Translate this page into:
Synthesis, photophysical, cyclic voltammetry properties, and molecular structure study of novel (5,10,15,20-tetratolylphenyl porphyrinato)zinc(II) with pyrazine
⁎Corresponding author. ra.soury@uoh.edu.sa (Raoudha Soury)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The precursor (5,10,15,20)-tetratolylporphyrin (H2TTP) (1) and Zn(II) complex (5,10,15,20)-tetratolylporphyrinato)zinc(II) [Zn(TTP)] (2) have been synthesized and utilized to afford a novel complex (3) bearing pyrazine as fifth coordination site on axial position, i.e. (pyrazine)(5,10,15,20-tetratolylporphyrinato)zinc(II) hemi-pyrazine hemidichloromethane solvate [Zn(TTP) (pyz)]0.0.5(pyz.CH2Cl2). Photophysical, electrochemical and X-ray diffraction were investigated. The single-crystal X-ray analysis indicated that in complex (3) (i) Zn(II) ion is penta coordinated, (ii) slight displacement of Zn atom by −0.28 Å towards the axial ligand (pyrazine) and (iii) the crystal lattice is made up of two dimensional layers stabilised by C−H…Cg intra- and intermolecular interactions (Cg is the centroid of pyrrole ring). Optical absorption studies revealed a redshifted of the Soret (B) and Q bands of the Zn-porphyrins complexes. Contrarily, a hypsochromic shift of Q bands in emission spectra of the complexes is noted as compared to the H2TPP. Lifetime of the electrons in (1–3) were determined and compared. Overall, this study would assist in understanding the impact of incorporation of pyrazine at the axial position on optical and electronic properties. Cyclic voltammetry (CV) was applied for the study of the electrochemical behavior of both (2) and (3) complexes in 0.2 M Tetra-n-butyl ammonium perchlorate (TBAP) solution in CH2Cl2. The results of electrochemical confirmed the potentials redox peaks shift more negative ca 60 mV to that is caused by the coordination of pyrazine ligand.
Keywords
Synthesis
Zn-porphyrin
Photophysical studies
Cyclic voltammetry properties
Solid-state structure
1 Introduction
Porphyrin and metalloporphyrin are π-conjugated macrocyclic scaffolds that are ubiquitously found in nature (Nath et al., 2016; Lu and Kobayashi, 2016). This class of compounds is known for its unique and tuneable photophysical properties (such as broad and intense absorption spanning visible region and colourful emission), high chemical and thermal stability (Sommer et al., 2011). Indeed, this is attributed to the planar and electron-rich four pyrrolic units connected via methylene unit (Jusélius and Sundholm, 2000). Besides, high rigidity, delocalisation of 22 π-electrons around the large ring system, and the presence of chemical modification sites (meso and β-positions) are added advantage with this class of chromophore. Owing to these features, a range of substituted porphyrins and their corresponding complexes have been reported (Lu and Kobayashi, 2016; Imahori et al., 2009; Chatterjee et al., 2016). Interestingly, many of them have shown potential candidateship for optoelectronic (Imahori et al., 2009; El-Nahass et al., 2014; Lind et al., 2009), sensing (Gujarathi, 2020; Park et al., 2013), catalysis (Barona-Castaño et al., 2016), bioimaging (Haque et al., 2017), and other intriguing applications (Haque et al., 2018).
It has been demonstrated that the energy levels, absorption/emission properties and applications can be significantly modulated by varying the substituents present at the periphery (meso and β-positions) and metal at the centre (Soury et al., 2018a, 2019; Chatterjee et al., 2016; El-Nahass et al., 2014). For example, it is found that the substituent present at meso position controls the catalytic activity of the complexes (Barona-Castaño et al., 2014). Similarly, when a suitable organic spacer is linked to the meso positions via alkynyl unit, electronic communication and photo-sensitization effect can be observed (Haque et al., 2018). In addition, a plethora of studies have demonstrated that photophysical properties of porphyrin and metalloporphyrin is a direct function of the spacer attached to it. Regarding the metals, it is found that the insertion of late transition metal like Zn(II) changes the symmetry of the free ligand and thus the optical properties(Gouterman, 1959). Also, the extent of shifting of the Q-bands has been related to the nature of the metal centre, the meso substituents and the porphyrin macrocycle undergoes non-planar distortion (Soury et al., 2018a, 2019, 2018b). During the literature survey, we noted that Zn(II) complexes bearing substituted porphyrin has already been reported (Lu et al., 2016; Chatterjee et al., 2016). However, those bearing pyrazine as fifth coordination site is yet to be explored (Chart 1).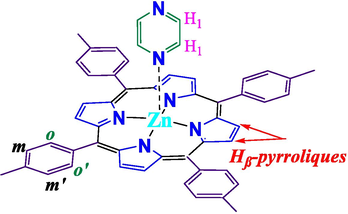
Chemical structure and numbering scheme for complex (3).
Therefore, it would be interesting to see the effects of insertion of Zn(II) cation and the axial ligands in the meso porphyrin on the electronic, redox, structural and photophysical properties. Based on these notions, we herein report the synthesis, characterization, optical and dielectric properties of a free base (5,10,15,20)-tetratolylporphyrin (H2TTP) (1) and corresponding Zn(II) complexes (5,10,15,20)-tetratolylporphyrinato)zinc(II) [Zn(TTP)] (2) and hemi-pyrazine hemidichloromethane solvate [Zn(TTP) (pyz)]0.0.5(pyz.CH2Cl2) (3). The IR and 1H NMR, optical and emission spectroscopy, as well as Electrospray mass spectrometry are used to characterize ligands as well as complexes. The structure of one of the complexes (3) is also evidenced by single crystal X-ray molecular structure.
2 Experimental
2.1 Materials and methods
2.1.1 Materials
The solvents and reagents are of grade quality and used as received.
2.1.2 Preparation of H2TTP (1) and [Zn(TTP)] (2)
Ligand H2TTP (1) and Zn(II) complex [Zn (TTP)] (2) were prepared following the procedure reported in literature (Adler et al., 1967; Hoard and Smith, 1975). The structure of the synthesized materials was confirmed using state of the art analytical technique prior to use for subsequent steps.
2.1.3 Synthesis and crystallization of [Zn(TTP)(pyz)]0.0.5(pyz.CH2Cl2) (3)
[Zn(TTP)] (2) (25 mg, 0.020 mmol) and pyrazine (90 mg, 1.125 mmol) were dissolved in 5 ml of dichloromethane (CH2Cl2). A purple to green–blue colour changes of the mixture was observed. Dark purple crystals of complex (3) were acquired by n-hexane slow diffusion in the CH2Cl2 solution. (in 89% yield). Anal. Calc. For [Zn(TTP)(pyz)]0.0.5(pyz.CH2Cl2): C53.25H41.5N6.5Cl0.5Zn (855.6 g/mol).C, 6.27; H, 4.80; N, 75.97%. Found: C, 6.31; H, 4.76; N, 75.81%. UV–Vis [CH2Cl2, λmax in nm (logξ)]: 425 (5.47), 560 (4.27), 604 (4.03). 1H NMR (CDCl3, 300 MHz): δ (ppm) = 8.89 (S, 8H, Hβ), 8.04 (d, 8H, Ho,o’); 7.53(d, 8H, Hm,m’) ; 6.35(d, 4H, H1) ; 2.3(s, 12H, CH3-Mésithy. IR (cm−1): 3020–2930: ν[CH(porph)]; 952: δ[CCH(porph)];700: δ [CC(phenyl porphy)], 3130: ν[CH(pyz)], 1500: ν[CN(pyz)].
2.2 Characterization
2.2.1 1H NMR, absorption and emission properties
The Bruker 300 Ultrashield spectrometer was used to perform the room temperature1H NMR spectra. UV–vis spectra and titration were obtained by using WinASPECT PLUS. Emission spectra were also detected on Horiba Scientific Fluoromax-4 spectrofluorometer. The 1 cm path length quartz cuvettes were used to analysed samples. Emission measurements were observed after irradiation at λ = 400 nm observed by the second harmonic of a titanium laser with a replication rate of 8 MHz. To detect the decay acquisition, Fluotime 200 (AMS technologies) is used with a GaAs microchannel plate photomultiplier tube (Hamamatsu model R3809U-50) followed by a Picoquant temporal correlation single photon counting system (PicoHarp300). The eventual time resolution of the setup is proximate to 30 ps. Picoquant-FLUOFIT software, was used to accomplish luminescence decays. The optically diluted method was used to detect quantum emission efficiencies in CH2Cl2 solutions at 25 °C (Yang et al., 1999). [Zn (TPP)] in a dichloromethane solution equilibrated in air was used as a quantum yield standard (Ф = 0.031). Here are the experimental uncertainties: 20% for the emission lifetimes; 2 nm and 5 nm for the absorption maxima and the emission maxima, respectively.
2.2.2 Electrochemistry
Cyclic voltammetry (CV) experiments were carried out with a CH-660B potentiostat (CH-instrument) at room temperature under an argon atmosphere (argon stream), using three-electrode electrochemical cell, in a standard one-compartment. Tetra-n-butylammonium perchlorate (TBAP) was utilized as a support electrolyte (0.2 M) in dichloromethane (CH2Cl2) after distillation with calcium hydride under argon. An automatic ohmic drop compensation procedure was systematically realized prior to recording the CV data in electrolytic solutions containing the studied compound at a concentration ̴10-3 M. CH-instrument vitreous carbon (Ф = 3 mm) working electrodes were polished with 1 µm diamond paste before each recording. As a reference electrode was used the redox couple Ag/Ag+ (10−2 M + TBAP 0.2 M in CH2Cl2).
2.2.3 X-ray characterisation
Data collection for compound (3) were performed at 298 K, the data are obtained by the Xcalibur, Sapphire 2, Eos, Gemini ultra. The monochromatized, λ = 0.71073 Å, MoKα graphite radiation was used. The data intensity were detected via a narrow-frame procedure. The CrysAlis RED (Oxford Diffraction, 2010) software was used to obtain the unit cell parameters, reflections are scaled and corrected for absorption (Oxford Diffraction, 2010). SIR-2004 via a direct method was used to elucidate the trial structure (Altomare et al., 1994). The final structural refinement was made against F2 data with the program SHELXL-97 (Sheldrick, 2008).
3 Results and discussion
3.1 Optical absorption investigation
The UV–visible spectra of the compounds (1–3) were collected in dichloromethane at a concentration of ca10-6M and 10-5 M, for the intense band (Soret band) and other bands (Q-bands), respectively (Fig. 1). The λmax values of these species were compared to other meso-porphyrins and Zn(II)-metalloporphyrins and are gathered in Table 1.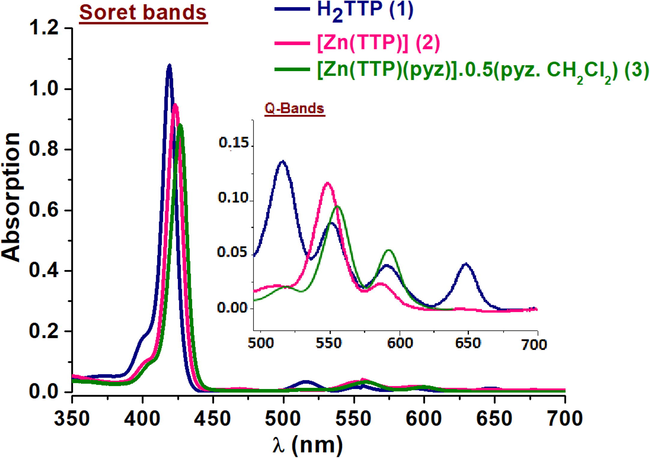
UV–Vis spectra in dichloromethane of (1–3) compounds.
Porphyrin/Metalloporphyrin
Soret band λ (nm) (logε)
Q bands λ (nm) (logε)
Eg (eV)
Log Kass
Ref.
H2TTPa (1)
417 (5.95)
515 (5.82) 553 (4.28) 598 (4.17) 646 (4.10)
1.88
–
C.W
[Zn(TTP)]a (2)
420 (5.75)
550 (4.31) 593 (3.86)
2.03
–
C.W
[Zn(TTP)] a
420 (-)
549, 588
–
–
[Kim and Shin, 2003]
Zn(TTP)(pyz)]0.0.5(pyz.CH2Cl2)a (3)
425 (5.47)
560 (4.27) 604 (4.03)
2.01
3.01 ± 0.2
C.W
[Zn(TTP)(4,4′-bpy)] a
428 (5.71)
562 (4.26) 602 (4.04)
2.02
5.14 ± 0.2
[Soury et al., 2018b]
[Zn(TTP)(mbpy ∼ py)]c
428 (-)
563 (-), 603 (-)
–
–
[Kim and Shin, 2003]
[Zn(TPP)(1-MeIm)]d
428 (-)
564 (-) 604 (-)
5.3 ± 0.2
[Paul et al., 2003]
[Zn(TPP)(2-MeIm)] d
428 (-)
564 (-) 604 (-)
5.4 ± 0.2
[Paul et al., 2003]
ain dichloromethane, bin toluene, c:mbpy ∼ py = N-methyl- 2,2′-bipyridinium ∼ pyridine, d in cyclohexane, 1-MeIm: 1-methyl-imidazole and 2-MeIm : 2-methyl-imidazole.
Fig. 1 shows the absorption spectral changes of complexes (1–3).We noticed; (i) the reduction of the number of Q bands of low intensity from four (between 515 and 645 nm) to two bands (between 550 and 600 nm) on going from the free porphyrin (1) to complexes (2–3). This reduction in number of bands can be attributed to the change in symmetry (D2h for free ligand vs D4h for Zn-complexes) (Gouterman et al., 1959; Sharma et al., 2019); (ii) bathochromic shift of the values of λmax of the soret and Q bands for (2–3) complexes are compared to the free base porphyrin. The shifts are 4 nm and 45 nm for the soret and Q-bands, respectively. This is supported by the earlier reported works (Soury et al., 2018a, 2019, 2018b).
3.2 Optical gap
The optical band gap is a fundamental property that characterize semicondutors physics. Fig. 2 depicts the optical band gap Eop-gap of the ligands and complexes and are determined using the Tauc plot method (Tauc et al., 1966; Polat et al., 2020). As it is clear from the Fig. 2, the straight line portion of (αhυ)2 vs photon energy (E = hυ) plot until null absorption.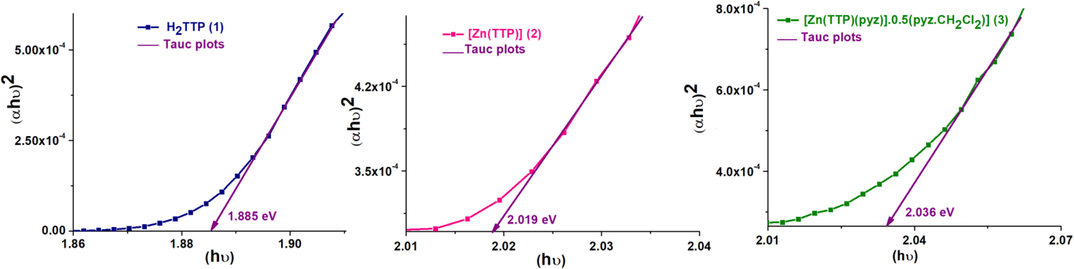
Plotsof (αhυ)2 vs photon energy E of (1–3) compounds. α is the absorption coefficient.
The Eop-gap values of (1–3) compounds are 1.885, 2.019 and 2.036 eV, respectively. Notably the Eop-gap of free base is at higher value than that of the Zn(II)-metaloporphyrins and are close to 2.00 eV. It is noteworthy that the optical gap does not change significantly for the studied complexes, but their low optical gap could be suitable for potential use in the development of new optoelectronic organic semiconducting materials and related compounds.
3.3 Titration
Zn(II)-porphyrin are known to bind N-donors (imidazole, pyridine etc) through axially and forming five-coordinated complexes (Lin et al.,2002), with association constant (Kass) in the range of 104 (Flamigni et al., 2006). In this work, we titrated complex (2) with a monodentate pyrazine ligand and change was monitored using UV/vis spectroscopy (porphyrin concentration values are: 10-6 M, 10-5 M for soret band and Q bands, respectively. Thepyrazine concentration values are: 10-4, 10-3, 10-2, 0.1 and 0.2 M). Fig. 3, shows a typical change observed for (3) upon addition of pyrazine. The addition of incremental amounts of pyrazine to the solution of the second compound (2) induces: (i) colour change from purple to green (ii) the soret and Q-bands are considerably red-shifted (Lin et al., 2002) by 5 nm and 10 nm, respectively. This red-shift was complemented by forming a new compound 1:1 (pyrazine: [Zn(TTP)]) binding stoichiometry (Paul et al., 2003) (iii) the appearance of a sharp isobestic points around 424 nm and 580 nm-590 nm for the soret band and Q-bands, respectively.![UV–visible data for comlex (2) upon adding pyrazine showing left the Soret band shift 10-6 M in CH2Cl2.The changes around 426 nm with a 1:1 fit (0–5000 equiv.), are presented in the inset. The left side of the Figure showed the Q bands shift 10-5 M in CH2Cl2. The changes around 560 nm with a 1:1 [Zn(TTP)]/pyrazine fit, are presented in the inset. The study was carried out at 298 K in dilute dichloromethane solution.](/content/185/2021/33/3/img/10.1016_j.jksus.2021.101364-fig4.png)
UV–visible data for comlex (2) upon adding pyrazine showing left the Soret band shift 10-6 M in CH2Cl2.The changes around 426 nm with a 1:1 fit (0–5000 equiv.), are presented in the inset. The left side of the Figure showed the Q bands shift 10-5 M in CH2Cl2. The changes around 560 nm with a 1:1 [Zn(TTP)]/pyrazine fit, are presented in the inset. The study was carried out at 298 K in dilute dichloromethane solution.
These results indicate equilibrium between pyrazine and Zn(II) in compound (2). Titration data are determined to calculate the value of the association constant Kass using the fitting procedure provided by the program GWBASIC. The value of binding constant in this study was found to be 1.5 × 103 M−1, which is quite lower for Zn(II)-porphyrin bearing 4,4′-byp, 1-methyl-imidazol and 2-methyl-imidazole as axial fragment. This is most likely due to the strong basicity of the pyrazine donor (Soury et al., 2018a, 2019, 2018b; Flamigni et al., 2006; Paul et al., 2003). Spectroscopic data for Zn-complex are summarized in Table 1, along with some porphyrinic species with N-donor axial ligands.
3.4 Photoluminescence studies
Porphyrins and related compounds are known to exhibit two types of emission: (a) high energy and a strong transition to the second excited (S2 → S0 transition state) between 380 and 440 nm called Soret or bands . Such types of bands are comparatively rare, and (b) low energy and a weak transition (Q bands), which are assigned to the S1 → S0 transitions. The Q(x,y) are designated as bands, where “x” is the vibrational quantum number in the electronically excited state (S1) and “y” in the electronic ground state (S0). Hence, the individual bands correspond to the (0,0) and (0,1) transitions with respect to vibrational states (Fig. 4). As shown in Fig. 4, major hypsochromic shift about ca 50 nm in the Q (0,0) band and about ca 70 nm in the Q (0,1) band are between the free base porphyrin H2TTP and the zinc porphyrins. The Q (0,0) emission bands of [Zn(TTP)] and the Q (0.1) emission bands of [Zn(TTP)pyz] 0.5(pyz.CH2Cl2) wavelengths are around 600 nm and 648 nm, respectively.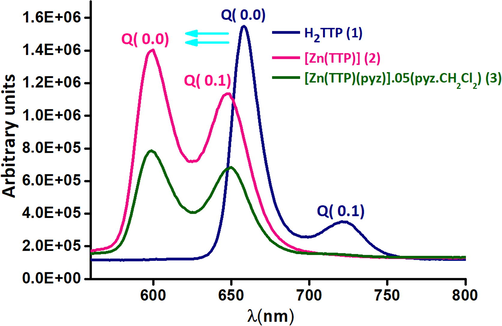
The emission spectra of (1–3) species. The spectra are obtained in CH2Cl2,having almost10-6 M concentration.
The decrease of the value of the fluorescence quantum yields (Φf): (Φf)(H2TTP) = 0.045 > (Φf) ([Zn(TTP)] = 0.039 > (Φf) ([Zn(TTP)(pyz)] 0.5(pyz.CH2Cl2) = 0.030 are caused by the insertion of the zinc metal on the free-base porphyrin and the adding of the pyrazine as a axial ligand. The values of fluorescent lifetime of (1–3) compounds are 7.9, 1.6 and 1.6, respectively. The (τf) of H2TTP is remarkably greater than those of the zinc-porphyrins complexes (Kim and Shin, 2003; Soury et al., 2018b; Oberda et al., 2013). The fluorescence τf depends on the insertion of the zinc metal, unaffected by the nature of the axial ligand.
3.5 X-ray crystallography of complex 3
The crystal was grown by slow diffusion of non-solvent (hexane) in solvent (dichloromethane). Key bond parameter data were listed in Table 2. The complex crystallizes in the monoclinic system (space group C 2/c). The parameters of the unit cell are a = 43.0761(17)Å, b = 9.44945(19) Å, c = 24.3292(11) Å, β = 121.806(2) °, V = 8416.6 (8) Å and Z = 8. The asymmetric unit is composed of one [Zn(TTP)(pyz)] complex, one half pyrazine molecule and one half dichloromethane molecule. Fig. 5a, represents the molecular structure of [Zn(TTP)(pyz)] complex, the zinc metal is coordinated to the four nitrogen atoms of the porphyrinato core ring and the nitrogen atom of the pyrazine axial ligand, forming a distorted square-based pyramid as coordination polyhedron for zinc(II).
Parameters
Complex (3)
CCDC
1,834,753
Empirical formula
C53.25H41.5N6.5Cl0.5Zn
Formula weight
855.6
Cryst. Sym
Monoclinic
Space group
C 2/c
a(Å)
43.0761(17)
b(Å)
9.44945(19)
c(Å)
24.3292(11)
β (°)
121.80(6)
V (Å3)
8416.6(8)
Z
8
ρcalc (g/cm3)
1.056
µ (mm−1)
0.662
Max./min. trans.
0.944/ 0.753
F(0 0 0)
3560
Crystal size (mm3)
0.55 × 0.46 × 0.11
T (K)
298
Θ range (°)
3.162–26.00
Limiting indices
−53 ≤ h ≤ 53,-11 ≤ k ≤ 11, −30 ≤ l ≤ 30
Reflec. collec/unique
32,187 / 8258
Parameters
575
S [Goodness of fit]
1.056
R1a, wR2 [Fo > 4σ(Fo)]
R1 = 0.047, wR2 = 0.139
R1, wR2b [all data]
R1 = 0.060, wR2 = 0.144
Min./max. res. (eÅ−3)
0.558 and −0.716
![Molecular structure of [Zn(TTP(pyz)].](/content/185/2021/33/3/img/10.1016_j.jksus.2021.101364-fig6.png)
Molecular structure of [Zn(TTP(pyz)].
a : b :
Zn(II) cation is coordinated to the four nitrogen atoms of the porphyrin ring with average equatorial distance value (Zn-Np) of 2.061 (2) Å and the nitrogen N5 atom of the pyrazine axial ligand with the Zn-N(pyridyl) bond length value of 2.203(2) (Å). The dihedral angle (Φ) between the “Np-M−NL” plan (Np is the closest pyrrole nitrogen atom and NL is the N atom of the pyrazine ligand axial) and the pyridyl plane of the axial ligand is 28.49 (2)° and the value of the dihedral angle between the pyrazine and the porphyrin core is 84.63(5)°. As shown in Fig. 5b, the formal diagram of the porphyrinato core of complex (3), where the metal is displaced by −0.28 Å toward the pyrazine axial ligand. This diagram shows that (3) presents a moderate ruffling (ruff) and doming deformations. The first deformation is indicated by the displacement of the meso carbons above and below the porphyrin mean plane (Jentzen et al., 1998). The doming (dom) deformation is often observed in five-coordinate complexes when the axial ligand causes a displacement of the metal center out of the mean plane, and the nitrogen atoms are also displaced toward the axial ligand (Scheidt and Lee, 1987).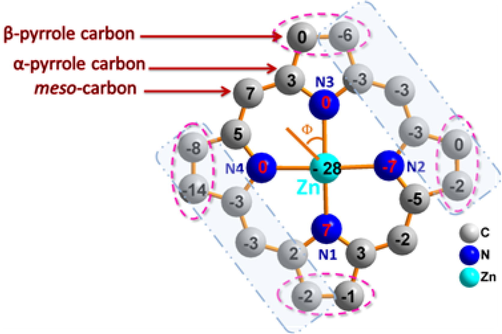
Formal diagram of the porphyrnato core of complex (3) illustrating the displacements of each atom from the 24-atoms core plane in units of 0.01 Å. Orientation of the pyz ligand vis-à-vis of the nearest N atom of the porphyrin ring (Φ dihedral angle) is also shown.
In the crystal structure of the title compound [Zn(TTP)(pyz)] molecules are connected with each other in such way as to make a pair of layers, parallel to [0 1 0] direction, which are parallel to other pairs with an interlayer distance of 8.170 Å, while layers are spaced apart with 8.160 Å (Fig. 5c), where each 2D chain is stabilized by weak C–H…Cg intermolecular interactions.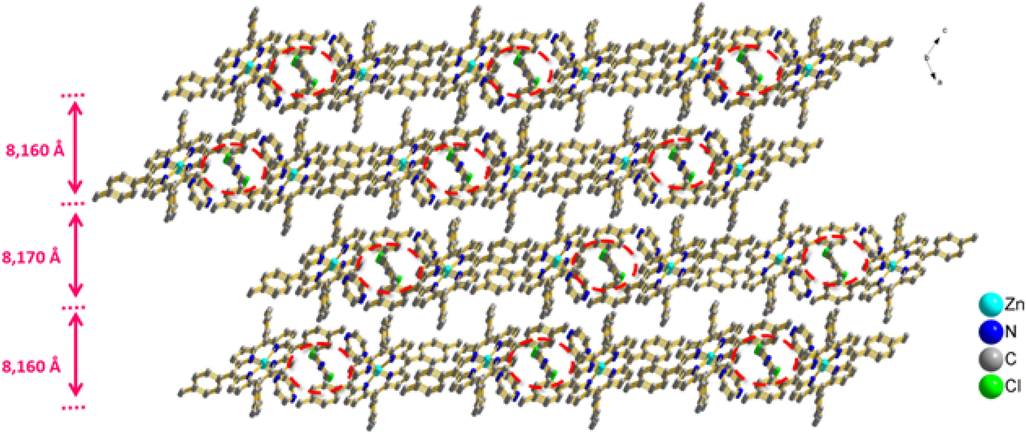
The packing of (3) viewed along the b axis showing the two-dimensional superstructure formed by pairs of layers containing the pyrazine free molecules.
Within a layer, the linkage of (3) is accomplished by two weak C-H…Cg intermolecular interactions between the carbon atom C47 and C29 of a phenyl ring of one TTP porphyrinate and the centroide Cg2 and Cg4 of a pyrrole ring of an adjacent [Zn(TTP)(pyz)] molecule and vice versa. The C47__H47…Cg2 and the C29__H29…Cg4 π intermolecular interactions are 3.469(4) Å and 3.444(4) Å, respectively (Fig. 5d).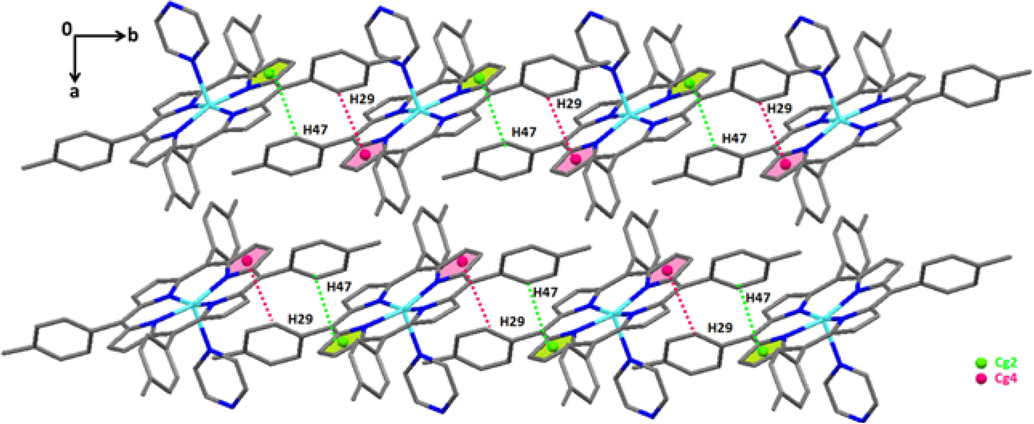
Drawing representing of the C47__H47…Cg2 and the C29__H29…Cg4 π intermolecular interactions in complex (3).
As can be seen from Fig. 5e, the presence of voids with radius of 1.2 Å and a grid of 0.7 Å are located between the 2D layers.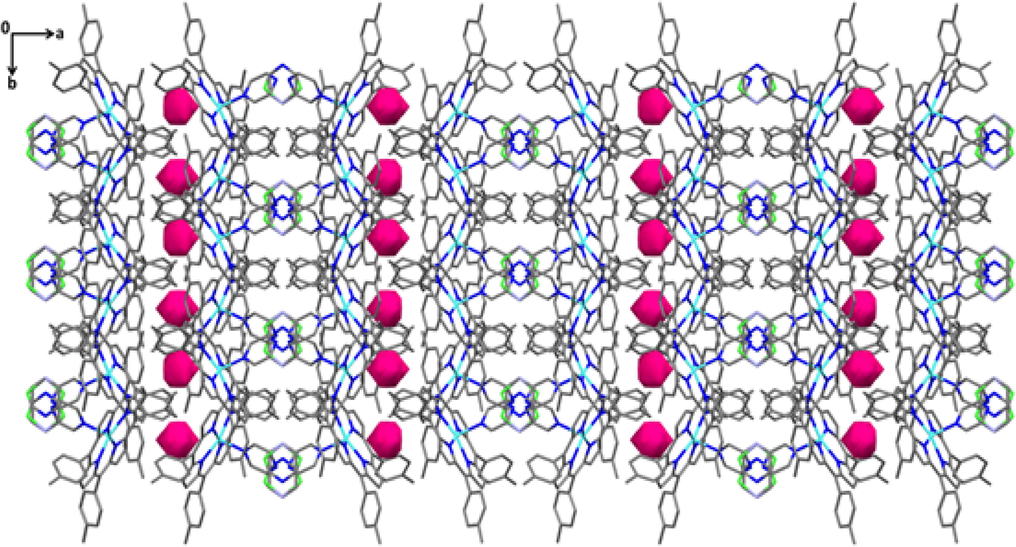
Packing diagram of (3) showing voids (represented in purple) calculated for a ball radius of 1.2 Å and a grid of 0.7 Å.
3.6 Cyclic voltammetry
The electro-oxidation of [Zn(TTP))] (2) and [Zn(TTP)(pyz)]0.0.5(pyz·CH2Cl2) (3) were studied using cyclic voltammetry in 0.2 M Tetra-n-butyl ammonium perchlorate (TBAP) solution in CH2Cl2. All the experiments were carried under argon at vitreous carbon electrode at room temperature. The onset potentials that are quoted versus Ag/AgCl are given in the Table 3. The cyclic voltammetry of [Zn(TTP)] exhibits two successive reversible one-electron oxidation processes (Table 3). The two steps are obtained at potentials E1/2 = 0.91 and.1.29 V versus Ag|AgCl that formally correspond to the first and second porphyrin ring oxidation. The cyclic voltammetry of complex (3) is shown in Fig. 6. The behavior is similar to that of [Zn(TTP)] and the both reversible one-electron waves appears at E1/2 = 0.84 and 1.23 V versus Ag|AgCl. Clearly, the addition of pyrazine coordinating group on the metal center cause a slightly shift (60 mV) to the more negative potentials comparing to [Zn(TPP)]. The electroreduction of both (2) and (3) complexes was examined under the same conditions. The results are shown in Fig. 6. The cyclic voltammetry of [Zn(TTP)] presents successive quasi-reversible reduction peaks at potential E1/2 = − 1.22 V and an irreversible wave at Ep = − 1.34 V, whereas [Zn(TTP)(pyz)]0.0.5(pyz·CH2Cl2) shows a shift to the more positive reduction peaks (60 mV) that is caused by the coordination of pyrazine group. It fact, the two waves appears at E1/2 = − 1.29 V and − 1.4 0 V vs. Ag|AgCl. It is worth mentioning that the second reduction wave of both (2) and (3) complexes exhibits a clear 2e reduction that could be a mix between Zn and porphyrin ligand reduction.
Complex
Oxidation
Reduction
1E1/2
2E1/2
1E1/2
2Ep
[Zn(TTP)] (2)
0.91
1.29
−1.22
−1.34
[Zn(TTP)(pyz)]0.0.5(pyz·CH2Cl2) (3)
0.84
1.23
−1.29
−1.40
![Electro-oxidation and reduction of [Zn(TTP)(pyz)] in 0.2 M (TBAP) at RT in CH2Cl2. Scan rate = 0.1 V/s.](/content/185/2021/33/3/img/10.1016_j.jksus.2021.101364-fig11.png)
Electro-oxidation and reduction of [Zn(TTP)(pyz)] in 0.2 M (TBAP) at RT in CH2Cl2. Scan rate = 0.1 V/s.
4 Conclusion
In this research article,the meso-porphyrin H2TTP (1), [Zn(TTP)] (2) and a novel pyrazine-zinc-porphyrin [Zn(TTP)(pyz)]0.0.5(pyz·CH2Cl2) (3) are synthesized and characterized. Photophysical studies indicated that four and five coordinated Zn(II) complexes possess markedly different absorption and emission properties. However, the fluorescence values of the free base porphyrin H2TTP is found to be higher than Zn(II) complexes. Titration experiment indicated equilibrium between pyrazine and Zn(II) in [Zn(TTP)] complex with moderate association constant (Kass) values. In solid-state for compound (3), Zn atom is five-coordinated with the pyrazine as an axial ligand. Moderate ruffling distortion and doming deformations are exhibited by porphyrin core. The supramolecular structure is formed with a two-dimensional chain, pairs parallel to the b axis in which each 2D chain is stabilized by weak intermolecular interactions C-H… Cg (The Cg is the centroid of pyrrole ring). In addition, this measurement is also suggestive of occurrence of electron transfer. The fact that the complexes (2) & (3) have high Eg values (≥2 eV), they could be used as component in semiconducting devices. Electrochemical properties of both (2) and (3) complexes were studied using cyclic voltammetry. The results showed the redox potentials of [Zn(TTP)(pyz)]0.0.5(pyz·CH2Cl2) ca 60 mV negative of the [Zn(TTP)] that is caused by the coordination of pyrazine axial ligand.
Acknowledgments
Dedicated to the memory of Professor Mohamed Salah Belkhiria (1951–2017) and Dr. Yassine Belghith (1982–2014), University of Monastir, Tunisia.
References
- Adler, A.D., Longo, F.R., Finarelli, J.D., Goldmacher, J., Assour, J., Korsakoff, L., 1967. A simplified synthesis for meso-tetraphenylporphine. J. Org. Chem. 32, 476–476.
- SIRPOW. 92–a program for automatic solution of crystal structures by direct methods optimized for powder data. J. Appl. Cryst.. 1994;27(3):435-436.
- [Google Scholar]
- Porphyrins as catalysts in scalable organic reactions. Molecules. 2016;21(3):310.
- [CrossRef] [Google Scholar]
- Temperature and frequency dependencies of AC and dielectric characterizations of copper tetraphenyl porphyrin thin films. Vacuum. 2014;99:153-159.
- [Google Scholar]
- A versatile bis-porphyrin tweezer host for the assembly of noncovalent photoactive architectures: a photophysical characterization of the tweezers and their association with porphyrins and other guests. Chem. Eur. J.. 2006;12(3):701-712.
- [Google Scholar]
- Study of the effects of substitution on the absorption spectra of porphin. J. Chem. Phys.. 1959;30(5):1139-1161.
- [Google Scholar]
- Next generation NIR fluorophores for tumor imaging and fluorescence-guided surgery: a review. Bioorg. Med. Chem.. 2017;25(7):2017-2034.
- [Google Scholar]
- Rise of conjugated poly-ynes and poly(metalla-ynes): from design through synthesis to structure-property relationships and applications. Chem. Rev.. 2018;118(18):8474-8597.
- [Google Scholar]
- Porphyrins and Metalloporphyrins, by KM Smith. Amsterdam: Elsevier; 1975. p. :317.
- Large π-aromatic molecules as potential sensitizers for highly efficient dye-sensitized solar cells. Acc. Chem. Res.. 2009;42(11):1809-1818.
- [Google Scholar]
- The aromatic pathways of porphins, chlorins and bacteriochlorins. PCCP. 2000;2(10):2145-2151.
- [Google Scholar]
- Conservation of the conformation of the porphyrin macrocycle in hemoproteins. J. Biophys.. 1998;74(2):753-763.
- [Google Scholar]
- Noncovalently linked zinc porphyrin-Ru (bpy)∼ 3 dyad assembled via axial coordination. Bull. Korean Chem. Soc.. 2003;24:1490-1494.
- [Google Scholar]
- Optically active porphyrin and phthalocyanine systems. Chem. Rev.. 2016;116(10):6184-6261.
- [Google Scholar]
- A spectroscopic and DFT study of thiophene-substituted metalloporphyrins as dye-sensitized solar cell dyes. PCCP. 2009;11(27):5598.
- [CrossRef] [Google Scholar]
- Substituent and axial ligand effects on the electrochemistry of zinc porphyrins. J. Electroanal. Chem.. 2002;531(2):155-162.
- [Google Scholar]
- Metal organic frameworks mimicking natural enzymes: a structural and functional analogy. Chem. Soc. Rev.. 2016;45(15):4127-4170.
- [Google Scholar]
- Oxford Diffraction, 2010. CrysAlis PRO, CrysAlis CCD and CrysAlis RED. Oxford. Diffraction Ltd, Yarnton, Oxfordshire, England.
- A novel complex of zinc tetraphenylporphyrin with two dioxane molecules in a rare attachment. Crystal structure, spectroscopy and theoretical calculations. Polyhedron. 2013;51:61-69.
- [Google Scholar]
- Atomic imaging of the irreversible sensing mechanism of NO2 adsorption on copper phthalocyanine. J. Am. Chem. Soc.. 2013;135(39):14600-14609.
- [Google Scholar]
- Induced fit process in the selective distal binding of imidazoles in zinc (II) porphyrin receptors. Inorg. Chem.. 2003;42(12):3779-3787.
- [Google Scholar]
- An investigation of the optical properties of YbFe1-xIrxO3- (x=0, 0.01 and 0.10) orthoferrite films. Vacuum. 2020;173:109124.
- [Google Scholar]
- Recent advances in the stereochemistry of metallotetrapyrroles. Struct. Bonding (Berlin). 1987;64:1-7.
- [Google Scholar]
- Tetrakis(ethyl-4(4-butyryl)oxyphenyl)porphyrinato zinc complexes with 4,4’-bpyridin: synthesis, characterization, and itscatalyticdegradation of Calmagite. RSC. Adv.. 2018;8(36):20143-20156.
- [Google Scholar]
- Meso-tetrakis (3, 4, 5- trimethoxyphenyl) porphyrin derivatives: Synthesis, spectroscopic characterizations and adsorption of NO2. Chem. Eng. J.. 2019;375:122005.
- [CrossRef] [Google Scholar]
- Synthesis of the (4, 4′-bipyridine)(5, 10, 15, 20- tetratolylphenylporphyrinato) zinc (II) bis (4, 4-bipyridine) disolvate dehydrate and evaluation of its interaction with organic dyes. J. Mol. Liq.. 2018;264:134-142.
- [Google Scholar]
- Photophysical properties of near-infrared phosphorescent π- extended platinum porphyrins. Chem. Mater.. 2011;23(24):5296-5304.
- [Google Scholar]
- Parameters dependent synthesis of zinc stannate nanowires using CVD and its porphyrin dye loaded optical studies. Vacuum. 2019;161:201-208.
- [Google Scholar]
- Optical properties and electronic structure of amorphous germanium. Phys. Stat. Sol.. 1966;15(2):627-637.
- [Google Scholar]
- J. Porphyrins. Phthalocyanines. 1999;3:117-147.







