Translate this page into:
Synthesis of Ag/TiO2 composites by combustion modified and subsequent use in the photocatalytic degradation of dyes
⁎Corresponding author. huemantzin.ortiz@inin.gob.mx (H.B. Ortiz-Oliveros)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this work, the synthesis of Ag/TiO2 photocatalysts by the modified combustion method for the degradation of dyes in water is presented. Three composite photocatalysts were prepared using silver concentrations of 0.05 mmol, 0.1 mmol, and 0.2 mmol. To evaluate the photocatalytic performance, methylene blue was tested as a preliminary reaction to simulate the degradation of dye compounds. Finally, a comparative analysis between the Ag/TiO2 photocatalyst with the best photocatalytic efficiency versus the TiO2-P25 Degussa without chemical treatment is presented. The results obtained show that all composites present a phase change from anatase to rutile. Photocatalysis experiments showed that Ag(0.05mmol)/TiO2 composite presented 98.9% degradation efficiency in concentrations below 15 mgL−1 of methylene blue and that the degradation efficiency was less than 60.6% in concentrations of 30 mgL−1. On the other hand, the Scanning Electron Microscopy micrographs show that the Ag(0.05mmol)/TiO2 compound does not present significant morphological changes after the degradation process. The fact that Ag(0.05mmol)/TiO2 has not presented significant morphological changes could suggest that it can be reused during the degradation process of organic compounds. The results show that the Ag(0.05mmol)/TiO2 composite obtained by combustion method has the potential to be used in the degradation of dyes mainly present in the textile industry.
Keywords
Combustion
Dye degradation
TiO2 composites
Titanium oxide
Silver composites
1 Introduction
In all sectors of society, from domestic activities to the various industrial activities of private and public services, waste is generated that is disposed into the aquifers (Sahunin et al., 2006). The manufacturing processes of the metallurgical, refining, chemical, and textile industries are mainly those that generate the greatest discharges of wastewater into bodies of water (Ramachandran et al., 2020). The textile industry is the one with the greatest source of pollution because it uses a large amount of water (21–377 m3/ton of textile) and energy in its processes, in addition to having pigments and colourants as the main pollutants in its wastewater discharges, reporting concentrations of approximately 1 ppm of these compounds, these compounds have been reported to be toxic, causing serious problems for public health and aquatic organisms (Ben Ayed et al., 2021).
Different economic and environmentally friendly methods have been proposed for the treatment of colored wastewater, for example ion exchange, membrane filtration, solar evaporation of water, electrochemical processes, advanced oxidation processes, among others. Among the most used treatment processes are advanced oxidation processes, where heterogeneous photocatalysis has shown to be a very promising technique (Azam et al., 2021). The photocatalysis process can produce oxidation–reduction reactions, which generate highly reactive free radicals, which react with the species that surround them, breaking molecular bonds and reducing or oxidizing the contaminants (Ayed et al., 2021).
Semiconductors (e.g., TiO2, ZnO, Fe2O3, CdS, and ZnS) can act as sensitizers for light-reduced redox processes due to its electronic structure, which is characterized by a filled valence band and an empty conduction band (Amadelli et al., 2008). Some authors have reported that the limitation of the rate of photocatalytic degradation is due to the recombination of photon-generated electron-hole pairs (Koe et al., 2020). In photocatalysis, the addition of semiconductors to noble metals can change the photocatalytic process. For example, platinum, gold, silver, or palladium enhancement the photoconversion of H2O to H2 and O2, increasing the H2 production (Ahmad and Saeed, 2019). Additionally, the transition metal doping species increase the trapping of electrons to inhibit electron-hole recombination during illumination (Koe et al., 2020).
Several methods to deposit noble metals on semiconductors have been studied, such as deposition–precipitation (Dozzi et al., 2009), photodeposition method (Gomathi Devi and Mohan Reddy, 2011), gas-phase deposition method (Wang et al., 2012), amongst others. Recently, the combustion synthesis method is promising for the preparation of multiple ceramic powder composites, since it allows the compounds to be obtained quickly and in less than 5 min (Cruz et al., 2018). Likewise, the desired products are obtained directly and without any subsequent purification processes, these characteristics have allowed it to be defined as a low-cost method (Cruz et al., 2015). Technically, the combustion synthesis is a solid-state preparation method that is based on the explosive decomposition of nitrate reagents and fuel mixtures, using the instantaneous heat generated by the chemical reaction between the desired metal nitrate used as the oxidizing agent, and a reducing agent, which is called fuel (e.g., glycine, urea, alanine, carbohydrazide) (Cheng et al., 2006).
Therefore, in this work presents the direct production of Ag/TiO2 composites by a modified combustion method, as a low-cost and direct synthesis alternative. In the synthesis by the combustion method, generally, nitrates are the reagents used plus glycine and/or urea as fuel. The importance of the modified method is that it directly uses oxides as reflectors to obtain another oxide (Cruz et al., 2018, 2015). Likewise, the photocatalytic performance of the different Ag/TiO2 composites obtained is studied. Methylene blue was used as a model pollutant, as a first approach for the use of the Ag/TiO2 composites in the degradation of dyes.
2 Material and methods
2.1 Materials
Ag/TiO2 composites were prepared using the following analytical grade reagents: titanium oxide (Degussa Germany), silver nitrate (Golden Bell, 98.2%), and urea (CH4N2O powder, 99.5%). Methylene blue (reagent grade, HYCEL) solutions of 10 mgL−1, 15 mgL−1, 20 mgL−1, and 30 mgL−1 were prepared with deionized water.
2.2 Methods
2.2.1 Preparation of Ag/TiO2 composites
The preparation of the composites was carried out by the modified combustion method previously studied (Cruz et al., 2018, 2015). To prepare the Ag(0.05mmol)/TiO2, Ag(0.1mmol)/TiO2 and Ag(0.2mmol)/TiO2 composites, 0.2 g of TiO2, 0.99 g of CH4N2O, and 0.0085 g of AgNO3, 0.2 g of TiO2, 0.99 g of CH4N2O, and 0.0164 g of AgNO3, 0.2 g of TiO2, 0.99 g of CH4N2O, and 0.034 g of AgNO3 were used respectively. In each case, the reagents were mixed in 5 mL of distilled water. The blending obtained was vigorously stirred and dried at 70 °C for 30 min and subsequently calcined in a muffle furnace (FELISA, model FE361) at 800 °C for 5 min.
2.2.2 Characterization of Ag/TiO2 composites
Representative samples of Ag/TiO2 powder composites were analyzed by X-ray diffraction. XRD patterns were recorded using a D8 Discover diffractometer and a Cu-Kα1 radiation. The diffraction spectra were obtained at 35 kV and 25 mA, from 20° to 80° (angle 2θ). A JEOL JSM-6610LV scanning electron microscopy (SEM) equipped coupled with an Energy Dispersive X-Ray Spectroscopy (EDS) probe was used to study the morphology and chemical composition of the samples. The thermogravimetric analyzes (TGA) were performed with a heating interval of 10 °Cmin−1 up to 1000 °Cmin−1, and N2 flow 50 mLmin−1 (STA449 F3 Jupiter, Thermogravimetric Analyzer) to determine the weight loss at high temperatures.
2.2.3 Photocatalysis experiments
All photocatalysis experiments were performed in batch, in a horizontal photoreactor (100 mL) coupled to a UV lamp (15 W, UV λmax = 254 nm, manufactured by Philips, The Netherlands). The following experiments were carried out in this work: 1) the variation of the concentration of methylene blue with respect to time in the three Ag/TiO2 composites; 2) determination of the photocatalytic efficiency of the different composites; 3) tests for degradation of methylene blue (at different concentrations) with respect to time, using the composite with the best photocatalytic efficiency; and 4) a comparative analysis of the photocatalytic efficiency of the Ag/TiO2 composite obtained by the modified combustion method with respect to the TiO2-P25 Degussa.
Methylene blue solutions of different concentrations were mixed with 0.1 g of Ag/TiO2 composites at different silver concentrations (0.05 mmol, 0.1 mmol, and 0.2 mmol). During the irradiation period, an oxygen flow was maintained in the photoreactor to keep the suspension homogeneous. Four 5 mL samples of the suspension were extracted every hour, and subsequently, the samples were centrifuged at 3500 rpm for 15 min. The residual concentration of methylene blue was determined by means of UV–Vis spectrophotometry in a Thermo scientific GENESYS 10S UV–Vis spectrophotometer. The emission spectra of methylene blue were recorded from 400 nm to 800 nm, where the maximum wavelength of methylene blue is between 663 nm and 668 nm.
The photocatalytic efficiency of the composites obtained was estimated with Equation (1) (Azam et al., 2018).
Finally, the estimation of the rate constants was performed assuming a first-order reaction (Whang et al., 2009), according to Equation (2):
3 Results and discussions
3.1 Characterization of Ag/TiO2 composites
The TiO2-P25 Degussa used as Ag/TiO2 precursors was characterized by XRD. The spectra obtained (not shown) indicate that the precursor was composed of 80% of the anatase phase and 20% of the rutile phase and impurities, as has been reported by various authors (Ammari et al., 2020).
Fig. 1 shows the XRD patterns of the Ag/TiO2 composites obtained by the modified combustion method. In this, seven intense peaks at 2θ, 27.46°, 36.06°, 39.18°, 41.21°, 54.31°, 56.6°, and 68.99° are observed. The spectrum was compared to the JCPDS (Joint Committee on Powder Diffraction Standards) cards, confirming that the reflections obtained correspond to the tetragonal structure of the rutile (JCPDS 01–089-4202). The thermal treatment above 800 °C that occurs during the combustion process, promotes the phase change from anatase to rutile. Hanaor and Sorrell (2011) have reported that the anatase to rutile phase transition usually occur between temperatures from 400 °C to 1200 °C, but this temperature range depends on the raw materials and processing methods used (Hanaor and Sorrell, 2011).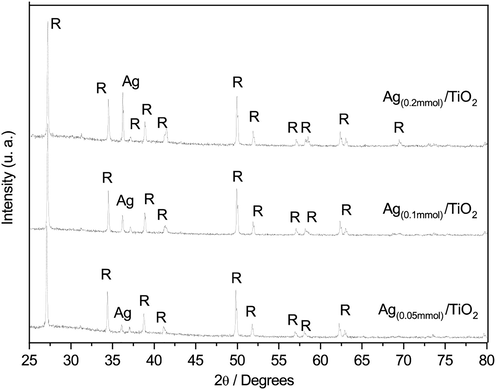
XRD patterns of a) Ag(0.05mmol)/TiO2, b) Ag(0.1mmol)/TiO2 and c) Ag(0.2mmol)/TiO2; R-Rutile.
Likewise, four intense reflections located at 2θ: 38.1°, 44.2°, 64.17°, and 77.3°, corresponding to the presence of Ag metallic (JCPDS 03–065-2871, cubic structure) are identified. Diffraction patterns suggest that the particles of Ag were not incorporated into the TiO2 structure, and a particle deposition of Ag occurs on the surface of TiO2. Additionally, the XRD spectra obtained, and the detection limit of the equipment used suggest that the silver content is at least 0.1% w/w of the analyzed sample.
The micrographs of the Ag/TiO2 material obtained at 15,000x are shown in Fig. 2, where the TiO2 with a granular appearance and an average size between 0.1 µm and 1 µm. Silver particles with sizes between 0.294 µm and 0.506 µm on the TiO2 surface were observed. The elemental composition obtained by EDS (not shown) indicates the presence of C, O, Ti, and Ag. The amount of carbon decreases with increasing concentration of Ag in the sample, which could be due to the excess of an Ag reaction with CO2 to form Ag2CO3, however, this last compound was not observed by X-ray diffraction (Li et al., 2002). Another possible explanation is that by increasing the amount of AgNO3 in the precursor mixture, it reacts more efficiently with urea. It has been reported that the concentration of fuel nitrate affects the formation of the products obtained and the ignition temperature reached during the process (Li et al., 2002). In the same, Fig. 2 shows the grain growth, suggesting a sintering process of the material due to the increase in temperature. Mazaheri et al. (2008), reported densification and grain growth similar to the conventional sintering process at high temperature (∼850 °C).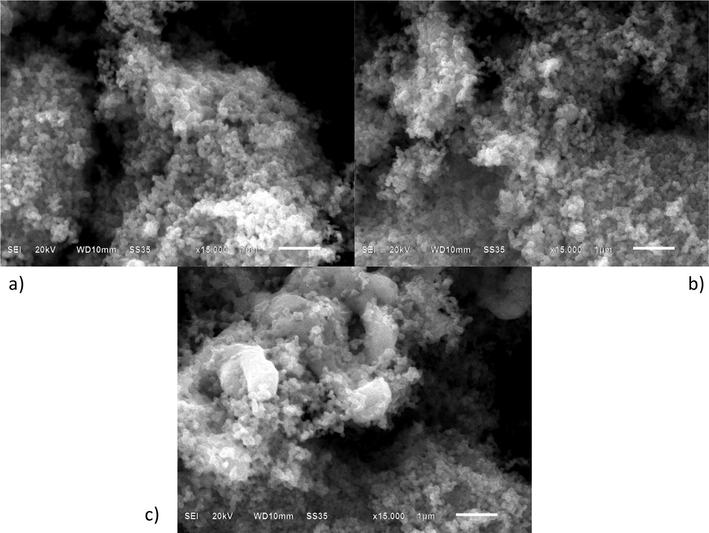
SEM analysis of a) Ag(0.05mmol)/TiO2, b) Ag(0.1mmol)/TiO2, and c) Ag(0.2mmol)/TiO2.
Fig. 3 presents the thermogravimetric analysis of Ag(0.05mmol)/TiO2, Ag(0.1mmol)/TiO2, and Ag(0.2mmol)/TiO2. Results show that in the temperature range from 25 °C to 1000 °C there is a weight loss smaller than 1% associated with dehumidification of the materials. This behaviour confirms that the composites prepared by the combustion method are thermostable in the tested temperature range.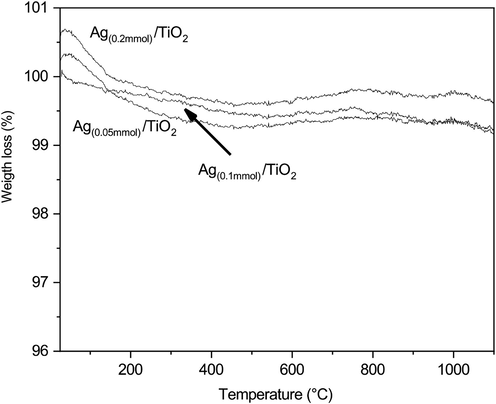
TGA curves of a) Ag(0.05mmol)/TiO2, b) Ag(0.1mmol)/TiO2, and c) Ag(0.2mmol)/TiO2.
3.2 Photocatalysis experiments
Fig. 4 shows the absorbance variation over time of the methylene blue solution (30 mgL−1) in contact with the composites: Ag(0.05mmol)/TiO2, Ag(0.1mmol)/TiO2 and Ag(0.2mmol)/TiO2.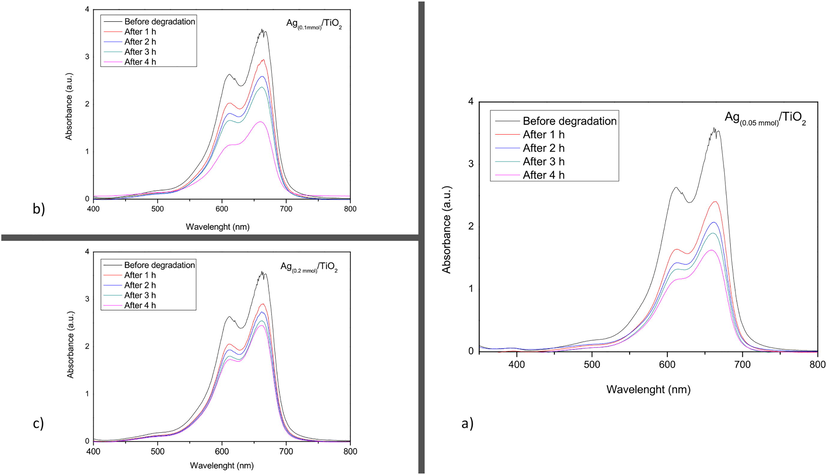
Variation of absorbance (over time) of methylene blue (30 mgL−1) in contact with 0.1 g of composites: a) Ag(0.05mmol)/TiO2, b) Ag(0.1mmol)/TiO2, and c) Ag(0.2mmol)/TiO2.
According to some recent studies, this photocatalytic process begins by exciting the ground state of silver through the transition of a photon to its singlet excited state. Silver triplet status can be generated by the cross-system process (see Equation 3). Both excited states can produce highly reactive radicals such as •O2− and •OH by UV irradiation and these are responsible to oxidize and decompose the intermediates formed in the solution, as noted in Equations 4 and 5 (Azam et al., 2016).
In the same degree, in Fig. 5, the concentration variation of methylene blue with respect to time is presented. The figure shows that in the three Ag/TiO2 composites tested, the concentration decreases over time. In the case of Ag(0.05mmol)/TiO2, 50% (15 mgL−1) of the methylene blue degradation was achieved at approximately 3 h after contact. The maximum levels of degradation (60.6%, equivalent to 11.8 mgL−1) were obtained at 4 h. On the other hand, Ag(0.1mmol)/TiO2 and Ag(0.2mmol)/TiO2 composites showed the levels of degradation are similar in the first 3 h. At 4 h, the Ag(0.1mmol)/TiO2 reaches a residual concentration of 11.9 mgL−1, very close to that of the Ag(0.05mmol)/TiO2 composite, without this being conclusive. Composite Ag(0.2mmol)/TiO2 obtained the lowest removal percentage, equivalent to a residual concentration of methylene blue of 19.4 mgL−1.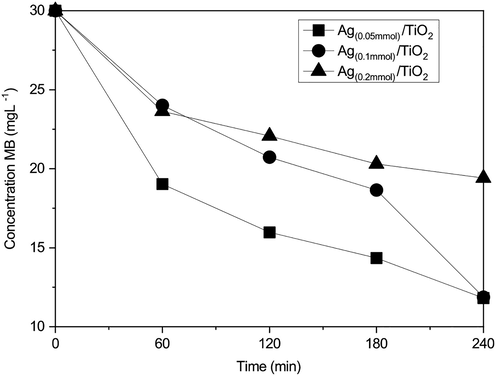
Change in concentration of methylene blue with time of the studied composites.
The described behaviour is evident when comparing the photocatalytic efficiency for each one of the tested composites. In Table 1, the photocatalytic efficiency of the different studied composites is presented, and it is estimated with Equation (1). As evidenced in this table, the material that showed the highest photocatalytic efficiency is the Ag(0.05mmol)/TiO2 composite. This composite presents a percentage of degradation (at 4 h) of 60.6%. It is possible, therefore, to set that increasing the silver concentration does not improve the photocatalytic properties of Ag/TiO2 material. The reduction of photocatalytic properties of the Ag/TiO2 composite by increasing Ag on the surface of TiO2 can be explained as follows, the presence of Ag can cause a retarding recombination reaction which occurs after excitation of the semiconductor with UV light increasing the rate of reductive photo-processes. However, it has been reported that increasing Ag on the surface of TiO2 has an effect controversial on the oxidation of organic compounds, because the photocatalyst surface itself may be strongly modified by silver deposition, causing an intrinsic decrease in photocatalytic activity, as was shown in the photodegradation results (Dozzi et al., 2009). Indicating that there is no linear relation of the photocatalyst ability with the content of Ag in TiO2.
Photocatalyst
Ag(0.05 mmol)/TiO2
Ag(0.1 mmol)/TiO2
Ag(0.2 mmol)/TiO2
Initial concentration (mgL−1)
30
30
30
Final concentration (mgL−1)
11.8
11.9
19.4
Photocatalytic efficiency (%)
60.6
60.4
35.3
Subsequently, the degradation tests were performed using the Ag(0.05mmol)/TiO2 material and varying the concentration of methylene blue (10 mgL−1, 15 mgL−1, and 20 mgL−1) as a function of time. Results show the best photocatalytic efficiencies of 99.5% and 98.9% are achieved in the lower concentrations of methylene blue solution (10 mgL−1 and 15 mgL−1), equivalent to a residual concentration of 0.05 mgL−1 and 0.17 mgL−1, respectively. At higher concentrations of methylene blue, the efficiency decreases from 90.2% to 60.6% for the 20 mgL−1 and 30 mgL−1 solutions, respectively.
Behnajady et al. (2007) have reported that the rutile phase has a small photocatalytic activity, however, in the present work, Ag/TiO2 (rutile) showed values of 90% or greater in the degradation of methylene blue for the concentrations of 10 mgL−1, 15 mgL−1 and 20 mgL−1. This can be explained through the model proposed by Bickley et al. (1991) and Hurum et al. (2003) where they showed that the band gap of rutile and anatase are Eg = 3.0 eV and Eg = 3.2 eV, respectively, and due to the above, the light absorbed by anatase is reduced compared to rutile (Bickley et al., 1991; Hurum et al., 2003).
On the other hand, rutile shows a direct or indirect band gap, whilst anatase has a lower indirect band gap than the rutile phase (Giocondi et al., 2007). This causes the lifetime of charge carriers to be longer in anatase than in rutile, however it should also be taken into account that only excitons that efficiently diffuse to the surface during their lifetime have an adequate oxidation–reduction behaviour (Xu et al., 2011). A measure of exciton mobility is reported to be the effective mass of the polaron. Dou and Persson, (2013) have reported effective electron (mc) and hole masses (mv) at the conduction band (CB) and valence band (VB) for rutile of 2.43 and 8.05 respectively (Thulin and Guerra, 2008). Whilst for anatase has been reported an mc = 0.75 and mv = 4.12. The above allows to explain the photocatalytic results obtained using Ag/TiO2 (rutile).
The experimental data analysis for the degradation of methylene blue with time was carried out assuming a first-order model according to the Equation (2) described above. Fig. 6 shows the fitting results of the experimental data using the first-order model. Rate constants and the correlation coefficients were obtained graphing ln(Co/C) vs t. The slope of the linear fit corresponds to the degradation rate constant. The rate constants and the correlation coefficients are presented in Fig. 6. As can be seen in Fig. 6, in all cases, the experimental data fitting has a statistical coefficient r2 greater than 0.9, showing that the model explains the experimental data adequately. Likewise, it can be observed that when the concentration of methylene blue decreases, the degradation rate increases. It has been reported that as the concentration of the pollutant increases, the order of the reaction tends to zero (Whang et al., 2009), as observed in the methylene blue solution with the highest concentration (30 mgL−1).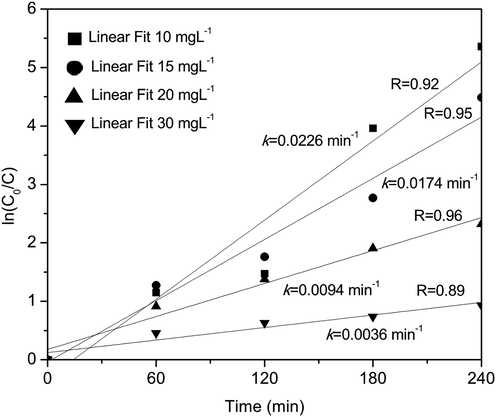
Fitting results of the experimental data using the first-order model.
On the other hand, the photocatalytic efficiency of the Ag(0.05mmol)/TiO2 composite versus the TiO2-P25 Degussa was determined. In this context, Fig. 7 shows the degradation of methylene blue (expressed in absorbance) in contact with TiO2-P25 Degussa. The results obtained indicate that the TiO2-P25 Degussa has an absorbance of 3.6 equivalent with a photocatalytic efficiency of 97.6% and higher than achieved by Ag(0.05mmol)/TiO2 of 60.6% (see Table 1 and Fig. 5a). Later, the photocatalyst materials used in the comparative analysis were recovered to study the morphological changes after the degradation process. The micrographs of the studied material are presented in Fig. 8. As shown in Fig. 8a and 8b, the Ag(0.05mmol)/TiO2 composite had not appreciable morphological changes after the degradation process. Since Ag(0.05mmol)/TiO2 shows no appreciable morphological changes, it supports the potential reuse of the material and, at the same time, shows the advantages of the modified combustion method in the preparation of photocatalysts. However, the results obtained by SEM of the TiO2-P25 Degussa after the degradation process (see Fig. 8d), show evident surface morphological changes such as agglomeration, apparent compaction, relatively dense and sintering of the material. These morphological changes suggest that the possible reuse of TiO2-P25 Degussa would be limited. It has been reported that after the second use, the photocatalytic activity of TiO2-P25Degussa decreases because more dye is adsorbed on the photocatalyst surface, covering the active sites with dye ions, and decreasing the formation of •OH radicals, which resulted in a decrease in the rate of degradation (Tichapondwa et al., 2020). Morphology control has been widely and extensively studied, because the photocatalytic properties of semiconductors are highly dependent on their morphology and particle size (Hoffman et al., 1994). Therefore, since Ag(0.05mmol)/TiO2 shows no appreciable morphological changes, it supports it’s the potential use as a candidate material in the degradation process of dyes (Wang et al., 2017).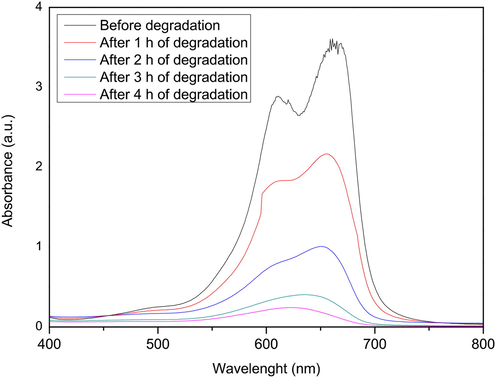
Absorbance spectra of methylene blue at 30 mgL−1 in contact with 0.1 g of TiO2-P25 Degussa.
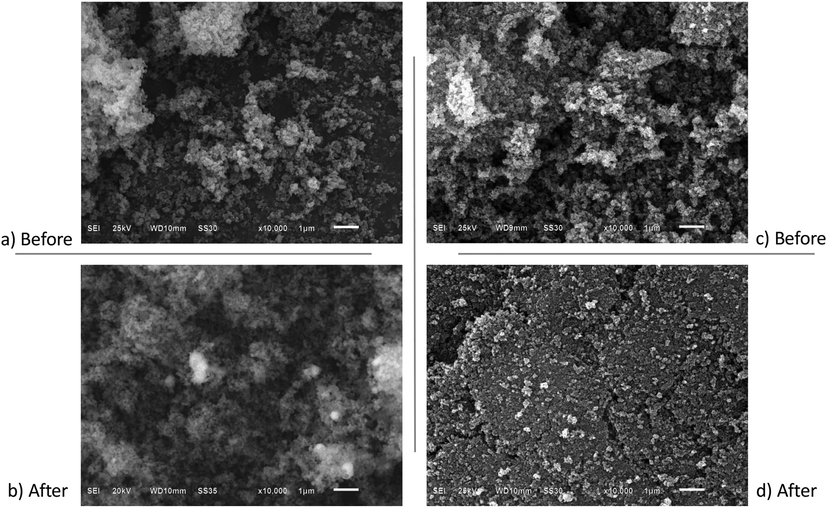
Micrographs obtained at 10000x, before and after of the degradation of methylene blue: a) and b) Ag(0.05mmol)/TiO2; c) and d) TiO2-P25 Degussa.
In general, the Ag(0.05mmol)/TiO2 synthesized reaches degradation efficiencies of 98.09%, much higher than those reported by other authors, under similar conditions of contact time, initial concentration of methylene blue and the amount of the photocatalyst (Quiñones et al., 2010; Saggioro et al., 2011; Shimizu et al., 2007; Whang et al., 2009).
Likewise, the evidence obtained shows that the TiO2-P25 Degussa material showed a photocatalytic efficiency of 97.6% higher than Ag(0.05mmol)/TiO2 material of 60.6% (30 mgL−1 of methylene blue, 4 h of radiation), however, the Ag(0.05mmol)/TiO2 material did not show appreciable morphological changes after degradation process. The foregoing supports the potential use of the combustion method in the preparation of photocatalysts. Furthermore, at concentrations below 30 mgL−1 of methylene blue, the Ag(0.05mmol)/TiO2 (rutile phase) composites have a high degradation efficiency comparable to that observed in TiO2-P25 Degussa (anatase phase).
As evidenced, the phase change from anatase to rutile and the morphological characteristics of Ag(0.05mmol)/TiO2 synthesized by the modified combustion method, favours the catalytic properties of the material. The difference in the results between other authors and the present investigation could be explained considering what deposits of Ag particles on the TiO2 surface favour a strong absorption of surface plasmon resonance (Gomathi Devi and Mohan Reddy, 2011). This absorption produces a collective oscillation due to the absorption of light by the deposited metal and an optical excitation facilitates the conduction of free electrons within the conduction bands of silver particles in oxidation/reduction processes.
In other words, it increases photoactivity in the visible light range, decreasing its prohibited bandwidth from 3.2 eV (380 nm) for the anatase phase to 3.0 eV (410 nm) for the rutile phase (Bickley et al., 1991; Hurum et al., 2003). Based on studies by other authors that use methylene blue as a model for the degradation of dye compounds present in wastewater from hospitals, pharmaceutical and textile industries, etc. (Cuerda-Correa et al., 2020), it is possible to assume that the Ag(0.05mmol)/TiO2 material could probably be used in the degradation of dyes generated in medical, pharmaceutical and textile applications.
Previous results indicated that Ag-doping has an incredibly significant effect on the inhibition of electron-hole recombination because the photoexcited electron could be trapped by Ag particles, which acted as an electron storage sink on the TiO2 surface. Ultraviolet light radiation promotes the formation of an electron-hole pair, in this case, the electron is transferred from TiO2 to silver while the holes remain in TiO2 (see Fig. 9). The results obtained suggest that electrons can interact with oxygen by ionosorption, stimulating oxygen depletion. On the other hand, the holes produce •OH radicals which together with the superoxides attack the C-S and C-N bonds which are very active in the MB molecule (Ahmed et al., 2020). Likewise, these can cause that the intermediate compounds formed (during degradation) to mineralize forming CO2, S and N (heteroatoms) that can form sulphate, nitrate, and ammonium ions, among others (Alshehri et al., 2017).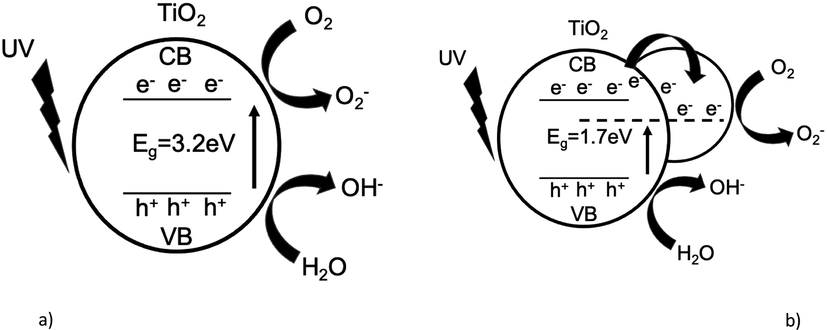
Schematic diagrams of the band energy structure and photocatalytic activity of (a) TiO2-P25 Degussa and (b) Ag(0.05mmol)/TiO2.
4 Conclusions
The structural characterization established that the modified combustion method allows to obtain the Ag(0.05mmol)/TiO2, Ag(0.1mmol)/TiO2, and Ag(0.2mmol)/TiO2 composites with the appropriate morphological characteristics. In addition, it showed that temperatures above 800 °C at which the combustion process occurs promote the phase change from anatase to rutile, the sintering of the material and that the prepared compounds are thermostable.
The Ag(0.05mmol)/TiO2 material showed the highest photocatalytic efficiency and the increase in the silver concentration did not improve the photocatalytic properties of the Ag/TiO2 composites. On the other hand, the experiments showed that the first-order model provides a good description and interpretation of the experimental data of the degradation of methylene blue by the Ag(0.05mmol)/TiO2 material with time.
As evidenced, the phase change from anatase to rutile and the morphological characteristics of Ag(0.05mmol)/TiO2 synthesized by the modified combustion method, favour the catalytic properties of the material, over TiO2-P25 Degussa.
Based on the evidence obtained, it is possible to conclude that the Ag/TiO2 composites obtained in this investigation could probably be used in the degradation of dye compounds that form part of wastewater generated in medical, pharmaceutical and textile applications. However, more photocatalytic efficiency studies should be performed using different types of dyes to verify the degradability of these materials.
5 Availability of data and material
The results of all experiments described are available at the request of the corresponding author.
6 Code availability
Not applicable.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
D. Cruz: Conceptualization, Investigation, Supervision, Data curation, Methodology, Formal analysis, Writing – original draft. H.B. Ortiz-Oliveros: Conceptualization, Data curation, Validation, Investigation, Writing – original draft, Writing – review & editing. R.M. Flores-Espinosa: Methodology, Writing – review & editing. P. Ávila Pérez: Writing – review & editing. I.I. Ruiz-López: Formal analysis. K.F. Quiroz-Estrada: Formal analysis.
Acknowledgements
The present work was financially supported by the Autonomous University of Puebla. Furthermore, the authors thanks to Dr Efrain Rubio and Dr Antonio Rivera for their support for XRD, SEM, TGA analyses and UV-vis, respectively.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis of Metal/Silica/Titania Composites for the Photocatalytic Removal of Methylene Blue Dye. J. Chem.. 2019;2019:9010289.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, and significant photochemical performances of delafossite AgFeO2 nanoparticles. J. Sol-Gel Sci. Technol.. 2020;94:493-503.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, and enhanced photocatalytic properties of NiWO4 nanobricks. New J. Chem.. 2017;41:8178-8186.
- [CrossRef] [Google Scholar]
- Preparation, characterisation, and photocatalytic behaviour of Co- TiO2 with visible light response. Int. J. Photoenergy. 2008;2008:853753
- [CrossRef] [Google Scholar]
- Elimination of a mixture of two dyes by photocatalytic degradation based on TiO2 P-25 Degussa. Mater. Today:. Proc.. 2020;22:126-129.
- [CrossRef] [Google Scholar]
- Local iron ore identification: Comparison to synthesized fe3 o4 nanoparticles obtained by ultrasonic assisted reverse co-precipitation method for auramine o dye adsorption. Desalin. Water Treat.. 2021;220:446-458.
- [CrossRef] [Google Scholar]
- Cd(II) complex constructed from dipyridyl imine ligand: Design, synthesis and exploration of its photocatalytic degradation properties. Inorganica Chim. Acta. 2018;471:698-704.
- [CrossRef] [Google Scholar]
- Structural elucidation and physicochemical properties of mononuclear Uranyl(VI) complexes incorporating dianionic units. Sci. Rep.. 2016;6:32898.
- [CrossRef] [Google Scholar]
- Removal of Chromium(III) and Cadmium(II) Heavy Metal Ions from Aqueous Solutions Using Treated Date Seeds: An Eco-Friendly Method. Mol. 2021;26(12):3718.
- [Google Scholar]
- Photocatalytic degradation of an azo dye in a tubular continuous-flow photoreactor with immobilized TiO2 on glass plates. Chem. Eng. J.. 2007;127:167-176.
- [CrossRef] [Google Scholar]
- Cationic Dye Degradation and Real Textile Wastewater Treatment by Heterogeneous Photo-Fenton, Using a Novel Natural Catalyst. Catalysis 2021
- [CrossRef] [Google Scholar]
- A structural investigation of titanium dioxide photocatalysts. J. Solid State Chem.. 1991;92:178-190.
- [CrossRef] [Google Scholar]
- Synthesis of bamboo-like carbon nanotubes by ethanol catalytic combustion technique. Trans. Nonferrous Met. Soc. China. 2006;16:s435-s437.
- [CrossRef] [Google Scholar]
- Modified combustion synthesis of γ-LiAlO<inf>2</inf> using metal oxides. Trans. Nonferrous Met. Soc. China (English Ed.. 2018;28
- [CrossRef] [Google Scholar]
- Effect of urea on synthesis of ceramics materials by the modified combustion method. Acta Phys. Pol. A. 2015;128:336-339.
- [CrossRef] [Google Scholar]
- Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2020
- [CrossRef] [Google Scholar]
- Comparative study of rutile and anatase SnO2 and TiO2: Band-edge structures, dielectric functions, and polaron effects. J. Appl. Phys.. 2013;113:83703.
- [CrossRef] [Google Scholar]
- Effects of gold nanoparticles deposition on the photocatalytic activity of titanium dioxide under visible light. PCCP. 2009;11:7171-7180.
- [CrossRef] [Google Scholar]
- The origin of photochemical anisotropy in SrTiO3. Top. Catal.. 2007;44:529-533.
- [CrossRef] [Google Scholar]
- Photocatalytic performance of silver TiO2: Role of electronic energy levels. Appl. Surf. Sci.. 2011;257:6821-6828.
- [CrossRef] [Google Scholar]
- Review of the anatase to rutile phase transformation. J. Mater. Sci.. 2011;46:855-874.
- [CrossRef] [Google Scholar]
- Photocatalytic Production of H2O2 and Organic Peroxides on Quantum-Sized Semiconductor Colloids. Environ. Sci. Technol.. 1994;28:776-785.
- [CrossRef] [Google Scholar]
- Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B. 2003;107:4545-4549.
- [CrossRef] [Google Scholar]
- An overview of photocatalytic degradation: photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res.. 2020;27:2522-2565.
- [CrossRef] [Google Scholar]
- Combustion synthesis of γ-lithium aluminate by using various fuels. J. Nucl. Mater.. 2002;300:82-88.
- [CrossRef] [Google Scholar]
- Two-Step Sintering of Nanocrystalline ZnO Compacts: Effect of Temperature on Densification and Grain Growth. J. Am. Ceram. Soc.. 2008;91:56-63.
- [CrossRef] [Google Scholar]
- Methylene blue photoelectrodegradation under UV irradiation on Au/Pd-modified TiO2 films. Appl. Surf. Sci.. 2010;257:367-371.
- [CrossRef] [Google Scholar]
- Efficient degradation of organic dye using Ni-MOF derived NiCo-LDH as peroxymonosulfate activator. Chemosphere 2020128509
- [CrossRef] [Google Scholar]
- Use of Titanium Dioxide Photocatalysis on the Remediation of Model Textile Wastewaters Containing Azo Dyes. Mol. 2011;16(12):10370-10386.
- [Google Scholar]
- Treatment of textile dyeing wastewater by photo oxidation using UV/H 2O2/Fe2+ reagents. ScienceAsia. 2006;32:181-186.
- [CrossRef] [Google Scholar]
- Sonocatalytic degradation of methylene blue with TiO2 pellets in water. Ultrason. Sonochem.. 2007;14:184-190.
- [CrossRef] [Google Scholar]
- Calculations of strain-modified anatase ${\text{TiO}}_{2}$ band structures. Phys. Rev. B. 2008;77:195112
- [CrossRef] [Google Scholar]
- Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys. Chem. Earth, Parts A/B/C. 2020;118–119:102900
- [CrossRef] [Google Scholar]
- Recent Progress on Visible Light Responsive Heterojunctions for Photocatalytic Applications. J. Mater. Sci. Technol.. 2017;33:1-22.
- [CrossRef] [Google Scholar]
- Size and Structure Matter: Enhanced CO2 Photoreduction Efficiency by Size-Resolved Ultrafine Pt Nanoparticles on TiO2 Single Crystals. J. Am. Chem. Soc.. 2012;134:11276-11281.
- [CrossRef] [Google Scholar]
- Laser-Induced Silver Nanoparticles on Titanium Oxide for Photocatalytic Degradation of Methylene Blue. Int. J. Mol. Sci.. 2009;10(11):4707-4718.
- [Google Scholar]
- Photocatalytic Activity of Bulk ${\mathrm{TiO}}_{2}$ Anatase and Rutile Single Crystals Using Infrared Absorption Spectroscopy. Phys. Rev. Lett.. 2011;106:138302
- [CrossRef] [Google Scholar]







