Translate this page into:
Synthesis, spectroscopic characterization and pharmacological studies on novel sulfamethaxazole based azo dyes
⁎Corresponding author. jathikeshvayya1959@gmail.com (J. Keshavayya)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University
Abstract
The present work describes the synthesis of novel heterocyclic azo dyes by general diazo-coupling reaction of sulfamethoxazole with the various coupling compounds 5-methyl-2-phenyl-2, 4-dihydro-3H-pyrazol-3-one, 6-hydroxy-4-methyl-2-oxo-1,2-dihydropyridine-3-carbonitrile and 1-ethyl-6-hydroxy-4-methyl-2-oxo-1,2-dihydropyridine-3-carbonitrile with good yield. The molecular structures of the newly synthesized compounds were confirmed by different spectroscopic techniques such as UV–Visible, FTIR, 1H NMR, Mass and elemental analysis. The in vitro biological screening of the synthesized compounds were tested against various microbial strains and results showed good activity compared with the standard drug. All the compounds exhibited promising anti-tubercular activity against Mycobacterium tuberculosis. The anticancer activity of the target compounds were screened against MCF-7, and compound A1 was found to be a potential anticancer agent with IC50 of 11.07 µg/mL. Also, the synthesized compounds studied for cleavage studies against CT-DNA by gel electrophoresis technique and all the compounds exhibited complete cleavage activity against CT-DNA.
Keywords
Sulfamethaxazole
Azo dyes
Diazotization and anticancer activity
1 Introduction
Azo dyes are the most important class of organic compounds having constantly received the attention of the researchers due to their versatile applications in various fields (Sahoo et al., 2015). In recent years, the growing interest in the synthesis of azo dyes having heterocycles in their structures have led to the design of optical recording systems and liquid crystalline devices (LCDs) (Wang et al., 2000; Peters and Gbadamosi, 1992; Qian et al., 2007; Shridhar et al., 2016). This is due to their high degree of brightness compared to azo dyes derived from aniline (Qiu et al., 2007; Kraska and Sokołowska-Gajda, 1987). The S and N containing heterocyclic azo dyes have showed potential applications in number of biological reactions such as inhibition of RNA, protein synthesis and nitrogen fixation (Rizk et al., 2017; Gopi et al., 2017). On the other hand, azo compounds have also played an essential role as antibacterial, antifungal, anticancer (Gouda et al., 2016) and antituberculosis agents etc. (Yazdanbakhsh et al., 2012). There has been a development of potentially active drugs and are available in the market. However, there is a growing resistance in strains such as Mycobacterium tuberculosis that causes pulmonary infection disease (Correia et al., 2014). Also, the anticancer drugs tend to have a certain limitations such as adverse side effects, high toxicity as well as the intrinsic and acquired resistance (Kamal et al., 2010; Bueno, 2016; Lai et al., 2016; Sahoo and Paidesetty, 2016). Due to these challenges, there is needed to design novel drugs with multiple curative properties (Moriarity et al., 2016). Furthermore, sulfonamide drugs are well-established antibiotics for human bacterial infections. Among the studied sulfonamides, sulfamethoxazole have showed excellent pharmacological properties due to its less toxicity, low cost and distinctive activity against various diseases (Singh et al., 2004; Dai et al., 2011).
In this direction, we have focused on the synthesis of some novel azo dyes derived from sulfamethoxazole and studied their pharmacological properties. The molecular structures of the newly synthesized dyes were confirmed by various physico-chemical techniques. The antimicrobial, antitubercular, anticancer and DNA cleavage activities of the azo dyes were studied in order to explore their potentiality to inhibit the respective pathogens.
2 Experimental
2.1 Methods and materials
All the reagents, sulfamethoxazole, 5-methyl-2-phenyl-2, 4-dihydro-3H-pyrazol-3-one, ethyl acetoacetate, ethyl cyanoacetate, ammonia, and ethylamine were of analytical grade and purchased from Sigma Aldrich Chemical Company. All the solvents used in the present study were purified by following the standard procedures. Melting points were measured from the open capillary method and are uncorrected. UV–vis spectra were recorded on Elico-SL 164 double beam spectrometer in the range 200–800 nm using ca. 10−6 M solution in Tetrahydrofuran (THF), N, N-dimethyl formamide (DMF) and Dimethyl sulfoxide (DMSO). IR spectra of the synthesized compounds were recorded as KBr pellets on a Perkin- Elmer-spectrum RX-IFTIR instrument in the region 4000–400 cm−1. The 1H NMR spectra were recorded on the FT-NMR spectrometer model Bruker Avance II, 400 MHz using DMSO-d6 as the solvent. ESI-MS was recorded on a mass spectrometer equipped with electrospray ionization (ESI) source having mass range 4000 amu in quadruple and 20,000 amu in Tof and elemental analysis was obtained from Vario EL III CHNS analyzer.
2.2 General procedure for the synthesis of azo dyes (A1-A3)
A well-stirred solution of sulfamethoxazole (1) (0.50 g, 2 mmol) in 5 mL of conc. HCl was cooled in an ice-salt bath and diazotized with a cold solution of sodium nitrite (0.19 g, 2.7 m mol) in 2 mL H2SO4. The above reaction mixture was stirred for two hours at the same temperature. The cold diazonium salt solution obtained was added to the well-stirred solution of coupling compounds (a-c) in dilute KOH solution. The resulting solution was stirred for additional three hours at 0–5 °C, while the pH of the reaction mixture was adjusted to 6–7 by adding required amount of sodium bicarbonate solution. The progress of the reaction was monitored by TLC, and the crude product was filtered off, washed with hot water, dried and recrystallized from ethanol (Scheme 1).
Synthetic route for the preparation of azo dyes A1-A3.
2.2.1 Synthesis of N-(5-methyl-1, 2-oxazol-3-yl)-4-[(E)-(3-methyl-5-oxo-1-phenyl-4, 5-dihydro-1H-pyrazol-4-yl) diazenyl] benzenesulfonamide (A1)
This dye was isolated as light orange yellow colour solid with 78% yield, m. p. 183–185 °C. IR (KBr, cm−1): 3238 (NH), 2900 (Ar-CH), 1687 (C⚌O), 1615 (C⚌N), 1517 (N⚌N). 1H NMR (DMSO-d6): δ 11.26 (s, 1H, NH), 7.92–7.88 (m, 4H, Ar-H of sulfamethoxazole ring), 7.73–7.71 (d, 2H, Ar-H), 7.43–7.41 (d, 2H, Ar-H), 7.21–7.17 (t, 1H, Ar-H), 6.10 (s, 1H, Ar-H attached to pyrazole ring), 2.33 (s, 3H, CH3 attached to oxazole ring), 2.31 (s, 3H, CH3 attached to pyrazole ring), 2.30 (s, 1H, CH of the oxazole ring). ESI-MS: m/z (%) = 439 [M + 1] +. Anal. Calcd. (%) For C20H18N6O4S: C, 54.79; H, 4.14; N, 19.17; S, 7.31. Found (%): C, 54.22; H, 3.92; N, 19.12; S, 7.28.
2.2.2 Synthesis of 4-[(E)-(5-cyano-2-hydroxy-4-methyl-6-oxo-1, 6-dihydropyridin-3-yl) diazenyl]-N-(5-methyl-1, 2-oxazol-3-yl) benzenesulfonamide (A2)
This dye was isolated as yellow colour solid with 81% yield, m. p. 178–180 °C. IR (KBr, cm−1): 3478 (OH), 3375, 3164 (NH), 2984 (Ar-CH), 1687 (C⚌O), 1646 (C⚌N), 1512 (N⚌N). 1H NMR (DMSO-d6): δ 14.61 (s, 1H, OH), 12.15 (s, 1H, NH attached to pyrazole ring), 11.30 (s, 1H, NH attached to benzene ring), 7.93–7.31 (m, 4H, Ar-H of sulfamethoxazole ring), 6.08 (s, 1H, Ar-H of pyrazole ring), 2.56 (s, 3H, CH3 of pyrazole ring), 2.31 (s, 3H, CH3 of oxazole ring). ESI-MS: m/z (%) = 415 [M + 1] +. Anal. Calcd. (%) For C17H14N6O5S: C, 49.27; H, 3.41; N, 20.28; S, 7.74. Found (%): C, 48.98; H, 3.16; N, 20.22; S, 7.65.
2.2.3 Synthesis of 4-[(E)-(5-cyano-1-ethyl-2-hydroxy-4-methyl-6-oxo-1, 6-dihydropyridin-3-yl) diazenyl]-N-(5-methyl-1, 2-oxazol-3-yl) benzenesulfonamide (A3):
This dye was isolated as light yellow colour solid with 85% yield, m. p. 180–182 °C. IR (KBr, cm−1): 3388 (OH), 3225 (NH), 3007 (Ar-CH), 1654 (C⚌O), 1612 (C⚌N), 1555 (N⚌N). 1H NMR (d6-DMSO): δ 14.45 (s, 1H, OH), 11.41 (s, 1H, NH), 7.92–7.90 (d, 2H, Ar-H), 7.85–7.83 (d, 2H, Ar-H), 6.12 (s, 1H, Ar-H attached to pyrazole ring), 3.95–3.89 (q, 2H, CH2CH3), 1.18–1.15 (t, 3H, CH2CH3), 2.52(s, 3H, CH3), 2.30 (s, 3H, CH3). ESI-MS: m/z (%) = 443 [M + 1] +. Anal. Calcd. (%) For C19H18N6O5S: C, 51.58; H, 4.10; N, 18.99; S, 7.25. Found (%): C, 51.42; H, 3.92; N, 18.24; S, 7.27.
2.2 Pharmacological activity
2.2.1 Antibacterial activity
Newly synthesized azo-compounds (A1-A3) were screened for antibacterial activity against Escherichia coli (ATCC 25922) and Bacillus subtilis (ATCC 19659) was evaluated by agar disc diffusion assay (Bauer et al., 1966). Briefly, 48 h old cultures of the selected bacteria were spread in 20 mL Muller Hinton Agar in Petri plates. Whatman No. 1 filter paper discs (5 mm in diameter) impregnated with the test compound (20 µL/disc) was placed on the plates. The inoculated plates were incubated for 24 h at 37 °C and the developed zone of inhibition was measured in millimeters. All the tests are performed in triplicate.
2.2.2 Antifungal activity
Antifungal activity of the target compounds against Aspergillus niger (ATCC627) and Candida albicans (ATCC 10231) were performed by poisoned food technique as described earlier (Sadana et al., 2003). The percentage of inhibition of the fungal growth was calculated by using the formula:
Where IP is the percentage of inhibition, C and T are the average of three replicates of mycelial growth (cm) of control and treated petri dishes respectively.
2.2.3 Anti-mycobacterial activity
The anti-mycobacterial activity of azo dyes A1-A3 was studied against Mycobacterium tuberculosis (H37RV strain) by Microplate Alamar Blue Assay (MABA) (Mangalam et al., 2017; Kirubavathy and Chitra, 2017). Briefly, 100 µL of the Middlebrook 7H9 broth were put into the 96-well plate, and the compounds were serially diluted on the plate (100–0.2 µg/mL). The plates were covered with Para-film and incubated at 37 °C for five days. After incubation, 25 µL of freshly prepared 1:1 mixture of Almar blue reagent and 10% Tween-80 were added to the plate and incubated for another 24 h. A blue color observed in the wells indicates no bacterial growth whereas pink color stipulates the development of the bacteria. The minimum inhibitory concentration (MIC) of the compounds was determined by observing the colour change.
2.2.4 Anti-cancer activity
The in vitro anticancer activity of the azo dyes A1-A3 was studied against breast cancer cell line MCF-7 by MTT assay (Doyle and Griffiths, 2000; Anjomshoa and Torkzadeh-Mahani, 2015). The cancerous cells were cultured in 96-well plate containing minimum essential media (MEM) with 10% inactivated fetal calf serum. The supernatant was removed from the plate, and fresh MEM solution was added and treated with synthesized compounds of concentrations 10–50 µg/mL and incubated for 48 h. After incubation, a stock solution of MTT (5 mg/mL) was added to each well. After 4 h incubation the DMSO was added to solubilise the MTT formazan. The optical density (OD) of each well was measured at 570 nm and the relative cell viability values are calculated according to the following formula:
The IC50 values of the target compounds were determined from the plot: 50% viability against the concentration of the compounds.
2.2.5 DNA cleavage studies
The efficiency of the newly synthesized azo dyes to cleave Calf-thymus DNA was studied to understand the drug mechanism. The DNA cleavage activity of the target compounds was studied against commercially available supercoiled CT DNA (Bangalore Genei, Bengaluru, and Cat. No 105850) as described earlier (Kirubavathy and Chitra, 2017; Sambrook et al., 1989). Agarose gel electrophoresis was employed for the determination of cleavage efficiency by the synthesized compounds.
3 Results and discussion
The synthetic path adopted for the preparation of novel disperse azo dyes having sulfamethoxazole core is depicted in Scheme 1. The target compounds A1-A3 were synthesized by coupling of 5-methyl-2-phenyl-2, 4-dihydro-3H-pyrazol-3-one (a), 6-hydroxy-4-methyl-2-oxo-1, 2-dihydropyridine-3-carbonitrile (b) and 1-ethyl-6-hydroxy-4-methyl-2-oxo-1, 2-dihydropyridine-3-carbonitrile (c) with diazotized sulfamethoxazole at 0–5 °C. The physical and analytical data of the azo dyes were displayed in Table 1. The structures of the newly synthesized compounds were characterized by UV–Visible, FT-IR, 1H NMR and mass spectroscopic techniques. The IR, 1H NMR and mass spectral data were found to be in good agreement with the newly synthesized azo dyes.
Compounds
Colour
M.P. (°C)
Mol.Wt.
Mol. Formula
Elemental analysis (%) Calcd. (Found)
C
H
N
S
A1
Orange
183–185
438.45
C20H18N6O4S
54.79 (54.22)
4.14 (3.92)
19.17 (19.12)
7.31 (7.28)
A2
Yellow
178–180
414.39
C17H14N6O5S
49.27 (48.98)
3.41 (3.16)
20.28 (20.22)
7.74 (7.65)
A3
Light yellow
180–182
442.44
C19H18N6O5S
51.58 (51.42)
4.10 (3.92)
18.99 (18.24)
7.25 (7.27)
3.1 Electronic absorption spectra and substituent effect
To explore the impact of solvent on the absorption spectra of the synthesized azo dyes A1-A3, we recorded their electronic absorption spectra (Figs. 1–3) in the region 200–800 nm. The three different solvents THF, DMF, and DMSO, were used at a concentration of 10−6 M, in which the solvents are arranged in the order of decreasing polarity. As depicted in Table 2, the absorption spectra of the azo dyes in different solvents exhibited maximum absorption band in the range of 388–438 nm which can be assigned to n → π∗ or π → π∗ transitions of azo (—N⚌N—)group. From these results, we can observed that increase in the solvent polarity has caused a bathochromic shift in the absorption maxima of all the dyes. This effect is due to the interaction between solvent molecules with a lone pair of electrons on the nitrogen atom of synthesized dyes that causes extended conjugation via increased polarity of the dyes. Further, it is evident that introduction of phenyl, methyl, ethyl and —H groups on the diazo component, the absorption spectra of each azo dye illustrated a lower in energy band in the visible region. The presence of cyano group shifts the absorption maxima to higher energy band, i.e., towards shorter wavelength and it can be explained by substituent effect. Introduction of electron donor group into diazo component produces increased bathochromic shifts, whereas electron acceptor group produces hypsochromic shifts.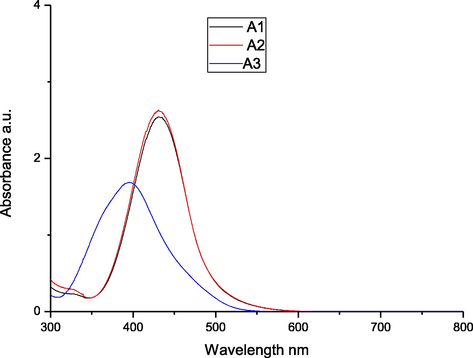
UV–Visible spectra of the azo dyes A1-A3 in DMF.
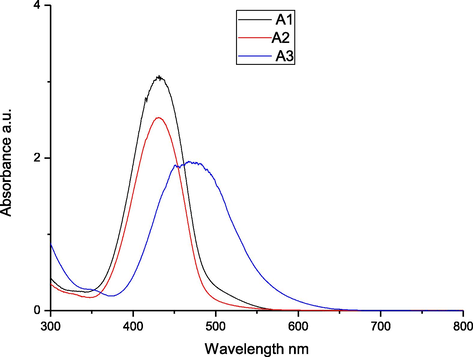
UV–Visible spectra of the azo dyes A1-A3 in DMSO.
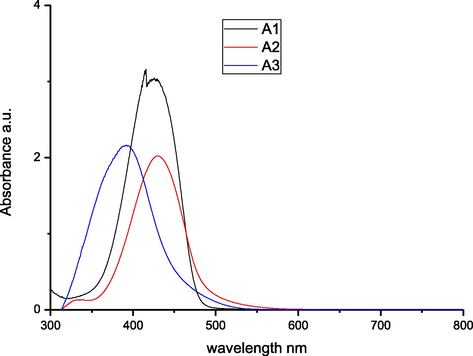
UV–Visible spectra of the azo dyes A1-A3 in THF.
Compounds
λmax (nm)
Logε
DMSO
DMF
THF
DMSO
DMF
THF
A1
431
438
427
4.18
4.09
4.03
A2
388
396
391
3.93
3.92
4.03
A3
429
431
426
4.09
4.11
4.00
3.2 IR spectral data
IR spectra of the compounds were recorded as KBr pellets in the region 4000–400 cm−1. Important IR bands exhibited by azo dyes were displayed in Table 3. Strong absorption bands appeared in the area 3480–3300 cm−1 and 3380–3100 cm−1 assigned to phenolic OH and NH groups respectively. The aromatic C–H stretching vibrations were appeared at 3007–2900 cm−1 and a high-intensity band at 1687–1654 cm−1 due to carbonyl function. Also, weak bands observed at 1646–1612 cm−1 and 1555–1512 cm−1 due to the presence of ʋ(C⚌N) and ʋ(N⚌N) groups respectively.
Compounds
ʋOH
ʋNH
ʋAr-CH
ʋC=O
ʋC=N
ʋN=N
A1
–
3238
2900
1687
1615
1517
A2
3478
3375, 3164
2984
1687
1646
1512
A3
3388
3225
3007
1654
1612
1555
3.3 1H NMR spectral data
1H NMR spectra of the synthesized compounds A1, A2 and A3, were recorded in DMSO-d6 at ambient temperature. 1H NMR spectra confirm the structures of all the synthesized azo dyes. The hydrogen atom of the NH group in all the compounds was observed in the range 11.26–12.15 ppm as a singlet. The phenolic OH in compound A2 and A3 appeared as a singlet at 14.61 and 14.45 ppm, respectively. The aromatic protons are resonated as multiplet in the region 6.08–7.90 ppm. The aliphatic protons of all the synthesized compounds were observed in the region 3.95–1.15 ppm.
3.4 Mass spectral data
The ESI mass spectra of the synthesized compounds exhibited their molecular ion peaks equivalent to their molecular mass and confirms the proposed molecular formula. The ESI mass spectra of the compounds A1, A2, and A3, showed [M + 1] peaks at m/z 439, 415 and 443 respectively which corresponds to the molecular weight of the compounds. The proposed fragmentation pattern of the synthesized compounds is presented in the Schemes 2–4 respectively.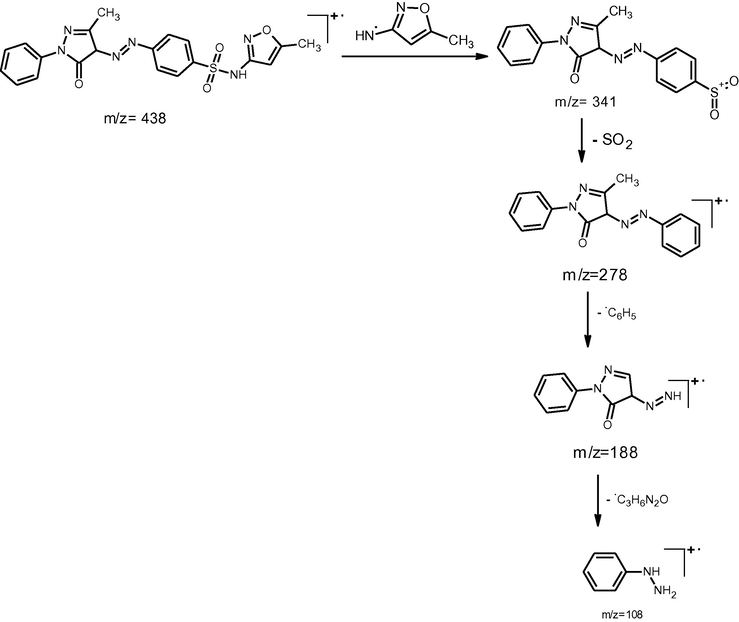
Mass spectral fragmentation of compound A1.
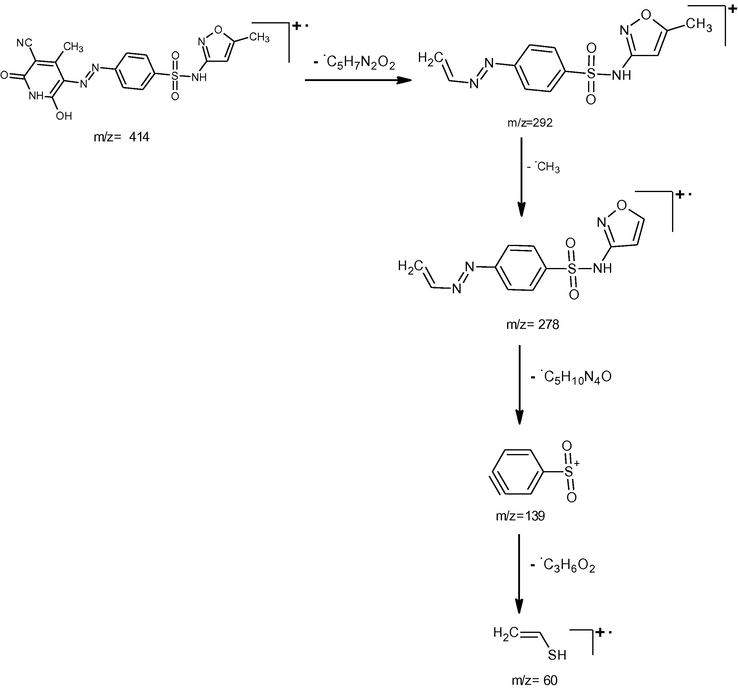
Mass spectral fragmentation of compound A2.
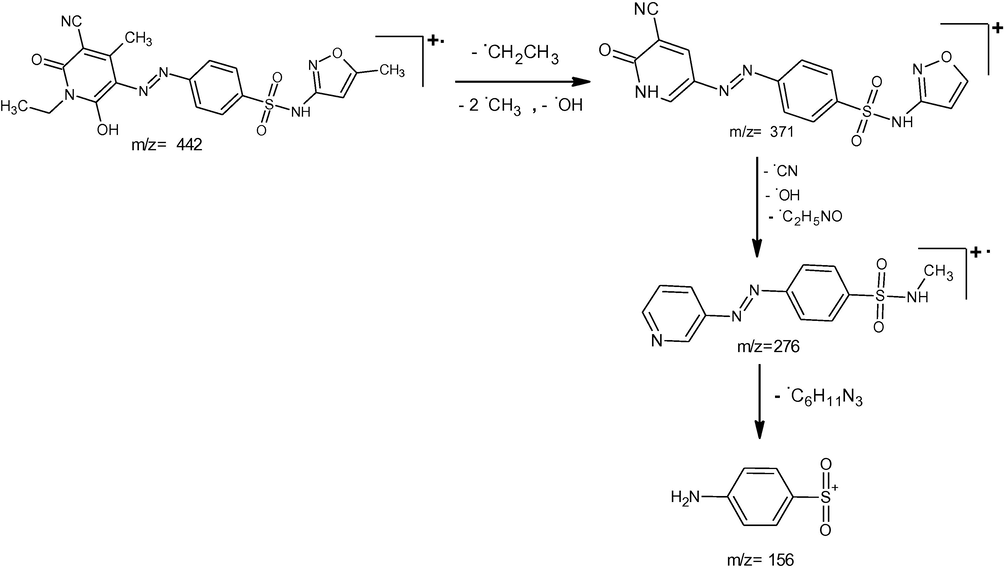
Mass spectral fragmentation of compound A3.
3.5 Antimicrobial activity
The azo dyes containing heterocyclic rings involved in the biological reactions have continued to attract more attention as potential drugs for therapeutic intervention in various diseases. In the present study, the results of the newly synthesized azo compounds tested for their antibacterial activity against pathogenic strains (E. coli and B. subtilis) as compared to standard drug streptomycin are illustrated in Table 4. Among the tested compounds, A1 and A3 exhibited the highest zone of inhibition against B. subtilis, while, the weak effect was observed in E. coli. However, the less inhibitory effect was seen in A2 against the tested strains. Further, the antifungal activity of the target compounds studied against A. flavus and C. albicans is shown in Table 4, and the activity can be ordered as A1 > A3>A2. In general, the tested compounds showed an excellent bactericidal, as well as fungicidal activities and this effect, could be attributed to their charge density distribution (Zhang et al., 2006). *Values are represented as the mean ± SEM. *Values are significant for the standard at 0.005 level of significance.
Compounds
Bacteria
Fungi
E. coli
B. subtilis
A. flavus
C. albicans
25 mg/mL
50 mg/mL
25 mg/mL
50 mg/mL
25 mg/mL
50 mg/mL
25 mg/mL
50 mg/mL
A1
1.8 ± 0.1
2.1 ± 0.30
1.9 ± 0.24
2.4 ± 0.29
44
74
39
78
A2
1.5 ± 0.6
1.9 ± 0.8
1.4 ± 0.3
1.6 ± 0.5
25
53
38
46
A3
1.5 ± 02
1.8 ± 0.5
2.0 ± 0.12
2.4 ± 0.12
44
70
55
64
Streptomycin
1.9 ± 0.25
2.5 ± 0.28
2.0 ± 0.32
2.6 ± 0.35
–
–
–
–
Fluconazole
–
–
–
–
37
78
39
81
3.6 Anti-mycobacterial activity
Tuberculosis is an infectious disease caused by Mycobacterium tuberculosis, and it is among the leading causes of death worldwide (Bedewi et al., 2017). Various drugs have been developed in recent times for the treatment of the disease (Kirubavathy and Chitra 2017). However, it remains a significant subject of concern for pharmaceutical industries to overcome the challenge due to the development of multidrug-resistant strain. Accordingly, there is an urgency for the development of effective anti-mycobacterial drugs. Therefore, the azo compounds in the present study were screened for their anti-mycobacterial activity against M. tuberculosis, and the results are tabulated in Table 5 and Fig. 4. From the findings, it is evident that all the tested compounds have noticeable inhibitory effect against the strain. Among these compounds, A1 was found to have high antitubercular activity with the MIC of 6.25 µg/mL which correlates to standard drug streptomycin used. Further, A3 and A2 exhibited good to moderate activities with the MIC of 50 and 100 µg/mL, respectively. S – Sensitive. R – Resistant.
Compounds
100 µg/mL
50 µg/mL
25 µg/mL
12.5 µg/mL
6.25 µg/mL
3.12 µg/mL
1.6 µg/mL
0.8 µg/mL
A1
S
S
S
S
S
R
R
R
A2
S
R
R
R
R
R
R
R
A3
S
S
R
R
R
R
R
R
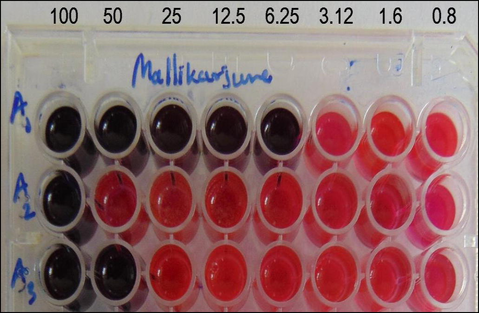
Anti-mycobacterial activity results of the azo dyes A1-A3.
3.7 Anti-cancer activity
Although there are various drugs for the treatment of cancer, still it remains the second cause of death worldwide, due to limitations such as adverse side effects, high toxicity, and development of multidrug resistance (Sabet et al., 2010). With these distinct challenges, there is a need for new anticancer drugs with less toxicity and side effects. And thus, recent studies have focused on the designing of potent compounds having heterocyclic rings as chemotherapeutic agents (Kumar et al., 2014). In this direction, we have studied the cytotoxic activity of the synthesized compounds A1-A3 against breast cancer (MCF 7) cell line by MTT assay. The synthesized compounds were found to have potential cytotoxic activity at different concentrations as shown in Table 6 and Fig. 5. Among the tested compounds, A1 and A3 were found to be more efficient with IC50 of 11.07 and 30.52 µg/ml respectively, whereas A2 showed moderate activity with IC50 of 78.61 µg/ml.
Compounds
IC50 (µg/mL)
A1
11.07
A2
78.61
A3
30.52
Doxorubicin
6.02

Anticancer activity of the synthesized azo dyes A1-A3 against MCF-7.
3.8 DNA cleavage studies
The results of all the above studies encouraged us to investigate the interaction of the synthesized azo dyes against CT-DNA at 100 µg/L concertation by an agarose gel electrophoresis method. The gel picture showing cleavage is presented in Fig. 6. Form the results; it is evident that the intensity of DNA was diminished after electrophoresis, was observed which is due to cleavage of DNA by the tested compounds. This observation indicates the role of the target compounds in the cleavage reactions. The difference which was observed in the bands of azo dyes (A1-A3) compared to control DNA due to molecular weight difference. This shows control DNA did not alone show any visible cleavage. Since the compounds were observed to cleave DNA, it can be concluded that the compounds inhibit the growth of a pathogenic organism by cleaving the genome.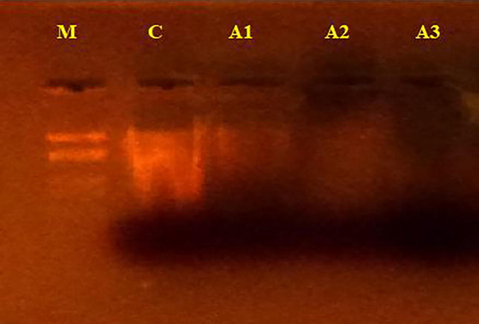
DNA cleavage activity of Calf-thymus DNA: M: Standard DNA, C: Control DNA (untreated Calf-thymus DNA), A1: compound A1, A2: compound A2, A3: compound A3.
4 Conclusion
The present study describes the synthesis of novel disperse azo dyes derived from sulfamethoxazole by conventional diazo-coupling reaction. From the results, the excellent colour brightness properties exhibited by the molecules are due to the presence of chromophores in their structures. Their molecular structures were confirmed by various spectroscopic techniques like UV–Visible, FTIR, 1H NMR and Mass spectrometry. The substituent effect indicates that the presence of electron releasing group on the diazo component has significant influence (bathochromic shift) on the absorption spectra of these dyes. The increasing solvent polarity, also, made effective shift in the electronic spectra of the dyes. Further, the newly synthesized azo dyes were proved to be potential antimicrobials against different pathogenic strains viz E. coli, B. subtilis, A. flavus and C. albicans. The analysis of anti-mycobacterial activity of the target compounds showed A1 to be having higher inhibitory effect against M. tuberculosis with IC50 of 6.25 µg/mL. Similarly, the anticancer activity of the synthesized compounds against MCF-7 cell line was found to have potential anticancer activity. Among the studied compounds, A1 exhibited higher activity with IC50 of 11.07 µg/mL compared to the standard drug doxorubicin. The observed results of the anticancer activity, it is evident that having five membered heterocycles in the molecule could be used as lead compounds for the development of potential anticancer drugs in future. Also, the DNA cleavage properties of the azo dyes were studied against CT DNA by gel electrophoresis and all the compounds showed potential cleavage efficiency. In summary, recently established approach of merging two or more pharmacophores in a single molecule has proved useful in curing multiple diseases. Therefore, it is paramount to develop novel drugs with multiple curative properties. From the present study, it is evident that the synthesized compounds have proved to be efficient in inhibiting multiple diseases. Hence, they may be used in pharmaceuticals for developing potential drugs.
Acknowledgements
Authors are grateful to the Chairman, Department of Chemistry, Kuvempu University, Shankaraghatta for providing laboratory facility. Authors are also thankful to SAIF, Panjab University, Chandigarh for spectral data and Maratha Mandal Central research laboratory for providing biological studies.
References
- In vitro DNA and BSA-binding, cell imaging and anticancer activity against human carcinoma cell lines of mixed ligand copper (II) complexes. Spectrochim. Acta A. 2015;150:390-402.
- [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol.. 1966;45(4):493.
- [Google Scholar]
- Antimicrobial adjuvants drug discovery, the challenge of avoid the resistance and recover the susceptibility of multi-drug resistant strains. J. Microb. Biochem. Technol.. 2016;8:169-176.
- [Google Scholar]
- Hydroxyquinoline derived vanadium (IV and V) and copper (II) complexes as potential anti-tuberculosis and anti-tumor agents. J. Inorg. Biochem.. 2014;141:83-93.
- [Google Scholar]
- Divergent C-H functionalizations directed by sulfonamide pharmacophores: late-stage diversification as a tool for drug discovery. J. Am. Chem. Soc.. 2011;133(18):7222-7228.
- [Google Scholar]
- Doyle A., Griffiths J.B., eds. Cell and Tissue Culture for Medical Research. Wiley; 2000.
- Design, synthesis, spectroscopic characterization and anti-psychotic investigation of some novel Azo dye/Schiff base/Chalcone derivatives. EJBAS. 2017;4(4):270-287.
- [Google Scholar]
- Synthesis and antitumor evaluation of thiophene based azo dyes incorporating pyrazolone moiety. J. Saudi Chem. Soc.. 2016;20(2):151-157.
- [Google Scholar]
- Synthesis and properties of monoazo disperse dyes derived from 3-amino-5-nitro [2, l] benzisothiazole. Dyes Pigm.. 1987;8(5):345-352.
- [Google Scholar]
- Synthesis of 3, 3-diindolyl oxyindoles efficiently catalyzed by FeCl3 and there in vitro evaluation for anticancer activity. Bioorg. Med. Chem. Lett.. 2010;20(17):5229-5231.
- [Google Scholar]
- Structural, theoretical investigations and biological evaluation of Cu (II), Ni (II) and Co (II) complexes of mercapto-pyrimidine Schiff bases. J. Mol. Struct.. 2017;1147:797-809.
- [Google Scholar]
- Synthesis, antimicrobial, anticancer, antiviral evaluation and QSAR studies of 4-(1-aryl-2-oxo-1, 2-dihydro-indol-3-ylideneamino)-N-substituted benzenesulfonamides. Arab. J. Chem.. 2014;7(4):396-408.
- [Google Scholar]
- Palliative chemotherapy in advanced colorectal cancer patients 80 years of age and older. Curr. Oncol.. 2016;23:144-153.
- [Google Scholar]
- Synthesis, stereochemical, structural, and biological studies of a series of N′-(2r, 4c-diaryl-3-azabicyclo [3.3. 1] nonan-9-ylidene) pyrazine-2-carbohydrazides. J. Mol. Struct.. 2017;1129:305-312.
- [Google Scholar]
- Current targeted therapies in the treatment of advanced colorectal cancer: a review. Ther. Adv. Med. Oncol.. 2016;8:276-293.
- [Google Scholar]
- 5, 6-(6, 7-) dichlorobenzothiazolylazo dyes for synthetic-polymer fibers. Dyes Pigm.. 1992;18(2):115-123.
- [Google Scholar]
- The first-order molecular hyperpolarizability and thermal stability of charge-transfer azo diol and azo aldimine. Dyes Pigm.. 2007;75(2):460-465.
- [Google Scholar]
- Synthesis and properties of the polymer containing azo-dye chromophores for nonlinear optical applications. Dyes Pigm.. 2007;75(2):454-459.
- [Google Scholar]
- Synthesis, dyeing performance on polyester fiber and antimicrobial studies of some novel pyrazolotriazine and pyrazolyl pyrazolone azo dyes. Arab. J. Chem.. 2017;10:S3303-S3309.
- [Google Scholar]
- Synthesis and biological activities of Bis alkyl 1,3,4-oxadiazole incorporated azo dye derivatives. Arab. J. Chem.. 2016;9:S1643-S1648.
- [Google Scholar]
- Synthesis, spectral characterization of some new 3-heteroaryl azo 4-hydroxy coumarin derivatives and their antimicrobial evaluation. J. Taibah. Univ. Sci. 2015;9(2):187-195.
- [Google Scholar]
- Medicinal interest of azo based organic compounds: a review. Asian J. Pharm. Clin. Res.. 2016;9:33-39.
- [Google Scholar]
- Synthetic, magnetic, spectral, antimicrobial and antifertility studies of dioxomolybdenum (VI) unsymmetrical imine complexes having a N∩ N donor system. Transition Met. Chem.. 2004;29(1):70-74.
- [Google Scholar]
- Hypervalent iodine mediated synthesis of 1-aryl/hetryl-1,2,4-triazolo [4,3-a] pyridines and 1-aryl/hetryl 5-methyl-1,2,4-triazolo [4,3-a] quinolines as antibacterial agents. Eur. J. Med. Chem.. 2003;38(5):533-536.
- [Google Scholar]
- Molecular Cloning:A Laboratory Manual. New York: Cold Spring Harbor Press; 1989.
- QSAR study of isatin analogues as in vitro anti-cancer agents. Eur. J. Med. Chem.. 2010;45(3):1113-1118.
- [Google Scholar]
- Synthesis, spectroscopic and thermal properties of a series of azo metal chelate dyes. Dyes Pigm.. 2000;44(3):195-198.
- [Google Scholar]
- Synthesis, spectral characterization and antimicrobial activity of some new azo dyes derived from 4, 6-dihydroxypyrimidine. J. Mol. Liq.. 2012;169:21-26.
- [Google Scholar]
- Inhibitory study of some novel Schiff base derivatives on Staphylococcus aureus by microcalorimetry. Thermochim. Acta. 2006;440(1):51-56.
- [Google Scholar]
- New Microbes New Infect.. 2017;17:69-74.







