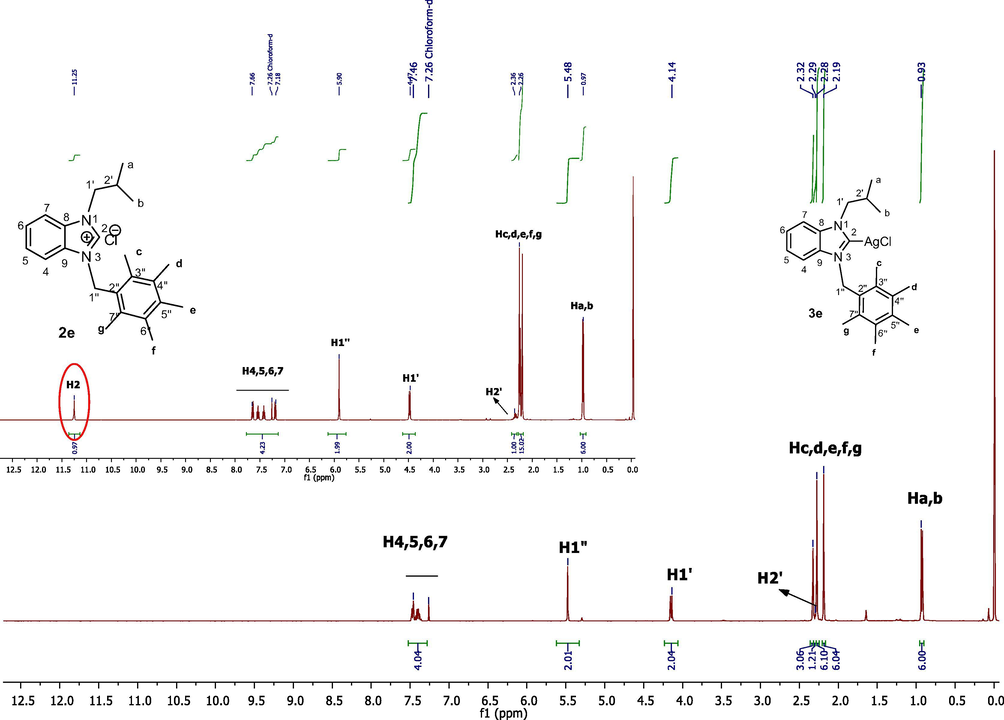
1H NMR spectra of silver complex 3e in CDCl3.
Therefore, in the 1H NMR spectrum Ag(I)-NHC complexes (3a-j), the disappearance of an acidic proton is evidence of formation complex. In the13C NMR spectra of salts (2a-j) the characteristic signals of carbon (NCHN) were seen as singlets at 144.5, 142.8, 144.02, 142.66, 143.78, 142.39, 143.96, 142.91, 141.91 and 143.04 ppm, respectively for benzimidazolium salts while for complex (3a-j) a signal of carbon (NCN) have shifted greatly downfield region compared to the corresponding benzimidazolium salt (2a-j) and were observed at 189.35, 189.06, 185.29, 187.95, 168.65, ppm respectively for complexes 3b, 3e, 3f, 3h and 3i however, the rest of carbon signal for complexes 3a, 3b, 3e, 3g and 3j were not observed. Fig. 4. These values, and the lack of the carben peak are in agreement with reported data for similar Ag-NHC complexes (Pytkowicz et al., 2001). At the same time, formation of the Ag(I)-NHC complexes (3a-j) was proven by IR spectra, which showed CN bond vibrations at 1466, 1400, 1450, 1466, 1458, 1591, 1468, 1458, 1400, 1468 cm−1, respectively.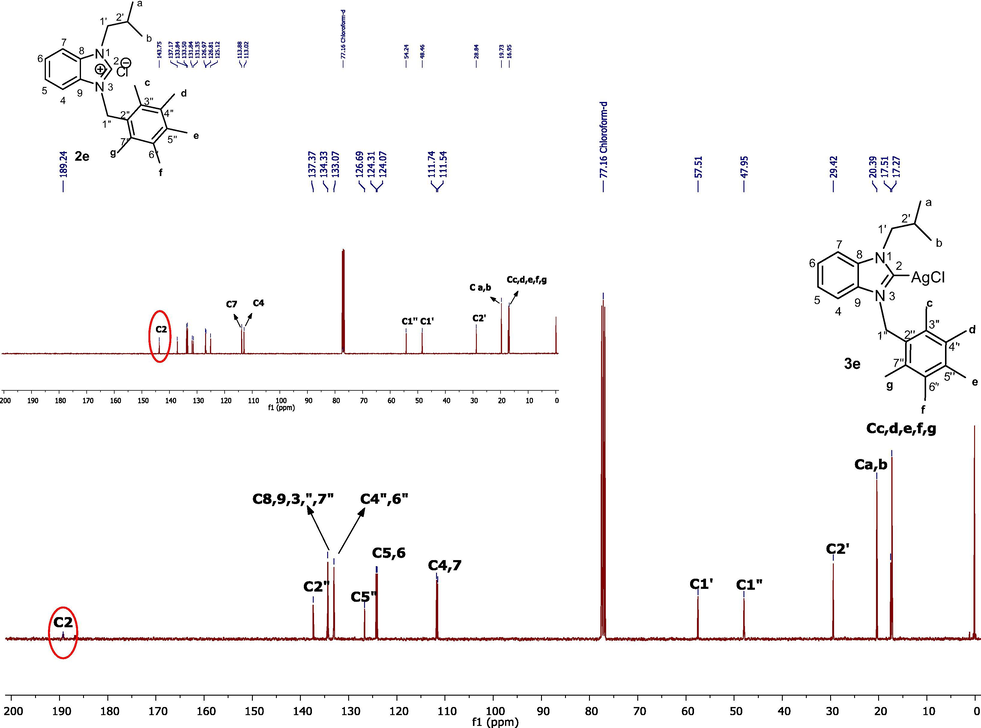
13C NMR spectra of silver complex 3e in CDCl3.
All the synthesized benzimidazolium salts (2a-j) and their corresponding silver(I) complexes (3a-j) were tested for antibacterial and antioxidant activities as per details given in the following text.
4 Biological activities
All the synthesized benzimidazolium salts (2a-j) and their corresponding silver(I) complexes (3a-j) were investigated for antibacterial against the both gram (+)/(−) bacterials. The DMSO did not exhibit any antimicrobial activity as reported earlier (Shahini et al., 2017; Patil et al., 2010a,b; Gleeson et al.,2008). The antimicrobial activities of the NHC precursors (2a-j) and their corresponding silver complexes (3a-j) are reported in Table 1. Minimum inhibitory concentration (MIC) determination
Microorganisms Compounds
Micrococcus luteus LB 14,110
Listeria monocytogenes ATCC 19,117
Salmonella Typhimurium ATCC 14,028
Staphylococcus aureus ATCC 6538
Pseudomonas aeruginosa
3a
20 ± 0.6
14 ± 0.5
18 ± 0.54
16 ± 0.25
16 ± 0.13
3b
22 ± 0.6
15 ± 0.6
18 ± 0.5
17 ± 0.3
17 ± 0.14
3c
35 ± 0.5
16 ± 0.2
18 ± 0.5
18 ± 0.5
22 ± 0.2
3d
30 ± 0.5
14 ± 0.5
16 ± 0.10
18 ± 0.11
16 ± 0.19
3e
25 ± 0.33
22 ± 0.5
18 ± 0.5
18 ± 0.18
20 ± 0.45
3f
36 ± 0.2
16 ± 0.3
18 ± 0.5
20 ± 0.1
20 ± 0.4
3g
28 ± 0.32
16 ± 0.5
22 ± 0.44
18 ± 0.15
22 ± 0.5
3h
30 ± 0.4
16 ± 0.2
16 ± 0.2
20 ± 0.2
18 ± 0.2
3i
38 ± 0.2
22 ± 0.2
22 ± 0.3
22 ± 0.2
20 ± 0.4
3j
34 ± 0.44
22 ± 0.5
22 ± 0.15
22 ± 0.3
20 ± 0.25
2a
20 ± 0.22
–
22 ± 0.22
18 ± 0.05
18 ± 0.22
2b
18 ± 0.2
20 ± 0.2
16 ± 0.3
20 ± 0.2
18 ± 0.2
2c
16 ± 0.2
18 ± 0.3
18 ± 0.22
16 ± 0.0
16 ± 0.5
2d
22 ± 0.2
16 ± 0.2
14 ± 0.2
20 ± 0.2
16 ± 0.2
2e
18 ± 0.2
18 ± 0.22
18 ± 0.33
18 ± 0.23
18 ± 0.22
2f
30 ± 0.4
22 ± 0.7
30 ± 0.4
25 ± 0.2
18 ± 0.22
2g
22 ± 0.3
16 ± 0.4
22 ± 0.4
18 ± 0.2
18 ± 0.2
2h
10 ± 0.4
14 ± 0.5
12 ± 0.10
14 ± 0.15
16 ± 0.10
2i
32 ± 0.32
–
16 ± 0.15
18 ± 0.1
18 ± 0.15
2j
20 ± 0.4
18 ± 0.5
18 ± 0.24
18 ± 0.5
18 ± 0.16
Compound 3i is more active than compounds 3h and 3c against the Micrococcus luteus LB 14110. The complexes have shown antibacterial activity to different extents, according on the typr of ligand. The silver complexes have shown enhanced activity compared with the benzimidazolium salts. The complexes exhibited enhanced antibacterial activity, which could be explained by the increased lipophilicity due to the synergistic effect of the complexes. Observed antibacterial activity of these complexes is comparable to that of our previous silver complexes (Achar et al., 2018a,b).
The MIC values of tested silver complexes and their starting material against Listeria monocytogenes ATCC 19117, Salmonella Typhimurium ATCC 14,028 and Micrococcus luteus are presented in Tables 2.
Microorganismindicator
Compounds
MIC (mg/ml)
Listeria monocytogenes ATCC 19,117
2h
1,25
2j
0,625
3f
0,0048
Ampicillin
0.039
Salmonella Typhimurium ATCC 14,028
2h
1,25
2j
0,039
3f
0,0024
Ampicillin
0,625
Micrococcus luteus
2h
0,3125
2j
0,3125
3f
0,0024
Ampicillin
0,0195
Silver complex 3f showed good activity than that of ampicillin against Micrococcus luteus with a MIC of 0.0024 mg/mL. while a MIC of 0.048 mg/mL was observed in the case of Salmonella Typhimurium for the silver complex 3f. MICs of other compounds remained within the tested range.
4.1 Antioxydant activities
The scavenging activity of the synthesized of the NHC precursors (Scheme 3) and silver complexes with DPPH (1,1-diphenyl-2-picrylhydrazyl) is represented in Scheme 4.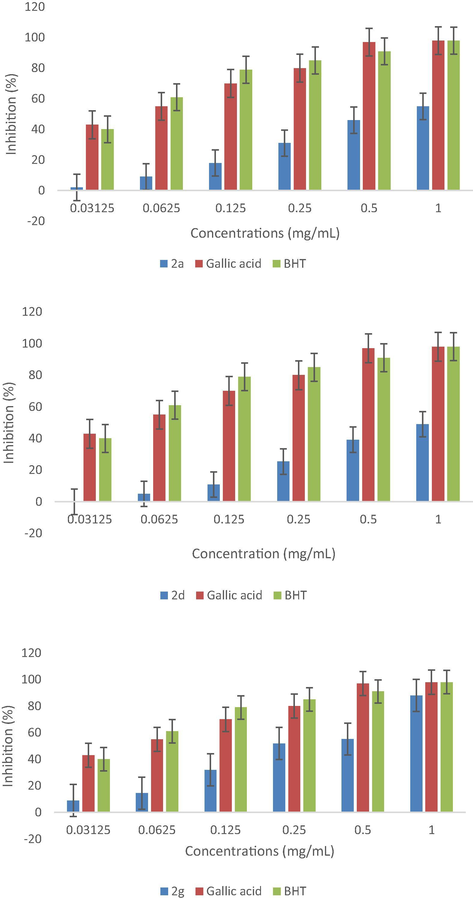
DPPH radicals scavenging activity of benzimidazoles salts, 2a, 2d, 2g.
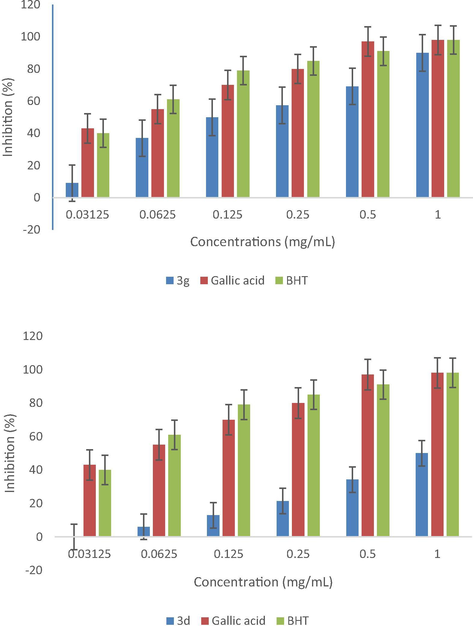
DPPH radicals scavenging activity of (Ag-NHC) complexes, 3d, 3g.
The analysis of the results showed that the profiles of obtained antiradical activity of the tested synthetic products 3g and 3d have a very important anti-radical activity and showed higher antioxidant activity than other products. For a used concentration (0.0625 mg/ml), the product 2d has the lowest radical activity comparing to the gallic acid and BHT (butylated hydroxytoluene). On the same way the compounds 2a, 2g, and 3d for a concentration equal to 0.0625 mg/ml had a lower radical activity than gallic acid and BHT (butylated hydroxytoluene). At a concentration of 1 mg/ml, these products 2a, 2d, 2g, 3g and 3d revealed a significant activity of DPPH compared with the synthetic antioxidants.
Cytotoxic Activities
In order to evaluate their cytotoxicity, the benzimidazoles salts 2a-j and silver–NHC complexes 3a-j were screened against the two human cancer cell lines, MDA-MB-231 and MCF7. The results are presented in Table 3 Values are mean value ± standard deviation of three different replicates.
benzimidazoles salts 2a-j and silver–NHC complexes 3a-j
Anticancer activity LC50 in µg/ml
3a
MCF7
MDA-MB-231
3b
4.2 ± 3.6
2.5 ± 4.3
3c
3.1 ± 3.1
2.6 ± 5.9
3d
1.7 ± 3.1
16 ± 2.8
3e
4.3 ± 1.8
0.0 ± 00
3f
0.68 ± 3.2
1.93 ± 2.6
3g
1.3 ± 4.1
3.3 ± 2.9
3h
2.0 ± 3.2
2.8 ± 2.9
3i
0.62 ± 3.1
1.95 ± 2.5
3j
1.3 ± 4.1
3.4 ± 2.9
2a
2.0 ± 3.2
2.7 ± 2.8
2b
NA
NA
2c
3.1 ± 5.9
6.3 ± 3.2
2d
NA
NA
2e
0.6 ± 2.9
3.1 ± 5.9
2f
Higher than 100 µg/ml
Higher than 100 µg/ml
2g
Higher than 100 µg/ml
Higher than 100 µg/ml
2h
Higher than 100 µg/ml
Higher than 100 µg/ml
2i
Higher than 100 µg/ml
Higher than 100 µg/ml
2j
Higher than 100 µg/ml
Higher than 100 µg/ml
Tetracyclinea
NT
NT
As shown in Table 3, the cytotoxicity of 3i and 3f were much greater in MCF7 with IC50 values 0.68 and 0.6 µg/ml, respectively as compared to their activity on MDA-MB-231 cells. The cytotoxicity of compound 3j in MCF7 and MDA-MB-231 cells was 2.3 and 3.4 µg/ml, while the IC50 values of compound on 2a were 2 and 2.7 µg/ml against MCF7 and MDA-MB-231, respectively. For the compounds 2f-j their IC50 values were more than 100 µg/ml. The compound 2d was inactive.
5 Conclusion
In this study six benzimidazolium salts and their silver(I)–NHC complexes have been prepared and characterized by 1H NMR, 13C NMR, IR and elemental analysis. In addition, these compounds showed significant activities compared with standard antibiotic. In addition, the silver(I)–NHC complexes showed significant antitumor activity against the cell lines MCF-7 and MDA-MB-231. In the aim to determine the antimicrobial activity specificity and spectra of action, studies are now in progress including a larger collection of bacteria of different species in order to provide possible application in different fields. Also advanced investigations focusing on new Au and Ag–NHC complexes as metallopharmaceutical compounds are in process.
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this research group No (RG-1435-023) and İnönü University support by Research Fund (İÜ‐BAP: FOA-2018-1135)
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ether and coumarinefunctionalized (benz)imidazolium salts and their silver(I)-Neheterocyclic carbene complexes : synthesis, characterization, crystal structures and antimicrobial studies. J. Organomet. Chem.. 2018;854:64-75.
- [Google Scholar]
- Achar, G., Shahinia, Patil, S.A., Malecki, J.G., Pan, S.H., Lan, A., Chen, X.R., Budagumpi, S., 2018. Sterically modulated silver(I) complexes of coumarin substituted benzimidazol–2–ylidenes: Synthesis, crystal structures and evaluation of their antimicrobial and antilung cancer potentials. J. Inorg. Biochem., 183, 43–57.
- Syntheticstrategy of diflurophosphate-bridgedbimetallic N-heterocycliccarbenecomplexes:synthesis, structures and photoluminescence of picolyl-substitutedalkylbenzimidazolylidene ligands. Inorg. Chem. Acta.. 2012;384:239-246.
- [Google Scholar]
- Anchez, O.S., Gonzalez, S., Higuera-Padilla, A.R., le on, Y., Coll, D., Fern andez, M., Taylor, P., Urdanibia, I., Rangel, H.R., Ortega, J.T., Castro, W., Goite, M.C., 2016. Remarkable in vitro anti-HIV activity of new silver(I)e and gold(I)-N-heterocyclic carbene complexes. Synthesis, DNA binding and biological evaluation. Polyhedron, 110, pp. 14–23.
- Homoleptic carbine silver(I) and carbine-copper(I) complexes. Organometallics. 1993;12:3405-3409.
- [Google Scholar]
- Poly(l-lactide) initiated by silver N-heterocyclic carbene complexes: synthesis, characterization and properties. Polym. Bull.. 2013;70:3475-3485.
- [Google Scholar]
- Sulfonated water-soluble N-heterocyclic carbine silver(I) complexes: behavior in aqueous medium and as NHC-transfer agent to platinum(II) Organometallics. 2013;32:2814-2826.
- [Google Scholar]
- N-heterocyclic carbene-Pd (II)-PPh3 complexes a new highly efficient catalyst system for the Sonogashira cross-coupling reaction: synthesis, characterization and biological activities. J. Coordinat. Chem.. 2018;71:133-189.
- [Google Scholar]
- Steric and electronic effects in the bonding of N-heterocyclic ligands to transition metals. J. Organomet. Chem.. 2005;690(24):5407-5413.
- [Google Scholar]
- Synthesis and structural characterization of alkyne-functionalized N-heterocyclic carbine complexes of ruthenium, palladium and rhodium. Inorg. Chim. Acta.. 2017;467:33-38.
- [Google Scholar]
- Synthesis and structure of Imine-N-heterocyclic carbine palladium complexes and their catalytic behavior in norbornene polymerization. Organometallics. 2013;32:4507-4515.
- [Google Scholar]
- “On water”-promoted direct alkynylation of isatins catalyzed by NHC-silver complexes for the efficient synthesis of 3-hydroxy-3-ethynylindolin-2-ones. Green Chem.. 2011;13:549-553.
- [Google Scholar]
- Silver-catalyzed carbomagnesiation of terminal aryl and silylalkynes and enynes in the presence of 1,2-dibromoethane. Chem. Commun.. 2009;2009:1115-1117.
- [Google Scholar]
- N-functionalized benzimidazol-2-ylidene silver complexes: synthesis, characterization, and antimicrobial studies. Turk. J. Chem.. 2013;37:1007-1013.
- [Google Scholar]
- Synthesis and characterization of oxygen-functionalised-NHC silver(I) complexes and NHC transmetallation to nickel(II) Dalton Trans.. 2014;43:4700-4710.
- [Google Scholar]
- Synthesis, crystal structures, characterization and biological studies of nitrile-functionalized silver(I) N-heterocyclic carbene complexes. Inorg. Chim. Acta.. 2015;433:35-44.
- [Google Scholar]
- Group 11 metal complexes of N-heterocyclic carbene ligands nature of the metal-carbene bond. Organometallics. 2004;23(4):755-764.
- [Google Scholar]
- Potential of silver against human colon cancer:(synthesis, characterization and crystal structures of xylyl (Ortho, meta, & Para) linked bis-benzimidazolium salts and Ag (I)-NHC complexes: in vitro anticancer studies) Chem. Cent. J.. 2013;7(1):27.
- [Google Scholar]
- Macrophage and colon tumor cells as targets for a binuclear silver(I) N-heterocyclic carbene complex, an anti-inflammatory and apoptosis mediator. J. Inorg. Bochem.. 2015;146:1-13.
- [Google Scholar]
- Bimetalic Cu(I) complex with a pypiridine-bridged bis(1,2,3-triazole-5-ylidene) ligand. Dalton Trans.. 2016;45:5713-5719.
- [Google Scholar]
- Synthesis of chiral silver(I) diaminocarbene complexes from(R, R)-4,5-di-tert-butylimidazoline. J. Organomet. Chem.. 2001;631:157-163.
- [Google Scholar]
- Karatas, M.O., Olgundeniz, B., Gunal, S., Ozdemir, I., Alici, B., cetinkaya, E., 2016. Synthesis, characterization and antimicrobial activities of novel silver(I) complexes with coumarin substituted N-heterocyclic carbene ligands. Bioorg. Med. Chem., 24, 643–650.
- Ag(I) complexes of benzimidazol-2- ylidene ligands: a study of catalytic efficiency towards three-component coupling reactions. Turk. J. Chem.. 2016;40:681-687.
- [Google Scholar]
- Synthesis of N-heterocyclic carbene silver and palladium complexes bearing bis(pyrazol-1-yl)methyl moieties. J. Organomet. Chem.. 2013;745–746:106-114.
- [Google Scholar]
- Macrocyclic quinoline bridged mercury and silver bis(N-heterocyclic carbene) complexes: synthesis, structure, and spectroscopic studies. Anorg. Allg. Chem.. 2013;639:881-885.
- [Google Scholar]
- Metal complexes with di(N-heterocyclic carbine) ligands bearing a rigid ortho-, meta or para-phenylene bridge. Dalton Trans.. 2016;45:9540-9552.
- [Google Scholar]
- Preparation of a series of coinage metal complexes with pyridine-based bis(N-heterocyclic carbine) ligands including transmetalation to palladium compleses. J. Organ met. Chem.. 2016;103:67-72.
- [Google Scholar]
- Easily prepared air- and moisture-stable Pd–NHC (NHC=N-Heterocyclic Carbene) complexes: a reliable, user-friendly, highly active palladium precatalyst for the suzuki-miyaura reaction. Chem. Eur. J.. 2006;12:4743-4748.
- [Google Scholar]
- Synthesis, characterization and antimicrobial activity of new silver complexes with N-heterocyclic carbene ligands. Inorg. Chem. Acta.. 2010;363:3803-3808.
- [Google Scholar]
- Synthesis, cytotoxicity and antibacterial studies of p-methoxybenzyl-substituted and benzyl-substituted N-heterocyclic carbene-silver complexes. Eur. J. Inorg. Chem.. 2010;7:1020-1031.
- [Google Scholar]
- Synthesis, cytotoxicity and antibacterial studies of novel symmetrically and nonsymmetrically 4-(methoxycarbonyl)benzyl-substitued N-heterocyclic carbine silver acetate complexes. Helvetica Chim. Acta. 2010;93:2347-2364.
- [Google Scholar]
- A cationic (N-heterocyclic carbene) silver complex as catalyst for bulk ring-opening polymerization of L-lactides. Eur. J. Inorg. Chem.. 2006;2006:2975-2984.
- [Google Scholar]
- NonesymmetricallypenitrobenzylesubstitutedNeheterocycliccarbeneesilver(I) complexes as metallopharmaceutical agents. Appl. Organomet. Chem.. 2017;31:3819.
- [Google Scholar]
- Non–symmetrically p–nitrobenzyl–substituted N–heterocyclic carbene–silver(I) complexes as metallopharmaceutical agents. Appl. Organomet. Chem.. 2017;31:3819.
- [Google Scholar]
- Song, X., et al. 2010. Development of PLGA Nanoparticles Simultaneously Loaded with Vincristine and Verapamil for Treatment of Hepatocellular Carcinoma. J. Pharm. Sci., 99, 4874–4879.
- Photoluminescence of self-assembled Ag(I) and Au(I) N-heterocyclic carbene complexes. Interplay the aurophilic, hydrogen bonding and hydrophobic interactions. RSC Adv.. 2017;7:14611-14617.
- [Google Scholar]
- A new application area for AgNHCs: CO2 fixation catalyst. ChemCatChem.. 2017;4:831-835.
- [Google Scholar]
- Transition metal complexes of an (S, S)-1,2-Diphenylethylamine-Functionalised N-heterocyclic carbine: a new member of the asymmetric NHC ligand family. Organometallics. 2016;35:1604-1612.
- [Google Scholar]
- Silver(I), nickel (II) N-heterocyclic carbene complexes base on bidentate bis-imidazolium salt with a quinoxaline linker: syntheses, structures, and characterization. J. Coord. Chem.. 2017;70:615-625.
- [Google Scholar]
- Silver-catalyzed highly regioselective formal hydroboration of alkynes. Org. Lett. 2014;16:3512-3515.
- [Google Scholar]
Further reading
- Coumarin-substituted 1,2,4-triazole-derived silver(i) and gold(i) complexes: synthesis, characterization and anticancer studies. New J. Chem.. 2019;43:1216-1229.
- [Google Scholar]
- Binuclear luminescent silver(I) e N-heterocycliccarbenecomplexderivedfrom1-picolyl-3-pyrimidylbenzimidazoliumhexaflurophosphate. J. Mol. Struct.. 2013;1042:123-128.
- [Google Scholar]
- Palladium (II)-NHC complexes containing benzimidazole ligand as a catalyst for C-N bond formation. Appl. Organomet. Chem.. 2011;25:163-167.
- [Google Scholar]
- Catalytic activity of N-heterocyclic carbene silver complexes derived from imidazole ligands. Nano Metal Chem.. 2017;47:462-466.
- [Google Scholar]
- Pugh, A.A., Danopoulos, 2007. Metal complexes with ‘pincer’-type legends incorporating N-heterocyclic carbine functionalities. Coord. Chem. Rev., 251, 610–641.







